Peripheral venous and arterial blood gas analysis in adults: are they comparable? A systematic review and meta-analysis
Abstract
Peripheral venous blood gas (PVBG) analysis is increasingly being used as a substitute for arterial blood sampling; however, comparability has not been clearly established. To determine if the pH, PCO2 and PO2 obtained from PVBG analysis is comparable with arterial blood gas (ABG) analysis. A search was conducted of electronic databases as well as hand-searching of journals and reference lists through December 2012 to identify studies comparing PVBG with ABG analysis in adult subjects. A systematic review was conducted in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses statement. A meta-analysis using a random effects model was used to calculate the average difference (bias) and the limits of agreement for the venous and arterial pH, PCO2 and PO2. A total of 18 studies comprising 1768 subjects were included in the meta-analysis. There was considerable heterogeneity between studies with I2 approaching 100%. There was little difference between the pH obtained from the PVBG and the ABG, with the arterial pH typically 0.03 higher than the venous pH (95% confidence interval 0.029–0.038). The venous and arterial PCO2 were not comparable because the 95% prediction interval of the bias for venous PCO2 was unacceptably wide, extending from −10.7 mm Hg to +2.4 mm Hg. The PO2 values compared poorly, the arterial PO2 typically 36.9 mm Hg greater than the venous with significant variability (95% confidence interval from 27.2 to 46.6 mm Hg). PVBG analysis compares well with ABG analysis for pH estimations in adults but not to the PCO2 or PO2. These differences are sufficiently large to be of clinical significance.
Abbreviations:
-
- ABG
-
- arterial blood gas
-
- ED
-
- emergency department
-
- LOA
-
- limit of agreement
-
- PVBG
-
- peripheral venous blood gas
-
- SD
-
- standard deviation
Introduction
The direct measurement of PO2, PCO2 and pH by arterial blood gas analysis (ABG) has long been the reference standard for blood oxygen, carbon dioxide and acidity. In addition, derived values such as HCO3, base excess, anion gap and the alveolar-arterial gradient are obtained from the ABG. These variables provide important and timely clinical information about a patient's metabolic and respiratory function that is vital for patient diagnosis and treatment. While ABG analysis is rapid and reliable, some argue that an arterial puncture carries a risk of haemorrhage and other vascular complications, is painful, and is no longer necessary for diagnosing respiratory failure because of the widespread use of pulse oximetry for measuring oxygen saturations. For these and other reasons (such as ease of collection), peripheral venous blood gas (PVBG) analysis is increasingly being used as a replacement to the ABG, especially in the emergency department (ED).1, 2
There are conflicting views concerning the reliability of the PVBG and whether it is sufficiently comparable with the ABG to justify widespread clinical use.3-5 One would expect the PVBG to have a lower PO2 and pH but a greater PCO2 than the ABG; however, it is not clear whether this relationship is either constant or predictable. It is also plausible that disease states affecting venous and arterial flow would result in a greater disparity between the measured variables in the PVBG and ABG. Conditions that affect venous return and cardiac output (and consequently venous and arterial blood flow) include cardiac failure, circulatory shock of any cause, respiratory failure and obesity, all of which are common in patients presenting to the ED.6 The site of PVBG collection is also important, as the measured values may differ depending on the metabolic activity of the tissues distal to the point of collection. The use of a tourniquet and the time from tourniquet application to sample collection may also be relevant, as induced local ischaemia can affect metabolism.7
The aim was to determine whether the pH, PCO2 and PO2 obtained from PVBG sampling of adult patients are comparable (i.e. have good agreement) with those obtained by ABG so as to justify the replacement of ABG in routine clinical use.
