Lessons learnt from revisiting decades of seagrass restoration projects in Cockburn Sound, southwestern Australia
Author contributions: GAK, JJV, MvK conceived of and designed study, performed research, analyzed data, wrote, and edited the paper; RA performed research, analyzed data, wrote, and edited the paper; GF analyzed data, wrote, and edited the paper.
Abstract
Seagrass loss is impacting coastal communities globally, and significant efforts are being spent to address this loss through restoration. Yet, the success of restoration projects and methodologies has rarely been assessed over decades. In this case study, we reviewed past and continuing seagrass restoration projects (66 restoration sites from 1990s to 2020s) in Cockburn Sound and Owen Anchorage, temperate Western Australia, to (1) address whether they were successful in rehabilitating seagrasses, (2) whether seagrasses could be restored at appropriate scales, and (3) what the requirements for successful seagrass restoration were. In 2022, 28 individual restoration sites were revisited to establish long-term restoration success. Methods of seagrass restoration included shoots (as sprigs, plugs, cores, and sods), seedlings, and seeds. Approximately 70% of sites revisited in 2022 showed demonstrable success in restoring seagrasses. Project extent ranged from meters to hectare scales, including a study that restored 3 ha using sprigs. In the 2010s, seed-based restoration research became a major success at hectare scales. Pre-existing environmental conditions and processes were extremely important in determining restoration success, which was both site-and time-specific and influenced the choice of restoration methods. Restoration required the environment to be suitable for natural seagrass revegetation, or it needed modification. Researchers' focus on small-scale experiments testing methods across a range of environments has prepared us for scaling up to hectares. In long-lived seagrasses, decades of hysteresis were overcome with restoration, as it assisted natural recovery.
Implications for Practice
- Address the suitability of the environment to be restored through the development of restoration suitability modeling and mapping (including using pilot restoration experiments) that incorporates an understanding of the biological and physical processes that impact restoration.
- Select restoration units to target the environmental conditions of areas to be restored. Sprigs are effective in shallow, semi-sheltered waters. Seeds are more effective at greater depths, away from the effects of local waves and swell. In oceanic settings, only mechanical transplanting of larger sods from meadows will be effective.
- Understand the environmental bottlenecks in the seagrass life history to enhance the survival of restoration units.
- Build community engagement in restoration that will influence the political and economic imperatives to restore seagrasses.
Introduction
Historically, there have been major limitations in the size (spatial scale) and success of seagrass restoration across the globe (van Katwijk et al. 2016). Seagrass restoration rarely has occurred at the scale of seagrass loss, although a recent exception shows it is possible (Orth et al. 2020). Seed-based restoration of Eelgrass (Zostera marina) to the coastal bays of Virginia, United States (Orth et al. 2020) has been successful at scales of 1000 s of hectares over a period of 20 years. Suitability of the habitat for restoration is a major limitation with anthropogenically impacted coastal environments. Light plays a major role in the loss of seagrasses when declines in available light are associated with dredging and port activities (Wu et al. 2017), nutrient pollution, algal blooms, and epiphyte smothering (Cambridge & McComb 1984) and can impact the success of restoration programs. Hydrodynamics and the effect of disturbance on restoration are also major limiting factors to success (van Keulen et al. 2003; Statton et al. 2017a). Habitat suitability for restoration has recently been a focus of modeling and mapping (summarized in Bertelli et al. 2022). Many of these suitability assessments address temperature, hydrodynamics, and the light climate, as well as water and sediment health (e.g. Hu et al. 2021; Oncken et al. 2022). It is also important to factor in natural recruitment in restoration programs, and this requires an understanding of demographic and genetic connectivity (Ruiz-Montoya et al. 2015; Kendrick et al. 2017; Sinclair et al. 2018).
Global comparisons of restoration monitoring indicated a median length of 6 months to 2 years (Statton et al. 2012; van Katwijk et al. 2016) suggesting we have made little investment in quantifying the ecological value of the restoration activity. This has resulted in poor social perceptions of success that drive social license and cost to restore (Abelson et al. 2020). Recent reviews of seagrass restoration in Australia have included social perceptions and citizen science, as well as addressing cost effectiveness (Tan et al. 2020; Sinclair et al. 2021). Decadal reassessment of restoration success has been limited to a few studies (Rezek et al. 2019) and basic, long-term research is crucial for the development and implementation of coastal marine ecological restoration (Abelson et al. 2020). Here we present a case study that addresses long-term restoration outcomes for Cockburn Sound, Western Australia.
Cockburn Sound was declared a future industrial zone and major port for Western Australia in the 1950s. Rapid industrial development and uncontrolled effluent release from industry and from the southern metropolitan sewage outfall at Woodmans Point resulted in a collapse of ecosystem function, including the loss of 80% of seagrasses (approximately 3300 ha) on shallow banks that ring the sound by 1978 (Cambridge & McComb 1984). The seagrass species lost from Cockburn Sound were predominantly the meadow-forming, slow-growing seagrasses Posidonia sinuosa (98%) and P. australis (2%) (Kendrick et al. 2002). Other species that were observed in video surveys with lesser cover were Amphibolis antarctica, A. griffithii, P. coriacea, Halophila ovalis, Heterozostera nigricaulis, and Syringodium isoetifolium (Kendrick et al. 2002). The loss of seagrasses resulted in declines in fish stock and closures of fisheries, declining water quality, and a shift from a benthic to a pelagic-dominated ecosystem.
