Tree logs for grassland restoration? Lessons from an unintentional experiment
Author contributions: ABP, RGR, GEO study design; ABP, RGR, PAT data collection; ABP, LSM, GEO analyzed data; ABP, RGR, LMS, PAT, GDSS, GEO wrote the manuscript.
Abstract
For subtropical Brazilian grasslands, few restoration techniques are established, and seeds of native grassland species are not available on the market. Here, we evaluated the effect of physical barriers (PB) on vegetation recovery in a degraded grassland. We considered species that exhibit attributes in their diaspores that facilitate dispersal as well-dispersed species (WDS) and compared the proportion of WDS at our site to their proportion in the regional species pool. To analyze plots near and distant from the PB, we calculated the extrapolated metrics by an asymptotic estimator (double sample size of each distance). We found 74 species in the degraded area, with higher richness and Shannon diversity values closer to logs, but higher Simpson values with larger distance from logs. Almost half of the species in the degraded area are WDS, more than in the regional species pool. While effects of logs on microsite conditions may also play a role (e.g. logs accumulating organic material or increasing soil moisture, providing shade), our data indicates that the PB works as a seed trap favoring the occurrence of WDS. It seems promising to develop applications in restoration, especially when associating PB with other restoration techniques to increase availability of adequate germination and establishment conditions.
Implications for Practice
- Physical barriers, in our case wooden logs, can work as seed traps for native species in degraded grasslands and possibly create microsite conditions that are favorable for vegetation establishment.
- The species that seemed to be most benefited by interception of logs were those that exhibit structures in their seeds that facilitate dispersal, as Andropogon spp., Aristida spp., and Asteraceae species.
- Development of artificial seed traps appears to be a cheap and simple way to enhance seed arrival in degraded grasslands, in particular when other ways of seed introduction have not been established.
Introduction
In temperate grassland restoration, hay transfer, direct seeding and topsoil application have been used to increase seed recruitment (Kiehl et al. 2010; Klimkowska et al. 2010; Rydgren et al. 2010). Restoration of tropical and subtropical grasslands and savannas also is limited by seed availability (Buisson et al. 2019), making passive restoration difficult (e.g. Koch et al. 2016; Torchelsen et al. 2019). Degradation in these ecosystems due to human action, such as afforestation or agricultural activities, among others, also may affect soil features (e.g. physical and chemical characteristics; soil seed bank). This makes active restoration necessary as the natural community has low resilience to severe degradation (Buisson et al. 2019; Nerlekar & Veldman 2020). Removal of vegetation that leads to the loss of belowground structure makes re-establishment of native species especially difficult (Buisson et al. 2019).
For subtropical grasslands in Brazil, studies that tested techniques of seed introduction still are scarce (e.g. Thomas et al. 2019a, 2019b; Porto et al. 2022) and few generalizations can be made about effectiveness of different seed introduction techniques. At the same time, seeds of native grassland species are not available on the market (Rolim et al. 2022), making restoration projects challenging. Hence, it is important to investigate techniques that modify the environment in a way that seed arrival and plant establishment from seeds in degraded areas is enhanced.
In forest restoration, perches are successfully used to increase seed rain at restoration sites (Guidetti et al. 2016). In grasslands, seed dispersal by animals also may play a role in restoration (see, e.g. Minervini Silva & Overbeck 2021 for the role of endozoochory), but perches should be less successful as few grassland plant species are dispersed by birds. The most common species in subtropical grassland belong to the Poaceae and Asteraceae (Andrade et al. 2019), plant families that contain many species that exhibit anatomical adaptations in their diaspores that facilitate dispersion by wind or surface dispersal (e.g. pappus in Asteraceae; silky hairs on dispersal units, lightweight seeds, awns, dispersal as “tumbleweeds”; Cheplick 1998, Cavanagh et al. 2020). This seed rain can be intercepted by physical barriers (PBs), increasing seed establishment, which may promote vegetation development at degraded sites (Fick et al. 2016). In addition, PBs can also increment plant establishment by changes of site conditions, creating safe site for germination, due to shading, accumulation of organic material and retention of sediment and litter (Fick et al. 2016).
Here we took advantage of an “unplanned experiment” to analyze the potential of PBs, created by wooden logs, to trap seeds and facilitate the establishment of grassland species. At a site originally covered by natural grassland where the topsoil had been removed, wooden logs had been placed to prevent further degradation due to human action in the area. Seven years later, we observed some recovery of the native species. We compared plots closer to and distant to the logs in terms of species richness and diversity to verify if logs served as seed traps. We identified species that exhibit attributes in their diaspores that facilitate dispersal (well-dispersed species [WDS]) in our sample and in the regional species pool and compared the percentage of WDS with these attributes in the degraded area with those that could potentially colonize.