Methods
Literature search
A systematic review and meta-analysis using the Preferred Reporting Items for Systematic Reviews and Meta-Analyses guidelines was performed; a formal review protocol was not registered.8 A search of the literature identified all studies published prior to December 2012 that compared the PVBG analysis with the ABG. Electronic databases from the Cochrane library, Cochrane register of diagnostic test accuracy studies, PubMed, KoreaMed, Google Scholar and TRIP databases were searched using a combination of following terms: venous blood gas, arterial blood gas, PVBG, ABG, VBG. The Boolean operators; AND as well as OR were used where appropriate. In addition, a manual search of the following journals published between January 1995 and December 2012 was performed; Respirology, Annals of Emergency Medicine, European Journal of Emergency Medicine, Journal of Emergency Medicine, Emergency Medical Journal and Thorax. The reference lists of publications included were checked for additional studies that may be relevant to the meta-analysis.
Inclusion and exclusion criteria
Only case–control or consecutive series studies that compared peripheral venous to ABG obtained from human subjects over the age of 16 years were included. We excluded single case reports, studies that did not compare peripheral venous to arterial samples and those that enrolled children or animals. We included studies reporting the mean and standard deviation (SD) for one or more of pH, PCO2 or PO2, for paired, sequentially obtained (with minimal delay) peripheral venous and arterial blood samples. Included studies clearly identified the study population, number of samples and subjects collected (and if any were excluded from the analysis), the method of sample collection and the type of blood gas analyser used for the analysis of each sample. Studies in which venous blood was obtained centrally or during cardiopulmonary bypass were excluded because of the significant physiological differences to venous blood that occur in these situations.
Data extraction
A standardized data collection method was used to record the following information from each publication: first author name, publication year, variables assessed (pH, PCO2, PO2), number of subjects, study population and geographical location. Paired (VBG and ABG) pH, PCO2, and PO2 values (mean and SD) from all included publications were also extracted. The HCO3 was not included in the analysis as it is a derived value (from the pH and pCO2 using the Henderson Hasselbalch equation) and therefore was thought unlikely to provide any additional information. The full text and any supplementary articles were reviewed prior to data extraction. Three investigators performed the search and data extraction independently and any disagreements were resolved by discussion.
Statistical analysis
The Bland–Altman analysis is the most common method to assess the comparability of methods of measurement, in this instance PVBG and ABG.9 With the availability of paired PVBG and ABG individual patient data, a consistent tendency for one method to exceed another is denoted as the bias and estimated by the mean difference (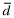 ) of paired values; the preferred bias is zero. The SD (
) of paired values; the preferred bias is zero. The SD (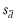 ) of these differences defines the limits of agreement (LOA). Assuming uniform bias and homoscedasticity across the range of measurement, then the LOA (
) of these differences defines the limits of agreement (LOA). Assuming uniform bias and homoscedasticity across the range of measurement, then the LOA (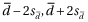 ) will encompass about 95% of the differences. If the bias is small and differences within the range of the LOA are not clinically important, then there is interchangeability of two measurements.
) will encompass about 95% of the differences. If the bias is small and differences within the range of the LOA are not clinically important, then there is interchangeability of two measurements.
However, the availability of only summary data from each study (the absence of individual patient data) prevented a full Bland–Altman approach. Meta-analytic methods for summary values from method comparison studies suggested by Williamson et al. were used.10 Only weighted averages of bias and LOA across studies can be estimated.
Within each study and for each of pH, PCO2 and PO2, the bias (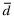 ) with SD (
) with SD (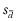 ) between the venous and arterial methods was calculated; as the venous and arterial values are correlated, the SD of the bias is estimated from the venous SD, the arterial SD and the correlation coefficient—
) between the venous and arterial methods was calculated; as the venous and arterial values are correlated, the SD of the bias is estimated from the venous SD, the arterial SD and the correlation coefficient—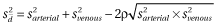 . If the correlation coefficient (ρ) was not reported, a value was imputed. A summary estimate of bias with 95% confidence intervals across included studies was estimated using a random effects meta-analysis; this provided an unconditional estimate of bias from all possible studies comparing peripheral venous and arterial blood values. Ninety-five per cent approximate credibility intervals were also estimated. This is the interval in which the bias of 95% of comparison studies would fall in the hypothetical population of all studies; this represents random variation within and across studies.11
. If the correlation coefficient (ρ) was not reported, a value was imputed. A summary estimate of bias with 95% confidence intervals across included studies was estimated using a random effects meta-analysis; this provided an unconditional estimate of bias from all possible studies comparing peripheral venous and arterial blood values. Ninety-five per cent approximate credibility intervals were also estimated. This is the interval in which the bias of 95% of comparison studies would fall in the hypothetical population of all studies; this represents random variation within and across studies.11
Mixed effects models meta-regressing the effects of moderators (study characteristics of included studies) on bias were also estimated. An omnibus test of the significance of moderators was by permutation tests (10 000 iterations). A summary LOA across studies was also estimated.
