Molecular breath analysis supports altered amino acid metabolism in idiopathic pulmonary fibrosis
Data availability statement: The raw data of the real-time breath measurements for all subjects is available indefinitely to anyone who wishes to access the data archived in the Research Collection of the ETH Zurich (www.research-collection.ethz.ch) at https://doi.org/10.3929/ethz-b-000306409.
, , , , , , , , . Molecular breath analysis supports altered amino acid metabolism in idiopathic pulmonary fibrosis; ETH Zurich Research Collection; https://doi.org/20.500.11850/306409
ABSTRACT
Background and objective
Diagnosis of idiopathic pulmonary fibrosis (IPF) is complex and its pathogenesis is poorly understood. Recent findings indicate elevated levels of proline and other amino acids in lung tissue of IPF patients which may also be of diagnostic value. Following these findings, we hypothesized that such altered metabolic profiles would be mirrored in exhaled breath and could therefore be captured non-invasively in real time.
Methods
We aimed to validate these results using real-time exhaled breath analysis by secondary electrospray ionization-mass spectrometry, which can provide a non-invasive, painless and fast insight into the metabolism. Breath analysis was performed in a matched 1:1 case–control study involving 21 patients with IPF and 21 control subjects.
Results
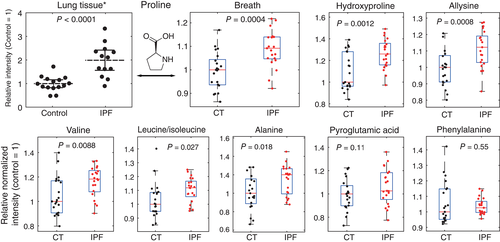
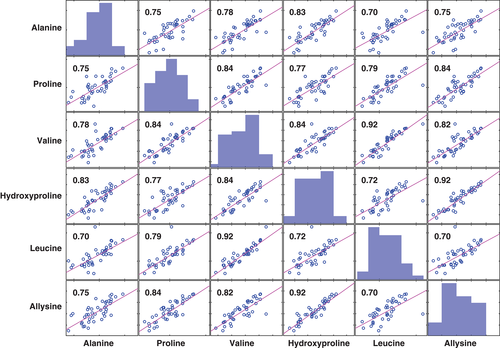
We found significantly (P < 0.05) elevated levels of proline, 4-hydroxyproline, alanine, valine, leucine/isoleucine and allysine in breath of IPF patients, whereas pyroglutamic acid and phenylalanine did not show significant differences. This coincides with the amino acid's abundance in pulmonary tissue indicating that our observations reflect progressing fibrosis. In addition, amino acid levels correlated across subjects, further supporting a common underlying pathway. We were able to obtain a cross-validated area under the curve of 0.86, suggesting that these increased amino acid levels in exhaled breath have the potential to be used as biomarkers for IPF.
Conclusion
We could validate previous findings of elevated lung tissue amino acid levels in IPF and show that online breath analysis might be a practical tool for a rapid screening for IPF.
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is a chronic progressive lung disease of unknown origin that is characterized by the irreversible loss of lung function leading to progressive dyspnoea.1 It is associated with a poor prognosis with a mean survival time of 2.5–5 years.2 In a large database of healthcare claims, its incidence was estimated to lie between 6.8 and 16.3 per 100 000 individuals.3 The incidence of the disease increases with advancing age, most patients are in the sixth or seventh decade at the time of diagnosis.4 The disease seems to affect more men than women, and many patients have a history of cigarette smoking.5
IPF is associated with a distinct radiological finding and the histopathological pattern of usual interstitial pneumonia (UIP). The disease is characterized by the presence of reticular opacities, traction bronchiectasis and honeycombing on high-resolution computed tomography scans (HRCT). The histopathological diagnostic criterion is a heterogeneous structure in which areas of fibrosis with scarring and honeycomb change alternate with areas of less affected or normal parenchyma. The so-called fibroblast foci are also consistently found.1
Diagnosis of IPF requires the exclusion of other forms of interstitial pneumonia, including other interstitial lung diseases associated with environmental exposure, medication or systemic disease. Diagnostic criteria and treatment recommendations have been published for patients with suspected IPF.1 In many cases, the diagnosis of IPF can be assumed on the basis of a fitting medical history and pathognomonic HRCT findings (predominantly subpleural fibrosis with honeycombing and traction bronchiectasis). In cases where HRCT shows only some features consistent with IPF, surgical lung biopsy is recommended by international guidelines.1, 6 In addition, a careful multidisciplinary discussion between pulmonologists, radiologists and pathologists is required in cases where a confirmative diagnosis is difficult to make. For this reason, a more detailed phenotyping of the disease would improve its characterization, understanding and ultimately its diagnosis.
