Pirfenidone and nintedanib for pulmonary fibrosis in clinical practice: Tolerability and adverse drug reactions
ABSTRACT
Background and objective
The real-world tolerability of pirfenidone and nintedanib in non-clinical trial patients is unknown. Many patients with pulmonary fibrosis have significant medical co-morbidities or baseline characteristics that exclude them from clinical trial participation.
Methods
We conducted a retrospective chart review study on subjects prescribed nintedanib or pirfenidone for pulmonary fibrosis treatment (any aetiology) from September 2014 to February 2016. A total of 186 subjects were included: 129 received pirfenidone and 57 were prescribed nintedanib and followed up for mean observation periods of 52 ± 17 weeks for pirfenidone and 41 ± 15 weeks for nintedanib. The primary outcome was drug discontinuation as a result of an adverse event.
Results
Subjects had significant respiratory impairment at baseline, 63% required home oxygen therapy and mean diffusion capacity of carbon monoxide (DLCO) was 36 ± 14% predicted. Drug discontinuation as a result of an adverse event occurred in 20.9% of subjects on pirfenidone and 26.3% on nintedanib. Drug discontinuation rates for both pirfenidone and nintedanib did not significantly differ from corresponding large clinical trials (ASCEND/CAPACITY and INPULSIS 1 and 2, respectively). Adverse events that occurred with highest frequency on pirfenidone were nausea (26.4%), rash/photosensitivity (14.7%) and dyspepsia/gastroesophageal reflux disease (GERD) (12.4%). Diarrhoea (52.6%) and nausea (29.8%) were reported most often with nintedanib therapy.
Conclusion
Patients with pulmonary fibrosis treated with nintedanib or pirfenidone in routine clinical practice had drug tolerability and adverse event profiles comparable with subjects enrolled in clinical trials despite having a greater degree of respiratory impairment and a high prevalence of co-morbid medical conditions.
Abbreviations
-
- 6MWT
-
- 6-min walk test
-
- ATS/ERS
-
- American Thoracic Society/European Respiratory Society
-
- BMI
-
- body mass index
-
- CPFE
-
- combined pulmonary fibrosis and emphysema
-
- DLCO
-
- diffusion capacity of carbon monoxide
-
- EMR
-
- electronic medical record
-
- FDA
-
- Food and Drug Administration
-
- FGF
-
- fibroblast growth factor
-
- FVC
-
- forced vital capacity
-
- GERD
-
- gastroesophageal reflux disease
-
- GI
-
- gastrointestinal
-
- HRCT
-
- high-resolution computed tomography
-
- ILD
-
- interstitial lung disease
-
- IPF
-
- idiopathic pulmonary fibrosis
-
- OR
-
- odds ratio
-
- PDGF
-
- platelet-derived growth factor
-
- PFT
-
- pulmonary function test
-
- TNF-α
-
- tumour necrosis factor alpha
-
- UIP
-
- usual interstitial pneumonia
-
- VEGF
-
- vascular endothelial growth factor
INTRODUCTION
Idiopathic pulmonary fibrosis (IPF) is the most common form of idiopathic interstitial pneumonia.1 IPF demonstrates a progressive and debilitating disease course with a median survival of 2.5–3.5 years from the time of diagnosis.2 The recent Food and Drug Administration (FDA) approval of nintedanib and pirfenidone has transformed the treatment of IPF, which was previously limited to supportive care and lung transplantation.3 Pirfenidone, a drug demonstrating both anti-inflammatory and antifibrotic properties, has been shown to reduce levels of TNF-α, platelet-derived growth factor (PDGF) and transforming growth factor-β amongst others.4 Nintedanib, an intracellular tyrosine kinase inhibitor, exhibits antifibrotic effects by acting on receptors for PDGF, vascular endothelial growth factor (VEGF) and fibroblast growth factor (FGF).5-7 Randomized clinical trials for nintedanib and pirfenidone both showed a reduction in the rate of decline of lung function with acceptable adverse effect profiles and drug tolerability.8-10