The two tests were said to be comparable if the average bias was less than the error defined by standard laboratory performance criteria (pH ± 0.04, PCO2 ± 5 mm Hg and PO2 ± 7 mm Hg),12 and the 95% approximate credibility interval was less than twice that of the laboratory error (pH 0.08, PCO2 10 mm Hg and PO2 14 mm Hg), as this was considered by the authors to be clinically unimportant.13, 14 Heterogeneity across the studies was tested using the Cochrane Q-test and the I2 test.15 Heterogeneity was said to be significant if P < 0.10 for the Q statistic. A Knapp and Hartung adjustment was used because of the uncertainty of standard error estimates; significance tests were t tests and F tests. Statistical significance was declared for P < 0.05. The software package METAFOR version 1.8-0 running in R version 3.0.1 was used.
Results
Characteristics of the studies
The procedure for identifying included studies is shown in Figure 1. The combined search performed in December 2012 identified 7733 records potentially suitable for inclusion. Of these, 7695 were excluded as they failed to meet the inclusion criteria (most were not relevant to the study question as there was no direct comparison of VBG and ABG analyses, others were performed on paediatric populations or the VBG sample was central or mixed venous). The remaining 32 full-text articles were examined for eligibility, and a further 14 studies were excluded (nine studies had incomplete data required for the meta-analysis, four studies were not PVBG samples, and one study was performed on cardiopulmonary bypass).6, 16 The remaining 18 separate studies were included in the qualitative synthesis and contributed data to the meta-analysis (Fig. 1); 16 assessed PCO2, 15 assessed pH, and 11 assessed PO2. Correlation coefficients were reported in just under half of included studies (29 of 59). In order to make maximum use of the data, a correlation coefficient of 0.85 was imputed for the analyses when one was not provided (0.85 was consistent with the general range of reported correlations).

Flow chart demonstrating the selection process of studies that were included in the meta-analysis for pH, PO2 and PCO2. ABG, arterial blood gas; PVBG, peripheral venous blood gas; SD, standard deviation; VBG, venous blood gas.
Comparison of pH estimations
For the assessment of pH, there were 1747 subjects from 15 studies (comprising 21 datasets) that compared PVBG with ABG.1, 4, 5, 13, 17-28 All studies provided details on sampling technique and the time between PVBG and ABG analysis (universally described as ‘minimal’). The populations ranged from healthy control participants to those that were acutely ill. Statistical heterogeneity was considerable (I2 = 85%, Chi2 = 158, df = 20, P < 0.0001).
Given the known clinical heterogeneity between subjects enrolled, we used a random-effects model for pooling results across these studies. The mean arterial pH for individual studies ranged from 7.15 to 7.46 and the mean venous pH between 7.10 and 7.43. The mean differences between arterial and venous values for all studies, along with 95% confidence intervals are shown in Figure 2. The estimated mean difference between the venous and arterial pH was 0.033 (0.029–0.038), lower in the venous samples and significantly different from zero.