Subsequent public outcry resulted in the state government funding a multidisciplinary scientific study, the Cockburn Sound Environmental Study (Cockburn Sound Environmental Study 1979) that recommended stronger management and tighter environmental regulation of effluent entering the Sound to control nitrogen and pollutants. The annual loading of nitrogen decreased from 2000 t N per year in 1978 to less than 500 t N per year in 1997 (Kendrick et al. 2002). Seagrasses did not recover to pre-industrialization levels (2929 ha in 1967), and losses continued, with a total of 740 ha of seagrass meadows observed in Cockburn Sound in 1987 that further reduced to 661 ha in 1995 (Kendrick et al. 2002). Possible causes of the hysteresis include sediment legacies of nutrients and pollutants. Recent studies have focused on the role of a sediment legacy in pollutants and the effect of those pollutants and increased organic matter loading in sediments on sulfur phytotoxicity (Fraser & Kendrick 2017; Fraser et al. 2023).
The loss of seagrasses in Cockburn Sound and Owen Anchorage from the 1950s through to the 1990s set up a political incentive for maintaining seagrasses in these shallow coastal marine ecosystems, to remediate environmental impacts and to rehabilitate seagrass meadows. Large investments and opportunities for seagrass restoration have occurred, including industry-funded programs that scaled up restoration across Success and Parmelia Banks in the 1990s (Paling et al. 2001a, 2001b, 2007). Another outcome was the Seagrass Research and Rehabilitation Program (2004–2013; BMT Oceanica 2013) that resulted in a best practice seagrass manual and a comprehensive report on restoration activities from 2004 to 2012. It was a unique document as it addressed how to restore temperate Australian species rather than temperate Northern Hemisphere species, like Zostera marina, that accounted for 80% of published literature in seagrass restoration at that time.
Seagrass restoration programs in the Cockburn Sound region (Figs. 1 & 2) first focused on the wave-swept Success and Parmelia Banks to the north (1996–2002), where transplant survival was heavily impacted by wave-driven oscillatory and directional currents. This led to the development of plugs, cores, and sods. Cores and plugs were approximately 100 cm circular sections cut from existing meadows and transplanted into the restoration site, with and without the polyvinyl chloride (PVC) sleeve, respectively. To improve survival further, large submerged heavy machinery was used to transplant larger sods. Seagrass sods were 0.25 and 0.55 m2 rectangular areas, 0.5 m deep, cut from the existing meadow (Paling et al. 2001a, 2001b). Sprig-based restoration, consisting of plant fragments with rhizomes and four to five shoots, then became the focus in more sheltered environments within Cockburn Sound (2004–2012) with a 3 ha area successfully transplanted with sprigs (BMT Oceanica 2013). Seed-based restoration became a focus of research in the 2010s (Sinclair et al. 2021) and included assessment of the use of land-based nurseries to grow out seedlings (Statton et al. 2013; Campbell 2016) and studies into direct seeding and recruitment bottlenecks (Statton et al. 2017a; Johnson et al. 2018). The work focused on seeding from the water surface onto unvegetated areas with the direct developing seeds of Posidonia spp. These seeds have no dormancy and are released from fruits with a leaf and root already developing. This led to the development of the Ozfish “Seeds for Snapper” community seed-based seagrass restoration program now active for 6 years, with a goal to restore lost P. australis meadows to Cockburn Sound and Owen Anchorage. OzFish is an Australian non-government organisation (NGO) that focuses on habitat recovery and restoration to enhance recreational fishing and fish stocks, specializing in rewilding rivers and estuaries, restoring seagrass and shellfish reefs, and removal of introduced species.
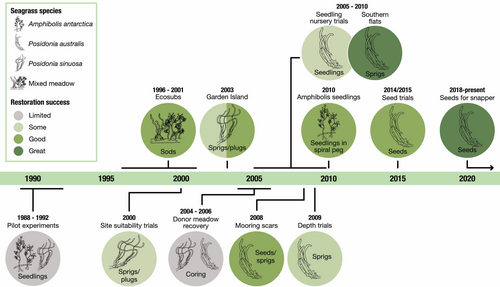
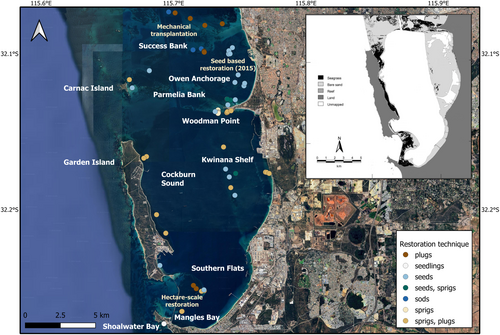
This paper summarizes the history of seagrass restoration in Cockburn Sound and Owen Anchorage, comparing the scale of restoration, the methods used, and reported success over a period spanning from the 1990s to the present day. In austral summer 2022 (February to April), we also revisited 28 individual restoration sites varying in age from 2 months to 26 years and assessed cover, survival, and density of transplants and seedlings. We conclude with an assessment of the drivers of success in restoration, the major restoration techniques used, and considerations for future seagrass restoration.
