Rapamycin inhibition of eosinophil differentiation attenuates allergic airway inflammation in mice
Abstract
Background and objective
The mammalian target of rapamycin (mTOR) signalling pathway regulates immune responses, and promotes cell growth and differentiation. Inhibition of mTOR with rapamycin modulates allergic asthma, while the underlying molecular mechanisms remain elusive. Here, we demonstrate that rapamycin, effectively inhibits eosinophil differentiation, contributing to its overall protective role in allergic airway inflammation.
Methods
Rapamycin was administered in a mouse model of ovalbumin-induced allergic airway inflammation, and the eosinophil differentiation was analysed in vivo and in vitro.
Results
Rapamycin significantly attenuated allergic airway inflammation and markedly decreased the amount of eosinophils in local airways, peripheral blood and bone marrow, independently of levels of interleukin-5 (IL-5). In vitro colony forming unit assay and liquid culture demonstrated that rapamycin directly inhibited IL-5-induced eosinophil differentiation. In addition, rapamycin reduced the production of IL-6 and IL-13 by eosinophils. Rapamycin was also capable of reducing the eosinophil levels in IL-5 transgenic NJ.1638 mice, again regardless of the constitutive high levels of IL-5. Interestingly, rapamycin inhibition of eosinophil differentiation in turn resulted in an accumulation of eosinophil lineage-committed progenitors in bone marrow.
Conclusions
Altogether these results clearly demonstrate a direct inhibitory role of rapamycin in eosinophil differentiation and function, and reemphasize the importance of rapamycin and possibly, mTOR, in allergic airway disease.
Abbreviations
-
- AHR
-
- airway hyperresponsiveness
-
- BALB/c
-
- BALB/cAnNCrl
-
- BALF
-
- bronchoalveolar lavage fluid
-
- BM-Eo
-
- bone marrow-derived eosinophils
-
- CMP
-
- common myeloid progenitor
-
- Eo-CFU
-
- eosinophil colony forming unit
-
- EoP
-
- eosinophil lineage-committed progenitor
-
- GMP
-
- granulocyte/macrophage progenitor
-
- IL
-
- Interleukin
-
- LT-HSC
-
- long-term haematopoietic stem cells
-
- mTOR
-
- the mammalian target of rapamycin
-
- NAMNC
-
- non-adherent mononuclear cells
-
- OVA
-
- ovalbumin
-
- Rapa
-
- rapamycin
Introduction
Allergic airway inflammation in asthma is characterized by eosinophil infiltration. Previous studies have demonstrated that a causative relationship exists between eosinophils and the development of allergic asthma,1 and that eosinophils participate in a variety of vital activities, including antigen presentation, cytokine production, chemokine production, secretion of granule mediators and leukotriene secretion.2 Eosinophils differentiate from a common myeloid progenitor (CMP) in mice through an intermediate granulocyte/macrophage progenitor (GMP) and then via an eosinophil lineage-committed progenitor (EoP),3 which is mediated by lineage-specific growth factors, such as interleukin-5 (IL-5).4
Rapamycin is a macrolide product of Streptomyces hygroscopius that was initially discovered in a soil sample from Easter Island (Rapa Nui) in the early 1970s.5 Its major cellular target, mammalian target of rapamycin (mTOR) is an atypical serine/threonine protein kinase that belongs to the PI3K-related kinase family and interacts with several proteins to form two distinct complexes named mTOR complex 1 (mTORC1) and 2 (mTORC2). mTOR is one of the central regulators of cell growth that integrates nutrient and hormonal signals and regulates cell metabolism, growth/differentiation and survival in many cell types.6
Previous works using rapamycin in animal models of allergic asthma showed controversial results. In some studies, rapamycin has been suggested to inhibit pathological changes of allergic asthma, including airway hyperresponsiveness (AHR), eosinophilic airway inflammation, goblet cell hyperplasia and IgE production;7-10 however, other works have also shown that it has little effects on the allergic airway inflammation and AHR.11-13 Most importantly, the underlying mechanisms that mediated the effects of rapamycin in the process of allergic asthma remain largely unknown.