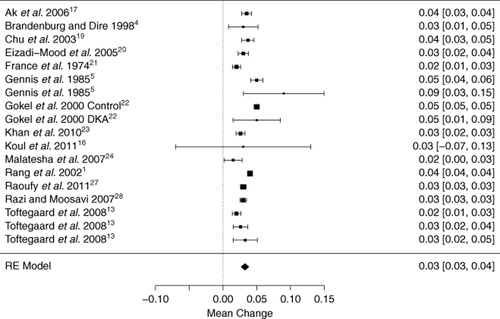
Forest plot of all studies that assessed pH; indicating the mean difference of the arterial-venous pH for each study population with 95% confidence intervals. DKA, diabetic ketoacidosis, RE model, random effects model. Subgroups—Gennis 1985a: ED patients requiring arterial blood gas, 1985b: acute resuscitation patients (post-arrest);5 Toftegaard 2009a: COPD and haemodynamically unstable patients, 2009b: no COPD and haemodynamically stable, 2009c: no COPD and haemodynamically unstable.13
The credible interval, or 95% interval of mean pH differences to be expected in future studies, was (0.015 to 0.051). A subgroup analysis by study setting (ED, intensive care unit or operating theatre) and patient characteristics (chronic obstructive pulmonary disease, metabolic disturbance, healthy volunteer) failed to explain this high degree of heterogeneity between studies. A summary LOA across studies was wide (−0.023 to 0.090).
Comparison of PCO2 estimations
There were 16 studies (21 datasets) with a total of 1768 subjects that compared PCO2 obtained from PVBG and ABG analysis.1, 5, 13, 17, 19-21, 23-31 There was no appreciable difference between peripheral venous and arterial collection technique other than anatomical site.
The meta-analysis for PCO2 results demonstrated a wide range of values. The mean partial arterial carbon dioxide concentration of each study ranged from 29.6 to 75.9 mm Hg, and the mean PvCO2 varied between 34.9 and 82.5 mm Hg. The bias was −4.15 mm Hg (standard error 0.67; 95% confidence interval −5.54 to −2.77 mm Hg) (Fig. 3, n.b. figure analysis excludes O'Connor26, due to incomplete data). Heterogeneity was statistically significant; the I2 of 99.6% showed that most variability was across studies. The 95% approximate credibility interval extended from −10.7 to 2.4 mm Hg, and the partial arterial carbon dioxide concentration would typically be 4.1 mm Hg less than the venous PCO2. However, using the lower and upper bounds of the credibility interval, a venous PCO2 of 55 mm Hg could actually be associated with a partial arterial carbon dioxide concentration between 44.3 mm Hg (normal) and 57.4 mm Hg (type 2 respiratory failure). A subgroup analysis by study setting and patient characteristics (given previously) failed to explain the high degree of study heterogeneity. A summary LOA across studies was wide (−5.9 to −2.4).
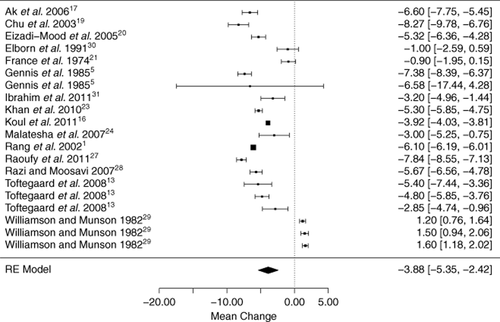
Forest plot of all included studies that assessed PCO2, indicating the mean difference of arterial-venous pCO2 for each study population with 95% confidence intervals. RE model, random effects model. Subgroups—Gennis 1985a: emergency department patients requiring arterial blood gas, 1985b: acute resuscitation patients (post-arrest);5 Toftegaard 2009a: chronic obstructive pulmonary disease (COPD) and haemodynamically unstable patients, 2009b: no COPD and haemodynamically stable, 2009c: no COPD and haemodynamically unstable;13 Williamson 1982a: general anaesthesia and isoflurane, 1982b: general anaesthesia and enflurane, 1982c: general anaesthesia and halothane.29 N.B. excludes O'Connor26 due to incomplete data, but if included, bias -4.15 (-5.54 to -2.77).
