Effects of Spartina alterniflora invasion and subsequent mangrove restoration on soil nitrogen mineralization in Quangang, China
Author contributions: JF conceived and designed the research; JF, JG, YC, CY performed the experiments; JF, JG analyzed the data; JF, JG, YC, NH, CY, RL wrote and edited the manuscript.
Abstract
The Spartina alterniflora has severely invaded the coastal mangrove ecosystems in China. Here, we attempt to evaluate the invasion effect of S. alterniflora and the restoration effect of mangrove wetlands from the perspective of the nitrogen cycle characteristics. The inorganic nitrogen content, soil nitrogen mineralization, ammonification, and nitrification rate of uninvaded mudflats with those of invaded S. alterniflora site and native Kandelia obovata site at different restoration stages were compared. The results showed that the NH4+–N was the dominant form of soil inorganic nitrogen at all sites. Owing to its higher ammonification rates, the nitrogen mineralization rates at S. alterniflora site were highest in spring and summer among all sites except for mudflat. The young mangrove (1 year and 8 years) had significantly higher soil inorganic nitrogen content but lower nitrogen mineralization than the mature mangrove, indicated the short-term response to restoration. The S. alterniflora invasion did not enhance soil nitrogen mineralization comparing with preinvaded mudflat, but it used more NH4+–N relative to K. obovata due to its higher soil nitrogen mineralization than mangrove communities in germination and growing seasons.
Implications for Practice
- The NH4+–N was the dominant form of soil inorganic nitrogen at all sites in Quangang coastal wetland.
- Spartina alterniflora did not enhance soil nitrogen mineralization comparing with preinvaded mudflat.
- S. alterniflora showed higher soil nitrogen mineralization than the restored mangroves in Spring and Summer.
Introduction
Coastal wetlands, which receiving large amount of nitrogen loading from industrial and agricultural activities (Gao et al. 2019b), are considered to be sources, sinks, and transformation zone of nitrogen, and can affect the nutrients of surrounding environment through the processes of nitrogen absorption, removal, and release (Jia et al. 2017). Nitrogen is also one of the important limiting factors affecting the photosynthesis processes and primary production of wetland vegetation (Bisbing & D'Amore 2018). The organic nitrogen and inorganic nitrogen are the main forms of soil nitrogen in the soil of wetland, however, the proportion of inorganic nitrogen is very low (Mou et al. 2011; Smyth et al. 2012). Since inorganic nitrogen is a form of nitrogen that plants can be used directly, the conversion process of organic nitrogen to inorganic nitrogen (nitrogen mineralization) largely affects the supply of soil available nitrogen, which in turn affects the primary productivity of the wetland system (Smyth et al. 2012; Jia et al. 2017).
As an important invasive plant in the coastal wetlands in China, Spartina alterniflora, which was introduced from the United States in 1978, has reached an area of 545.80 km2 in 2015 (Liu et al. 2018). The mangrove ecosystem also had been severely invaded by S. alterniflora in China (Li et al. 2016b; Feng et al. 2018). At present, several methods had been developed to control the S. alterniflora and our previous research found that the ecological replacement method using native mangrove to restore S. alterniflora invaded areas was feasible and effective (Feng et al. 2018, 2019). Moreover, mangrove restoration not only inhibits the spread of S. alterniflora and increases the area of the mangroves, but it also reverses the changes in the diet of the benthic macrofauna caused by the invasion of S. alterniflora (Feng et al. 2018), increases the soil carbon content (Feng et al. 2017, 2019) gradually.
The rapid spread and growth of S. alterniflora is not only associated with its special reproduction strategy (Li et al. 2009), but may also be closely related to the eutrophication condition of marine environment in China (Zhao et al. 2015; Jia et al. 2016; Cui et al. 2017). As a woody community, mangrove forests have different nitrogen utilization mechanism compared to the S. alterniflora community. However, little information was reported for the impact of invasive S. alterniflora on the soil mineralization in mangrove ecosystems. Therefore, we investigated the invasion effects of S. alterniflora on the soil inorganic nitrogen contents and nitrogen mineralization in mangrove wetlands and tried to clarify whether the ecological replacement of mangroves can gradually remediate the invasion impact of S. alterniflora.