A History of Restoration Research and Development
The timeline of restoration activities demonstrates that decades of restoration have occurred in this region and that methods developed from limited success pre-1990s have led to some (a mix of success and failure), good (success over a limited area) to great (restoration scaled up to hectare areas) outcomes demonstrating the time required to adapt standard practices to the regional and local context to build successful restoration outcomes (Fig. 1). A total of 15 research papers and one major synthesis report have been published on seagrass restoration in Cockburn Sound, Owen Anchorage, Success, and Parmelia Banks, south of the metropolitan area of Perth, Western Australia (Table S1). They describe restoration activities across 66 restoration sites (Fig. 2). Restoration activities in Cockburn Sound have focused on persistent, meadow-forming species from the genera Posidonia and Amphibolis. This manuscript follows the last major synthesis by 12 years (BMT Oceanica 2013) and includes the development of seed-based restoration, a restoration technique not included in the previous syntheses.
Pre-1990s: Exploring the Field of Seagrass Restoration
Until the mid-1990s, many seagrass transplantation techniques used successfully elsewhere in the world were attempted in Cockburn Sound (Table S1) and across southern Australia, but with limited success. Kirkman (1998) documented numerous unsuccessful transplantation experiments, using a variety of seagrass species (Posidonia australis, P. sinuosa, P. coriacea, Amphibolis antarctica, and Halophila ovalis) and a variety of transplanting methods (seeds, seedlings, and sprigs). Paling et al. (2000) noted that, of some 7500 transplants (plugs) of A. griffithii planted in Cockburn Sound, most had washed away due to high levels of wave impact associated with winter storms. Many other early studies were not formally published and existed only in the gray literature and were difficult to access.
1990s: the Rise of Mechanical Transplantation
The 1990s focused on whether seagrass restoration could be successful at larger scales in wave-exposed shallow environments in Owen Anchorage and Success and Parmelia Banks to the north of Cockburn Sound (Table S1). These areas were being commercially dredged for lime sands, and restoration offered the opportunity to rescue and replant seagrasses before they were lost to dredging. The physical movement of sediment and the scale of orbital motion were excessive, 200–1000 cm2.second−1 under wind-driven waves and up to 5000 cm2.second−1 under severe storm conditions, challenging traditional shoot-based restoration approaches. Researchers found that in areas of higher water movement, larger circular plugs cut from seagrass beds performed better than anchored sprigs (shoots) (van Keulen et al. 2003). Larger plugs of A. griffithii and P. sinuosa survived better, as there was reduced disturbance to the roots and rhizomes when they were cut from the donor meadows (van Keulen et al. 2003). Improved survival of both plugs and sprigs was also achieved by transplanting them into existing meadows of colonizing species, such as H. nigricaulis (van Keulen et al. 2003). It was also found to be possible to transplant seagrasses to greater depths than donor sites, although wave energy (Paling et al. 2003) and sediment stabilization (van Keulen et al. 2003) were important factors in transplant survival.
This preliminary work gave rise to the concept of mechanical transplantation of large square meter blocks (sods) of seagrass (Paling et al. 2001a, 2001b). The ECOSUB project (I and II) used mechanical engineering to transplant large cubes (called sods, 0.25 and 0.55 m2 in seagrass area, respectively) of seagrass over a 5-year period between 1996 and 2001 (Paling et al. 2001b). The mechanical harvesters, ECOSUB I and II, were designed to transplant into hydrodynamically active environments where up to 30 cm of sediment burial and erosion driven by ocean swell and storm waves and currents were recorded (van Keulen et al. 2003). ECOSUB I was able to transplant 18 sods. day−1, or 4.3 m2, but the actual transplantation rate was 100–120 sods, or 430–516 m2, per month. The theoretical maximum output of ECOSUB II was 75 sods, or 40 m2, per day (Paling et al. 2001a, 2001b). A total of 2040 sods were planted by ECOSUB I between September 1996 and November 1999, and ECOSUB II planted 414 sods between early 2000 and July 2001 for a total area of 760 m2 transplanted and 4550 m2 of seagrass habitat created (Table S1). Sods were planted in either rows 0.5 m apart or in grids with varying distances apart to test infilling rates. Initial survival of transplanted sods was generally high, although this varied depending on planting season, and losses increased with time. After 3 years, sod survival ranged from 40 to 80%. Note that sods were only harvested in areas that were to be dredged and were therefore utilized to rescue seagrasses under threat. We would suggest this method of sod extraction would not be appropriate unless donor beds were to be lost through extractive activities.
2000s to 2010s: Focus on Environmental Variables and Planting Methods to Improve Success
The 2000s saw an increase in focused effort to understand impacts associated with harvesting sprigs, plugs, and cores, as well as studying environmental constraints to restoration success through an industry-funded, multi-institutional Seagrass Research and Restoration Program (BMT Oceanica 2013) (Table S1). With the growth of sprig, plug, and sod transplanting, a concern arose about the viability of sourcing planting units (sprigs, plugs, and cores) from nearby donor meadows. Investigations in Owen Anchorage and Garden Island in 2004 and 2005 showed source meadows of seagrasses P. sinuosa and P. australis recovered within 24 months (Verduin et al. 2012). Shoot densities in harvested areas were similar to controls that were not harvested for both species.
The influence of water depth on transplantation success was studied on Parmelia Bank, northern Cockburn Sound. Over 36 months, the survival of sprigs and increases in shoot density generally declined with depth. Greater survival (>70%) and increases in shoot density (approximately 30 times) were observed at the shallowest depths (2 m) but at 9 m, survival was less than 40% after 3 years (BMT Oceanica 2013). Depth was also a limiting factor on the survival and growth of seagrass sprigs in Cockburn Sound (Paling et al. 2007) as light is a key factor influencing depth limits (Cambridge & Hocking 1997).
