Detection and localization of regucalcin in spermatozoa of water buffalo (Bubalus bubalis): A calcium-regulating multifunctional protein
Contents
Regucalcin (RGN) is a calcium-regulating, anti-apoptotic, antioxidative and antiproliferative multifunctional protein predominantly seen in liver and kidney. All these functions are very crucial during spermatogenesis and sperm maturation process until fertilization of the ovum. Although many studies have reported the wide distribution of regucalcin in the male reproductive tract of the rat, human and bovine, its presence in spermatozoa is yet to be demonstrated wherein calcium has a pivotal role in the transport, capacitation, acrosomal reaction and further fusion with ova. Here, we detected the expression of regucalcin mRNA and protein in buffalo spermatozoa using real-time PCR and Western blot, respectively. The study detected two new regucalcin isoforms of 44 kDa and 48 kDa size along with the reported 34-kDa, 28-kDa and 24-kDa isoforms, wherein the 34-kDa isoform was found to be membrane associated in spermatozoa. Further, immunocytochemistry study localized the regucalcin protein in the acrosomal region of the caudal and ejaculated buffalo spermatozoa while it was detected in both cytoplasm and acrosomal region of testicular spermatozoa. This discovery of RGN in spermatozoa and localization in the acrosomal region will help to focus researchers to see its role in calcium-related functions like capacitation, acrosomal reaction and membrane fusion. Overall, regucalcin may be a new fertility marker in buffalo and can be utilized for infertility treatments.
1 INTRODUCTION
Regucalcin (RGN) is a 299-amino acid protein having a molecular weight of approximately 34 kDa, seen in cytoplasm, peri-nuclear space, nucleus (Ishigami, Handa, Maruyama, & Supakar, 2003; Maia, Santos, Schmitt, & Socorro, 2008; Tsurusaki, Misawa, & Yamaguchi, 2000) and mitochondrial fractions (Arun, Aleti, Parikh, Manne, & Chilukuri, 2011) of cells. It is also named as Senescence Marker Protein-30 due to its molecular weight and characteristic downregulation with ageing in rat liver (Fujita, Shirasawa, Uchida, & Maruyama, 1992). RGN is demonstrated to have multiple roles like calcium homoeostasis, L-ascorbic acid biosynthesis, antioxidant, antiprolific as well as anti-apoptotic functions (Correia, Vaz, Silva, Cavaco, & Socorro, 2017; Correia et al., 2014; Jeong et al., 2008; Kondo et al., 2008; Yamaguchi, 2005; Yamaguchi & Daimon, 2005). The location of regucalcin gene (RGN) on the X-chromosome and the highly conserved nature of RGN throughout evolution, from invertebrates to vertebrates, indicate its role in important basic biological functions (Laurentino et al., 2011). The in vitro existence and in vivo existence of two isoforms of RGN (28 and 24 kDa) due to proteolysis were demonstrated (Arun et al., 2011). Two alternatively spliced mRNA variants of regucalcin originated by exon skipping mechanisms have also been reported in liver and kidney of humans (Murata & Yamaguchi, 2014). Alternative splice variants of RGN lacking exons 4 and 5 were also described in breast and prostate tissues (Maia, Santos, Schmitt, & Socorro, 2009). Another study has reported four RGN immunoreactive bands of 30, 56, 78 and 131 kDa in rat mammary gland and two immunoreactive bands of 56 and 30 kDa in prostate in Western blot analysis (Maia et al., 2008).
RGN was shown to be expressed in liver, kidney (Shimokawa & Yamaguchi, 1992; Yamaguchi & Isogai, 1993), brain (Yamaguchi, 2000), heart (Yamaguchi & Nakajima, 2002), bone (Yamaguchi, Misawa, Uchiyama, Morooka, & Tsurusaki, 2002), lung (Mori et al., 2004), submandibular gland (Ishii et al., 2005), ovary (Fayad, Lévesque, Sirois, Silversides, & Lussier, 2004) and breast (Maia et al., 2008). In the recent past, RGN expression was reported from the male reproductive tract. Noteworthy, RGN expression in all testicular cell types in rat, human and bovine was confirmed by PCR, Western blot and immunohistochemistry (Cucuzza, Divari, Mulasso, Biolatti, & Cannizzo, 2014; Laurentino et al., 2011). Additionally, it is detected in accessory sex glands namely seminal vesicles, bulbourethral and prostate glands of male reproductive tract of the rat, bovine and human (Cucuzza et al., 2014; Laurentino et al., 2011) and also in seminiferous tubule fluid (Laurentino et al., 2011), epididymal fluid (Laurentino et al., 2011) and in seminal vesicular fluid (Harikrishna, Shende, Reena, Thomas, & Bhure, 2016; Harikrishna, Shende, Parmar, et al., 2016). Its wide distribution in male reproductive tissues and in their secretions suggests an important role of this protein in male reproduction.