S. alterniflora can cause a series of invasive effects on the cycling of the biogenic elements in the coastal wetland ecosystems (Yu et al. 2015; Yang et al. 2017; Feng et al. 2019). For example, the invasion of S. alterniflora was pointed out to change the content of organic carbon, including particulate organic carbon and the microbial biomass carbon, as well as the content of nitrogen and phosphorus of wetland soil in the Yangtze River Estuary, Yellow River Estuary, and Minjiang Estuary of China (Mou et al. 2011; Li et al. 2016a; Jia et al. 2017). S. alterniflora was reported to increase the rate of the nutrient cycle through litter decomposition (Smyth et al. 2012) and can uptake dissolved inorganic N from tidal subsidies (Peng et al. 2011) to obtain a large quantity of nitrogen resources, which further favored its invasion ability. Several soil physicochemical factors, including the temperature, water content, salinity, and pH also were changed owing to the invasion of S. alterniflora (Feng et al. 2017, 2019), thus indirectly affected the nitrogen soil mineralization.
The impact of S. alterniflora on nitrogen mineralization may be related to the region, vegetation type, and growing seasons. For example, the soil nitrogen mineralization was not significantly changed by the S. alterniflora in the Yangtze River Estuary, while opposite results were found in the Minjiang Estuary where the soil nitrogen mineralization was considerably changed due to the invasion (Peng et al. 2011; Li et al. 2016a). The perennial S. alterniflora had seasonal variation of growth characteristics and biomass, which can change the soil physicochemical characteristics and microbial communities (Lin et al. 2021). These might further influence the soil inorganic nitrogen dynamics, for example, the inorganic nitrogen content in the S. alterniflora community was lower than that of the native plant community during the growing season in Yangtze Estuary, China (Peng et al. 2011). Different to the saltmarsh ecosystems, S. alterniflora mainly invades the bare mudflats outside the mangrove forest and changes the vegetation type and element cycle (Li et al. 2016b; Feng et al. 2017, 2019). The soil bulk density, water content, and some other soil properties in mangroves may be lower than those in the S. alterniflora community (Yu et al. 2015), which might lead to the differences in the nitrogen mineralization (Sardans et al. 2017; Bisbing & D'Amore 2018). Our previous results showed that S. alterniflora and mangroves can increase the carbon and nitrogen content in the surface soil (Lin et al. 2021; Huang et al. 2022) and then could promote available substrates for microbes to influence element cycling processes and pools for entire ecosystems (Lin et al. 2021). However, soil carbon and nitrogen dynamics are also related to geographical features (e.g., longitude and latitude), forest age, and tidal elevation. Therefore, it is of great significance to study the spatial and temporal effect of S. alterniflora invasion and mangrove restoration on nitrogen mineralization in coastal wetland for the interpretation of invasive mechanism and maintenance of the wetland ecosystem (Bisbing & D'Amore 2018).
Here we compared the inorganic nitrogen content, soil nitrogen-mineralization rate, and soil nitrification rate of uninvaded mudflats with those of a S. alterniflora invaded area, and mangrove at different restoration stages and evaluated the invasion effect of S. alterniflora and the restoration effect of mangrove wetlands from the perspective of the nitrogen cycle characteristics. We propose the following hypotheses. (1) The soil nitrogen mineralization of S. alterniflora community will be higher compared with those of bare mudflats and native mature mangroves. (2) The soil nitrogen mineralization of S. alterniflora will exhibit seasonal differences. owing to its growing characteristics. (3) The soil nitrogen mineralization level will be changed with the restoration ages of mangrove. We attempt to reveal the impact of S. alterniflora in the mangrove area from the perspective of the soil nitrogen supply to provide a basis for the protection and management of coastal wetlands.
Methods
Study Area
The study was conducted in the costal wetland (Fig. 1. 24°56′30.77″N, 118°41′42.47″E) near the Pannan Saltern in the Quangang District, Quanzhou City, Fujian Province. This area has a subtropical monsoon climate with the annual average temperature of 17–21°C, and the average rainfall of 1,400–2,000 mm, which occurs mainly in May to June. It has a regular semidiurnal tide, with an annual mean tidal variation of 5.12 m. The water salinity ranged from 17.98‰ to 26.46‰, with the average salinity of 22.22‰.The mangrove plants in Quanzhou are mainly Kandelia obovata, Aegiceras corniculatum, and Avicennia marina. S. alterniflora invasion occurred in 1980s in Quanzhou and spread throughout Quanzhou Bay by 2005. In 2002, plantation of K. obovata has been carried out after S. alterniflora was cut. Around 2008, large areas of S. alterniflora were cut and then were replaced by native mangroves (K. obovata) to control the spread of S. alterniflora.