Similarly, mooring scars are major disruptors to meadow formation, and once moorings are removed, they offer experimental units for exploring restoration methodologies and are a focus for restoration projects engaging local community groups in New South Wales, Australia (Ferretto et al. 2021). At Woodmans Point, Northern Cockburn Sound, shoots of the seagrasses P. australis and P. sinuosa were transplanted into large mooring scars to test their capacity to enhance natural seagrass shoot growth, seedling colonization, and meadow infilling (BMT Oceanica 2013). In 2012, the researchers also investigated the use of sandbags and artificial seagrass to enhance the survival of sprigs and shoots. Sandbags and artificial substrata have been used extensively to modify terrestrial environments to enhance restoration. Also in South Australia, sandbags are exclusively used as a substrate for Amphibolis and Posidonia restoration (Tanner 2015). Artificial seagrass was also trialed, as it has also been used as a hydrodynamic buffer and to protect against high levels of grazing (Statton et al. 2013). Results were equivocal, with significant differences in survival between mooring scars after 5 months. At the more wave-exposed mooring scar, treatments with sandbags and artificial seagrasses had a positive effect on transplant survival (40–60%) in comparison to the bare sand control (30%) after 5 months, whereas at the more sheltered mooring scar, only 20–30% survived in the treatments versus 50% survived in the bare sand control. The differences were attributed to intense crab grazing around the sandbags and artificial seagrass in the more sheltered site.
Hectare-Scale Restoration
Hectare-scale shoot-based seagrass restoration occurred at Southern Flats (depth: 1.5–3 m) over four separate summers between 2004 and 2008, yielding a total area of 3.1 ha (Table S1). The greater biomass of P. australis made it more robust than P. sinuosa and was the target species for this restoration program. Donor sprig material was harvested from an area on Parmelia Bank that was to be lost to industrial dredging and from a depth of 5–6 m (BMT Oceanica 2013).
Initially, two hectares were planted out with 25–35 cm long sprigs made from four to five shoots anchored to the substratum by metal pins at 1 m spacing between transplants. This resulted in 20,000 sprigs being deployed in the 2005/2006 austral summer (BMT Oceanica 2013). However, due to poor sprig survival and the desire to maintain a 2 ha restored site, 36,000 sprigs were added to the 2 ha site in 2006, increasing density from 1 sprig.m−2 (i.e. 1 m spacing) to 4 sprigs.m−2 (i.e. 0.5 m spacing). A further 1 ha plot was planted at 4 sprigs.m−2 in the summer of 2007/2008. Initially, the transplanting was performed by commercial divers, but community volunteers were enlisted in transplanting after the first year.
In July 2010, survival of the eastern hectare was 23.3%. In comparison, the middle and western hectares (planted between 2005 and 2008) showed survival rates of 86 and 87%, respectively, with shoot densities of 103.2–109.6 shoots.m−2, respectively. These results approximate naturally occurring shoot densities of 100.8 ± 7.08 shoots.m−2 from nearby P. australis meadows. Many sprigs were recorded flowering for the first time in July 2010.
P. sinuosa and P. australis Seedling Nurseries
Land-based aquaculture nurseries were used to investigate the growth requirements of seagrasses at the seedling stage and assessed their value as propagules for restoration (Statton et al. 2013). Seedlings of both P. australis and P. sinuosa were able to be grown from direct developing (recalcitrant) seeds (Statton et al. 2017a). The outcomes of this study were that P. australis seedlings represent a potentially useful source of propagules for seagrass restoration programs. Growing seedlings in tanks prior to transfer to the field reduced seedling mortality and improved establishment. Sediment composition (unsorted carbonate sediments with 1.5% dry organic matter added by weight) had a greater influence on seedling establishment (i.e. initial anchorage success) than adding inorganic nutrients (N and P) (Statton et al. 2013). The optimal light condition for growing seedlings in tank culture was 70% of the surface irradiance. Seedlings had greater biomass and larger root systems than seedlings grown in higher and lower light regimes. Growing seedlings in low water movement was more desirable for the production of larger seedlings with greater root development. Seedlings were heavily reliant on seed stores in their early stages of growth. Seed starch content may be used as a surrogate for assessing seedling health, where healthier seedlings retain the greatest amount of starch (Statton et al. 2013). Site selection played an important role in determining suitable sites for seed-based restoration methods. Successful seedling establishment was linked to sediment characteristics, water depth, and water motion (growth and anchorage potential) (Statton et al. 2017a). The abundance of invertebrate herbivores (e.g. Orth et al. 2006; Statton et al. 2015) and bioturbators (Johnson et al. 2018) were also considerations when selecting seedling restoration sites. In sheltered waters, seedling grazing was highest and enclosures increased levels of grazing, acting as refugia from predation for the grazers (Statton et al. 2017a).
