Invasive diagnostic techniques in idiopathic interstitial pneumonias
Abstract
Fibrosing interstitial lung diseases (f-ILDs) represent a heterogeneous group of disorders in which the aetiology may be identified or, not infrequently, remain unknown. Establishing a correct diagnosis of a distinct f-ILD requires a multidisciplinary approach, integrating clinical profile, physiological and laboratory data, radiological appearance and, when appropriate, histological findings. Surgical lung biopsy is still considered the most important diagnostic tool as it is able to provide lung samples large enough for identification of complex patterns such as usual interstitial pneumonitis (UIP) and nonspecific interstitial pneumonitis. However, this procedure is accompanied by significant morbidity and mortality. Bronchoalveolar lavage is still a popular diagnostic tool allowing identification of alternative diagnoses in patients with suspected idiopathic pulmonary fibrosis (IPF) when an increase in lymphocytes is detected. Conventional transbronchial lung biopsy has a very low sensitivity in detecting the UIP pattern and its role in this clinical-radiological context is marginal. The introduction of less invasive methods such as transbronchial cryobiopsy show great promise to clinical practice as they can be used to obtain samples large enough to morphologically support a diagnosis of IPF or other idiopathic interstitial pneumonias, along with fewer complications. Recent advances in the field suggest that less invasive methods of lung sampling, without significant side effects, in combination with other diagnostic methods could replace the need for surgical lung biopsy in the future. Indeed, these new multidisciplinary procedures may become the main diagnostic work-up method for patients with suspected idiopathic interstitial pneumonia.
Abbreviations
-
- BAL
-
- bronchoalveolar lavage
-
- CT
-
- computed tomography
-
- DIP
-
- desquamative interstitial pneumonia
-
- f-ILDs
-
- fibrosing interstitial lung diseases
-
- FNA
-
- fine needle aspiration
-
- HP
-
- hypersensitivity pneumonitis
-
- HRCT
-
- high-resolution computed tomography
-
- IPF
-
- idiopathic pulmonary fibrosis
-
- NSIP
-
- nonspecific interstitial pneumonitis
-
- SLB
-
- surgical lung biopsy
-
- TBB
-
- transbronchial lung biopsy
-
- UIP
-
- usual interstitial pneumonitis
Introduction
The diagnostic approach to fibrosing interstitial lung diseases (f-ILDs) is a step-by-step process, and invasive procedures are considered only when clinical scenario, laboratory tests and high-resolution computed tomography (HRCT) scan features are inconclusive.1-3 Pulmonologists can utilize a large array of invasive procedures including bronchoalveolar lavage (BAL), endobronchial biopsy, transbronchial lung biopsy with conventional forceps (TBB) or cryoprobes (c-TBB), blind or ultrasound-guided transbronchial biopsies/aspiration (TBNA, EBUS), oesophageal echoendoscopic biopsies/aspiration (EUS),4-6 trans-thoracic biopsies (computed tomography (CT) or ultrasound guided) or medical thoracoscopy.1 Surgical lung biopsy (SLB) provides substantial lung tissue but complications related to this procedure are considerable.
The Multidisciplinary Approach
Interstitial pneumonias represent a group of disorders characterized by heterogeneous clinical profiles, laboratory and CT scan features.3 The majority of these disorders are chronic and patients present with subtle symptoms at diagnosis, mainly limited to the lungs. A minority may present with rapidly progressive symptoms/signs not limited to the lungs, leading rapidly to respiratory failure. The disease can derive from a systemic disorder that involves the lung or may be linked to an identifiable triggering cause. When clinical features or serologic tests are suggestive of but not definitive for connective tissue disease, the designation of interstitial pneumonia with autoimmune features has been suggested.7 Only when specific systemic background or causes cannot be identified should the term idiopathic or cryptogenetic be used. Currently, it is thought that a combination of clinical profile, laboratory tests and HRCT scan features may be diagnostic in more than 50% of patients in the context of an idiopathic entity8 (Table 1) (Fig. 1). HRCT can have a pivotal role: bibasilar subpleural honeycombing is pathognomonic of usual interstitial pneumonitis (UIP) pattern8 and subpleural sparing is characteristic of nonspecific interstitial pneumonitis (NSIP).8, 9 However, accurate identification of honeycombing is sometimes difficult due to the presence of other conditions that can mimic honeycombing (e.g. emphysema, traction bronchiectasis).10 When honeycombing changes are not detectable, subpleural bibasilar reticulation is considered suggestive of UIP pattern, but further information (mainly provided by a SLB) is deemed necessary3, 8-10 (Fig. 2). The role of less invasive procedures (mainly c-TBB) in documenting complex morphological patterns is debated due to a lack of experience in the majority of centres and a lack of high-quality evidence.4, 11
| Clinical profile | HRCT scan features | |
|---|---|---|
| IPF | Dyspnoea/dry cough/chronic onset/digital clubbing/elderly former smokers (predominantly males) | Bibasilar honeycombing in the subpleural sites |
| NSIP | Dyspnoea/cough/low-grade fever/subacute onset/middle-aged nonsmoker females | Ground glass/subpleural sparing/perilobular pattern (Fig. 1) |
| RB-ILD |
Middle-aged or young smokers Mild symptoms/mild DLCO impairment |
Centrilobular nodules in the upper lobes |
- DLCO, diffusing capacity of the lung for carbon monoxide; HRCT, high-resolution computed tomography; IPF, idiopathic pulmonary fibrosis; NSIP, nonspecific interstitial pneumonitis; RB-ILD, respiratory bronchiolitis – interstitial lung disease.