In this article, we report that rapamycin can attenuate allergic airway inflammation likely through a direct inhibition of eosinophil differentiation from eosinophil progenitor cells, which appears to be independent of the levels of IL-5.
Methods
Mice
C56BL/6 mice (♀, 6–8 weeks old) were purchased from the Slac Laboratory Animal of Shanghai, and IL-5 transgenic mice (line NJ.1638) were acquired from Mayo Clinic. These mice were housed in specific pathogen-free facility in Zhejiang University. This study was approved by the Animal Ethics Committee at Zhejiang University, China.
Animal treatment, sample collection, lung histopathology, enzyme-linked immunosorbent assay, Western blot and quantitative reverse transcriptase polymerase chain reaction
The methods are described in the Supplementary Appendix S1.
Flow-cytometric analysis
EoP were identified as Lin−Sca1−CD34+IL-5Ra+c-kitlo cells, and were analysed according to published protocols.3 The methods of intracellular staining and apoptosis are described in the Supplementary Appendix S1.
Methylcellulose colony forming assays
Mouse bone marrow non-adherent mononuclear cells (NAMNC) were collected as described previously.14, 15 A total of 200 000 bone marrow NAMNCs were cultured in 0.9% methylcellulose (StemCell Technologies, Vancouver, British Columbia, Canada) according to manufacturer's instructions (as detailed in the Supplementary Appendix S1).
Liquid culture of mouse bone marrow-derived eosinophils
Bone marrow cells were cultured according to published protocols4 (as detailed in the Supplementary Appendix S1). Briefly, bone marrow NAMNC was cultured at 106/mL in medium supplemented with stem cell factor and FLT3 ligand from days 0 to 4. On day 4 and day 8, the medium was replaced with fresh medium containing recombinant murine interleukin-5 (rmIL-5).
Statistical analysis
A statistical software package (GraphPad Prism, San Diego, CA) was used for statistical analysis. Data were analysed with Student's t-test (two-tailed) or one-way analysis of variance. All data are expressed as mean ± SEM. A P < 0.05 was considered statistically significant.
Results
Rapamycin attenuates OVA-induced allergic airway inflammation and mucus production
Rapamycin administration markedly reduced the OVA-induced total count of inflammatory cells and the number of eosinophils in bronchoalveolar lavage fluid (BALF) (Fig. 1a,b). In rapamycin treated group, HE staining of the lung tissues revealed that the inflammatory cell infiltration was significantly decreased, and the periodic acid–Schiff staining also demonstrated that the levels of goblet hyperplasia were remarkably reduced, compared with the OVA group (Fig. 1c,d). These findings altogether demonstrate that rapamycin is also capable of attenuating OVA-induced allergic airway inflammation and mucus hyper production in C57BL/6 mice.
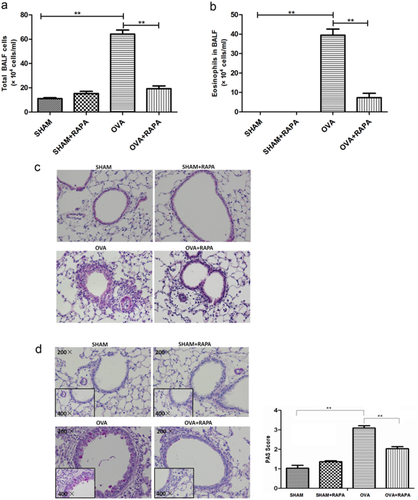
Rapamycin attenuates the severity of eosinophilic airway inflammation and mucus hyper production. OVA/alum-sensitized C57BL/6 mice were challenged with OVA for three times. Rapamycin was administrated 1 h prior to each OVA challenge. The animals were divided into four groups as follows: Saline/DMSO (SHAM), Saline/Rapa (SHAM/RAPA), OVA/DMSO (OVA) and OVA/Rapa (OVA/RAPA). (a) The total inflammatory cells and (b) eosinophils were counted respectively according to Wrights–Giemsa staining. (c) Representative images of histologic analysis of the lung sections with HE staining (original magnification ×200) and (d) periodic acid–Schiff staining and quantification of mucus production under Olympus microscope. Data are presented as mean ± SEM, n = 5–6 animals per group. *P < 0.05; **P < 0.01. Results are representative of three independent experiments. (BALF, bronchoalveolar lavage fluid; OVA, ovalbumin; Rapa, Rapamycin; SEM, standard error of mean).