2010s–2020s: Scaling up Using Seed-Based Restoration and Involving Local Communities
In the early 2010s, conversations started around using seeds directly, rather than seed nursery approaches (e.g. Statton et al. 2013). Like most plants, seagrasses have a type III survivorship curve where millions of seeds are required for 1000s to 10,000 seedlings to persist at hectare scales (Kendrick et al. 2017). Posidonia australis was chosen as the initial test seagrass for seed-based restoration as it was under threat in parts of its distribution (Evans et al. 2014) and it produced many fruits and seeds (Kendrick et al. 2023). The resulting multidisciplinary research program addressed: restoration and population genetics of P. australis and how this may affect seed and shoot sourcing for restoration (Sinclair et al. 2014); demographic approaches to seed-based restoration (Ruiz-Montoya et al. 2012, 2015; Sinclair et al. 2018); seed-based restoration experiments (Statton et al. 2017a); use of seed-based methodologies with other seagrass species (Statton et al. 2017b, 2018; Waite et al. 2021); and scaling up seed-based restoration to hectares (Tan et al. 2020; Sinclair et al. 2021).
The “Seeds for Snapper” Project
The “Seeds for Snapper” project started in 2018 led by community coordinators at Ozfish (https://ozfish.org.au/projects/seeds-for-snapper/) and researchers at The University of Western Australia (Sinclair et al. 2021). Fruits were collected by hand and net using free divers and SCUBA divers. The fruit were then transferred into aquaculture tanks that were circulated and aerated with pumped seawater. When the fruit splits open (dehisced), the seeds sank and were collected from the bottom of the tanks and placed in holding aquaria until counted (volumetrically) and bagged for delivery to the restoration sites where they are dispersed by hand at the surface of the water from boats. Activities were highly targeted to fruit maturation and release and occurred every day over a 4-week period between early-November to early-December.
The 2021 season demonstrated that restoration using seeds could be scaled up with a dedicated community group. Between 18th November and 15th December 2021, the OZFISH “Seeds for Snapper” Community group collected 1.18 million fruits. They obtained 375,000 viable seeds that were dispersed to six restoration sites. A total of 42 dive sessions were conducted with over 300 individual collection dives done by the community. Over 1000 hours were volunteered during this time.
The experience with seed-based approaches in Cockburn Sound and Owen Anchorage suggests that they offer the opportunity to scale-up seagrass restoration through community involvement to address the loss of seagrasses in Australia at the ecosystem scale. At the same time, this initiative implemented the social-ecological restoration approach that increased community connection and stewardship while effectively restoring the habitat, leading to affirmative political action and increased resourcing of this conservation activity (Abelson et al. 2020). The “Seeds for Snapper” program developed recurring funding from local water and gas authorities, the State Department of Transport and local industries in the region and receives media attention and promotion from the partners. Seed-based approaches also address the cost of more traditional seagrass restoration approaches, seen to be excessive by some (Bayraktarov et al. 2016), through their development of integrated community-based restoration approaches (Tan et al. 2020; Sinclair et al. 2021).
Revisiting Past Restoration Programs in 2022
We revisited 28 restoration and rehabilitation sites across Cockburn Sound and Owen Anchorage in early 2022. We focused on seed, sprig, plug, and sod-based restoration methods and a timeline from 0.5 to 26 years (Tables 1 & S2; Fig. 1). Cockburn Sound is dominated by temperate, slow-growing seagrasses, primarily Amphibolis and Posidonia species, that can take decades to grow into sizable meadows (Carruthers et al. 2007). However, most projects only monitor for 1–3 years, which makes it difficult to determine if they have been successful at the ecosystem scale (van Katwijk et al. 2016). To further complicate this, there are also several different ways to measure success, and the most ecologically relevant method changes with time. For Posidonia spp. and Amphibolis spp., the relevant measure of success in the first few years is survival, shoot growth, and rhizome expansion. After 5+ years, an increase in seagrass cover is a better indicator of restoration success. A proportion of the old restoration sites we revisited also were either indiscernible from natural recolonization and/or there was no evidence of previous restoration activities at that site. Please note that the restoration sites we visited were originally sand patches that showed low to no natural recovery of seagrasses since seagrass was lost across Cockburn Sound in the 1960s and 1970s (Kendrick et al. 2002). At each site, evidence that there had been restoration was first identified. These ranged from identifying a site from metal boundary stakes, metal pins holding sprigs, tags and labels, rope lines, and regular rectangular boundaries to seagrass patches and meadows. Then the extent of the restored meadow was measured along its longest and shortest axis, followed by estimates of shoot density and cover using 0.0625 m2 quadrats. Seedlings were also counted, and the number of shoots on each seedling was also determined.
| Location | Sites | Species | Restoration method | Timing of restoration | Time monitored (years) | Max age (years) | Success in 2022 |
|---|---|---|---|---|---|---|---|
| Cockburn Sound | 2 | Posidonia australis | Seeds | 2022 | Ongoing | 0.12 | Yes |
| Owen Anchorage | 6 | P. australis | Seeds | 2018–2022 | Ongoing | 5 | Yes |
| Owen Anchorage | 2 | P. australis | Seeds | 2014–2015 | 7.5 | 7.5 | Yes |
| Cockburn Sound | 1 | P. australis | Seeds | 2014 | 7.5 | 7.5 | Yes |
| Woodman Point | 1 | P. australis | Seeds | 2014 | 7.5 | 7.5 | Yes |
| Woodman Point | 3 | P. australis | Sprigs | 2008–2009 | 3 | 13 | Yes |
| Woodman Point | 2 | P. sinuosa, P. australis | Sprigs | 2008 | 4 | 14 | Yes |
| Southern Flats | 3 | P. sinuosa, P. australis | Sprigs | 2004–2008 | 6 | 15.8 | Yes |
| Cockburn Sound | 6 | P. sinuosa | Sprigs, Plugs | 2000 | 2 | 22 | No |
| Success Bank | 2 | P. sinuosa, P. coriacea, Amphibolis griffithii | Sods | 1996–2001 | 1.5+ | 26 | Yes |
| Total | 28 |
There were 22 examples of small (10–100 m2) to medium (1000 m2—1 ha) scale, partial or full success and six complete failures (Tables 1 & S2). Natural recruitment in areas of restoration was higher, suggesting either restoration had enhanced recruitment or environmental pressures restricting natural recruitment were reduced independent of the restoration activity. Separating these contrasting models of seagrass recovery is difficult. We describe the outcomes for three different revisited restoration programs in greater detail. All these programs show larger-scale restoration success (Fig. 1) and have developed dense patches and meadows at meter to hectare scales over 6.5–26 years.