Although RGN has been assigned to many physiological functions, it is known principally to control cellular Ca2+ level by regulating the activity of Ca2+ pumps and channels (Takahashi & Yamaguchi, 1993, 1999; Yamaguchi & Mori, 1989). Several studies have shown that elevation of sperm intracellular Ca2+/Ca2+ influx regulates the motility, hyperactivation, chemotaxis, capacitation and acrosome reaction, thus facilitates the spermatozoa in reaching and fertilizing the oocyte (Foresta & Rossato, 1997; Kwon, Park, Mohamed, & Pang, 2013; Lishko & Kirichok, 2010; Mannowetz, Naidoo, Choo, Smith, & Lishko, 2013; Suarez, 2008). These Ca2+-dependant functions of spermatozoa and wide distribution of RGN in male reproductive tract motivated us to explore the presence of RGN in spermatozoa. There are no published reports of RGN expression in spermatozoa of any species. The research intervention to improve buffalo fertility is gaining importance as these animals remain underutilized despite having immense genetic potential. The main reason behind this are reproductive problems like poor reproductive efficiency and infertility (Moaeen-ud-Din, 2014). Thus, the aim of the present study was to detect and localize RGN in the spermatozoa of buffalo which can be used as a fertility marker and used for infertility treatment in buffaloes.
2 MATERIALS AND METHODS
All experiments were performed in accordance with the Institutional Animal Ethics Committee (I.A.E.C), Indian Veterinary Research Institute (IVRI), Izatnagar, India.
2.1 Sample collection
Fresh semen ejaculates of healthy breeding Murrah buffalo bulls (n = 3) maintained at Germ plasm Centre (Animal Reproduction Division, Indian Veterinary Research Institute, Izatnagar, India) were collected using the artificial vagina method and directly processed. Caudal semen samples were collected from mature buffalo testis procured from slaughter house (Bareilly, Uttar Pradesh, India) immediately after slaughter and transported to laboratory (n = 5). The epididymis was separated from the testis after thoroughly washing with PBS, and epididymal ducts were exposed by trimming the superficial tissue. Using a 16-gauge hypodermic needle, the caudal region was punctured at many points and subsequently squeezed to collect the spermatozoa-rich fluid in PBS. Samples containing at least 75% of motile spermatozoa and less than 20% morphological abnormality were used for the study. For studying testicular sperms, fresh testis (n = 5) collected from the slaughter house were used (four impression smears from each animal).
2.2 Primers
The forward (5′-GATCCGTTTGGATCCTGAGA-3′) and reverse primers (5′-TTGAAAATTCCACCAGCCTC-3′) for qPCR assay of buffalo RGN were designed using Gene Tool BioLite software from the published cDNA sequence of Bos taurus (Accession No: NM 173957.2). The theoretical length of the amplified product was ~197 bp.
2.3 Total RNA isolation and cDNA synthesis from spermatozoa
Three fresh ejaculates per buffalo were collected (n = 3), and 1 ml of each ejaculate was centrifuged at 700 g for 10 min at room temperature (28°C). The resulting pellet was reconstituted in 1 ml of PBS and purified using discontinuous 45/90% Percoll (Sigma, USA) in PBS (pH 7.4) to remove contaminating somatic cells, germ cells and leucocytes. Two millilitres of 45% Percoll was layered over 3 ml of 90% Percoll, and 1 ml of reconstituted sperm pellet was carefully layered on the top of this and centrifuged at 360 g for 30 min. The spermatozoa pellet was further used for total RNA and protein isolation.