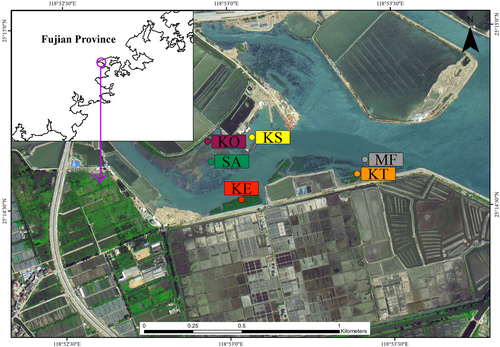
Sample Collection
In this study, an uninvaded bare mudflat (MF), a S. alterniflora invaded area (SA), a relative mature K. obovata wetland area (KO) were selected. Ecological replacement project in invaded area using native K. obovata were conducted in 2008 (KE), 2010 (KT) and 2017 (KS), thus these three sites were selected to investigate the temporal effect of restoration by space–time replace method. All the sites were adjacent to each other to minimize possible differences in environmental conditions. A in situ incubation method (Peng et al. 2011) using top-closed PVC tube was used to study the soil nitrogen mineralization in the above six sample sites. Four 10 m × 10 m quadrats were established in each sites, two PVC tube (inner diameter of 5 cm and length of 15 cm) was used to collect two soil samples at a depth of 0–15 cm and these samples were pooled together as a replicate and used for the determination of the initial NH4+–N and NO3−–N contents. Another two tubes were used to collect the top 15 cm soil samples and then the openings of the tube were covered with plastic film (gas-impermeable) and tied with plastic bands to prevent inorganic nitrogen exchange with the soil outside of the tube. Subsequently, the tube was inserted into the original hole and the top of the tube was kept at the same level of the soil. After incubation for 30 days, the two PVC tube from each quadrat were collected and soil samples were pooled together as a replicate to measure the NH4+–N and NO3−–N contents. According to the growth characteristics of S. alterniflora, three incubation periods were set, that is, the germination period of S. alterniflora April 2018–May 2018 (spring), the growing period of S. alterniflora August 2018–September 2018 (summer), and the withering period of S. alterniflora December 2018–January 2019 (winter). Therefore, the above procedure was repeated in the different seasons.
Sample Processing and Measurement
After collection, the soil samples were stored at −20°C and transported back to the laboratory as soon as possible. The soil samples were removed from the PVC tubes, put into airtight ziplock bags, and mixed evenly. The sodium salicylate-sodium hypochlorite spectrophotometric method method and the ultraviolet spectrophotometric method were used to determine the NH4+–N and NO3−–N contents of the fresh soil samples, respectively. After measurement of inorganic nitrogen, only the soil samples in summer were freeze-dried using a vacuum freezer (Boyikang, Beijing) and weighed to calculate water content () and bulk density(). Then subsamples were grounded into a fine powder using a mortar and pestle and passed through a 250 μm sieve. The soil samples were extracted by distilled water and the salinity (water:soil of 5:1) and pH (water:soil of 2.5:1) were measured using multipleparameter water analyzer (WTW Multi 3630 IDS). The total nitrogen (TN) were determined with an elemental analyzer (Elementar Macrocube).
Method of Calculating the Mineralization Rate
The net nitrogen mineralization rate of the soil is calculated based on the difference between the content of soil inorganic nitrogen (NH4+–N and NO3−–N) after incubation and before incubation using the following equations:
Rmin = (Ci − C0)/△t,
Rnit = (Cinit − C0nit)/△t,
Ramm = (Ciamm − C0amm)/△t.
Here, C0 and Ci are the soil inorganic nitrogen content before and after incubation (mg/kg), respectively; Cinitri and C0nitri are the soil NO3−–N contents before and after incubation, respectively; Ciamm and C0amm are the soil NH4+–N contents before and after incubation, respectively; and △t is the time incubated, respectively. Rmin is the net nitrogen mineralization rate [mg/(kg d)]; Rnitri is the net nitrification rate; and Ramm is the net ammonification.