Mechanical Transplantation Outcomes (1996–2001)
The sites restored using the mechanical transplantation approach were revisited after 21–26 years (March 2022). The surviving sods have grown extensively, in some cases merging with their neighboring sods, but interestingly they have kept their square shape in some areas (Fig. 3). As a very rough approximation, the total area of restored seagrass in 2022 was between 1094 and 2310 m2 of direct expansion from sods, assuming 40–80% survival of 760 m2 of sods (BMT Oceanica 2013) and a mean size of patch associated with the sods of 3.6–3.8 m2 (Fig. 3).
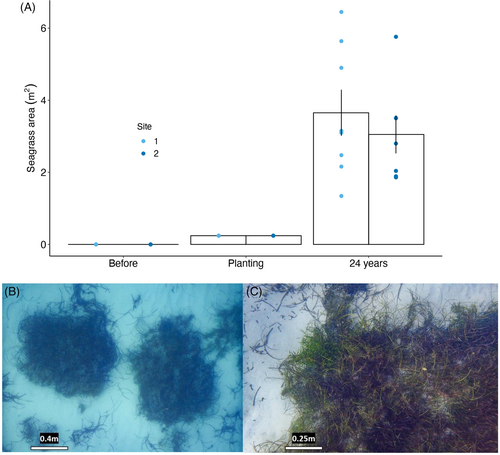
Measurements of replicate 0.25 × 0.25 m quadrats were taken from several sods randomly across the original transplant sites (Site 1 n = 32: Site 2: n = 28; Table 2). It is difficult to ascertain the actual planting size from records, so our interpretation is limited to observation of the present size of sods and whether sods have coalesced. Some sods have increased their width by up to 300%, and there has been some coalescence. They have also maintained the original mixed species composition, with shoot densities reaching up to 1108 shoots.m−2 for mixed Posidonia species (Posidonia coriacea and P. sinuosa) and up to 504 shoots.m−2 for Amphibolis species (Table 2).
| ECOSUB site | Row number | Mean shoot density | Range in shoot density | ||
|---|---|---|---|---|---|
| Posidonia coriacea | Amphibolis griffithii | P. coriacea | A. griffithii | ||
| 1 | 8 | 397 ± 116 | 230 ± 51 | 72–1108 | 16–380 |
| 2 | 7 | 498 ± 94 | 127 ± 127 | 0–752 | 8–504 |
Hectare Scale Restoration Outcomes (2006–2008)
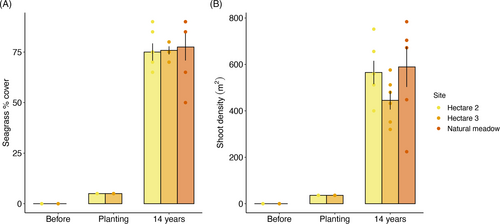
The hectare-scale restoration in Southern Flats (depth: 1.5–3 m) was revisited after 15.8 years (February 2022). This project demonstrated that large-scale (3 ha) seagrass restoration is possible, and meadows can be restored within a decade in Cockburn Sound (BMT Oceanica 2013). Interestingly, there was also high natural recruitment and growth during this time. In 2022, we compared natural seagrass meadows that had existed in 2008 with restored meadows. Shoot density and % cover were measured at two of the 1 ha plots (central [T2] and eastern [T3] and at a natural meadow nearby [C1]; Fig. 4A & 4B). Neither shoot density (one-way analysis of voariance (ANOVA), F = 1.5397, p = 0.2465, degrees of`freedom [df] = 2) nor % cover (one-way ANOVA, F = 0.0726, p = 0.9303, df = 2) in transplant sites were statistically significantly different compared to the natural meadow, with shoot densities varying from 445 to 589 shoots.m−2 and % cover from 75 to 78% (Fig. 4). The growth of transplants that were planted between 2006 and 2008 has resulted in the development of extensive meadows over the last 5–6 years and can be seen clearly from subtidal oblique imagery (Fig. 5). Sexual reproduction was also reported from the restored meadows, with flowers recorded in July 2010, 5 years after restoration. That the environment has improved to a point where a natural meadow that existed in 2008 and restored areas were not statistically different (shoot density, % cover) is an outcome of the initial restoration program. Restoration enhances and augments natural processes of recovery, and this case study demonstrates that restoration enhanced the natural recovery of southern flats by 3 ha.