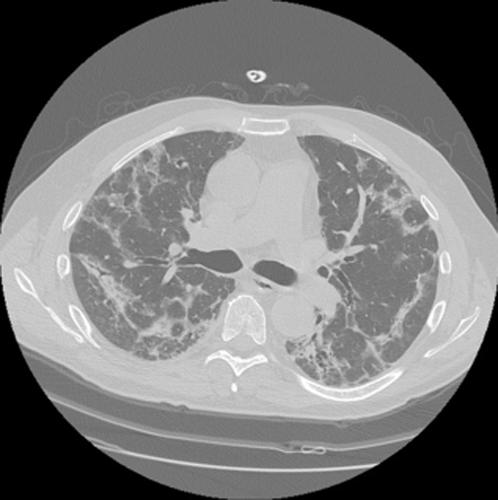
54-year-old female with subacute onset of dyspnea, dry cough and mild fever. High-resolution computed tomography scan features: bilateral perilobular pattern and little bronchiectasis in the periphery of middle zones of both lungs. Mild ground glass attenuation with a perilobular distribution is also present.
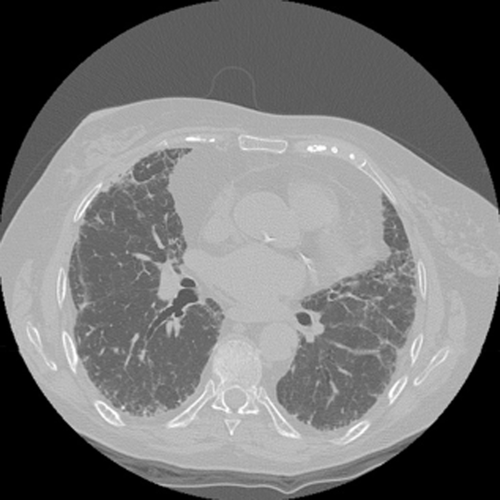
Possible usual interstitial pneumonitis pattern with a mild peripheral reticulation and moderate architectural secondary lobule distorsion in both lower lobes. In right lower lobe, a focal dendriform calcification is present. No honeycombing is visible.
In clinical practice the gap between the ‘perfect diagnosis’ (truth) and the ‘possible diagnosis’ for each patient is influenced by the relationship between risks/benefits of the diagnostic tools and therapeutic choices available.12 Such considerations need to be discussed with the patient and different approaches adopted. Specific or even genetic biomarkers may be used in specialized centres (telomerase mutations or MUC5B polymorphisms for patients with suspected idiopathic pulmonary fibrosis (IPF))13, 14 and may change the clinical perception of the disease. Clinical evolution may also be used to reach a diagnosis: functional decline, imaging features and laboratory tests changes during follow-up.3 When the patient's condition permits, observing the clinical development of the disease over a follow-up period can be considered a good compromise between disease progression and the need for more invasive procedures at the onset of the disease.