Rapamycin exerts no considerable effects on the various subtypes of T helper cells
The various subtypes of T helper cells in lung tissue were assessed to investigate further the mechanisms mediating the effect of rapamycin in this study. As shown in Figure 2, rapamycin administration failed to influence the frequency of Th2 cells, Th17 cells and Tregs in the lung. The number of neutrophils (Fig. 2d) and the expression of IL-10 (Fig. 2e) in BALF were increased after the OVA exposure, and both of them were slightly but not significantly reduced by the rapamycin, which were consistent with the levels of Th17 and Treg.
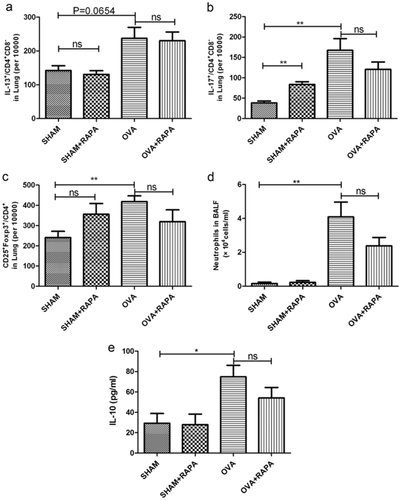
The level of various subtypes of T helper cells in lung. 24 h after the last OVA or PBS challenge, lymphocyte were isolated from lung tissue for flow cytometric analysis of (a) Th2, (b) Th17 and (c) Tregs. (d) Neutrophils in BALF were counted according to Wrights–Giemsa staining. (e) The level of IL-10 in BALF was detected by enzyme-linked immunosorbent assay. Data are presented as mean ± SEM, n = 5–6 animals per group. *P < 0.05; **P < 0.01; ns P > 0.05, no significant. Results are representative of three independent experiments. (BALF, bronchoalveolar lavage fluid; OVA, ovalbumin; PBS, phosphate buffer saline; SEM, standard error of mean).
Rapamycin inhibits eosinophil differentiation in vivo independent of IL-5
The above findings led us to explore further the possible mechanisms mediating the protective effect of rapamycin in allergic inflammation. As eosinophils are known to play a pivotal role in asthma pathogenesis, we next examined the numbers of eosinophils in peripheral blood and bone marrow, and found that they both were significantly reduced by rapamycin (Fig. 3a,b), which was in line with the results in BALF (Fig. 1b). Interestingly, treatment of rapamycin failed to attenuate the levels of IL-5 and IL-13 in the serum of asthmatic mice (Fig. 3c,d). These data suggested that rapamycin attenuation of allergic airway inflammation might be mediated through inhibition of eosinophil differentiation and independent of IL-5.