Seed-Based Restoration Outcomes (2015)
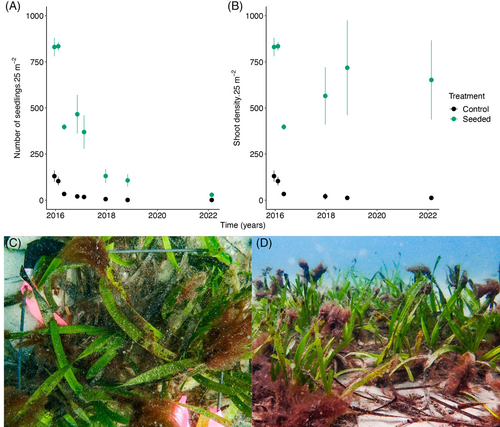
A site in Owen Anchorage (BA15) was restored using the seed-based approach described in Section 2.2.2 in 2015 and was monitored for 6.25 years (February 2022). In February 2022, we observed dense patches of seagrass that were in the process of merging into meadows in the 25 m2 seeded plots (Fig. 6C & 6D) whereas the natural seedling recruitment plots (controls) had few to no patches. Survivorship followed a type III survivorship curve, with mortalities highest in the first 2 years of seedling life in both seeded and naturally recruited experimental plots (Fig. 6A). In the 25 m2 seeded plots, 20–40 individuals, from 5000 seeds sown, survived 6.5 years, whereas in the naturally recruited plots, 0–2 individuals survived. Interestingly, after 6.5 years, shoot densities in seeded plots were between 5 and 25 times greater than those of naturally recruiting plots (Fig. 6B). Similarly, densities of shoots in seeded plots after 6.25 years were on average as high as those of 1 month-old seedlings. Between 2021 and 2023, 2.3 ha were seeded by the volunteer program, OZFISH Seeds for Snapper, and survivorship has been similar.
Discussion
This case study demonstrates that the big drivers of restoration success are that the environment needs to be suitable for seagrass colonization; that the selection of transplant unit, either sods (Paling et al. 2001a, 2001b), sprigs (BMT Oceanica 2013), seedlings, or seeds (Kirkman 1998; Statton et al. 2017a), needs to be made with consideration of biological (Johnson et al. 2018) and physical environmental drivers (van Keulen et al. 2003; Paling et al. 2007), and critical stages in the seagrass life history need consideration (Statton et al. 2017a), where predicted effects on the survival of transplant units should inform the goals and milestones of the program. We also demonstrate community-based citizen science and community restoration as the most effective approaches to increasing the scale of restoration (Sinclair et al. 2021). Similar assessments globally report that seagrass restoration success is influenced by scale (van Katwijk et al. 2016), cost (Bayraktarov et al. 2016), and environmental drivers (Bertelli et al. 2022). Also, the framework for seagrass restoration success has recently focused on scaling up seagrass restoration (e.g. van Katwijk et al. 2016) and the role that community, through volunteerism, can both reach the scaling goals, change attitudes and perceptions, and drive political change (Abelson et al. 2020).
Seagrass restoration at scale has been demonstrated to be possible in Cockburn Sound and Owen Anchorage (this paper; BMT Oceanica 2013). Seagrass restoration has been successful for more that 70% of the 28 restoration experiments and programs we revisited in Cockburn Sound and Owen Anchorage. Methodologies have developed from small experiments of 1–10 s/m2 in size testing species, methodology, donor meadow impacts, and the effect of waves, sediment movement, and light attenuation with depth. Seagrasses have been restored into a range of different environments in relation to water flow, hydrodynamics and light. Both existing sprig and seed-based restoration programs have developed viable methods to use for revegetation of hectares of unvegetated seafloor.
Our revisiting of seagrass restoration programs demonstrated that natural colonization had also occurred, sometimes obscuring the restoration results. In Cockburn Sound, seagrasses have taken decades to recover, and published accounts up to 1999 have reported continued losses (Kendrick et al. 2002). Recent unpublished mapping exercises (Hovey 2025, University of Western Australia) show that seagrass cover has increased in areas where restoration has been active, but for total seagrass cover in Cockburn Sound, losses from other areas have balanced increases such that there has been no net gain in seagrass cover since 661 ha were reported in 1999 (Kendrick et al. 2002). Restoration has addressed the need to reduce hysteresis and increase the rate of recovery of seagrasses into areas lost, thus augmenting the recovery of impacted seagrass habitats and therefore building resilience in seagrass-dominated coastal ecosystems (Kilminster et al. 2015). The issue of hysteresis and the persistence of alternate states has a large influence on the rate of recovery after disturbance in seagrass ecosystems, as demonstrated in Cockburn Sound (Kendrick et al. 2002), Shark Bay (Kendrick et al. 2019) and globally (McGlathery et al. 2013; O'Brien et al. 2018). With a significant reduction or removal of the environmental drivers that caused seagrass loss and system collapse in Cockburn Sound, restoration programs can create the nucleus to alter ecosystem states and break hysteresis to promote the natural recovery of seagrasses (BMT Oceanica 2013).
