Melatonin supplementation improved cryopreserved Thai swamp buffalo semen
Abstract
The production of reactive oxygen species (ROS) during cryopreservation process impairs the sperm characteristics and fertilizing ability. However, melatonin, an antioxidant, could protect spermatozoa against this cell damage during cryopreservation. Therefore, we attempted to evaluate whether the melatonin supplementing in the semen extender could improve the sperm quality of swamp buffalo during cryopreservation. The semen collected from six swamp buffalo bulls were diluted with tris-citrate egg yolk extender supplementing with 0, 0.1, 0.5, 1.0, 2.0 and 3.0 mM of melatonin. The parameters of sperm viability and motility were evaluated using computer-assisted semen analyser (CASA) after cryopreservation on days 1, 7, 15 and 30. The group supplemented with 1.0 mM melatonin exhibited the higher viability after cryopreservation on days 1, 7, 15 and 30 with 58.346 ± 2.1a, 57.586 ± 2.0a, 55.082 ± 1.8a and 55.714 ± 1.8a, respectively, and showed the best results of motility parameters. However, higher concentration of melatonin at 3.0 mM impaired all the parameters. In conclusion, the addition of melatonin at 1 mM to semen extender could exert the best protection against sperm damage in swamp buffalo bull during cryopreservation.
1 INTRODUCTION
Semen cryopreservation is an important procedure used to preserve sperm for a specific period of time. The achievement of semen cryopreservation depends on many factors such as species and age of animals, dilution of semen before freezing, and cooling and freezing process (Andrabi, 2009; Rasul et al., 2000; Sansone et al., 2000). Moreover, the storage duration in liquid nitrogen is important on the quality of cryopreserved semen. In the previous studies, it was reported that the prolonged storage has effects to sperm motility, mitochondrial function, plasma membrane integrity, abnormalities of chromatin structure and DNA integrity (Chatterjee & Gagnon, 2001; Fraser et al., 2014). The low quality of cryopreserved semen would result in poor conception rate by artificial insemination (Andrabi, 2009; Andrabi et al., 2008). Generally, because of cold shock, cooling rate, ice formation, diluent composition, osmotic stress and other factors influencing functional status of survivors (e.g., membrane stability, oxidative damage, membrane receptor integrity and nuclear structure), the cryopreservation process could reduce semen quality by more than 50% (Andrabi, 2009; Hu et al., 2011; Watson, 2000; Zhang et al., 2015). In addition, reactive oxygen species (ROS) and oxidative stress during freezing process may lead to serious sperm damage such as apoptosis, membrane lipid peroxidation, disruption of mitochondria and DNA damage (Anger et al., 2003).
Many studies have recently reported that antioxidants, such as carnitine, CoQ10, vitamin C, vitamin E, zinc, cysteine, glutathione, melatonin and selenium, can improve the semen quality of domestic animals (Ahmadi et al., 2016; Foote et al., 2002; Li et al., 2012). Melatonin (N-acetyl-5-methoxytryptamine) is an indole derivative endogenous compound and regulates the circadian clock, immunological enhancement, sexual behaviour and seasonal reproduction in animals (Arnao & Hernández-Ruiz, 2006; Li et al., 2012). In addition, melatonin by promoting their oxygen radical scavenging activities becomes an intracellular antioxidant to protect the cells from ROS-mediated damages under oxidative stress (ChaithraShree & Ingole, 2019). Indeed, the supplementation of melatonin as a potent antioxidant was used throughout semen cryopreservation in mammals for increasing the semen quality such as improvement of motility, progressive motility, viability and DNA integrity, reduction in membrane lipid peroxidation and modulation of sperm capacitation (Pang et al., 2016).
It is well-accepted that assisted reproductive technology, particularly semen cryopreservation and artificial insemination, greatly promotes the animal breeding and production. However, compared with dairy cattle, it was shown that the sperm of buffalo is more susceptible to damage during freezing and thawing process than that of cattle (El-Regalaty, 2017; Garg et al., 2009; Nair et al., 2006). In the light of the above information, the study was conducted to evaluate the effect of melatonin supplementation in semen extender on frozen-thawed sperm quality of Thai swamp buffalo.