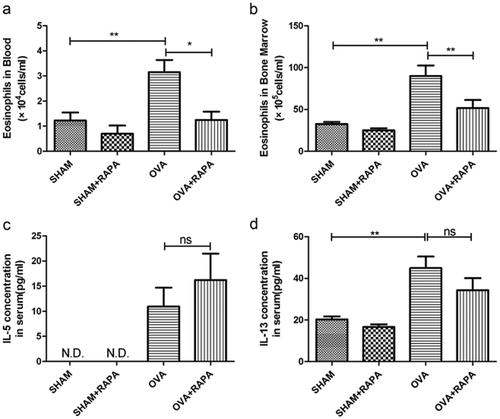
Rapamycin inhibits eosinophil differentiation in vivo independent of IL-5. (a) Levels of Eosinophils in peripheral blood, and (b) bone marrow were counted by Wrights–Giemsa staining. (c) Levels of IL-5 and d) IL-13 in serum was assayed by enzyme-linked immunosorbent assay. Data are presented as mean ± SEM, n = 5–6 animals per group. *P < 0.05; **P < 0.01; ns P > 0.05, no significant, N.D., not detectable. Results are representative of three independent experiments.
Rapamycin inhibits eosinophil differentiation and function in vitro
To explore whether mTOR is involved in eosinophil differentiation, the levels of phosphorylated S6 ribosomal protein6 and autophagy marker LC3B,16 two critical downstream signals of mTOR, were analysed (Fig. 4a). During eosinophil differentiation, the mTOR activity was decreased on day 6 and was increased back on day 8, suggesting a possible role of mTOR in regulation of eosinophil differentiation.

Rapamycin inhibits eosinophil differentiation and function in vitro. (a) Western blot analysis and corresponding quantification of phosphorylated S6 ribosomal protein (P-S6) and autophagy related protein LC3B during bone marrow eosinophil differentiation. (b) Bone marrow NAMNC were cultured in methylcellulose semi-solid medium for 14 days with IL-5 (10 ng/mL) in the different concentration of rapamycin, the number of Eo-CFU was counted and determined by expression of the eosinophil surface Ag Siglec F (>90%). (c) Liquid culture of mouse bone marrow-derived eosinophils, and rapamycin treatment inhibited the expression of eosinophil surface Ag Siglec F, representative histogram are shown for Siglec F+ cells (eosinophils) on day 10.  , control;
, control;  , Rapa. (d) Expression of mRNA encoding CCR3 was assessed by quantitative RT-PCR. The mRNA levels in untreated cells (Day 4) were adjusted to 1 and served as the reference values.
, Rapa. (d) Expression of mRNA encoding CCR3 was assessed by quantitative RT-PCR. The mRNA levels in untreated cells (Day 4) were adjusted to 1 and served as the reference values.  , control;
, control; 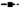 , Rapa. (e) BM-Eos(10 days in liquid culture) were plated in fresh complete medium containing recombinant murine interleukin-5 (rmIL-5) for 24 h, with or without rapamycin, cell-free supernatants were collected for the detection of IL-6 and IL-13 by enzyme-linked immunosorbent assay.
, Rapa. (e) BM-Eos(10 days in liquid culture) were plated in fresh complete medium containing recombinant murine interleukin-5 (rmIL-5) for 24 h, with or without rapamycin, cell-free supernatants were collected for the detection of IL-6 and IL-13 by enzyme-linked immunosorbent assay.  , control;
, control;  , Rapa. Media only served as control. Data are presented as mean ± SEM, n = 5–6 animals per group. *P < 0.05; **P < 0.01. Results are representative of three independent experiments. (BM-Eos, bone marrow-derived eosinophils; CCR3, C-C chemokine receptor type 3; Eo-CFU, eosinophil colony forming unit; mRNA, messenger ribonucleic acid; NAMNC, non-adherent mononuclear cells; RT-PCR, reverse transcriptase polymerase chain reaction; SEM, standard error of the mean).
, Rapa. Media only served as control. Data are presented as mean ± SEM, n = 5–6 animals per group. *P < 0.05; **P < 0.01. Results are representative of three independent experiments. (BM-Eos, bone marrow-derived eosinophils; CCR3, C-C chemokine receptor type 3; Eo-CFU, eosinophil colony forming unit; mRNA, messenger ribonucleic acid; NAMNC, non-adherent mononuclear cells; RT-PCR, reverse transcriptase polymerase chain reaction; SEM, standard error of the mean).