Invasive Procedures
BAL
Recognition of an inflammatory cellular pattern (increased lymphocytes, eosinophils or neutrophils) in the BAL frequently helps clinicians to narrow the differential diagnosis of f-ILDs, even though such patterns are non-specific. Increased lymphocytes suggest an alternative diagnosis to IPF15, 16 and may be observed in idiopathic NSIP (mainly in cellular NSIP or in NSIP where alveolar opacifications are present in the HRCT scan), cryptogenic-organizing pneumonia, hypersensitivity pneumonitis (HP) and lymphocytic interstitial pneumonia.1 Smoking-related ILDs, mainly desquamative interstitial pneumonia (DIP), are characterized by the presence of ‘smoker's’ macrophages and increased eosinophils, often at levels as high as seen in eosinophilic pneumonia.17 BAL lymphocyte subsets have been examined extensively in sarcoidosis and HP: a CD4/CD8 ratio >3.5 was reported to be very specific for sarcoidosis in a characteristic clinical-radiologic context;18, 19 subacute HP usually presents with a reduced CD4/CD8 ratio.1 Recent guidelines from the American Thoracic Society/European Respiratory Society on IPF suggest avoiding BAL in subjects with suspected IPF.20 This suggestion is however open to criticism: in a minority of cases with HRCT scan typical for UIP, an alternative diagnosis such as chronic HP or idiopathic NSIP may be considered after the detection of an increased lymphocyte count in BAL;16 when atypical clinical-radiological features are predominant, alternative diagnoses need to be considered and BAL could contribute to the collection of useful information with low risk for the patients. BAL is also useful to indicate unexpected diagnoses in cases mimicking f-ILDs such as disseminated lung malignancies including lymphomas, eosinophilic pneumonia, alveolar proteinosis, Langerhans cell histiocytosis, chronic alveolar haemorrhage, exogenous lipoidic pneumonia, dust exposure (asbestos exposure) and bronchiolitis.1, 19, 21-23 BAL has also a role in the diagnostic work-up of patients with f-ILD and may provide additional information in patients who cannot, or will not agree to, be subjected to more invasive procedures—in a minority of cases, the procedure may suggest an alternative diagnosis. BAL may also have a prognostic value:24 increased neutrophils with a low lymphocyte count suggest a bad prognosis in chronic HP and increased neutrophils have been reported to predict mortality in IPF.
TBB and transbronchial cryobiopsy
Andersen et al.25 are credited with the first description of TBLB following their observation that bronchial biopsy specimens of small airways harvested during rigid bronchoscopy often contained lung parenchyma. The forceps used in conventional TBLB reach lung tissue through the bronchial routes and specimens come from the centrilobular regions.1 The disorders that are centred around terminal and respiratory bronchioles or significantly involve these structures (OP) or are distributed along the lymphatic routes (sarcoidosis, carcinomatous lymphangitis) may be easily sampled by the forceps. In ‘lymphangitic’ disorders, bronchial biopsies may also contribute to increasing diagnostic accuracy. TBLB specimens may contain very specific and informative lesions (also using immunohistochemical staining) that are diagnostic by themselves (e.g. carcinomatous lymphangitis, other neoplasms, lymphangioleiomyomatosis or Langerhans cell histiocytosis). Alternatively, TBLB specimens may show characteristic, but not specific, features that may be considered diagnostic only after discussion of the clinico-radiologic context (e.g. OP, sarcoidosis, HP). Finally, they may not be informative when morphologic findings are completely incongruous with the clinical-radiologic context.26, 27 The diagnostic role of conventional TBLB in IPF and other idiopathic fibrotic interstitial pneumonias is extremely important. Berbescu et al.28 reported that the hallmarks of UIP (honeycombing, patchy fibrosis and fibroblastic foci) may be identified in TBLB samples. Tomassetti et al.29 confirmed this observation but showed that the sensitivity of the procedure is very low and that other patterns such as NSIP and DIP provide a very low negative predictive value (i.e. when these patterns are identified in tiny TBB specimens, the probability remains high of a diagnosis of UIP in larger samples). The procedure has a low complication rate: pneumothorax occurs in up to 8% of cases,1, 29 major bleeding in less than 1% of cases and other complications (acute exacerbation of fibrotic lung disease, air embolism) even more rarely.30
More recently, cryoprobes have been used to obtain lung tissue4 and studies of their clinical role in f-ILDs are starting to show promising results. Samples obtained through c-TBB are usually 40–50 mm2 in size and contain peripheral structures of the secondary pulmonary lobules.31 Recognition of UIP pattern on these large specimens has good inter-observer variability index and may be recognized by pathologists with high confidence (Fig. 3).5 Immunohistochemical analysis may be carried out easily in samples obtained by cryobiopsy. In the near future new markers such as laminin-5-gamma2-chain, fascin, heat shock protein 27, beta-cateninn and MUC5b (Fig. 4) will provide additional useful information on the morphological background of IPF.13, 14 Less high-quality evidence studies are available for other patterns such as NSIP and DIP, but the combination of morphological information provided by c-TBB and BAL profiles might be considered diagnostic and thereby avoid a SLB. Indeed the value of c-TBB, in combination with other clinical findings in the context of a multidisciplinary discussion, was found to be similar to that reported for SLB.32
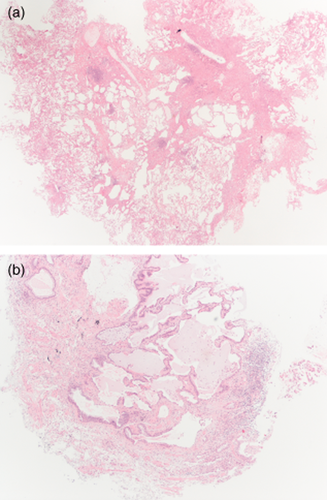
(a) Transbronchial cryobiopsy. Usual interstitial pneumonitis (UIP) pattern: normal lung abutting with fibrotic lung. Scattered fibroblastic foci are also present (HE, low power). (b) Transbronchial cryobiopsy. UIP pattern: subpleural honeycomb changes (HE, mid power).