The whole RNA isolation procedure was carried out in an area dedicated to RNA work after thoroughly cleaning with RNase Zap (Sigma, USA), using RNase/DNase-free water and RNase-free glass/plastic wares. The total RNA from sperm samples was isolated using the conventional TRIzol (Invitrogen, USA) method (Parthipan et al., 2015) with slight modification. TRIzol (Invitrogen, USA) pre-heated to 60°C was added to the sperm pellet and mixed well using a micropipette. Using a 26-G needle fixed to a syringe, the sperm cells were lysed by passing the contents through the needle several times, followed by incubation at 65°C for 30 min with intermittent vortexing at every 10 min. To facilitate complete lysis of spermatozoa and separation nucleoprotein complex, the contents were passed again through the needle for several times. Following the addition of 200 μl of chloroform for each ml of TRIzol used, the contents were mixed vigorously and incubated for 5 min at room temperature. The upper layer formed after centrifugation at 12,000 g for 15 min at 4°C was transferred into fresh tubes and incubated for 15 min at room temperature after adding 500 μl of nuclease-free isopropanol. The centrifugation step was repeated, and the RNA pellet formed was washed with 75% ethanol and air-dried in RNase-free environment. The RNA pellet was then reconstituted in nuclease-free water and treated with DNase I (Thermo Fisher Scientific, USA) as per manufacturer's instruction to remove any trace of DNA. The DNase was later inactivated by incubating at 65°C for 10 min. The quality and concentration of the isolated total RNA were assessed using the NanoDrop (Thermo Scientific, USA). The total RNA samples were then stored at −80°C for further analysis. Synthesis of cDNA was carried out from 2 μg of total RNA using Revert Aid™ H-Minus First-Strand cDNA Synthesis kit (Fermentas, USA) as per the protocol described by the manufacturer.
2.4 Real-time PCR
Following the manufacturer's instructions, the qualitative real-time PCR was performed using Maxima SYBR Green/ROX qPCR Mix (Fermentas, USA) in Mx3000P PCR cycler (Stratagene, the Netherlands). Reactions were set in triplicates for each batch of cDNA synthesized along with a non-template control (NTC). The qPCR was performed using 2 μl of cDNA (100 ng/μl), 12.5 μl of 2 × PCR master mix and 5 pmol of each primer in a final volume of 25 μl. Thermal cycling conditions were as follows: 10 min at 95°C followed by 40 repeats of 15 s at 95°C and 1 min at 60°C followed by 5 min at 72°C.
2.5 Western blot
Pure sperm pellet of three different buffalo bulls (three ejaculates per animal) obtained after Percoll purification as explained previously was treated with TPER solution (Thermo Fisher, USA) to lyse spermatozoa. After adding 100 μl of TPER solution to the pellet from 200 μl of semen, homogenized for 1–2 min over ice and centrifuged at 10,000 g for 15 min at 4°C. Total amount of particulate and supernatant fraction of sperm cell lysate were loaded and analysed by Western blot analysis.
Western blot of the particulate and supernatant fraction of sperm cell lysate was performed with primary anti-RGN monoclonal antibody (ab81721, Abcam, UK). The samples were resolved by a 12% SDS-PAGE after mixing with sample buffer containing 5% β-mercaptoethanol and denaturing for 10 min at 100°C. Resolved proteins were blotted onto a PVDF (polyvinylidene difluoride) membrane (Hybond-P, UK) by the semidry transfer method. Blotted membranes were later blocked overnight at 4°C with Tris-buffered saline containing 0.05% (v/v) Tween-20 and 5% (w/v) dry skimmed milk powder (Hi Media, India) and then incubated for 1 hr at 37°C with anti-RGN monoclonal antibody (1:2000). After washing out the unbound primary antibody, membrane was incubated with secondary antibody conjugated with horse radish peroxidase (1:5000; anti-mouse, Santa Cruz, USA) and developed for 5 min with DAB substrate (Sigma, USA).
To rule out the possibility of oligomer formation of RGN in the supernatant fraction, Western blotting was performed with a slightly modified procedure under strong reducing conditions (Berthold & Siedow, 1993). Supernatant fraction sample having 100 μg total protein was denatured for 10 min at room temperature in sample buffer containing 8.0 M urea. Later, β-mercaptoethanol was added to a final concentration of 5% and boiled for 15 min followed by the addition of 200 mM DTT just before loading on a 12% SDS-PAGE containing 2.5 M urea. The resolved proteins were further transferred and developed as explained before. The same experiment was performed using anti-RGN monoclonal antibody (1:2000) and anti-RGN polyclonal antibody (dilution 1:100) available in the laboratory for comparison (Harikrishna, Shende, Reena, et al., 2016).