Comparison of PO2 estimations
There were 11 studies (with 16 datasets) containing a total of 1151 subjects that compared PVBG O2 with ABG samples.13, 17, 20, 21, 23-29 All studies provided details on method of blood sampling and analysis. The fraction of inspired oxygen was not stated for any study. The I2 value was very high (99%) with a significant Q score for heterogeneity (P < 0.0001, df = 15). A random effects model meta-analysis demonstrated a wide range of PO2 values. The mean partial arterial oxygen concentration for each study ranged from 55.4 to 146.5 mm Hg, and the mean PvO2 varied from 33.1 to 107.1 mm Hg (Fig. 4, n.b. figure analysis excludes O'Connor26). The difference between the venous and arterial PO2 was very large.
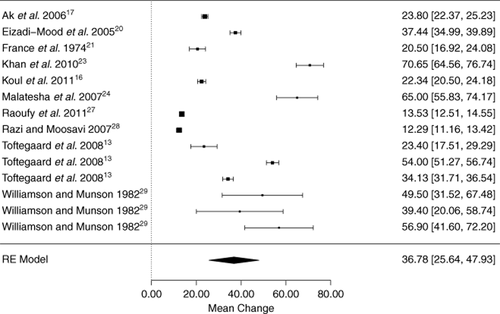
Forest plot of all studies that assessed PO2, indicating the mean difference of the arterial-venous PO2 for each study population with 95% confidence intervals. RE model, random effects model. Subgroups—Toftegaard 2009a: chronic onstructive pulmonary disease (COPD) and haemodynamically unstable patients, 2009b: no COPD and haemodynamically stable, 2009c: no COPD and haemodynamically unstable;13 Williamson 1982a: general anaesthesia and isoflurane, 1982b: general anaesthesia and enflurane, 1982c: general anaesthesia and halothane.29 N.B. excludes O'Connor26 due to incomplete data, but if included, bias 36.90 (27.18 to 46.62).
The average bias was high at 36.9 mm Hg with a standard error of 4.56 mm Hg, and the 95% confidence interval ranged from 27.2 to 46.6 mm Hg. The summary LOA across studies was also wide (21.2–52.6), and the credible interval was −2.5 to 76.3 mm Hg. In other words, the partial arterial oxygen concentration would typically be 36.9 mm Hg more than the venous PO2; however, using limits of the credibility interval, a venous PO2 of 20 mm Hg could actually correspond both to an arterial PO2 of 17.5 mm Hg (type 1 respiratory failure) and 96.3 mm Hg (normal). A subgroup analysis by study setting and patient characteristics failed to explain this high degree of heterogeneity between studies.
Discussion
The ABG allows the direct measurement of pH, PCO2 and PO2 as well as calculated values such as the HCO3 and base excess that define clinical paradigms such as ‘respiratory acidosis’ and ‘type 2 respiratory failure’. The diagnosis of these pathological states is used to initiate and guide treatments including titrated oxygen therapy, non-invasive and invasive ventilation, which have proved beneficial to patient survival.
The widespread availability of pulse oximetry has encouraged the interpretation of oxygen saturation as a crude estimate of the PO2 without the need for blood sampling and has resulted in fewer ABG analyses being performed.17 While the arterial oxygen saturation generally correlates well with the partial arterial oxygen concentration, there are important limitations. The sigmoid shape of the oxygen dissociation curve means that the relationship between pulse oximetry and PO2 is non-linear. It is not useful when monitoring for oxygen toxicity as the saturation levels are at the upper limits of the curve (i.e. large changes in PO2 will result in only small changes in arterial oxygen saturation that are not recognized as being significant).