Data Statistics
The one-way ANOVA was used to compare the differences in the soil physical–chemical properties, NH4+–N and NO3−–N contents before incubation, net nitrogen mineralization rate nitrification rate and ammonification rate among the different incubation periods, and different wetland types (p < 0.05 indicates a significant difference). A post hoc test was conducted to test differences using the Tukey test method. Data were log(x + 1)-transformed prior to statistical analysis when required to satisfy the assumption of the ANOVA. Nonparametric test was used when the assumption was not reached. All the above statistical tests were performed using SPSS 22.0 (IBM SPSS Statistics for Windows, IBM Corp., Armonk, NY, U.S.A.).
Results
Comparison of the Soil Physicochemical Characteristics and Inorganic Nitrogen Content among Different Sites
The SA site had higher water content (98.65 ± 10.13%) and lower bulk density (0.41 ± 0.06 g/cm3) than MF and KS (p < 0.05; Table 1), but did not show differences with other sites. The MF and KS sites had the highest pH (7.52 ± 0.05 for MF and 7.29 ± 0.08 for KS), while the KO had the lowest pH (p < 0.05). The youngest KS had the lowest soil TN contents (0.83 ± 0.05 mg/g) than the other sites, while no significant differences were found for TN contents among the other five sites.
| Site | Water content (%) | Bulk density (g/cm3) | pH | Salinity (mg/g) | TN (mg/g) |
|---|---|---|---|---|---|
| MF | 77.60 ± 5.30bc | 0.62 ± 0.05ab | 7.52 ± 0.05a | 19.13 ± 1.43ab | 1.2 ± 0.07a |
| SA | 98.65 ± 10.13a | 0.41 ± 0.06c | 7.17 ± 0.04bc | 15.13 ± 1.52bc | 1.6 ± 0.12a |
| KS | 64.28 ± 2.28c | 0.65 ± 0.05a | 7.53 ± 0.12a | 12 ± 0.35c | 0.83 ± 0.05b |
| KT | 87.11 ± 6.22ab | 0.49 ± 0.04abc | 7.29 ± 0.08ab | 22.38 ± 1.28a | 1.18 ± 0.05a |
| KE | 91.58 ± 4.593ab | 0.46 ± 0.03bc | 6.92 ± 0.12bc | 16.75 ± 1.27abc | 1.3 ± 0.04a |
| KO | 84.77 ± 5.49ab | 0.41 ± 0.02c | 6.75 ± 0.12c | 14 ± 1.88bc | 1.53 ± 0.2a |
The NH4+–N was the dominant form of inorganic nitrogen at all sites. Except for SA and KO, NH4+–N and SIN content at all the other sites changed significantly among seasons (Fig. 2; p < 0.05) that the highest values were found at spring or summer (except for KE). For NO3−–N content, all sites showed similar seasonal variation that the lowest values were observed in wither.
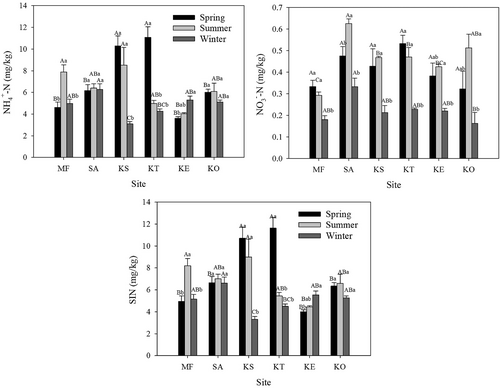
In the spring, the soil NH4+–N and SIN content of sites KS and KT before incubation were significantly higher than those of all the other sites (Fig. 2; p < 0.01). No significant differences in the soil NO3−–N content were found among all sites before incubation.
In summer, the soil NH4+–N and SIN content before incubation at MF and KS were significantly higher than those of KE (p < 0.01), while no significant differences were found among other sites. The SA had significantly higher soil NO3−–N content than other sites (p < 0.01) except for KO. Except for KE, all the mangrove communities showed higher soil NO3−–N content than MF (p < 0.01).
In winter, the lowest soil NH4+–N and SIN content before incubation was observed at KS (p < 0.05). SA had significantly higher soil NH4+–N and SIN content than MF and young KS and KT sites (p < 0.01). The soil NO3−–N content of SA was significantly higher than that of KO, and there was no difference for the soil NO3−–N contents among all the other sites.