2.6 Immunocytochemistry
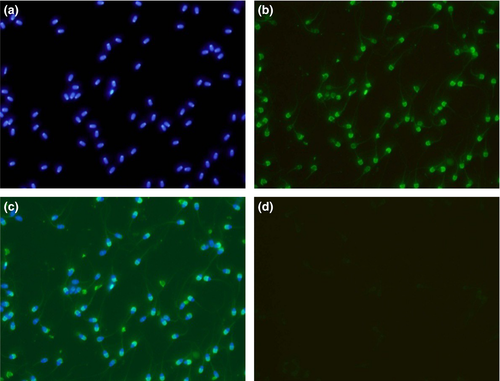
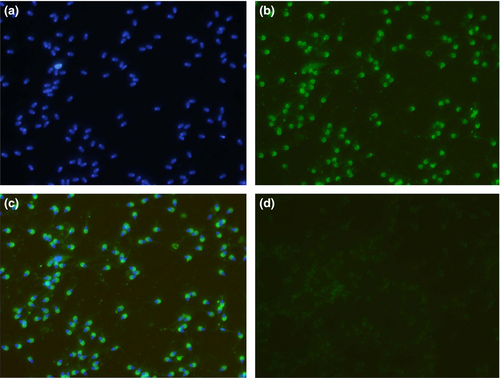
Immunocytochemistry of buffalo testicular (n = 5), caudal (n = 5) and ejaculated spermatozoa (n = 3; three ejaculates per animal) was performed using anti-RGN polyclonal antibodies available in the laboratory. Three smears per spermatozoa type along with one negative control were set for each experiment. A 100 μl of ejaculated semen as well as caudal semen was centrifuged at 300 g for 10 min at 25°C followed by washing twice with 1 ml of PBS at 300 g for 10 min. Pellets were reconstituted in PBS, and the cell count was adjusted roughly to 1 × 107 cells/ml using hemocytometer. Cell smears were prepared on clean grease-free cover slips and allowed to air-dry. For testicular spermatozoa, impression smears of the testes were made by cutting open the testis cross-sectionally and pressing the interior portion against clean grease-free cover slips. Cells were simultaneously fixed and permeabilized with acetone at −20°C for 20 min. Cover slips were washed three times for 5 min each with PBS. Non-specific sites were blocked with 10% normal goat serum in PBS by incubating for 30 min at room temperature. The cover slips were further washed thrice with 0.05% Triton X-100 in PBS of 5 min each. Later, cover slips were incubated with anti-RGN polyclonal antibodies (1:50 dilution) for 45 min at 37°C. Negative control was set for each sperm type by incubating with pre-immunized sera (1:50) instead of primary antibody. Washing step was repeated, followed by incubation with FITC-labelled rabbit anti-mouse IgGs (ab6724, Abcam, 1:5000) in PBS for 15 min, and unbound conjugate was washed thoroughly. The cover slips were mounted with Fluoroshield containing DAPI (F6057, Sigma, USA) and inverted on clean dry glass slides. Images were captured using a fluorescent microscope (IX 71, Olympus, Japan).
3 RESULTS
3.1 Regucalcin mRNA expression in spermatozoa
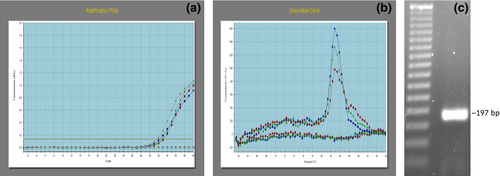
The mRNA expression of RGN in buffalo spermatozoa was confirmed by qPCR of the spermatozoa cDNA. Concentrations of total RNA isolated were in the range of 50–100 ng/μl with A260/280 values between 1.8 and 1.9. The RGN gene amplification plot in spermatozoa is observed (Figure 1a). A single peak in dissociation curve as well as no amplification in NTC (non-template control) showed the specificity of the amplified product (Figure 1b). Further, the amplified PCR product showed the expected 197 bp size on 2% agarose gel electrophoresis (Figure 1c).