In the two largest clinical trials evaluating pirfenidone in IPF, the CAPACITY and ASCEND trials, the most common adverse drug reactions reported were nausea, diarrhoea, dyspepsia and rash.8, 10, 11 The RECAP trial, an ongoing extension study providing long-term follow-up for patients who participated in the CAPACITY trial, showed similar adverse event rates with nausea occurring most frequently.12 In the INPULSIS 1 and 2 trials, the largest studies to date assessing the efficacy of nintedanib for treatment of IPF, gastrointestinal-related adverse reactions (i.e. diarrhoea) were most frequently reported.9 The INPULSIS-ON study, an ongoing open-label extension study for nintedanib, has reported preliminary results suggesting a similar safety and drug tolerability profile as INPULSIS 1 and 2.13
In clinical practice, many patients who receive antifibrotic therapy have significant medical co-morbidities or baseline characteristics that would have excluded them from clinical trial participation. For example, subjects with severe abnormalities on pulmonary function test (PFT) (forced vital capacity (FVC) < 50% predicted and diffusion capacity of carbon monoxide (DLCO) < 30% predicted) were excluded from the previously mentioned clinical trials. We hypothesized that the drug tolerability and adverse events of pirfenidone and nintedanib therapy would differ from prior studies in a more heterogeneous patient population including subjects with advanced disease, a high prevalence of co-morbid medical conditions and the inclusion of subjects with non-IPF pulmonary fibrosis in addition to IPF.
METHODS
Patients
We conducted a retrospective chart review study on 186 consecutive subjects prescribed pirfenidone or nintedanib therapy for pulmonary fibrosis between September 2014 and February 2016. All subjects received pulmonary care at the Temple Lung Center in Philadelphia, PA, USA. Inclusion criteria were age at least 30 years old, therapy with either pirfenidone or nintedanib and a diagnosis of pulmonary fibrosis (any aetiology) as determined by each subject's primary pulmonologist during routine clinical care. Subjects with pulmonary fibrosis from IPF, in addition to those with pulmonary fibrosis from other aetiologies (i.e. non-specific interstitial pneumonia, chronic hypersensitivity pneumonitis, connective tissue disease-associated interstitial lung disease (ILD), etc.) were included. Patients were excluded if lung transplantation, transition to hospice or death occurred prior to starting therapy. A diagnosis of IPF was determined by each subject's primary pulmonologist in accordance with american thoracic society/european respiratory society (ATS/ERS) guidelines incorporating HRCT characteristics, surgery lung biopsy data (when available) and clinical exclusion of alternative causes of ILD.
This study was performed in accordance with the Declaration of Helsinki. This human study was approved by the Office for Human Subjects Protections Institutional Review Board of Temple University (approval: 23215). Adult participant consent was not required because the research involved no more than minimal risk to the participants (waiver granted).
Study design
Subjects who met criteria for study inclusion were analysed depending on the antifibrotic therapy they received. The electronic medical record (EMR) for our healthcare system was used to collect data pertaining to baseline demographic information, medical co-morbidities, pulmonary fibrosis diagnosis, PFT, HRCT, surgical lung biopsy results and prescribing information for antifibrotic therapy. The most recent PFT and 6-min walk tests (6MWTs) performed prior to starting antifibrotic drug therapy were used for baseline values. A single radiologist from our institution with expertise in chest radiology reviewed all HRCT images for likelihood of usual interstitial pneumonia (UIP). The ATS/ERS criteria for evaluating HRCT for UIP pattern were followed.3 All outpatient encounters in the EMR including follow-up visits and telephone encounters were reviewed to collect information pertaining to adverse reactions and drug tolerability. In addition, correspondences from the subject's outpatient specialty pharmacy that dispensed nintedanib or pirfenidone were reviewed to confirm accurate start dates and end dates (in the event therapy was discontinued).