Real-time exhaled breath analysis is a technique to obtain insight into human metabolism in a fast, non-invasive and painless fashion, by detecting trace concentrations of metabolites in breath.7-9 Secondary electrospray ionization-mass spectrometry (SESI-MS)10 is a highly sensitive technique11-15 to analyse a variety of sample vapours at ambient pressure and has been used in this context to identify biomarkers,16-19 monitor pharmacokinetics20, 21 and metabolic circadian variation.22 Furthermore, it has proven its strength in obtaining information about metabolic pathways in several studies during recent years.23-25
Results from a recently published case–control study, in which metabolic profiling of lung tissue samples of IPF patients was performed, suggest altered levels of proline and other amino acids in IPF patients.26 Following these findings, we hypothesized that such altered metabolic profiles would be mirrored in exhaled breath. Thus, in this targeted study, we examined the levels of exhaled proline and other amino acids of IPF patients and healthy controls. Hence, we determined whether exhaled breath analysis by SESI-MS could support non-invasive IPF diagnosis by validating previous observations obtained in biopsied tissue specimens.
METHODS
Subjects and study design
The study was a case–control study with 21 IPF patients and 21 controls. Inclusion criteria for subjects with IPF were: age older than 18 years at study entry and confirmed diagnosis of IPF based on the American Thoracic Society (ATS) criteria.1 The extent of fibrotic changes (honeycombing and reticular changes) had to be greater than the extent of emphysema on HRCT scans. Only IPF patients without evidence or suspicion of an alternative diagnosis that may contribute to their interstitial lung disease were included. Exclusion criteria for IPF patients were: any other lung disease, acute exacerbation or inflammation of the airways within the last 6 weeks, acute or chronic hepatic disease and renal failure or renal replacement therapy. Exclusion criteria for control subjects were: any clinical signs of lung disease (based on spirometry and history), acute inflammatory disease of the airways (e.g. common cold) within the last 6 weeks, acute or chronic hepatic disease and renal failure or renal replacement therapy. Breath profiles of patients with IPF were compared with control subjects matched for age, gender and smoking state. The subject characteristics and regular medication are listed in Table 1. The study was carried out in accordance with the Declaration of Helsinki of the World Medical Association and was registered at ClinicalTrials.gov (NCT02437448). The local ethics committee approved the study (KEK-ZH-Nr. 2014-0573 and KEK-ZH-Nr. 2015-0187) and all participants gave their written informed consent to participate.
| IPF (n = 21) | Controls (n = 21) | |
|---|---|---|
| Gender (males/females) | 17/4 | 17/4 |
| Age (years) | 66 ± 9 | 67 ± 8 |
| BMI (kg/m2) | 28 ± 3 | 26 ± 5 |
| Non-smoker/ex-smoker | 11/10 | 11/10 |
| Pack-years | 26 ± 7 | 30 ± 17 |
| FEV1 (L) | 2.1 ± 0.6 | 3.0 ± 0.6 |
| FEV1 % predicted | 75 ± 22 | 102 ± 14 |
| FVC (L) | 2.5 ± 0.8 | 3.9 ± 0.7 |
| FVC % predicted | 73 ± 22 | 105 ± 17 |
| FEV1/FVC | 80 ± 6 | 77 ± 4 |
| DLCO (mmol/kPa/ min) | 3.9 ± 1.8 | N.D. |
| DLCO % predicted | 50 ± 21 | N.D. |
| Antihypertensive medication (beta-blocker, Ca-antagonist, ACE-inhibitor/AT-2 blocking agent, diuretics) | 10 | 10 |
| Antacids | 15 | 3 |
| Prednisone | 2 | 0 |
| N-acetylcysteine | 7 | 0 |
- Values are presented as mean ± SD, or sample numbers.
- ACE, angiotensin-converting-enzyme; AT-2, angiotensin II receptor; BMI, body mass index; DLCO, diffusion capacity for carbon monoxide; FEV1, forced expiratory volume in 1 s; FVC, forced vital capacity; IPF, idiopathic pulmonary fibrosis.