3.2 Regucalcin protein expression in spermatozoa
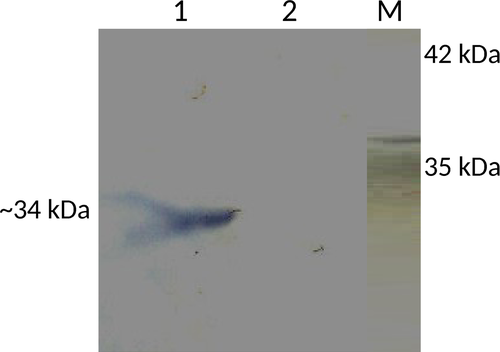
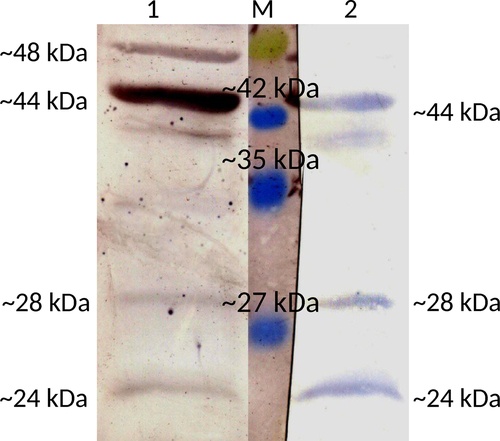
The presence of ~34-kDa isoform of regucalcin in the particulate fraction of a sperm cell lysate was confirmed by Western blot probed with anti-RGN monoclonal antibodies, whereas it was missing in the supernatant (Figure 2). The supernatant of the buffalo spermatozoa lysate showed the presence of other isoforms of regucalcin by Western blot analysis probed using both anti-RGN monoclonal and polyclonal antibodies. Three bands of 44 kDa, 28 kDa and 24 kDa sizes were obtained with monoclonal antibodies, whereas an additional band of 48 kDa along with the other three bands was visualized with polyclonal antibody. Further, the 44 kDa band appeared to be very prominent on the blot probed with polyclonal antibody (Figure 3).
3.3 Immunocytochemistry of spermatozoa
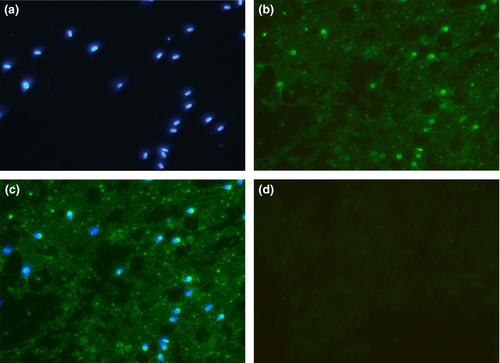
The immunofluorescence studies of spermatozoa from testicular, epididymal and ejaculate showed localization of RGN predominantly in the acrosomal region (Figures 4-6). However, the cytosol and acrosomal regions of the testicular spermatozoa showed immunofluorescence (Figure 4) whereas the immunofluorescence had been restricted to only acrosomal region in case of spermatozoa of cauda epididymis (Figure 5) and ejaculated spermatozoa (Figure 6). Slight fluorescence of the tail axonal region was also observed in few spermatozoa of all samples.
4 DISCUSSION
Here, for the first time, we are reporting the presence of RGN from the spermatozoa of any species. We detected the presence of RGN mRNA in spermatozoa of buffalo by qPCR. Thereafter, the RGN protein has been detected in spermatozoa using anti-RGN antibodies. As the most common isoform of RGN is of 34 kDa, we investigated its presence in ejaculated spermatozoa by Western blot, thereby detecting it in the particulate fraction of lysate but absent in the supernatant. As it has been reported as a cytosolic protein (Arun et al., 2011), we investigated for the presence of RGN by Western blotting under strong reducing conditions in the supernatant of spermatozoa cell lysate. Our study found bands of 44 kDa, 28 kDa and 24 kDa specific to RGN upon probing with monoclonal and polyclonal antibodies, and an additional band of 48 kDa with polyclonal antibody only. The 28 kDa and 24 kDa isoforms have been reported earlier (Arun et al., 2011). The isoforms of 44 kDa and 48 kDa are first reported in our study. The exact functions of these isoforms are not clear at this point. Both polyclonal and monoclonal antibodies raised against the 34 kDa isoform reacted with these new isoforms suggestive of common antigenic epitopes. The possibility of getting these higher molecular weight isoforms due to oligomerization of its lower molecular weight isoforms is less, as we performed the Western blot in strong reducing environment.