The primary outcome for the study was drug discontinuation as a result of an adverse drug event. Secondary outcomes were time to drug discontinuation, adverse events, clinical outcome if an adverse event occurred (i.e. dose reduction, drug discontinuation, etc.), severe bleeding events, myocardial infarction and acute exacerbation of pulmonary fibrosis. Severe bleeding was defined as a bleeding event requiring emergency department evaluation, hospitalization or blood transfusion. Acute exacerbations of pulmonary fibrosis were identified by each subject's primary pulmonologist and were defined as a worsening of baseline respiratory status in the absence of alternative aetiologies (i.e. infection, pulmonary embolism, aspiration, etc.)
Statistical analysis
Results are reported as a mean ± SD or as a number with percentage as appropriate. Statistical analysis was performed using Student's t-test and Fisher's exact test. Results from our cohort were compared with pooled results from the largest corresponding clinical trials (ASCEND and CAPACITY trials for pirfenidone, and INPULSIS 1 and 2 trials for nintedanib). A multivariate logistic regression analysis was performed to evaluate for clinical characteristics associated with antifibrotic drug discontinuation.
RESULTS
A total of 186 subjects from the 249 reviewed met inclusion criteria for the study. Subjects were excluded for the following reasons: 49 subjects opted out of antifibrotic therapy after the prior authorization paper work was initiated, 5 subjects were unable to obtain insurance approval for drug, 7 subjects died or transitioned to home hospice prior to drug initiation and 2 subjects underwent lung transplantation prior to starting drug. Of the 186 subjects included, 129 were prescribed pirfenidone and 57 received nintedanib. The mean observation period for subjects on pirfenidone was 52 ± 17 weeks, and for nintedanib was 41 ± 15 weeks.
Baseline characteristics for subjects in our cohort are listed in Table 1. Corresponding values from pertinent clinical trials are reported when available. One hundred fifty-two of the 186 subjects (81.7%) were prescribed antifibrotic therapy for IPF. Remaining subjects had fibrotic ILD without typical features of UIP on HRCT or surgical lung biopsy (in these subjects, pulmonary fibrosis was believed to be the driver of disease by the subject's primary pulmonologist). Overall, the study population was comprised of individuals with advanced pulmonary fibrosis as evidenced by the large proportion requiring home oxygen therapy (117 of 186 subjects or 63%) and a mean DLCO % predicted of 36 ± 14. Subjects in our study had a lower mean DLCO and FVC % predicted than subjects from corresponding clinical trials (Table 2). Medical co-morbidities with the highest prevalence in our cohort were coronary artery disease (50 of 186 subjects or 27%) and gastroesophageal reflux disease (GERD) (95 of 186 subjects or 51%). Evaluation of HRCT revealed either a definite or a possible UIP pattern in the majority of subjects (Table 2).
| Baseline characteristics | Pirfenidone group (n = 129) | Pirfenidone (CAPACITY and ASCEND trials) (n = 623) | Nintedanib group (n = 57) | Nintedanib (INPULSIS 1 and 2 trials) (n = 638) |
|---|---|---|---|---|
| Age (years) | 70 ± 9 | 67.2 | 71 ± 8 | 66.6 |
| Male, n (%) | 82 (63.6) | 74.3 | 34 (59.6) | 79.5 |
| Race, n (%) | ||||