Breath analysis
Breath analysis was performed in real time using SESI-MS. The setup featured a heated breath sampling line (stainless steel, i.d. 4 mm, T = 80°C), connected to a cylindrical SESI-chamber, which was coupled to a high-resolution TripleTOF 5600+ mass spectrometer (AB Sciex, Concord, Ontario, Canada). The subjects were sitting in an upright position and delivered four to six consecutive full exhalations through a disposable mouth piece with a pressure of 12 mbar (controlled via bio-feedback and a manometer visible to the subjects). All subjects refrained from eating, smoking, using chewing gum, tooth brushing and drinking anything except water within 1 h prior to the measurement. The sampling procedure was standardized across all subjects, to exclude confounding influences from varying exhalation manoeuvres.27 Positive ion mode was used, with a mass range of 40–450 Da.
Compound identification
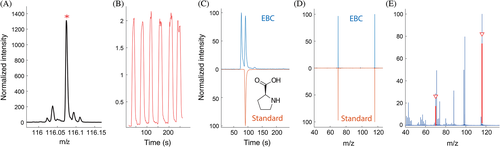
To confirm the identity of the detected compounds, ultra-high performance liquid chromatography–tandem MS (UHPLC–MS/MS) measurements of exhaled breath condensate (EBC) were compared to commercial standards as a complementary technique. For some compounds, additional real-time MS/MS experiments were conducted. EBC was solely used for identification of the compounds due to known confounding factors in EBC collection (e.g. dilution effects).28 The complete experimental details describing EBC collection and UHPLC–MS/MS methods can be found elsewhere.23, 29
Data analysis
After preprocessing of the raw data (see Appendix S1 (Supplementary Information) for details), P-values were calculated for all target compounds using a two-sample t-test. Relationship between metabolites as well as physiological parameters reflecting disease progression were investigated by calculating the pairwise Pearson correlation coefficients. To assess their predictive capabilities, we applied 1 million 10-fold cross-validations with correct and randomly shuffled labels. This enables the estimation of CI of the classification performance and prove that the algorithm is unbiased.30-32 To exclude any feature selection bias, all peaks were included as predictors.33
RESULTS
Initially, 23 patients with IPF were included. Two patients had to be excluded: one developed lung cancer during the study period and mass spectrometric data from another one were not usable. Five patients suffering from IPF were on pirfenidone treatment. Other medications are listed in Table 1. From 11 patients, a surgical lung biopsy was available.
Detected compounds
After data processing of the real-time breath measurements of all 42 subjects (Table 1), the mass spectrometric signals of amino acids were examined. Among the amino acids reported by Kang et al.,26 we could obtain good signals at the accurate mass of alanine, proline, valine, leucine/isoleucine, 4-hydroxyproline, pyroglutamic acid and phenylalanine. In addition, we included allysine, as it plays a key role in the cross-linking of collagen and elastin fibres.34 Therefore, we hypothesized that it may also be potentially altered. Most of the compounds could be confirmed in EBC [see Fig. 1, Appendix S3 and Figs S3-S9 (Supplementary Information)].
Altered metabolites
We observed significantly elevated levels of proline (P = 0.0004), 4-hydroxyproline (P = 0.0012), alanine (P = 0.018), valine (P = 0.0088), leucine/isoleucine (P = 0.027) and allysine (p = 0.0008) in exhaled breath of IPF patients, whereas pyroglutamic acid (P = 0.11) and phenylalanine (P = 0.55) did not show any significant differences (see Fig. 2, Table 2). Additionally, signal levels correlated strongly across all subjects (Fig. 3) and were mostly independent of lung function values [forced vital capacity and diffusion capacity for carbon monoxide see Figs. S10 and S11 (Supplementary Information)].
| Compound | Molecular formula | m/z | Δm (ppm) | P | Mean fold increase in IPF (95% CI) |
|---|---|---|---|---|---|
| Proline | [C5H9NO2 + H]+ | 116.0704 | −1.8 | 0.0004 | 1.09 (1.06–1.18) |
| Allysinea | [C6H11NO3 + H]+ | 146.0811 | −0.4 | 0.0008 | 1.12 (1.07–1.21) |
| 4-Hydroxyprolinea | [C5H9NO3 + H]+ | 132.0654 | −0.9 | 0.0012 | 1.15 (1.07–1.24) |
| Leucine/isoleucine | [C6H13NO2 + H]+ | 132.1017 | −1.9 | 0.0267 | 1.08 (1.01–1.14) |
| Valine | [C5H11NO2 + H]+ | 118.0860 | −1.8 | 0.0088 | 1.11 (1.04–1.20) |
| Alanine | [C3H7NO2 + H]+ | 90.0549 | −0.4 | 0.0176 | 1.13 (1.02–1.21) |
| Pyroglutamic acid | [C5H7NO3 + H]+ | 130.0499 | 0.2 | 0.1139 | 1.07 (0.98–1.13) |
| Phenylalanine | [C9H11NO2 + H]+ | 166.0863 | 0.2 | 0.5500 | 0.98 (0.91–1.04) |
- P-values were calculated using a two-sample t-test.