Methods
Study Area and Data Collection
The degraded grassland site is located in Saint'Hilaire Municipal Natural Park (−30.102319°S, −51.088843°W), in Viamão and Porto Alegre municipalities in Rio Grande do Sul State, Brazil (Fig. 1). The vegetation in Saint'Hilaire Park consists of a mosaic of grassland and forest patches. Climate is Cfa according to the updated Köppen system, that is, subtropical without a marked dry season and with hot summers (Peel et al. 2007). At the study site (total area 1700 m2) vegetation and topsoil had been removed to perform aeromodelling activities (flying of airplane models). To prevent this activity in the protected area, wooden (eucalypt) logs were placed at the site in 2012 (Fig. 1D). To evaluate the effect of the logs in enhancing vegetation development, we evaluated, in December 2019 (7 years after placement of logs), vegetation cover in 10 transects, established randomly perpendicular to pairs of logs.
Along each transect, the vegetation was surveyed in square plots of 0.25 m2. The number of plots varied according to the distance between logs, totaling 125 plots. All plant species were identified within plots and their ground cover was estimated using a decimal scale (Londo 1976). The species nomenclature followed the Tropicos website (Tropicos 2020) for exotic species and the Flora do Brasil (Flora do Brasil 2020) for native species.
Data Analysis
Due to different distances between logs, number of plots per transect varied, and shorter distances were better represented in our sample than longer distances (see Table S1). Based on observed species richness from each plot (S number of species) we randomly picked species form the regional pool (using function “sample” from package “base” in R). In other words, we simulated communities with the same observed richness from the plots but formed by random species that could potentially occur in the plots. For each simulated and observed community we calculated the percentage of WDS and we compared simulated and observed values using a Kruskal–Wallis test followed by a post hoc Nemenyi test for contrast between groups (Hollander et al. 2015). This approach is similar to the one used by de Bello et al. (2012), however, much simplified since we do not calculate functional diversity, instead we only calculate frequency of one functional group (WDS). Therefore, we extrapolated species richness (Hill numbers of order q = 0) and diversities (Shannon diversity and inverse of Simpson concentration corresponding of Hill numbers of order q = 1 and q = 2, respectively) using the iNEXT package (Chao et al. 2014; Hsieh et al. 2016) in the R Statistical Environment (R Core Team 2022). For this, we calculated the extrapolated metrics by an asymptotic estimator using the double sample size of each distance class, assuming a 95% confidence interval based on 999 replications.
All species were classified according to the presence or absence of attributes in their diaspores that facilitate dispersal, such as pappus, silky hairs on dispersal units or awns. We call these species well-dispersed species (WDS). To test if these species were preferentially trapped by the PBs, we compared the proportion of species with the same attributes from a reference site and from the regional species pool (i.e., considering all species that could potentially colonize the plots). To access the regional grassland species pool we gathered information from three databases: (1) SpeciesLink (CRIA 2022), looking for herbaria registers of grassland species that mentioned the Saint'Hilaire Municipal Natural Park as sampling local; (2) grassland species of the Flora of the Saint'Hilaire Municipal Natural Park (de Mello et al. 2018); and (3) a vegetation sampling carried out by us in a reference site nearby the degraded area. The reference site was a natural grassland area of 3.8 ha, sampled by 31 plots of 1 m2, where all species were recorded (PA Thomas, unpublished data).
The analyses were performed using ggplot2, PMCMRplus, and stats packages in R environment (Pohlert 2022; R Core Team 2022).
Results
A total of 74 species from 15 families were found in plots at the degraded site (Table S2). Nine individuals were not identified to the species level. Poaceae was the richest family with 26 species, followed by Asteraceae (22) and Rubiaceae (6). The species with highest mean cover (≥5%) were Aristida filifolia (Arechav.) Herter, Andropogon lateralis Nees, Eryngium horridum Malme, Galianthe fastigiata Griseb., and Chamaecrista repens (Vogel) H.S.Irwin & Barneby (Table 1).