2 MATERIALS AND METHODS
2.1 Ethics approval and consent to participate
The use of animals and the procedures for animal handling was approved by the Institutional Animal Use and Care Committee (IACUC) at Naresuan University, Thailand (no. NU-AG610303).
2.2 Animal management and semen collection
Six sexually mature swamp buffalo bulls at the age of 4–11 years were used in this study. All these bulls were maintained under optimal condition of feeding and management. Semen was regularly collected weekly for successive 6 weeks using artificial vagina at 42°C and then transferred to laboratory and kept at 37°C in water bath. The ejaculates of individual bulls were evaluated for volume, concentration and percentage of motility. The following minimum standards with at least 70% motility and 500 × 106 sperm/ml were used (Shahzad et al., 2016).
2.3 Preparation of semen extender
The components of extender media used for semen cryopreservation contained Tris-citric egg yolk extender (200 mM tris-(hydroxymethyl)-aminomethane, 70 mM citric acid, 55 mM fructose, 20% (v/v) egg yolk, 7% (v/v) glycerol, benzylpenicillin (1,000 IU/ml) and streptomycin sulphate (1,000 µg/ml); pH 6.9). Melatonin (M5250; Sigma-Aldrich) was dissolved in dimethyl sulphoxide (DMSO) and added to the extender to yield five different final concentrations: 0.1, 0.5, 1.0, 2.0 and 3.0 mM. Final concentration of DMSO in all samples was 0.1%. The control group contained the same DMSO concentration as that in the treatment groups.
2.4 Evaluation of sperm kinematic parameters
Since only those semen samples which the motility and sperm concentration is more than 70% and 5 × 108, respectively, were used in this study, no fresh sample was applied as the control group but only frozen-thawed semen without melatonin treatment as the control. Each specimen was divided into six aliquots and diluted with extenders containing different melatonin concentration to a final concentration of 2 × 107 sperms/ml. Diluted semen aliquots were packed into 0.25 ml straws, cooled to 4°C within 2 hr and equilibrated for 2 hr at 4°C. The semen straws were placed 5 cm above liquid nitrogen (LN2) for 15 min in styrofoam box and then immediately submerged in LN2 for storage at −196°C. After 1, 7, 15 and 30 days of storage in LN2 tank, semen straws (n = 3 per each freezing group) were thawed at 37°C for 30 s in water bath. The semen samples (n = 2,592 straws) were evaluated at post-thawed stage for sperm motility, velocity distribution and kinematics using computer-assisted sperm analysis (CASA; The IVOS II, Hamilton Thorne Inc.). The aliquots of 6 µl of thawed semen in each groups were loaded into pre-heated slides (Leja Slides, SCA®, Spain) at 37°C. The total motility (Tot motile), static sperm (Static), progressive motility (P motile), slow motility (Slow), straight-line velocity (VSL), curve-line velocity (VCL), average path velocity (VAP), linearity (LIN), straightness (STR), beat cross frequency (BCF), wobble (WOB) and amplitude of lateral head displacement (ALH) were measured. Moreover, sperm viability was evaluated by eosin-nigrosin staining. The stain procedure was followed by mixing 7 µl of semen with 7 µl of 1% eosin for 30 s, adding 7 µl of 5% nigrosin for 30 s on a pre-warmed slide (37°C) and immediately shedding with another slide. The viable and non-viable sperm were investigated using phase-contrast microscopy at magnification of 400×. Sperm with an unstained head were regarded as the live sperm and sperm having pink head were deemed as dead sperm.
2.5 Statistical analysis
The data were analysed statistically using ANOVA. Analysis of variance was performed with the general linear model procedure of SAS (SAS Enterprise Guide 4.1; SAS Institute Inc.). The significance of the differences was analysed by Duncan's multiple range test, and p < .05 was considered to be statistically significant.