To test whether rapamycin could directly inhibit eosinophil differentiation, in vitro eosinophil colony forming unit (Eo-CFU) assay and liquid culture of bone marrow NAMNCs were performed. Rapamycin markedly reduced the number of Eo-CFU in a dose-dependent manner and inhibited eosinophil production in liquid culture (Fig. 4b,c). In addition, C-C chemokine receptor type 3 was significantly increased during eosinophil differentiation, and it was attenuated by rapamycin (Fig. 4d).
Next, we questioned whether rapamycin could affect the functions of eosinophils. To address this, bone marrow-derived eosinophils (BM-Eo) were stimulated with rmIL-5 in presence of rapamycin for 24 h, and cytokines such as IL-13 and IL-6 were significantly decreased (Fig. 4e). These data altogether demonstrated that rapamycin inhibited eosinophil differentiation and functions, which might contribute to its overall protective effect in allergic airway inflammation.
In vivo inhibition of eosinophil differentiation by rapamycin results in accumulation of eosinophil progenitors in bone marrow
To test further the effect of rapamycin on bone marrow eosinophil differentiation, an ex vivo Eo-CFU assay was performed. To our surprise, both rapamycin and OVA alone remarkably increased the number of Eo-CFU relative to the sham controls, and rapamycin treatment further induced the Eo-CFU in OVA-treated mice (Fig. 5a). These data suggested that rapamycin inhibited eosinophil production but might result in an accumulation of the eosinophil progenitors in bone marrow.
Rapamycin induces accumulation of eosinophil progenitors in vivo. (a) Bone marrow cells were isolated after 24 h of the last ovalbumin (OVA) challenge from different groups of mice, and were also cultured in methylcellulose semi-solid medium with IL-5(10 ng/mL) for 14 days, and the number of Eo-CFU was counted. (b) Bone marrow cells were stained with antibodies against lineage markers, Sca-1, c-Kit, CD34 and IL-5Ra chain. EoP was identified as Lin−Sca-1−CD34+IL-5Ra+c-Kitlo cells. The number of Eop in bone marrow was increased in OVA and rapamycin group (per million cells). (c) Representative flow cytometry profiles are shown (gated on Lineage−Sca-1−CD34+ cells). The stratagem of EoP analysis is described in the online supplement. Data are presented as mean ± SEM, n = 5–6 animals per group. *P < 0.05; **P < 0.01. Results are representative of three independent experiments. (EoP, eosinophil lineage-committed progenitor; Lin, lineage).
To address further this possibility, we analysed the level of long-term haematopoietic stem cells (LT-HSC), CMP, GMP and EoP in the bone marrow of different mouse groups. OVA challenge or rapamycin treatment alone significantly increased the number of EoP in bone marrow (Fig. 5b). Moreover, rapamycin further enhanced the OVA-induced numbers of EoP (Fig. 5b) and CMP (Supplementary Fig. S1c), although statistically, the change of EoP was not significant (P = 0.0793). The enhanced number of progenitors by rapamycin treatment was most likely due to the decreased eosinophil differentiation, which in turn resulted in an accumulation of the progenitors. The numbers of LT-HSC, GMP and megakaryocyte/erythrocyte progenitors were not affected by rapamycin treatment (Supplementary Fig. S1d–f).