- † Compound not confirmed in exhaled breath condensate due to low intensity and/or lack of a standard.
- Δm (ppm), deviation from theoretical mass in parts per million; IPF, idiopathic pulmonary fibrosis; m/z, mass to charge ratio.
Classification results
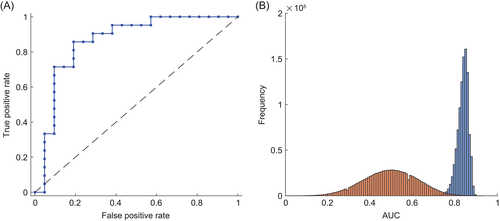
Using the signals of all target compounds, we assessed their diagnostic potential using 1 million 10-fold cross-validations, to estimate CI for the classification parameters. Taking the median score of each patient, we calculated an area under the receiver operating characteristic curve (ROC-AUC) of 0.86 (see Fig. 4A). The median ROC-AUC of all 10-fold cross-validations was 0.84 (0.78–0.88, 95% CI) with a median sensitivity of 0.71 (0.62–0.81), and specificity of 0.86 (0.76–0.90) (see Fig. 4B).
DISCUSSION
Several studies have investigated the pathogenesis of IPF at most -omics levels, including genomics, transcriptomics, epigenomics, proteomics and metabolomics, as recently reviewed by Kan et al.35 Despite all these investigations, no pharmacological cause could be identified for IPF and its pathophysiology is still poorly understood.1 A common characteristic of fibrotic diseases is the formation of fibrous connective tissue such as collagen and elastin. It is also known that turnover in the extracellular matrix is elevated in IPF and associated with disease progression.36, 37 Recent work has found increased levels of proline, a key constituent of collagen, in lung tissue of IPF patients compared to healthy controls, which was attributed to increased proline production via the ornithine aminotransferase (OAT) pathway.26 Also, a cross-validated ROC-AUC of 0.959 was reported26 using the metabolic pattern obtained from profiling with gas chromatography–mass spectrometry (GC-MS). While this revealed potential new physiological insights into IPF on a metabolic level, it was still based on invasive procedures (i.e. lung biopsy). The low prevalence of IPF and the high level of invasiveness of currently available diagnostic tools pose a great challenge for physicians. Therefore, a fast and non-invasive method that enables a better characterization of IPF and simplify the diagnostic process would be highly desirable. Given that some of the metabolites reported by Kang et al.26 are accessible in breath using sensitive instrumentation such as SESI-MS, we aimed to validate these results.
Using the comprehensive workflow described above, we could identify the presence of proline, alanine, lysine, isoleucine, phenylalanine, valine and pyroglutamic acid in EBC. In addition, the corresponding fragments were also detected in real-time tandem MS experiments for most compounds. This underscores the strength of our combined approach, as compound identification is rarely achieved in real-time exhaled breath analysis, due to insufficient resolution or the lack of a complementary technique using the same ionization method. The identification procedure for proline is illustrated in Figure 1 (see Appendix S1 (Supplementary Information) for other compounds).