3 RESULTS
3.1 Melatonin increases viability and motility of frozen-thawed sperm
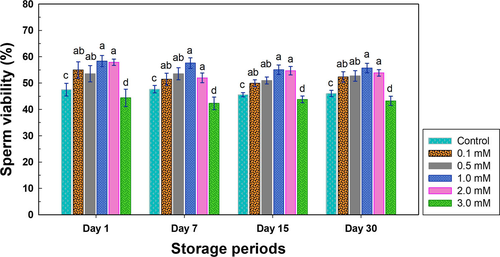
It was found that the percentages of sperm viability in the groups supplemented with 0.1, 0.5, 1.0 and 2.0 mM of melatonin are more than 50% after different cryopreservation duration (Figure 1). By mean, the groups supplemented with 0.1, 0.5, 1.0 and 2.0 mM of melatonin were 54.976 ± 3.1ab, 53.346 ± 3.4ab, 58.346 ± 2.1a and 57.878 ± 1.20a, respectively, after cryopreservation on day 1. After cryopreservation on day 7, the groups supplemented with 0.1, 0.5, 1.0 and 2.0 mM of melatonin were 51.438 ± 2.3ab, 53.544 ± 1.9ab, 57.586 ± 2.0a and 51.976 ± 1.84a, respectively. After cryopreservation on day 15, the groups supplemented with 0.1, 0.5, 1.0 and 2.0 mM of melatonin were 49.902 ± 1.3ab, 50.99 ± 2.11ab, 55.082 ± 1.8a and 54.684 ± 1.61a, respectively. After cryopreservation on day 30, the groups supplemented with 0.1, 0.5, 1.0 and 2.0 mM of melatonin were 52.314 ± 2.0ab, 52.72 ± 2.46ab, 55.714 ± 1.8a and 53.882 ± 1.22a, respectively. The group supplemented with 1.0 mM of melatonin showed the best results. Furthermore, the current results showed that supplementation of melatonin in semen extender significantly increased the viability of thawed sperm when compared with the control group (p < .05), except for the group of 3.0 mM melatonin, in which the viability was even significantly lower than that in the control group (p < .05; Figure 1).
In accord with the viability result, high concentration of melatonin, that is, 3.0 mM in the present study, has significantly deleterious effect on sperm mobility (Table 1). However, the rest of groups supplemented melatonin improved thawed sperm motility, and it indicated that the supplementation of 1.0 mM melatonin exhibited the significantly better parameters than those in the control group (p < .05; Table 1). Furthermore, the supplementation of 1.0 mM melatonin also exhibited the best results for significant improvement in all the sperm motility parameters when compared with the control group (p < .05). Again, the concentration of melatonin reaching 3.0 mM also impaired the sperm motility (Table 1).