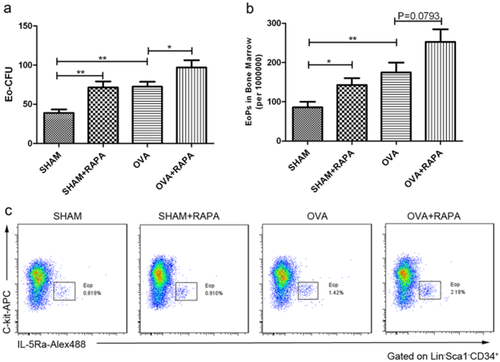
Rapamycin effectively decreased eosinophil levels in IL-5 transgenic NJ.1638 mice
To confirm further that rapamycin could inhibit eosinophil differentiation in vivo, we used NJ.1638 mice, in which IL-5 is constitutively overexpressed in plasma, and therefore higher eosinophilia exhibits in blood, bone marrow and nearly all organ systems. As shown in Figure 6, treatment of these mice with rapamycin for three consecutive days significantly decreased the levels of eosinophils in both blood (Fig. 6a) and bone marrow (Fig. 6b). Meanwhile, IL-5 concentrations in plasma were not affected by rapamycin treatment (Fig. 6c). Moreover, rapamycin inhibition of eosinophil differentiation also in turn resulted in an accumulation of EoP in bone marrow (Fig. 6d), which was in complete agreement with that in OVA-induced asthma model (Fig. 5b).
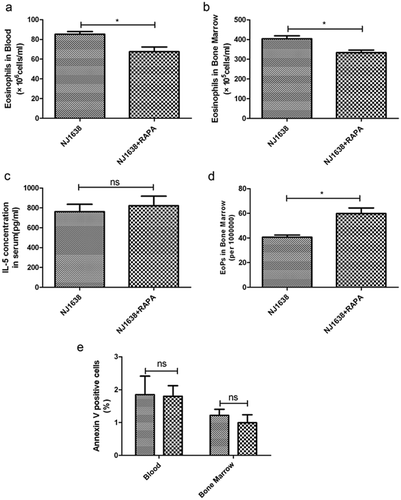
Rapamycin decreases eosinophil production in IL-5-transgenic mice. NJ.1638 mice were administered (i.p.) with rapamycin for three times at a 24 h interval. Twenty-four hours after the last rapamycin administration, bone marrow and peripheral blood were harvested. The number of eosinophils in (a) peripheral blood, and (b) bone marrow were counted by Wrights–Giemsa staining respectively. (c) Concentration of IL-5 in serum was detected by enzyme-linked immunosorbent assay. (d) EoP in bone marrow was increased in rapamycin treated mice. (e) Eosinophil apoptosis was evaluated by annexin V and DAPI staining in blood and bone marrow (gated on Siglec F+ cells), rapamycin appears to have no effect on eosinophil apoptosis. Data are presented as mean ± SEM, n = 5–6 animals per group. *P < 0.05; ns P > 0.05, no significant. Results are representative of three independent experiments.  , NJ 1638;
, NJ 1638;  , NJ 1638 + RAPA.
, NJ 1638 + RAPA.
However, there might be another possibility that the decreased eosinophils by rapamycin in vivo were due to an enhanced apoptosis of these leukocytes. We next attempted to examine whether rapamycin could promote eosinophil apoptosis. Mature leukocytes in blood and bone marrow of NJ.1638 mice were gated on Siglec F+, which showed eosinophil levels in these mice were increased to more than 70% of total white blood cells. Interestingly, rapamycin exerted inappreciable effects on the eosinophil apoptosis (Fig. 6e), suggesting that the decreased eosinophils in these mice were most likely due to the attenuated differentiation.
Discussion
In the present prevention study, we have demonstrated that rapamycin, an mTOR inhibitor, effectively inhibited eosinophil differentiation, contributing to its overall protective role in OVA-induced allergic airway inflammation in mice. Rapamycin markedly decreased the amount of eosinophils in local airways, peripheral blood and bone marrow independent on IL-5, and resulted in an accumulation of eosinophil lineage-committed progenitors in bone marrow. In vitro colony forming unit assay and liquid culture demonstrated that rapamycin directly inhibited IL-5-induced eosinophil differentiation. In addition, rapamycin reduced the production of IL-6 and IL-13 by eosinophils.