Differences in the Soil Nitrogen Mineralization Rates among Different Sites
Owing to the higher NH4+–N contents than NO3−–N contents, the nitrogen ammonification contributed largely to the TN mineralization rates at all sites. All the Ramm, Rnit, and Rmin values for all sites changed significant among different seasons (Fig. 3; p < 0.05) and the highest values were found at summer at most of the sites.
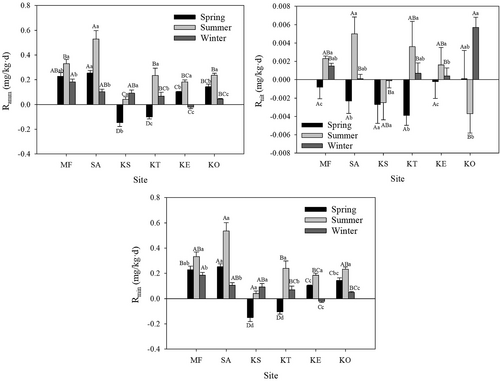
In spring, the Ramm and Rmin values of SA site was significantly higher than those of all sites except for MF site (Fig. 3; p < 0.01). The KS and KT had the lowest Ramm and Rmin values (p < 0.05). The Rnit values were all negative except for KO and no significant differences were found among all sites.
In summer, the Ramm and Rmin values at SA site was significantly higher than all sites except for MF (Fig. 3; p < 0.01), and the KS and KT had lowest values (p < 0.05). The SA had the highest Rnit values but only significantly higher than the KO sites (p < 0.05).
In winter, the Ramm and Rmin values was highest at MF and lowest at KE (Fig. 3; p < 0.05). The differences between site. The KO showed highest nitrification rate but no significant differences were found among all the other sites (p < 0.05).
Discussion
The productivity of most natural and constructed ecosystems are limited by the available nitrogen supply in terrestrial ecosystems (Jia et al. 2017; Bisbing & D'Amore 2018). The intensity of nitrogen mineralization in wetland soils determines whether the wetland ecosystem can effectively convert organic nitrogen into inorganic nitrogen, thereby increasing the productivity of the wetland ecosystem (Reich et al. 1997; Gao et al. 2012; Jia et al. 2017). Since the environment and organisms can affect each other, the changes of vegetation type, such as plant invasion will change the characteristics of the biogenic elements in the soil in coastal wetland ecosystem (Yu et al. 2015; Feng et al. 2017, 2019). The invasion of S. alterniflora is found to have increased the nitrogen mineralization in Minjiang Estuary wetland, China (Li et al. 2016a). The increased nitrogen mineralization would further improve the productivity of the ecosystem, which can promote the accumulation of carbon (Peng et al. 2011). This will stimulate the decomposition process of soil microorganisms, thus increasing the available nitrogen content within ecosystem and benefit for the growth and further spread of invasive plant (Sardans et al. 2017). However, the nitrogen mineralization rates of S. alterniflora were not increased comparing with preinvaded mudflat in our study, which might be attributed to the high requirement and rapid uptake of NH4+–N during its growing seasons.
It had been found that the inorganic nitrogen content in the S. alterniflora community was lower than that of the native plant community during the growing season in Yangtze Estuary, China (Peng et al. 2011). This may be due to the rapid growth characteristics of the invasive S. alterniflora, which has a higher nutrient utilization rate than the native plant (Sardans et al. 2017), leading to a decrease in the soil NH4+–N content. In this study, we also found that the soil NH4+–N content of the S. alterniflora community was lower than those of most of the mangrove communities before incubation, and the soil mineralization rate was higher than the other mangrove communities in spring and summer, but the soil NH4+–N content among them had no significant differences. This might mean that S. alterniflora uses more NH4+–N relative to K. obovata in its germination season and growing seasons. The invasion of S. alterniflora has weak effect on the nitrate nitrogen content in this study, which may be because the NO3−–N contents of all communities were relatively low and the NH4+–N is the dominant form of inorganic nitrogen in this study area. Another fact is that the periodic flooding at the coastal wetlands caused anoxic condition in the soil (Ha et al. 2018), leading to the low nitrate nitrogen content and the lack of considerable change.