| Parameters | Storage period | Concentration of melatonin | |||||
|---|---|---|---|---|---|---|---|
| Control | 0.1 mM | 0.5 mM | 1.0 mM | 2.0 mM | 3.0 mM | ||
| Tot motile (%) | Day 1 | 42.40 ± 2.92cd | 46.61 ± 2.48bc | 47.55 ± 2.58b | 53.61 ± 2.86a | 46.84 ± 2.77bc | 36.09 ± 2.52d |
| Day 7 | 40.78 ± 2.86cd | 44.18 ± 2.63bc | 45.81 ± 2.41b | 53.37 ± 3.38a | 43.09 ± 2.65bc | 35.79 ± 2.24d | |
| Day 15 | 38.01 ± 1.96cd | 41.82 ± 2.42bc | 46.06 ± 2.62b | 51.89 ± 3.01a | 45.33 ± 2.37bc | 35.97 ± 2.36d | |
| Day 30 | 40.93 ± 1.83cd | 42.73 ± 2.59bc | 47.63 ± 2.35b | 53.30 ± 2.70a | 43.32 ± 2.84bc | 36.06 ± 2.09d | |
| Static (%) | Day 1 | 57.59 ± 2.92cd | 53.39 ± 2.48bc | 52.45 ± 2.58bc | 46.63 ± 2.87a | 53.15 ± 2.77bc | 63.91 ± 2.52d |
| Day 7 | 59.22 ± 2.86cd | 55.85 ± 2.63bc | 54.19 ± 2.41bc | 46.64 ± 3.37 a | 56.91 ± 2.65bc | 64.21 ± 2.24d | |
| Day 15 | 61.99 ± 1.96cd | 58.13 ± 2.41bc | 53.88 ± 2.61bc | 48.11 ± 3.01a | 54.67 ± 2.37bc | 64.03 ± 2.36d | |
| Day 30 | 58.94 ± 1.86cd | 57.53 ± 2.63bc | 52.37 ± 2.42bc | 46.70 ± 2.67a | 58.01 ± 3.32bc | 63.94 ± 2.08d | |
| P motile (%) | Day 1 | 19.24 ± 1.60cd | 20.70 ± 1.70bc | 22.66 ± 2.04ab | 26.15 ± 2.07a | 22.24 ± 1.76bc | 16.65 ± 1.56d |
| Day 7 | 18.49 ± 1.54cd | 19.57 ± 1.45bc | 21.75 ± 1.63ab | 25.87 ± 2.14a | 20.51 ± 1.49bc | 17.03 ± 1.61d | |
| Day 15 | 17.00 ± 1.24cd | 19.25 ± 1.57bc | 21.08 ± 1.71ab | 24.03 ± 2.04a | 20.43 ± 1.57bc | 14.73 ± 1.29d | |
| Day 30 | 18.75 ± 1.36cd | 18.82 ± 1.64bc | 22.59 ± 2.12ab | 25.42 ± 1.85a | 19.51 ± 1.78bc | 15.16 ± 1.45d | |
| Slow (%) | Day 1 | 11.84 ± 0.87bc | 13.53 ± 0.77ab | 12.55 ± 0.72ab | 13.78 ± 0.85a | 11.99 ± 0.87ab | 10.04 ± 0.78c |
| Day 7 | 11.22 ± 0.72bc | 12.59 ± 0.96ab | 12.45 ± 1.00ab | 13.27 ± 1.05a | 11.69 ± 0.98ab | 9.90 ± 0.70c | |
| Day 15 | 11.43 ± 0.50bc | 11.21 ± 0.99ab | 12.98 ± 1.10ab | 14.31 ± 1.04a | 13.35 ± 1.13ab | 11.10 ± 1.01c | |
| Day 30 | 11.80 ± 0.72bc | 12.62 ± 0.76ab | 13.83 ± 0.53ab | 13.61 ± 0.71a | 12.59 ± 0.91ab | 11.38 ± 0.86c | |
- Abbreviations: P motile, percentage of progressive motility; Slow, percentage of slow motility; Static, percentage of static sperm; Tot motile, total motility.
- a,b,cValues in the row with different letters indicate significant differences (Duncan's multiple range test).
3.2 Melatonin has no effects on the kinematic parameters of frozen-thawed sperm
To further analyse sperm kinematic parameters such VAP, VSL, VCL, WOB, ALH, BCF, STR and LIN, it was shown that melatonin supplementation could not improve or enhance sperm kinematics after different cryopreservation duration (Tables 2 and 3), although it did significantly improve the motility of frozen-thawed sperm at certain concentrations. Surprisingly, high concentration of melatonin at 3.0 mM had no effect on kinematic parameters as it did in sperm motility.