Recent studies have found paradoxical effects of rapamycin on house dust mite (HDM)-induced asthma in BALB/cAnNCrl (BALB/c) mice. Mushaben et al. have shown that HDM-induced increases in AHR and inflammation were prevented by rapamycin administered simultaneously with intranasal HDM; however, rapamycin had no effect on inflammatory cell infiltration when given after sensitization and just prior to HDM exposure, despite having blocked the increase in AHR.8 Fredriksso et al. have shown that administration of rapamycin coincident with HDM exposure significantly attenuated eosinophilic airway inflammation, while in the treatment model, the airway inflammation was exacerbated.9 Yamaki et al. have found that both preventive and therapeutic administration of rapamycin attenuated the symptoms of food allergy.10 Actually, we also noticed that rapamycin exerted inconsiderable effects on the OVA-induced total number of inflammatory cells and eosinophils in BALF in a treatment model (Supplementary Fig. S2). Hence, the above studies demonstrated mixed effects of rapamycin in allergic asthma, probably depending on various stimuli, different treatment approaches and distinct study objects. More importantly, rapamycin may exert divergent effects on asthma pathogenesis that are dependent upon the temporal relationship between rapamycin administration and allergen sensitization/ exposure.9
A central role of mTOR in integrating cytokine signalling and regulating T effector lineage commitment and immune responses has been emphasized. Recent evidence has clearly identified that the mammalian target of rapamycin complex 1 (mTOR complex 1) signalling promotes Th1 and Th17 differentiation while mTORC2 signalling facilitates Th2 differentiation, and mTOR inhibition with rapamycin can selectively promotes Foxp3+ regulatory T cells differentiation while blunting Th17 differentiation and function.17, 18 Also, antigen-specific Th2 memory cells can be re-differentiated into Foxp3+ T cells by TGF-β when stimulated in the presence of all-trans retinoic acid and rapamycin, and the converted Foxp3+ T cells in turn suppressed the proliferation and cytokine production of Th2 memory cells and Th2 memory cell-mediated allergic asthma.19 In the current study, we found that treatment of rapamycin failed to change the levels of Th2, Th17 and Tregs induced by OVA. Recently Mushaben et al. have also observed that in HDM-treated BALB/c mice, rapamycin even further decreased the number of Tregs though the ratio of Tregs/conventional T cells was not different.8 These data altogether demonstrate that, although rapamycin is known to induce Tregs in vitro, it fails to upregulate Tregs in these allergic asthma models in vivo, and consequently, Tregs seem not to mediate the protective effect of rapamycin in allergic airway inflammation.
It is widely accepted that IL-5 undertakes the most critical role in the differentiation, migration, activation and survival of eosinophils.20 Rapamycin significantly inhibited IL-5-enhanced eosinophil survival and release of eosinophil cationic protein in a concentration-dependent fashion.21 As for differentiation, IL-5 is not restricted in action to the later stages of eosinophil differentiation but rather an enhancing factor for differentiation and proliferation of eosinophil progenitors.22 It is noteworthy that rapamycin inhibited the differentiation of eosinophils but without a significant influence on serum IL-5 expression in asthmatic mice, and it also reduced the number of eosinophils but again unaltered the IL-5 level in IL-5 transgenic NJ.1638 mice, obviously suggesting that the inhibitory effect of rapamycin in allergic inflammation is independent of IL-5 expression. We have previously observed that budesonide inhibits allergen-induced eosinophil production and mobilization from the bone marrow and subsequent trafficking through peripheral blood into the airways, but exerts inappreciable effect on the local or systemic levels of IL-5.23 Recently, we also noticed that IL-17 remarkably inhibited eosinophil differentiation in vivo without affecting the levels of IL-5.24 These studies altogether strongly suggest that targeting inhibition of eosinophil differentiation independent of IL-5 might be an effective approach in controlling allergic airway inflammation.