The immunocytological study suggests the localization of RGN in the acrosomal region of ejaculated buffalo spermatozoa. There have been reports of the localization of RGN protein in germ cells, mainly in the cytoplasm and weakly in the nucleus of rat and human testis (Laurentino et al., 2011), whereas it was only weakly in nuclei in bovine testes (Cucuzza et al., 2014). Our previous study also demonstrated the localization of RGN mainly in cytoplasm and weakly in nucleus of buffalo germ cells (Harikrishna, Shende, Parmar, et al., 2016). The presence of RGN mRNA suggests the possibility of RGN protein synthesis either in germ cells or spermatozoa or in both. Another possibility is by translocation of RGN into spermatozoa from the external source as it has been detected in the secretions of male reproductive tract (Harikrishna, Shende, Reena, et al., 2016; Laurentino et al., 2011), and there are reports of translocation of exogenous RGN to the nucleus of osteoblasts (Otomo & Yamaguchi, 2006) and liver cells (Omura & Yamaguchi, 1999). Further studies are needed to investigate whether the RGN associated with spermatozoa is of endogenous origin only or both endogenous and exogenous origin.
Further, experiments were conducted to document its expression and localization in different stages of the spermatozoan development by immunocytological studies. The RGN was detected in the cytoplasm and acrosomal region of testicular spermatozoa. However, it is predominantly localized to the acrosomal region of the caudal and ejaculated spermatozoa (Figure 7). Thus, it is observed from the present study that as the sperm advances towards the maturation, that is from testicular cells to ejaculated mature spermatozoa, the RGN appears to relocate from the cytosol to the acrosomal region. Furthermore, acrosome has been demonstrated as a potent calcium-storing organelle of spermatozoa (Costello et al., 2009; Ho & Suarez, 2001) and many studies have reported the requirement of Ca2+ for acrosome reaction (Thomas & Meizel, 1988; Yanagimachi, 1982; Yanagimachi & Usui, 1974). Several ultrastructural studies have demonstrated calcium-binding sites on the outer acrosomal membrane (Berruti, Franchi, & Camatini, 1986; Roomans, 1975; Watson & Plummer, 1986; Watson, Plummer, Jones, & Bredl, 1995). Thus, the localization of RGN to acrosome indicates its role in calcium regulation in spermatozoa. Yet, its exact functions remain to be identified. Interestingly, a recent study identified calcium-binding proteins of 64, 45, 43 and 39 kDa in the acrosomal membrane of bovine spermatozoa (Nagdas et al., 2013). This discovery of calcium-binding protein, RGN, in spermatozoa and particularly in the acrosomal region will help to focus researches on finding out its role in calcium-related functions like capacitation, acrosomal reaction and membrane fusion which can make it a good candidate of male fertility marker and infertility studies.
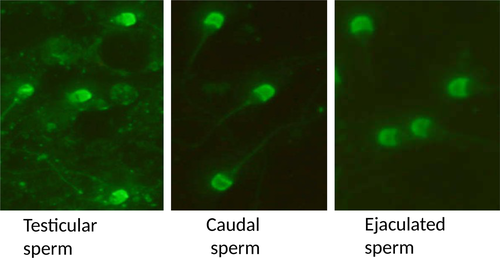
In conclusion, the study demonstrated the mRNA and protein expression of regucalcin in buffalo spermatozoa. To our knowledge, this is the first report of regucalcin from spermatozoa of any species. Additionally, we had detected two new regucalcin isoforms along with other reported isoforms in buffalo spermatozoa using Western blot. Present study also indicated the relocation of RGN from the cytosol to the acrosomal region during maturation of spermatozoa and its membrane association in spermatozoa.
ACKNOWLEDGEMENT
We thank CSIR for providing the fellowship for the first author. We also thank ICAR-IVRI for providing grant and other support for this study.
CONFLICT OF INTEREST
The authors declare that they have no conflict of interest.
AUTHOR CONTRIBUTIONS
We declare that all authors contributed to the research and manuscript as follows: Harikrishna P and S. K. Bhure contributed to the concept, design of the experiment and manuscript drafting. G. Taru Sharma and S. K. Ghosh contributed to data interpretation and analysis. A. M. Shende and Mehtab S. Parmar contributed to the immunocytochemistry study of the experiment. Jobin Thomas and Harikumar S contributed to the Western blot study and manuscript drafting.