| Parameters | Storage period | Concentration of melatonin | |||||
|---|---|---|---|---|---|---|---|
| Control | 0.1 mM | 0.5 mM | 1.0 mM | 2.0 mM | 3.0 mM | ||
| VAP (µm/s) | Day 1 | 65.97 ± 1.11 | 64.81 ± 1.25 | 66.01 ± 1.19 | 68.26 ± 1.33 | 68.16 ± 1.45 | 65.41 ± 1.13 |
| Day 7 | 64.76 ± 2.76 | 65.89 ± 1.76 | 66.61 ± 2.17 | 68.44 ± 1.85 | 67.60 ± 1.74 | 65.21 ± 1.52 | |
| Day 15 | 63.24 ± 0.96 | 65.56 ± 1.40 | 66.58 ± 2.13 | 65.54 ± 1.96 | 64.74 ± 2.05 | 63.89 ± 2.10 | |
| Day 30 | 64.80 ± 1.31 | 62.25 ± 1.33 | 64.01 ± 1.14 | 68.26 ± 1.34 | 63.21 ± 1.57 | 61.53 ± 1.57 | |
| VSL (µm/s) | Day 1 | 53.34 ± 1.59 | 51.93 ± 1.76 | 53.94 ± 1.76 | 55.11 ± 1.76 | 55.44 ± 2.08 | 53.21 ± 1.66 |
| Day 7 | 53.40 ± 1.70 | 52.74 ± 1.81 | 54.29 ± 2.29 | 55.52 ± 1.86 | 54.64 ± 1.96 | 53.00 ± 1.62 | |
| Day 15 | 50.78 ± 1.11 | 51.94 ± 2.28 | 53.51 ± 2.06 | 52.91 ± 1.88 | 52.19 ± 2.01 | 50.99 ± 2.11 | |
| Day 30 | 52.56 ± 1.52 | 50.64 ± 1.45 | 51.50 ± 1.23 | 55.06 ± 1.35 | 51.53 ± 1.81 | 49.40 ± 1.55 | |
| VCL (µm/s) | Day 1 | 115.25 ± 2.52 | 112.80 ± 2.38 | 116.46 ± 2.50 | 119.49 ± 2.94 | 120.72 ± 2.81 | 115.42 ± 2.33 |
| Day 7 | 115.55 ± 2.43 | 115.60 ± 2.71 | 113.52 ± 2.70 | 117.75 ± 2.50 | 115.65 ± 2.41 | 112.28 ± 1.81 | |
| Day 15 | 110.42 ± 2.29 | 113.30 ± 1.67 | 115.80 ± 2.79 | 113.05 ± 2.94 | 111.88 ± 2.86 | 111.19 ± 3.35 | |
| Day 30 | 112.55 ± 1.96 | 107.04 ± 2.00 | 111.12 ± 2.00 | 117.42 ± 2.42 | 108.12 ± 1.97 | 107.85 ± 2.65 | |
- Abbreviations: VAP, average path velocity; VCL, curvilinear velocity; VSL, straight-line velocity.
| Parameters | Storage period | Concentration of melatonin | |||||
|---|---|---|---|---|---|---|---|
| Control | 0.1 mM | 0.5 mM | 1.0 mM | 2.0 mM | 3.0 mM | ||
| WOB (%) | Day 1 | 59.18 ± 0.89 | 59.14 ± 0.83 | 59.51 ± 0.82 | 59.29 ± 0.75 | 59.41 ± 0.73 | 59.25 ± 0.75 |
| Day 7 | 59.59 ± 0.66 | 58.92 ± 0.87 | 60.48 ± 0.84 | 59.99 ± 0.75 | 60.18 ± 0.73 | 59.91 ± 0.77 | |
| Day 15 | 59.41 ± 0.75 | 59.59 ± 0.91 | 59.32 ± 0.87 | 60.06 ± 0.79 | 59.86 ± 0.81 | 59.46 ± 0.66 | |
| Day 30 | 59.46 ± 0.80 | 59.82 ± 0.78 | 59.43 ± 0.85 | 59.92 ± 0.80 | 59.93 ± 0.80 | 58.92 ± 0.72 | |
| ALH (µm) | Day 1 | 5.37 ± 0.18 | 5.33 ± 0.16 | 5.37 ± 0.16 | 5.61 ± 0.16 | 5.62 ± 0.14 | 5.29 ± 0.12 |
| Day 7 | 5.44 ± 0.12 | 5.48 ± 0.16 | 5.28 ± 0.14 | 5.49 ± 0.12 | 5.37 ± 0.15 | 5.23 ± 0.10 | |