Eotaxin plays a crucial role in eosinophil chemoattraction and activation in asthma pathogenesis.25 We have measured the levels of the eotaxin1 in BALF and eotaxin2 in lung tissues. These chemokines were increased in OVA-challenged mice, and rapamycin reduced their expression (Supplementary Fig. S3). The reduction of the eosinophil chemoattractants might be the consequence of a direct effect of rapamycin in protection of the airway damage, however, there is the another possibility that rapamycin decreased the eosinophilic airway inflammation through inhibition of eosinophil differentiation, which in turn decreased the local airway injury, thereby decreasing the expression and release of eotaxins. Currently, it is difficult to explain the effects of rapamycin on the levels of eotaxins, and further experiments might be warranted.
Although mTOR signalling has been demonstrated to play an important role in a plethora of cellular processes, its role in the regulation of haematopoiesis or myelopoiesis remains relatively unexplored. Suppression of the mTOR pathway, combined with activation of Wnt signalling, allows for the ex vivo maintenance of mouse LT-HSC under cytokine-free conditions, and increases the number of LT-HSC in vivo.26 Geest et al. have recently demonstrated that rapamycin-sensitive mTOR signalling plays an important role in the regulation of expansion of haematopoietic progenitors during myelopoiesis in a stage-specific manner utilizing a human ex vivo granulocyte differentiation system. Rapamycin treatment inhibited the expansion potential of committed CD34+ lineage-positive progenitors, but did not affect early haematopoietic progenitors.27 In our current study, we found that although rapamycin significantly inhibited the eosinophil differentiation, it increased the levels of EoP. The different conclusions between our study and those of Geest et al. remain unclear, probably because of the different research subjects and different identifications of progenitors. In their study, they used human CD34+ cells isolated from umbilical cord, and found that inhibition of mTOR activity significantly decreased proliferation of Lin+CD34+ haematopoietic cells, while we examined the number of Lin−sca1−CD34+IL-5Ra+c-kitlo cells that were regarded as eosinophil lineage-committed progenitors.
Rapamycin inhibition of eosinophil differentiation would undoubtedly contribute to its overall protective effect in allergic inflammation, though we could not rule out the possibility that other effector cells or inflammatory factors may also mediate this process. However, the increased amount of EoP by rapamycin strongly indicated an impaired eosinophil differentiation in vivo, as in general, the decreased inflammation or cytokines would not induce EoP production. Ideally, utilizing a bone marrow-specific mTOR-deficient mouse model or using mTOR knockout progenitors for a bone marrow transfer model would be the best to characterize the role of rapamycin and mTOR in regulation of eosinophil differentiation in asthma, which might be warranted in our future study. Interestingly, we have recently observed that bone marrow cells impaired with autophagic proteins exhibited an enhanced eosinophil differentiation, which indeed contributed to the allergic inflammation in vivo (unpublished data). Since rapamycin readily induces autophagy, it appears plausible that rapamycin inhibition of eosinophil differentiation is due partly to its suppression of mTOR and subsequent induction of autophagy.
In summary, the present study reveals that the protective role of rapamycin in asthma is, at least in part, due to its direct inhibition of the eosinophil differentiation from their progenitors, with an additional effect on eosinophil functions. This study reemphasizes the importance of rapamycin and mTOR in allergic inflammation and may lead to new therapeutic targets for asthma.
Acknowledgements
We would like to thank Yan JIN, Hong-Bin ZHOU, Luan-Qing CHE, Wang-Shu YU and Jia-Li QIAN of the Department of Respiratory and Critical Care Medicine, Second Affiliated Hospital, Zhejiang University School of Medicine for their technique assistant in this study. This work was supported by the National Science Foundation for Distinguished Young Scholars of China (30825019), the Key Program of National Natural Science Foundation of China (81130001), the Program for Zhejiang Leading Team of S&T Innovation (2011R50016), the National Key Technologies R&D Program for the 12th Five-year Plan (2012BAI05B01) and the National Nature Science Foundation of China (81100019).