| Family | Species | Mean cover (%) |
|---|---|---|
| Poaceae | Aristida filifolia (Arechav.) Herter | 41.12 |
| Poaceae | Andropogon lateralis Nees | 11.60 |
| - | Moss | 8.16 |
| Apiaceae | Eryngium horridum Malme | 6.00 |
| Rubiaceae | Galianthe fastigiata Griseb. | 5.76 |
| Fabaceae | Chamaecrista repens (Vogel) H.S.Irwin & Barneby | 5.36 |
| Poaceae | Schizachyrium sp. | 4.80 |
| Poaceae | Aristida laevis (Nees) Kunth | 4.48 |
| Poaceae | Andropogon selloanus (Hack.) Hack. | 3.68 |
| Poaceae | Dichanthelium sabulorum (Lam.) Gould & C.A. Clark | 2.64 |
| Poaceae | Aristida flaccida Trin. & Rupr. | 2.56 |
| Poaceae | Melinis repens (Willd.) Zizka | 2.40 |
| Asteraceae | Baccharis dracunculifolia DC. | 1.84 |
| Poaceae | Eragrostis polytricha Nees | 1.68 |
| Poaceae | Piptochaetium montevidense (Spreng.) Parodi | 1.60 |
| Rubiaceae | Borreria verticillata (L.) G.Mey. | 1.28 |
| Asteraceae | Gamochaeta simplicaulis (Willd. ex Spreng.) Cabrera | 1.12 |
| Turneraceae | Piriqueta taubatensis (Urb.) Arbo | 1.04 |
| Poaceae | Stenotaphrum secundatum (Walter) Kuntze | 1.04 |
| Asteraceae | Achyrocline satureioides (Lam.) DC. | 1.04 |
| Asteraceae | Disynaphia ligulifolia (Hook. & Arn.) R.M.King & H.Rob. | 0.96 |
| Fabaceae | Crotalaria tweediana Benth. | 0.96 |
| Onagraceae | Oenothera sp. | 0.96 |
| Poaceae | Eragrostis neesii Trin. | 0.96 |
| Campanulaceae | Wahlenbergia linarioides (Lam.) DC. | 0.88 |
| Asteraceae | Orthopappus angustifolius (Sw.) Gleason | 0.88 |
| Poaceae | Schizachyrium condensatum (Kunth) Nees | 0.80 |
| Rubiaceae | Richardia brasiliensis Gomes | 0.80 |
| Poaceae | Axonopus affinis Chase | 0.72 |
| Asteraceae | Baccharis crispa Spreng. | 0.64 |
| Poaceae | Axonopus suffultus (Mikan ex Trin.) Parodi | 0.56 |
| Poaceae | Paspalum plicatulum Michx. | 0.56 |
| Poaceae | Setaria parviflora (Poir.) Kerguélen | 0.56 |
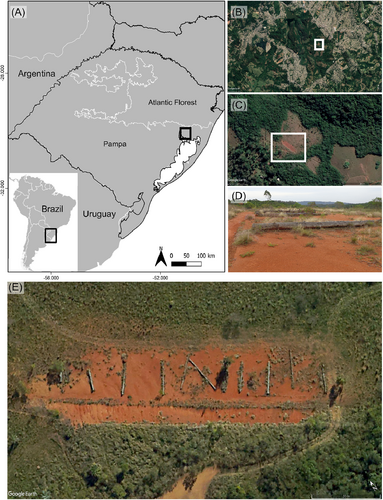
Species richness was highest closest to the PBs and generally decreased with distance from the log (Fig. 2A). For estimated Shannon diversity, the plot closest to the PB (0–0.5 m) presented the highest value compared to the other distances (Fig. 2B). Simpson's diversity showed higher values for those plots far from the logs (Fig. 2C). Furthermore, total ground cover was also negatively affected by distance from logs (Fig. S1).
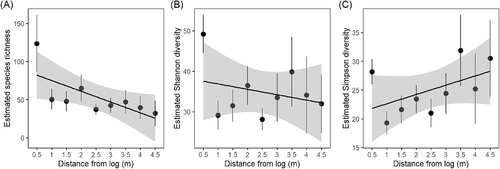
Our characterization of the species pool resulted in 318 species (Table S2). The richest families were Asteraceae with 90 species, followed by Poaceae (63) and Fabaceae (26). One third of these species (33%) had diaspores with attributes that facilitate dispersal (WDS). At the degraded site, 43.2% had these attributes, and these species also showed the highest cover values (Table 1). Logs trapped more WDS than expected, based on the regional species pool (Fig. 3, χ2 = 88.59, p = 0.18) or the reference grassland (χ2 = 5.86, p = 0.05).