| Day 15 | 5.25 ± 0.17 | 5.31 ± 0.15 | 5.45 ± 0.13 | 5.31 ± 0.14 | 5.28 ± 0.11 | 5.32 ± 0.14 | |
| Day 30 | 5.35 ± 0.15 | 5.13 ± 0.15 | 5.28 ± 0.17 | 5.51 ± 0.18 | 5.09 ± 0.16 | 5.26 ± 0.15 | |
| BCF (Hz) | Day 1 | 30.55 ± 0.76 | 30.18 ± 0.70 | 30.58 ± 0.76 | 30.13 ± 0.67 | 30.15 ± 0.57 | 30.46 ± 0.67 |
| Day 7 | 30.40 ± 0.57 | 30.13 ± 0.72 | 31.04 ± 0.76 | 31.13 ± 0.60 | 31.04 ± 0.71 | 30.79 ± 0.65 | |
| Day 15 | 30.56 ± 0.76 | 31.00 ± 0.89 | 30.65 ± 0.81 | 30.95 ± 0.84 | 30.54 ± 0.80 | 29.60 ± 1.12 | |
| Day 30 | 30.47 ± 0.72 | 30.58 ± 0.76 | 30.41 ± 0.72 | 30.61 ± 0.73 | 31.17 ± 0.83 | 29.58 ± 0.67 | |
| STR (%) | Day 1 | 80.20 ± 0.97 | 80.30 ± 0.96 | 80.49 ± 0.86 | 79.63 ± 0.68 | 79.73 ± 0.85 | 80.61 ± 0.85 |
| Day 7 | 79.66 ± 0.74 | 80.18 ± 0.87 | 81.11 ± 0.87 | 80.79 ± 0.77 | 80.25 ± 1.00 | 80.93 ± 0.82 | |
| Day 15 | 80.07 ± 0.85 | 80.62 ± 0.96 | 79.85 ± 0.77 | 80.50 ± 0.71 | 80.12 ± 0.79 | 79.61 ± 0.88 | |
| Day 30 | 80.57 ± 0.85 | 80.63 ± 0.80 | 80.01 ± 0.92 | 80.46 ± 0.85 | 81.19 ± 0.96 | 80.30 ± 1.02 | |
| LIN (%) | Day 1 | 49.04 ± 1.28 | 49.01 ± 1.24 | 49.41 ± 1.16 | 48.77 ± 1.00 | 48.96 ± 1.02 | 49.28 ± 1.08 |
| Day 7 | 49.15 ± 0.97 | 48.74 ± 1.24 | 50.60 ± 1.22 | 50.00 ± 1.05 | 49.92 ± 1.16 | 50.00 ± 1.09 | |
| Day 15 | 49.22 ± 1.10 | 49.65 ± 1.33 | 49.00 ± 1.15 | 49.92 ± 1.06 | 49.61 ± 1.09 | 48.98 ± 1.03 | |
| Day 30 | 49.45 ± 1.18 | 49.84 ± 1.14 | 49.18 ± 1.22 | 49.74 ± 1.22 | 50.23 ± 1.23 | 48.82 ± 1.14 | |
- Abbreviations: ALH, amplitude of lateral head displacement; BCF, beat-cross frequency; LIN, linearity (VSL/VCL); STR, straightness (VSL/VAP); WOB, wobble (VAP/VCL).
4 DISCUSSION
Semen cryopreservation is an important procedure in the field of assisted reproduction. However, this process including cooling, freezing and thawing negatively affects sperm functions by inducing osmotic stress, cold shock, intracellular ice crystal formation, excessive production of ROS and an alteration in antioxidant systems (Amidi et al., 2016; Chatterjee & Gagnon, 2001; Oehninger et al., 2000). Melatonin is an intracellular antioxidant which protects cells from ROS-mediated damages under oxidative stress (ChaithraShree & Ingole, 2019), and it can stimulate the activity of antioxidant enzymes such as superoxide dismutase, glutathione peroxidase and catalase (Jang et al., 2010; Karbownik & Reiter, 2000).