Thus, in hypercapnic respiratory failure when oxygen saturation is maintained (approaching 100%), titrating supplemental oxygen therapy downward is difficult as large changes in PO2 result in small changes to oxygen saturation. The curve also shifts to the right as blood pH is reduced; thus, a given arterial oxygen saturation will correspond to a lower PO2 in the presence of acidaemia. Despite the limitations of pulse oximetry, some institutions (particularly ED across Australia) have replaced the ABG with the combination of pulse oximetry and the PVBG, implying that the two tests are equivalent, if not superior to, the ABG.32
It is not at all clear that this assertion has been proved. Indeed, the results of this systematic review do not support this assertion. While the peripheral venous and arterial pH compared well (with narrow LOA) the same cannot be said for the PCO2 and PO2. The PvO2 compared very poorly with the partial arterial oxygen concentration with no meaningful or consistent relationship, and an unacceptably high variability between studies. The difference (average bias) in PCO2 was not as marked but was sufficiently large to potentially influence patient management.
Importantly, this means that the venous PCO2 is not able to be used to predict the arterial PCO2 by utilizing a ‘correction factor’. Therefore, the venous PCO2 is not comparable with the arterial PCO2 and should not be used as a substitute when an accurate PCO2 is required. Notably, the venous was not always greater than the arterial value. This may reflect the inherent inaccuracy of PVBG, sampling error, variation in analysis or dynamic change in patient pathophysiology because of the temporal heterogeneity of PVBG and ABG sampling times (although reported as minimal by these studies).
The PVBG might be useful in determining that the venous blood has a very low amount of CO2 and a very high O2 to exclude hypercapnia and hypoxia, respectively, and this is the subject of a separate formal Cochrane diagnostic test accuracy review.33
Our overall estimate of the differences between venous and arterial values is subject to a high degree of heterogeneity between studies that was not explained simply by the patient factors reported. The studies included patients with a range of clinical conditions, and it may be that predictable associations between arterial and venous values exist for at least some of them. It could be argued, however, that the patients included in this review are representative of those that are potential candidates for PVBG sampling (such as ED patients for example).
Publication bias is a potential limitation of our study, as there are no effective means available to search for unpublished, non-randomized observational studies. Another limitation, as mentioned in the statistical analysis, is the lack of individual patient data. This precluded the use of the Bland–Altman approach to the data analysis; however, our meta-analytic method using summary data is appropriate and common. Some studies have found good agreement between the two methods when both summary and individual patient data are available.34
As a consequence of metabolism in the tissues, one would expect that the venous blood will have a lower PO2, a lower pH and a higher PCO2 than the arterial blood. However, our meta-analysis suggests that for PO2 and PCO2, the relationships are neither linear nor constant and cannot justify a direct comparison between the two tests. While our subgroup analysis was unable to provide an explanation for this, several possibilities exist. In addition to the right shift of the sigmoid oxygen disassociation curve with acidosis, both the individual tissue metabolic rate and regional venous blood flow may also be relevant (including the site of venous collection and the effect of the use of a tourniquet). Among the studies that were excluded, it was noted that the greatest discordance between venous and arterial values occurred in subjects with cardiac failure (although this was from central venous and not peripheral blood).6 Further research that compares the site of PVBG collection with and without tourniquet and includes subgroups with cardiac failure may be useful.
Our analysis shows that the PCO2 and PO2 obtained from PVBG are not sufficiently comparable nor do they have a stable relationship with the ABG to allow the use of a conversion factor, as is proposed by some authors.17, 27 In addition, haemodynamically unstable patients and those with congestive cardiac failure may have greater discordance between the PVBG and ABG for measures of PO2 and PCO2. This has important implications for the use of PVBG as a replacement test for ABG analysis, especially among diverse and unwell patient populations such as those in the ED. The question of whether PVBG analyses is a clinically useful test for the diagnosis of respiratory failure is the subject of a separate Cochrane review under way at present.33
In conclusion, PVBG analysis compares well with ABG for pH estimations in adults but does not accurately reflect the partial arterial carbon dioxide concentration or partial arterial oxygen concentration. The differences between venous and arterial gas tensions are sufficiently large to be of clinical significance and suggest that venous and arterial blood gas analyses are not comparable.
Acknowledgements
The authors would like to acknowledge all of the medical librarians at the Prince of Wales Hospital who assisted in obtaining many of the original articles.