The present OZFISH “Seeds for Snapper” seed-based restoration program is cost effective as it does not invest in early life history survival, does not grow out seedlings in costly nurseries (e.g. Statton et al. 2013) and accepts that the greater than 90% natural losses of seedlings in the first year of life will also occur with seeds and transplanted seedlings in restoration programs (e.g. Statton et al. 2017a). Therefore, seed-based restoration is a numbers game where millions of seeds will be required to restore hectares of unvegetated sand. We also built in a process of seeding over multiple years to reduce the impacts of interannual biological and physical drivers of seed mortality (e.g. Statton et al. 2017a). The checks and balances in the restoration method and the cost effectiveness of this approach are advantageous for rapid recolonization of impacted areas in Cockburn Sound (Sinclair et al. 2021). There are limitations to seed-based restoration including: higher rates of mortality in the first 2 years of seedling establishment; dependence on high flowering and seed set to maintain high levels of collection of fruit and dispersal of seeds (Kendrick et al. 2023), and they are more influenced than sprigs by hydrodynamics (Statton et al. 2017a), grazing, and bioturbation (Orth et al. 2006; Statton et al. 2017a; Johnson et al. 2018). Seed-based restoration has restored 2 ha and present estimates of costs from 2018 to 2021 “Seeds for Snapper” seasons were approximately US$15,000 to $40,000 ha−1.
Sprig-based restoration programs were also successful at scales of 1000 m2 to hectares and can be used to target areas of high intrinsic value or areas where more environmental modification is required. Transplant success for restoring 3 ha using community volunteers has been shown at Southern Flats, Cockburn Sound (BMT Oceanica 2013). Sprig-based restoration also has the highest recorded survival, growth, and infilling, and return to ecological function of the methodologies we have reviewed. Sprig survival among the multiple sprig-based restoration programs has been highly variable, although it was 60–90% on Southern Flats, and their growth over 15–20 years has resulted in new meadows being formed. Sprig-based restoration in the 2000s cost approximately US$16,000 to US$34,000 ha−1 with community volunteers, or US$84,000 to US$168,000 ha−1 for commercial divers (Paling et al. 2009). Community volunteer estimates for costs using sprigs are comparable to seed-based restoration.
The sod-based restoration program (Paling et al. 2001a, 2001b) was well designed for wave-swept environments where 20–50 cm.second−1 oscillatory currents resulted in up to 30 cm sediment movement weekly to monthly (Paling et al. 2003). Sod-based restoration between 1996 and 2002 resulted in a total area of 760 m2 transplanted and 4550 m2 of seagrass habitat created and costed in the 1990s approximately US$1,000,000 ha−1 (Paling et al. 2009) so it is not a cost-effective approach, except in wave-impacted environments, and is also a promising method to rescue seagrass that is being destroyed for industrial development, mining, and dredging.
Monitoring for restoration outcomes regularly ignores the life history imperatives of natural populations. Seagrasses, like most plants, have a type III survivorship curve, an exponentially declining survivorship (Statton et al. 2017a; Kendrick et al. 2023). Determining the slope and intercept of the exponentially declining type III survivorship curve will be advantageous in planning and managing seed-based restoration efforts. Practitioners should not be concerned with substantial loss of transplants and seedlings in the first years after the restoration activity but should focus on maintaining expected losses as predicted from the survivorship curve. After 2 years, most sprig transplanting programs described in this review have had greater than 30% survival of restoration units, which fits expected survivorship, and we recommend that any survivorship greater than this level is the actual benefit the restoration program brings on top of natural recruitment and growth.
Modifications to enhance the survival of restoration units have also been trialed and need more detailed study. Some modifications include seed nurseries to grow out seeds before transplantation (Statton et al. 2013); hessian bags (Tanner 2015) or tubes (Statton et al. 2021) to both modify hydrodynamics and stabilize sediments, meshes (van Keulen et al. 2003; MacDonnell et al. 2022) and sediment capping to protect seedlings and sprigs during early development; plugs and cores to enhance survivorship and to stabilize sediments in wave-swept environments (van Keulen et al. 2003), and control of grazers and bioturbators in sheltered environments (Statton et al. 2015; Johnson et al. 2018).
Our revisit of historical restoration programs demonstrated that seed, sprig, and sod restoration has been successful in restoring hectares of seagrass over decades. Success has been underpinned by research at smaller scales that have focused on method development, impacts to donor beds, environmental suitability, and the use of modifications to the environment to enhance survival. These smaller experimental studies have led to the development of seed-, shoot-, and sod-based successes at 1000 m2 to hectare seagrass restoration. The research from the 1990s was in a highly dynamic oceanographic environment, and scaling up transplants to large sods was required for success. The research in the 2000s focused more on sheltered shallow (<5 m) subtidal seagrass habitat and was successful in restoring 3 ha of seagrass with sprigs (shoots). Since then, in the last decade, the focus has been on seeding deeper (>5 m) subtidal habitats with seeds and has also proved to be highly effective at hectare scales. We recommend a focus on the suitability of the environment for restoration by the use of modeling and experimental assessments; continued research and development of seed-, shoot-, and sod-based restoration for seagrasses with a focus on understanding the scaling of these methods to effectively restore seagrass-dominated seascapes; and to empower the development of a community of practice around partnership with community groups, like OzFish, to build seagrass restoration at scale.
Acknowledgments
Restoration described in this review was funded through the Seagrass Research and Rehabilitation Programme, supported by Cockburn Cement Pty Ltd. and the Western Australian Department of Industry and Resources, a series of Australian Research Council Industry Linkage grants partnering with Cockburn Cement/Adelaide Brighton Pty Ltd. (LP100200429, LP130100155, LP130100918, and LP160101011) and the Western Australia Marine Science Institution, Westport Research program, WWMSP project 2.3: Seagrass Restoration. We also thank J. Middleton and B. Martin from Ooid Scientific for designing Figure 1 in this manuscript. Open access publishing facilitated by The University of Western Australia, as part of the Wiley - The University of Western Australia agreement via the Council of Australian University Librarians.