Recently, the evidences about the beneficial effects of melatonin supplementation on protecting spermatozoa by reducing the number of free radicals during freezing and thawing process in animals and human have been emerged (Husna et al., 2017; Karbownik & Reiter, 2000; Karimfar et al., 2015; Meamar et al., 2016; Succu et al., 2011). This study also demonstrated the protective effect of melatonin supplementation on the quality of semen cryopreservation in Thai swamp buffalo after storing in LN2 for 1, 7, 15 and 30 days. Low concentration of 0.1 mM melatonin supplementation could improve the sperm viability, but it was not enough to exert positive effects on the sperm motility unless the melatonin concentration increased to 1.0 mM. In addition, it was found that the high concentration, that is 3.0 mM, of melatonin in the semen extenders did not protect sperm from cryodamage but impair the sperm quality.
The results showed that 0.1, 0.5, 1.0 and 2.0 mM of melatonin supplementation in semen extender exhibited the positive effects on sperm viability and motility, and the group supplemented with 1.0 mM melatonin showed the best results in all parameters. Interestingly, storage periods of frozen sperm in LN2 did not affect the sperm quality if melatonin had been supplemented. These results were consistent with the previous study demonstrating that supplementation of 1.0 or 2.0 mM melatonin in semen extender could improve the post-thawed quality of bull semen (Ashrafi et al., 2013). Effect of melatonin on post-thawed semen quality in ram, Nili-Ravi buffalo bull and Egyptian buffalo bull has also been reported (Husna et al., 2017; Mohamed, 2015; Succu et al., 2011). The semen extender supplemented with 0.1 mM melatonin significantly improves the post-thawed motility and viability of sperm in Egyptian buffalo bull (Mohamed, 2015), and the melatonin at 0.1 mM and 0.5 mM also performs the positive effects on post-thawed quality of sperm in Nili-Ravi buffalo bull (Husna et al., 2017). However, the ram sperm needs higher concentration of melatonin at 2.0 mM increasing viability and motility of post-thawed sperm (Succu et al., 2011).
It was speculated that melatonin could protect sperm from the cryodamage but not improve sperm kinematic parameters due to the function of antioxidation. If sperm survived after freezing-thawing procedure, the kinematic parameters will not be affected. In our preliminary analysis of sodium dodecyl sulphate–polyacrylamide gel electrophoresis (SDS-PAGE) on thawed sperm, it was noticed that the expression of 75, 100 and 125 kDa of proteins changed in accord with the concentrations of melatonin supplementation and freezing periods (Figure S1). It is not clear whether any important proteins are involved in the protection effect of melatonin. A further proteomic analysis would be conducted to identify the key proteins.
In sum, the concentration of melatonin supplementation used to protect sperm from damage during cryopreservation was related to animal species and freezing process. The results emerged from our study clearly demonstrated that 1.0 mM melatonin supplementation in semen extender could exert valuable effects on post-thawed semen quality in Thai swamp buffalo.
ACKNOWLEDGEMENTS
This study was financially supported from Agricultural Research Development Agency (Public Organization), Thailand (PRP6005011440).
CONFLICT OF INTEREST
The authors declare no conflict of interest.
AUTHOR CONTRIBUTIONS
Wilasinee Inyawilert designed the experiment. Wilasinee Inyawilert, Janyaporn Rungruangsak, and Vuttikai Paungsukpaibool performed the experiment. Wilasinee Inyawilert analyzed the data and drafted the manuscript. Wilasinee Inyawilert,Yu-Jing Liao, and Pin-Chi Tang revised and edited the manuscript.
Open Research
DATA AVAILABILITY
The data that support the findings of this study are available from the authors upon reasonable request.