| Caucasian | 101 (78.3) | 89.7 | 45 (78.9) | 56.4 |
| Black | 6 (4.7) | n/a | 5 (8.8) | 0.3 |
| Hispanic | 12 (9.3) | n/a | 3 (5.3) | 0 |
| Asian | 0 (0) | n/a | 2 (3.5) | 30.4 |
| Other | 10 (7.8) | n/a | 2 (3.5) | 12.9 |
| BMI (kg/m2) | 30.2 ± 5.8 | n/a | 29.2 ± 5.4 | 28.1 |
| IPF diagnosis, n (%)† | 106 (82.2) | 100 | 46 (80.7) | 100 |
| Surgical lung biopsy, n (%) | 26 (20.2) | 42.7 | 10 (17.5) | 22.6 |
| Time since IPF/ILD diagnosis (years) | 2.5 ± 1.9 | 1.1 | 2.0 ± 1.6 | 1.7 ± 1.4 |
| Smoking status, n (%) | ||||
| Former smoker | 93 (72.1) | 65.2 | 39 (68.4) | 68.2 |
| Never smoker | 35 (27.1) | n/a | 16 (28.1) | n/a |
| Active smoker | 1 (0.8) | n/a | 2 (3.5) | n/a |
| Home oxygen therapy | 80 (62) | 24.9 | 37 (64.9) | n/a |
| Co-morbid medical conditions, n (%) | ||||
| Pulmonary hypertension | 20 (15.5) | n/a | 11 (19.3) | n/a |
| Congestive heart failure | 10 (7.7) | n/a | 4 (7) | n/a |
| Coronary artery disease | 36 (27.9) | n/a | 14 (24.6) | n/a |
| CPFE | 24 (18.6) | n/a | 12 (21.1) | n/a |
| Diabetes mellitus | 31 (24) | n/a | 15 (26.3) | n/a |
| GERD | 64 (49.6) | n/a | 31 (54.4) | n/a |
| Concurrent medication use, n (%) | ||||
| Prednisone | 38 (29.5) | n/a | 10 (17.5) | 21.3 |
| Acid reflux medication‡ | 89 (68.9) | n/a | 37 (64.9) | n/a |
| Anticoagulant (i.e. warfarin) | 30 (23.3) | n/a | 7 (12.3) | n/a |
| Mycophenolate mofetil | 5 (3.9) | n/a | 5 (8.8) | n/a |
| Sildenafil/tadalafil | 9 (6.9) | n/a | 6 (10.5) | n/a |
- Data are presented as mean ± SD unless otherwise specified.
- † The remaining subjects without IPF had non-IPF pulmonary fibrosis with aetiologies that included non-specific interstitial pneumonia, connective tissue disease-associated ILD and chronic hypersensitivity pneumonitis. More information pertaining to these subjects can be found in Table S1 (Supplementary Information).
- ‡ Proton pump inhibitor and/or H2 blocker.
- CPFE, combined pulmonary fibrosis and emphysema; GERD, gastroesophageal reflux disease; ILD, interstitial lung disease; IPF, idiopathic pulmonary fibrosis; n/a, not applicable; SD, standard deviation.
| Baseline characteristics | Pirfenidone group (n = 129) | Pirfenidone (CAPACITY and ASCEND trials) (n = 623) | Nintedanib group (n = 57) | Nintedanib (INPULSIS 1 and 2 trials) (n = 638) |
|---|---|---|---|---|
| HRCT pattern, n (%) | ||||
| Definite UIP pattern | 56 (43.4) | n/a† | 18 (31.6) | n/a‡ |
| Possible UIP pattern | 42 (32.6) | n/a | 15 (26.3) | n/a |
| Inconsistent with UIP pattern | 27 (20.9) | n/a | 21 (36.8) | n/a |
| No HRCT available for review | 4 (3.1) | n/a | 3 (5.3) | n/a |
| PFT | ||||
| FVC (% predicted) | 68 ± 18 | 71.6 | 66 ± 17 | 79.8 |
| DLCO (% predicted) | 37 ± 14 | 45.6 | 35 ± 13 | 47.4 |
| 6-Min walk distance (m) | 268 ± 103 | 404 | 290 ± 77 | n/a |
- Data are presented as mean ± SD unless otherwise specified.
- † Subjects in the INPULSIS trials were required to have either a definite or a possible UIP pattern on HRCT if a surgical lung biopsy was not available.
- ‡ Subjects in the ASCEND and CAPACITY trials were required to have either a definite UIP pattern on HRCT or a possible UIP pattern on HRCT accompanied by a surgical lung biopsy for confirmation.
- DLCO, diffusion capacity of carbon monoxide; FVC, forced vital capacity; n/a, not applicable; PFT, pulmonary function test; SD, standard deviation; UIP, usual interstitial pneumonia.