The invasion of S. alterniflora can change the microbial communities in the soil (Yang et al. 2020) and the soil microbial activity can increased in summer under the high temperature (Zhang et al. 2016), which might improve the decomposition rate of organic matter in the soil and result in more inorganic nitrogen in the soil (Huang et al. 2016). Therefore, the S. alterniflora community showed higher ammonification and mineralization rates in the summer than those of the mangrove communities. It had been revealed that the nitrogen fixation on the surface litter of S. alterniflora can import nitrogen into the soil (Liao et al. 2010), increasing the nitrogen content and further mineralization rate of the wetland soil. This helps explain why the soil in the S. alterniflora community remain has a relatively high inorganic nitrogen content in winter.
The young restored KS and KT showed higher soil NH4+–N content than those old mangrove of KE and mature KO, which possibly was a short-term response to restoration. The mature mangrove community had high litter yield, resulting inrelatively higher nitrogen input into soil. It also can reabsorb nitrogen from the senescent leaves to increase the efficiency of nitrogen utilization (Cartaxana & Catarino 2002), leading to less absorption from the environment (Clough et al. 2000; Mandal et al. 2013). In addition, it is also possible that the self-thinning phenomenon has occurred in mature mangrove communities after a long period of development (Kamara et al. 2012), and the low tree density require less nitrogen, thus, leading to a higher soil inorganic nitrogen content than the young mangroves.
In the spring, the nitrogen mineralization rates in the mangroves restored in 2008 and the mature mangroves were considerably higher than those of the mangroves restored in 2010 and 2017. The well-developed buttress root system of relative older mangroves can intercept more of the sediments from the tidal water, which might promote soil nitrogen content and mineralization. Compared with young mangroves, the mature mangroves have higher total organic carbon and particulate organic carbon contents (Feng et al. 2019), indicating that the microorganisms will be able to obtain more nutrients to maintain their physiological activity, thereby promoting soil nitrogen mineralization.
Owing to the low nitrate nitrogen content, the S. alterniflora invasion and mangrove restoration did not considerably change the soils nitrification rate in this study. Although other research had shown that the invasion of S. alterniflora can enhance soil nitrification to a certain extent (Zhang et al. 2016), the soil nitrogen will be lost in the form of nitrogen gas with the increasing nitrification effect (Wrage et al. 2001). Gao et al. (2019a) showed that the denitrification rate of the S. alterniflora community is relatively high, suggesting that a large amount of nitrate nitrogen was eliminated through denitrification, resulting in a decrease in the nitrate nitrogen content and a low nitrification rate. Sun and Zhu (1989) found that there were large quantities of bacteria, actinomycetes, and fungi in the soil of S. alterniflora salt marsh, while the ammonifying bacteria was dominant (Sun & Zhu 1989). These results indicated that S. alterniflora mainly enhanced ammonification, but had weak effect on soil nitrification, which is consistent with this study.
In summary, the S. alterniflora community had similar soil nitrogen mineralization rates with the preinvaded mudflat but higher nitrogen soil mineralization rates than the mangrove forests in Spring and Summer. This indicated that the S. alterniflora used more NH4+–N relative to K. obovata in germination and growing seasons. The young mangrove community (KS) was greatly influenced by the restoration project, while the nitrogen mineralization rates changed with the mangrove restoration ages. Our previous research results showed that mangrove restoration can reverse the changes in the diet of benthic macrofauna in S. alterniflora invaded areas gradually and can enhance the soil organic carbon, nitrogen, and phosphorus contents of the soil to a certain extent (Feng et al. 2017, 2019). The environmental characteristics and ecological function of planted mangroves developed toward the mature mangrove forests after the invasive S. alterniflora was replaced. However, the vegetation and their forest ages were not the unique factor affecting soil inorganic nitrogen contents and nitrogen mineralization rates, especially in coastal wetlands receiving large amount of nitrogen loading from industrial and agricultural activities (Gao et al. 2019a). Therefore, further studies using control experiment that consider different exogenous nitrogen levels are needed to accurately evaluate the the effect of vegetation development on nitrogen mineralization process.
Acknowledgments
This research was supported by the National Natural Science Foundation of China (41976160, 41706090) and Fundamental Research Funds for the Central Universities of Sun Yat-sen University (17lgpy96). It was also partly funded by the Fundamental Research Funds for the Central Universities, Sun Yat-sen University (22qntd2202), the State Key Laboratory of Tropical Oceanography, South China Sea Institute of Oceanology, Chinese Academy of Sciences (LTO2205) and the Research Fund Program of Guangdong Provincial Key Laboratory of Marine Resources and Coastal Engineering.