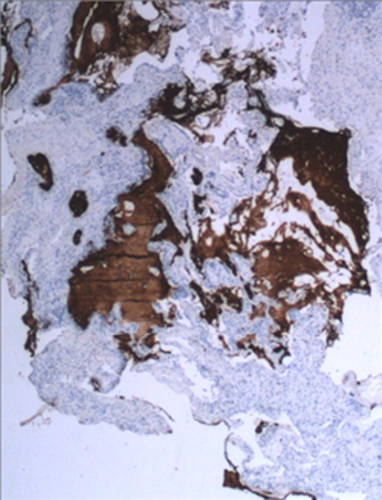
Usual interstitial pneumonitis pattern. Transbronchial cryopsy sample. Immunohistochemistry documenting accumulation of MUC5b mucin in the cystic spaces (anti MUC5b, mid power).
It is clear that transbronchial cryobiopsy instead of regular TBB can be considered a potential alternative to SLB. However, studies on the standardization of the procedure are still absent.31 The bioptic sample size is many times greater than that obtained by regular TBB and is representative also of the periphery of the secondary pulmonary lobule. Improved results are seen in ‘intubated patients’ and when biopsies are taken within 1 cm of the pleura. In studies to date, the sample size appears to be larger when larger cryoprobes or a freezing time >4 s are used. The routine use of bronchial blockers (e.g. Fogarty balloon or another blocker) appears to prevent the potentially dangerous effects of any major bleeding.33 The rate of pneumothorax varies in different studies and seems to be higher when biopsies are obtained just beneath the pleura or in subjects with the UIP pattern (a higher pneumothorax rate of 28% was reported by Casoni and coworkers, but in this study the biopsies were obtained at a maximum of 1 cm distance from the pleura and samples showed a UIP pattern in 75% of cases).5 The strategies used to collect samples are not yet standardized,31 with the majority of studies to date having retrieved lung tissue from one segment and only a minority have collected lung samples from different segments of the same lobe.4 No prospective studies have yet been published comparing these two approaches in terms of diagnostic yield and rate of complications. Even less information is available on biopsies performed in different ipsilateral lobes, although this approach seems reasonable in cases in which the CT scan pattern differs greatly in different lobes (i.e. pleuroparenchymal fibroelastosis in the upper lobes and reticulation in the lower lobes).