A summary of the statistical analysis is displayed in Table 2. The comparison of breath peak intensities with literature levels in lung tissue clearly shows agreement between the findings from lung biopsies and the real-time breath measurements (see Fig. 2). While proline and 4-hydroxyproline make up around 20% of human fibrillar collagen,38 elastin has high abundances of proline, valine, alanine, leucine and isoleucine.39-41 All signals corresponding to these metabolites showed a significant increase in breath of IPF patients. Furthermore, signal levels of those amino acids that have a very low abundance in both collagen and elastin (pyroglutamic acid and phenylalanine) were the only ones that did not show a significant difference between the groups within our cohort. This indicates that altered turnover of collagen and elastin is indeed the underlying process of our observations. The strong correlation between all significantly altered compounds (see Fig. 3) further suggests a shared metabolic process. It is especially noteworthy that the highest correlation can be observed between allysine and 4-hydroxyproline (r = 0.92). In contrast to the other compounds in this work, these amino acids are not genetically coded, but formed by post-translational modifications, and have their major abundance in humans in structural proteins such as collagen or elastin. Therefore, they can be expected to have high specificity for proline and elastin turnover. A correlation analysis to other physiological parameters revealed that the amino acid breath levels in IPF patients are largely independent of lung function parameters commonly used to stage IPF (forced vital capacity % predicted and diffusion capacity of the lung for carbon monoxide % predicted, see Appendix S4 (Supplementary Information).42 This suggests that our observations reflect ongoing fibrotic processes, independent of the extent of previously formed fibrous tissue, implying the potential to obtain insights in early IPF pathophysiology upon disease onset, which may not yet be reflected in an impaired lung function. We did not see an effect of antifibrotic treatment (pirfenidone), but the sample size (n = 5) was not sufficient to make a conclusive statement in this regard.
The estimation of the potential of these compounds as biomarkers in exhaled breath to diagnose IPF is summarized in Figure 4. The ROC-AUC of 0.86 indicates a good usability43 of real-time breath analysis to support IPF diagnosis. A comparison of the distributions of the 1 million calculated AUC using correct and random labels (see Fig. 4B, Appendix S2 , Fig. S1-S2 and Tables S1-S2 (Supplementary Information) also shows that the classification is highly significant.
Our study has some limitations. Although breath amino acid levels look promising for a rapid diagnosis of IPF, our study design included only IPF patients and healthy controls. Therefore, the differentiation of other fibrotic pulmonary diseases, such as nonspecific interstitial pneumonia (NSIP), remains to be investigated. Nonetheless, the possibility of a pre-screening for fibrotic pulmonary diseases would still reduce the number of unnecessary CT scans, minimizing X-ray exposure of patients and costs, even if the elevated amino acids were not specific for IPF. Additionally, our sample size is limited and an independent validation in a larger cohort needs to be conducted before making definitive statements. However, as this study builds on previous results in a targeted fashion, we expect the risk of overfitting to be low. Lastly, case–control studies do not establish a causal relationship and therefore imply the risk of a bias. However, a randomized controlled trial, which would be needed to resolve this issue, is not realistic as no therapy exists to date that can cure or significantly improve IPF.
In conclusion, we could validate previous findings from lung biopsies that collagen-related amino acids are increased in patients with IPF. To the best of our knowledge, this is the first external validation of metabolomic results in IPF. In addition, we obtained these consistent results in a non-invasive, fast and painless fashion using real-time breath analysis. This also allowed for a good discrimination of IPF patients and healthy controls, demonstrating the potential of real-time breath analysis with SESI-MS to support IPF diagnosis.
Data availability statement
The raw data of the real-time breath measurements for all subjects is available indefinitely to anyone who wishes to access the data archived in the Research Collection of the ETH Zurich (www.research-collection.ethz.ch) at https://doi.org/10.3929/ethz-b-000306409.44
Acknowledgements
This work was supported by a grant from the Swiss National Science Foundation (CR23I2_149617) and a Marie-Curie European Reintegration Grant (to P.M.-L.S.) within the 7th European Community Framework Programme (276860). The sponsor had no role in the design of the study, the collection and analysis of the data or in the preparation of the manuscript. This work is part of the Zurich Exhalomics project under the umbrella of ‘University Medicine Zurich/Hochschulmedizin Zürich’.
Disclosure statement
M.K. reports personal fees from Astra Zeneca, Boehringer Ingelheim, Roche, Novartis, Mundipharma, Vifor, CSL Behring and Bayer, outside the submitted work.
Abbreviations
-
- AUC
-
- area under the curve
-
- DLCO
-
- diffusion capacity for carbon monoxide
-
- EBC
-
- exhaled breath condensate
-
- FEV1
-
- forced expiratory volume in 1 s
-
- FVC
-
- forced vital capacity
-
- IPF
-
- idiopathic pulmonary fibrosis
-
- m/z
-
- mass to charge ratio
-
- MS
-
- mass spectrometry
-
- ROC
-
- receiver operating characteristic
-
- SESI
-
- secondary electrospray ionization
-
- UHPLC–MS/MS
-
- ultra-high performance liquid chromatography–tandem MS