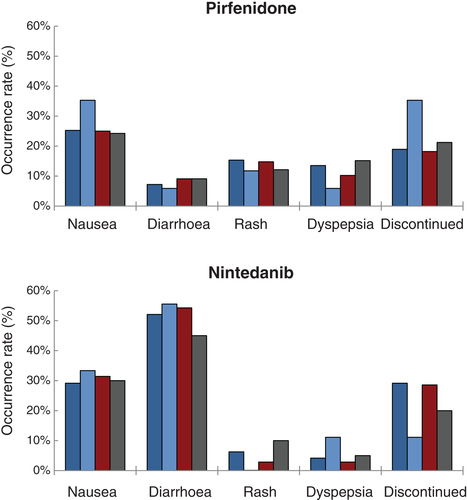
Adverse events occurring with the highest frequency for subjects on pirfenidone were nausea (26.4%), rash/photosensitivity (14.7%) and dyspepsia/GERD (12.4%). Anorexia, vomiting, diarrhoea and rash/photosensitivity occurred less frequently compared with pooled results from the CAPACITY and ASCEND trials (Table 3). On nintedanib therapy, diarrhoea (52.6%) and nausea (29.8%) were reported most often. Adverse reactions on nintedanib occurred with similar frequencies when compared with pooled data from the INPULSIS 1 and 2 trials.
| Outcome | Pirfenidone group (n = 129) | Pirfenidone (CAPACITY and ASCEND trials) (n = 623) | Nintedanib group (n = 57) | Nintedanib (INPULSIS 1 and 2 trials) (n = 638) |
|---|---|---|---|---|
| Observation period (weeks) | 52 ± 17 | 72 (CAPACITY), 52 (ASCEND) | 41 ± 15 | 52 |
| Adverse reaction | ||||
| Nausea, n (%) | 34 (26.4) | 36.1 | 17 (29.8) | 24.5 |
| Vomiting, n (%) | 4 (3.1)† | 13.3 | 3 (3.5) | 11.6 |
| Diarrhoea, n (%) | 9 (6.9)† | 25.8 | 30 (52.6) | 62.4 |
| Elevated transaminases, n (%) | 3 (2.3) | 3.5 | 3 (5.3) | 5 |
| Dyspepsia/GERD, n (%) | 16 (12.4) | 18.5 | 3 (5.3) | n/a |
| Anorexia, n (%) | 6 (4.7)† | 13.0 | 3 (5.3) | 10.7 |
| Rash/photosensitivity, n (%) | 19 (14.7)† | 30.3 | 3 (5.3) | n/a |
| Severe bleeding, n (%)‡ | 0 (0) | 1 (1.8) | ||
| Myocardial infarction, n (%) | 1 (0.8) | 0 (0) | ||
| Drug discontinuation, n (%) | 27 (20.9) | 14.6 | 15 (26.3) | 19.3 |
| Time to drug discontinuation (days) | 148 ± 96 | n/a | 89 ± 56 | n/a |
| Acute exacerbation on therapy, n (%) | 21 (16.3) | n/a | 6 (10.5) | 5.2 |
| Time to first acute exacerbation (days) | 247 ± 131 | n/a | 294 ± 179 | n/a |
- Data are presented as mean ± SD unless otherwise specified.
- † Frequency of adverse drug reaction reached statistical significance (defined as P value < 0.05) when compared with subjects from corresponding clinical trials (pooled results).
- ‡ Severe bleeding defined as a bleeding event requiring emergency department evaluation, hospitalization or blood transfusion.
- GERD, gastroesophageal reflux disease; n/a, not applicable; SD, standard deviation.
The outcomes for subjects experiencing any adverse reaction are shown in Figure 1. 66.7% of subjects tolerated pirfenidone without dose reduction nor drug discontinuation. In comparison, subjects treated with nintedanib tolerated therapy in 52.6% of cases. A dose reduction was necessary in 12.4% of subjects prescribed pirfenidone, and in 21.1% of subjects on nintedanib. None of these differences reached statistical significance.
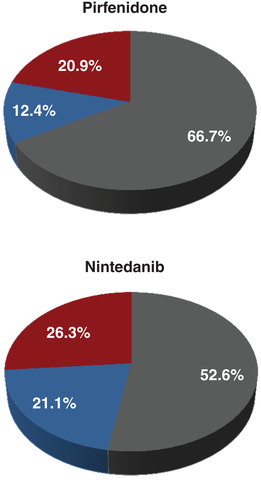
 , Tolerated pirfenidone;
, Tolerated pirfenidone;  , dose reduction needed;
, dose reduction needed;  , discontinued pirfenidone.