Mediastinal biopsies/aspiration and transcutaneous approach
Endobronchial ultrasonographic fine needle aspiration (FNA) and endo-oesophageal ultrasound FNA have good test performance for diagnosis of sarcoidosis, even in clinically unselected cohorts of patients with intrathoracic lymphadenopathy.6, 34, 35 Other ILDs that may present with hilar/mediastinal adenopathies may have a morphological confirmatory diagnosis through these approaches (mycobacterioses, fungal infections, lymphoproliferative disorders).36, 37 Interestingly, EUS may allow the sampling of subdiaphragmatic organs and thus lymphomatoid granulomatosis or other kinds of lymphoproliferative disorders may be diagnosed with biopsies performed in liver, spleen or even adrenal glands.38 Blinded FNA using 19-G needles is still useful in the same clinico-radiologic context, although its diagnostic yield appears significantly lower.39 The diagnostic role of these procedures in f-ILD is however irrelevant or minimal. In the majority of cases, enlarged hilar and mediastinal adenopathies may be found in CT scan films of patients with f-ILD, but such moderate enlargement is almost always due to non-specific inflammation.40 Biopsy of these lymph nodes is therefore of little clinical value except in cases of suspected cancer (as occurs in patients with IPF).41
The transcutaneous approach guided by imaging techniques (CT or ultrasound) is used in pulmonary nodules with a very high diagnostic yield in neoplastic lesions even when these lesions measure less than 1 cm. This approach is rarely used in the diagnostic work-up of patients with ILDs, although case reports and a few case series have reported good results mainly in alveolar opacification or when core biopsies with large needles are performed.42, 43
SLB
According to current guidelines, SLB is still recommended for diagnostic confirmation in a proportion of patients with suspected f-ILD, in particular in the diagnosis of IPF when the HRCT scan pattern is not typical.3, 20 However, two important points need to be considered. Firstly, SLB represents only a small sample of the whole lung and the minimal quantity of lung necessary to guarantee the maximum morphological information compared with the whole lung or lungs remains unknown. Secondly, surgical biopsy may be associated with a significant mortality at 90 days, mainly related to acute exacerbation. Kondoh et al. reported that 5 of 236 consecutive patients who underwent SLB experienced an acute exacerbation of IPF following the procedure, with two patients dying within 30 days of the SLB.44 Utz et al. reported a VATS 30-day mortality of 18.8%, with all deaths occurring in patients with UIP/IPF.45 In a meta-analysis conducted by Nguyen and Myer, a total of 1188 patients undergoing VATS were evaluated and, collectively, 24 patients (2.1%) died within 30 days of the procedure.46 Another meta-analysis showed that the median diagnostic yield was 95% (range, 42–100%), with IPF as the most frequent diagnosis (33.5%).46 Notably, neither biopsy site, biopsy number or SLB method were associated with diagnostic accuracy. The pooled post-operative mortality rate for included studies was 3.6% (95% confidence interval, 2.1–5.5), with significant heterogeneity observed. Subgroup analysis revealed that exclusion criteria based on immunocompromised status, mechanical ventilation and severe respiratory dysfunction (diffusing capacity of lung for carbon monoxide <35% or forced vital capacity <55% predicted), but not SLB technique or underlying interstitial lung disease subtype, were possible sources of heterogeneity. Other complications related to SLB are prolonged air leak, persisting pain, fistula and empyema.47 Given these potential risks and complications, performing a SLB is not always possible due to age, pulmonary disease severity, comorbidities or patient disinclination to undergo the procedure. However, new less surgical invasive approaches are under investigation, with reduced or no assisted ventilation and with expected reduction of acute lung injury mainly related to the lack of requirement for invasive ventilation.48
Conclusions
Semi-invasive or invasive procedures may increase the diagnostic accuracy in patients with suspected f-ILD in which HRCT features are not characteristic. However, any benefits need to be weighed against the risks of the procedures. BAL is the least risky diagnostic procedure. TBB is useful mainly in subjects with HRCT scan documenting alveolar opacification or perilymphatic lines/nodules, but should be avoided in subjects with ‘possible’ UIP HRCT scan features. Transbronchial cryobiopsy is a new bioptic approach in diffuse parenchymal lung diseases, and is the most likely ‘new star in the horizon’. SLB is the most invasive procedure for subjects with f-ILD. Future methodologies might be characterized by a decrease in the use of SLB (due to the high risks of this procedure, mainly in elderly or patients with comorbidities), the introduction of new diagnostic measures (biomarkers, genetic/epigenetic signatures) together with a more precise interpretation of HRCT scan13, 14, 49-53 and an increased role of morphology (using traditional or more sophisticated investigative tools). In particular, the morphologic information obtained via these new safer methods will be complemented by more informative pathogenetic analysis leading to the diagnosis of f-ILDs.13, 14, 54-56 Future studies are required in order to better compare patients' needs, risks and information likelihood provided by different semi-invasive or invasive procedures, the robustness of therapeutic opportunities available and an evaluation for each individual of risks/benefits ratios offered by different levels of diagnostic confidence.57 Figure 5 shows a possible diagnostic algorithm in which patient inclination, clinical profile (including pulmonary function tests), laboratory tests results and HRCT scan features are compared with the risks and benefits of different invasive procedures.
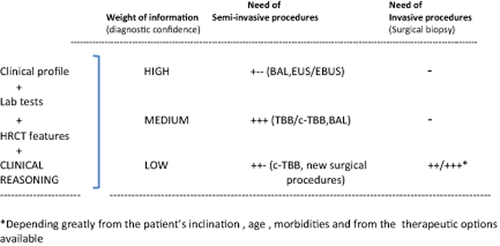
Diagnostic algorithm showing the role of semi-invasive and/or invasive procedures in the clinical-radiological scenario of patients with suspected fibrosing interstitial lung disease.