Discussion
Our data indicated that the presence of PBs enhanced the establishment of native plant species in a degraded area with bare soil. The most species-rich families were Asteraceae (22 species) and Poaceae (26 species), and the established community included WDS such as Aristida and Andropogon species. Rubiaceae (six species) was the third family in the degraded area, including several Richardia species (R. brasiliensis Gomes, R. grandiflora (Cham. & Schltdl.) Steud. and R. humistrata (Cham. & Schltdl.) Steud.). Species of this genus can be considered ruderal and are commonly found in anthropic environments and areas modified by humans (Lorenzi 1991; Cruz & Martins 2015). The presence of ruderal species in degraded grassland areas after highly impacting activities is common (Le Stradic et al. 2018; de Souza Vieira & Overbeck 2020), especially Richardia species (Vieira et al. 2022).
Fick et al. (2016) demonstrated the effectiveness of barrier structures to mitigate desertification processes and enhance plant recruitment in a degraded semiarid grassland. In their study, Fick et al. (2016) combined barriers with seed addition and soil disturbances, but the increased survival of grass seedlings was likely improved due to the barriers, which suggests that these structures may have functioned as artificial “nurse plants.” Importantly, barriers can have effects beyond enhancing seed arrival: the high cover of moss (mean value of 8.16% in all plots) in the plots closest to the logs in our study indicates that the logs also lead to a modification of local microclimate, maintaining higher levels of soil moisture. Furthermore, moss may also serve as seed traps, as shown in peat bogs and peatlands where this group of plants is recommended for restoration (Groeneveld et al. 2007). Planting nurse plants has been applied and evaluated in different open ecosystems (see Castellanos et al. 1994 and Rubio-Casal et al. 2001: saltmarshes; Hulvey et al. 2017: dryland in general, “restoration island”; Franks 2003: “coastal dunes”; Holl et al. 2021: planting patches, “applied nucleation”) and may lead to the establishment of “islands of fertility” (Gornish et al. 2021) where vegetation development is facilitated. However, the use of nucleation-based techniques in the restoration of grasslands is still little explored (Shaw et al. 2020).
The spatial patterns of vegetation at the degraded site demonstrates that the PBs provided a greater richness of individuals close to them with a gradual decrease as the distance increased. The Simpson index, which gives more weight to abundant species, indicate that the plots closest to the PBs showed a less even community composition in their abundances. Species of the genus Aristida had the highest cover values and occurred mainly closer to the logs. Aristida spp. form large tussocks which contributes to their dominance, reflected by the Simpson index. However, when we measured plant diversity using Shannon diversity, plots closest to the PBs appeared as more diverse, due to higher weight of species richness.
Despite the effectiveness of PBs to trap seeds, 7 years after they were installed (2013–2022, personal observation), a large portion of the soil remained exposed, especially further in all away from the logs, even though the study site is immersed in conserved natural grasslands. Apparently, the proximity of grasslands did not supply enough propagules. Alternatively, the site conditions were too severe (e.g. Fick et al. 2016) to promote fast establishment and vegetation cover, that is, there could be microsite limitation, wherein recruitment of individuals remains limited in spite of seed availability, often requiring disturbances, for example in soil surfaces to break physical crusts and facilitate seed burial (Fick et al. 2016). While the use of PBs appears to be promising, apparently it is not sufficient in the studied system and the creation of adequate conditions for plant establishment is also important. Nonetheless, the efficiency of PBs to increase seed rain in grassland restoration should be studied to disentangle the mechanism(s) at play (seed trap vs. change in microsite conditions). Needless to say, such barriers do not need to be heavy tree logs, but could be made of light and portable materials and thus could be a low-cost and easy to implement technique. Such experiments should also include the evaluation of factors such as the importance of wind direction and ideal height of barriers. Overall, improved knowledge on dispersal and recruitment processes in grasslands is important to find ways to increase seed rain in grassland restoration and thus facilitate restoration (Arruda et al. 2018; Török et al. 2021), even if seed introduction may remain necessary in many cases.
Acknowledgments
We thank staff at the Saint'Hilaire Municipal Natural Park for their availability to assist in field trips, especially to Francisco Carlos Carvalho da Silva, Gerson Luís Mainardi and Josimar Antunes Appel. A.B.P., R.G.R., P.A.T. received scholarships from the Coordenação de Aperfeiçoamento de Pessoal de Nível Superior - Brasil (CAPES) - Finance Code 001. P.A.T. received a “Bolsas Funbio – Conservando o Futuro” grant from Funbio and Instituto Humanize to develop this study. G.E.O. is supported by CNPq.