, discontinued pirfenidone.Drug discontinuation rates as a result of an adverse event were similar for pirfenidone and nintedanib (20.9% vs 26.3%, respectively). The rates of drug discontinuation did not significantly differ when compared with corresponding clinical trials (Table 3). Pirfenidone discontinuation resulted most often from a skin-associated adverse reaction (10 of 27 subjects) or a gastrointestinal-associated adverse reaction (10 of 27 subjects). In contrast, nintedanib was discontinued as a result of a gastrointestinal-related adverse event in the majority of cases (Fig. 2).

 , Malaise/anorexia;
, Malaise/anorexia;  , transaminitis;
, transaminitis;  , skin-associated adverse reaction;
, skin-associated adverse reaction;  , gastrointestinal-associated adverse reaction.
, gastrointestinal-associated adverse reaction.One subject on nintedanib had a severe bleeding event from a duodenal ulcer requiring hospitalization, multiple blood transfusions and an upper endoscopy with placement of two endoclips. Of note, this patient was 76 years old and on dual antiplatelet therapy with aspirin and clopidogrel at the time of the upper gastrointestinal bleed.
No patients on nintedanib had an acute myocardial infarction during the study. One subject on pirfenidone, a 71-year-old male with a known history of coronary artery disease, had a non-ST segment elevation myocardial infarction requiring placement of multiple stents. His hospitalization was also complicated by new-onset atrial fibrillation.
In our study, 16.3% of subjects on pirfenidone and 10.5% of subjects on nintedanib had at least one acute exacerbation of pulmonary fibrosis (Table 3). This trend towards fewer exacerbations in the nintedanib group as compared with pirfenidone did not reach statistical significance (P = 0.37). Patients receiving nintedanib had a trend towards prolonged time to first exacerbation compared with pirfenidone (mean duration: 294 days vs 247 days), but this also was non-significant (P = 0.72).
We performed a multivariate logistic regression analysis to evaluate for clinical characteristics associated with drug discontinuation. A history of congestive heart failure (OR: 4.54, 95% CI: 1.41–14.55, P = 0.01) and an age ≥70 years old (OR: 2.21, 95% CI: 1.04–4.69, P = 0.04) were the only characteristics associated with antifibrotic drug discontinuation. Of note, clinical characteristics associated with disease severity (FVC % predicted, DLCO % predicted and home oxygen use) had no association with drug discontinuation. Additionally, we compared subjects with non-IPF pulmonary fibrosis to subjects with IPF using propensity score matching to evaluate the association of ILD diagnosis on drug tolerability. After statistical matching, we found that subjects with non-IPF pulmonary fibrosis had similar drug discontinuation and adverse reaction rates to those with IPF (Table S2).
Subjects were compared for differences in drug tolerability with varying lung function. Using cut-off values of 50% predicted for FVC and 30% predicted for DLCO (inclusion criteria cut-off values in clinical trials), we found no statistically significant differences in the rates of common adverse reactions or drug discontinuation for nintedanib or pirfenidone (Fig. 3).
 , FVC ≥50 (n = 111);
, FVC ≥50 (n = 111);  , FVC <50 (n = 17);
, FVC <50 (n = 17);  , DLCO
≥30 (n = 88);
, DLCO
≥30 (n = 88);  , DLCO
<30 (n = 33); nintedanib:
, DLCO
<30 (n = 33); nintedanib:  , FVC ≥50 (n = 48);
, FVC ≥50 (n = 48);  , FVC <50 (n = 9);
, FVC <50 (n = 9);  , DLCO
≥30 (n = 35);
, DLCO
≥30 (n = 35);  , DLCO
<30 (n = 20).
, DLCO
<30 (n = 20).Fifteen subjects in the study crossed over and were prescribed the alternative antifibrotic drug after demonstrating intolerability to the first therapy (Table S3). Eleven of these subjects switched to nintedanib, while the other four transitioned to pirfenidone. Nine of the subjects (60%) tolerated the alternative therapy, three (20%) developed an adverse reaction necessitating a dose reduction and three (20%) discontinued the alternative drug as well.
DISCUSSION
Our study evaluated the drug tolerability and adverse effect profiles of nintedanib and pirfenidone for pulmonary fibrosis at a large ILD referral centre. Despite our subjects having greater baseline respiratory impairment and significant co-morbid medical conditions, adverse events and drug discontinuation occurred with a frequency similar to clinical trial data. In fact, in our study, subjects receiving pirfenidone reported anorexia, vomiting, diarrhoea and rash less frequently than subjects from the ASCEND and CAPACITY trials.
Subjects in our study who received nintedanib had poorer baseline lung function (lower mean DLCO and FVC % predicted) compared with subjects in the INPULSIS 1 and 2 trials.9 In addition, the majority of our subjects were on home supplemental oxygen suggesting advanced pulmonary fibrosis. Despite these traits, subjects from our cohort had comparable drug tolerability and adverse event rates to those from the INPULSIS 1 and 2 trials. Our findings suggest that nintedanib may be prescribed to patients with advanced pulmonary fibrosis without greater risk of drug intolerability.
Pirfenidone, which only gained FDA approval in 2014, has been approved for treatment of IPF in Japan since 2008 and in Europe since 2011. In subsequent years, several publications have reported on the tolerability of pirfenidone in both Europe and Japan. A study by Ogura et al. reported on the Japanese post-marketing surveillance for 1371 IPF patients treated with pirfenidone. The authors concluded that pirfenidone had a comparable safety profile to previous clinical trials in Japan with an adverse event drug discontinuation rate similar across all stages of IPF severity (22.9–26.7%).14 Conversely, a recent retrospective observational study utilizing surveys evaluated the clinical experience of pirfenidone in 502 patients from Japan showed a higher 1-year drug discontinuation rate of 37.1%.15 Multiple, small, single-centre studies in Europe have reported drug discontinuation rates of pirfenidone ranging from 13% to 19%.16-24 The discontinuation rate of pirfenidone in our study was 20.9%, which is within the range of previously published results.
We report in our study at least one acute exacerbation of pulmonary fibrosis in 16.3% of patients on pirfenidone and 10.5% of patients on nintedanib. The 1-year incidence of IPF exacerbations has varied widely in the literature (3.6–14.7%).9, 25, 26 The incidence of IPF exacerbations in our studies was higher than that reported in most previous studies. This could be a consequence of our population being compromised of subjects with more advanced disease comparatively and our inclusion of subjects with non-IPF pulmonary fibrosis.
One limitation of our study is that information regarding the primary and secondary outcomes was derived from review of the EMR and thus depended on both subjects to actively report adverse reactions to their healthcare providers and for providers to document events in the medical record. As a result, the true incidence of adverse reactions may have been underestimated in our cohort. In contrast, our drug discontinuation rates should not be susceptible to this issue as these events were confirmed by correspondences from the outpatient specialty pharmacies that dispensed nintedanib and pirfenidone. Second limitation was the single-arm design of the study that lacked a control or placebo group. Lastly, subject's diagnosis of IPF was determined by their primary pulmonologist during routine clinical care. This could have led to a confirmation bias with respect to a clinical diagnosis of IPF in some patients, as insurance companies in the United States currently require this diagnosis for coverage of pirfenidone and nintedanib.
In conclusion, our study evaluating the use of nintedanib and pirfenidone at a large ILD referral centre determined that both drugs had an adverse event profiles and drug tolerability rates comparable with previously published clinical trials. This held true despite our cohort having a greater degree of respiratory impairment, a high prevalence of co-morbid medical conditions and the inclusion of subjects with non-IPF pulmonary fibrosis in addition to IPF. While it is promising that each of these agents appear safe to use in a broader cohort of patients than previously included in large randomized controlled trials, future prospective studies are needed to elucidate the efficacy of treatment with expanded prescribing practices.




