Regional distribution and integrity of equine ovarian pre-antral follicles
Contents
The goal of this study was to determine the distribution of pre-antral follicles in the ovarian parenchyma of mares. For Experiment 1, each ovary was cut longitudinally at the greater curvature, performing two hemiovaries. After that, six fragments from each hemiovary were obtained, resulting in 12 fragments, which were divided into the innermost region of the parenchyma, the middle region and the outermost region. All the three obtained sections were cut transversally to obtain two fragments from each one. For Experiment 2, each ovary also submitted to a longitudinal cut on the greater curvature, forming two hemiovaries. Each hemiovary was sectioned into four symmetrical fragments, resulting in eight fragments per ovary. The fragments were related as being near to or far from the ovulatory fossa. The fragments of both experiments were immediately fixed in Carnoy for 12 hr and kept in 70% ethanol for 24 hr. Follicles were classified according to the stages of development and for morphological integrity according to oocyte morphology and granulosa cells. After the histological assessment, a total of 1,130 follicles were visualized from Experiment 1, being 1,054 (93.3%) primordial follicles and 76 (4.7%) follicles in development. The innermost region had the highest percentage of pre-antral follicles compared to the other regions (p < .05). The middle and outermost regions showed higher percentages of intact primordial and developing follicles than the innermost region (p < .05). Considering Experiment 2, 938 follicles were found, being 894 (95.3%) primordial and 44 (4.7%) follicles in development. The region near the ovulatory fossa presented higher (58.7%; 551 of 938) follicular concentration compared to the region far from the ovulatory fossa (41.3%; 387 of 938; p < .05). As a conclusion, distribution of pre-antral follicles in the equine ovary has a specific pattern through the parenchyma. Also, the follicular integrity differed in the studied ovarian areas.
1 INTRODUCTION
The mammalian ovary is composed of a follicular pool that contains thousands of pre-antral follicles, of which approximately 99.9% never reach ovulation due to the follicular atresia process Markström, Svensson, Shao, Svanberg, and Billig (2002). It is interesting to consider how useful the ovarian pool of pre-antral follicles could maximize offspring of high-value animals and also contribute to the elucidation of follicle activation (Gomes et al., 2015). All reproductive biotechnologies would be improved with a better knowledge about in vitro culture of pre-antral follicles. These follicles are located in the outer portion of ovarian tissue in most species; however, the equine species is not covered by this definition because it is assumed that the follicles are mainly located in the inner portion of the ovary (Charleston et al., 2007). However, little is known about the distribution of primordial follicles in the ovarian parenchyma.
The pre-antral follicle count in the ovaries of mammals provides an estimate of follicular population. Such a study is laborious as all the follicles present in the ovaries must be accounted for. Thus, only ovarian cortex has been studied (Schmidt, 2003; Stansfield, Nöthling, & Ansari, 2011). Most domestic species have been studied about the location and distribution of the ovarian follicular population. Some recent studies found peculiarities, such as the canine species, in which a different number of pre-antral follicles was found between the right and left ovaries (Lunardon et al., 2015). In the bovine species, a distinction in the population of pre-antral follicles in female Bos taurus taurus and Bos taurus indicus was observed (Silva-Santos et al., 2011).
Compared with similarly sized species, the equine ovary has larger dimensions and a lower number of follicles (Driancourt, Paris, Roux, Mariana, & Palmer, 1982; Szlachta & Tischner, 2008). Furthermore, the ovulatory fossa is an aspect that is present only in the equine ovaries, comparing to other species. These characteristics make it challenging to conduct studies of the follicular population in equine ovaries. Recent studies showed small amounts of follicles, even when collected from the inner portion of the ovary (Alves et al., 2015; Gomes et al., 2015). More recently, a new hypothesis was presented to elucidate the uneven distribution of follicles in the equine species. According to Alves et al. (2016), there is a variation in follicle quality, class distribution and follicle and stroma cell densities. These recent publications portray the growing interest of the scientific community in the mapping of the equine follicles. Therefore, this study proposes two investigations regarding the distribution of pre-antral follicles in the equine ovary: i) the presence and integrity of follicles in the innermost, middle and outermost regions of the ovary and ii) the presence and integrity of follicles in the regions near and far from the ovulatory fossa.
2 MATERIALS AND METHODS
This study was performed in accordance with the Animal Experimentation Ethics Committee at the State University of Londrina based on Federal Law 11.794.
2.1 Ovaries
Ovaries (n = 7) were collected from mares in seasonal anoestrus from a slaughterhouse located approximately 40 km (20 min) from the laboratory. Ovaries were transported and stored in a thermal plastic container at 21°C, containing saline solution (Gomes et al., 2012). At the laboratory, each ovary was carefully dissected with a scalpel to remove connective tissue. Ovaries containing corpus luteum (CL) were discarded. There was no repetition of the ovaries in the experiments.
2.1.1 Experiment 1
A total of three ovaries were used. Each ovary was subjected to a longitudinal cut performed on the greater curvature from the ovary to obtain two hemiovaries. Each hemiovary was sectioned vertically into six fragments, totalling 12 fragments that measured approximately 1.5 × 0.5 × 0.5 cm. These fragments were defined into three regions that include the extension of the ovary, termed as follows: the innermost region of the parenchyma (C1), the middle region (between the innermost region and the outermost region C2) and the outermost region (C3). After sectioning, each region (C1, C2 and C3) was cut transversally to obtain two fragments (Figure 1).
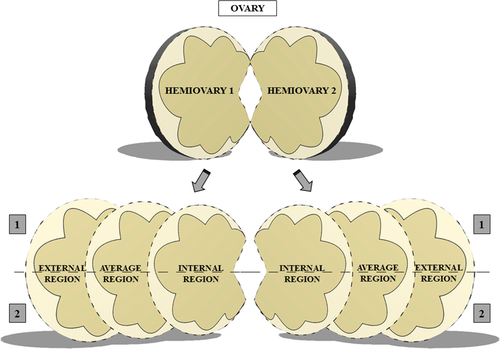
2.1.2 Experiment 2
For this experiment, four ovaries were used. Each ovary was submitted to a longitudinal cut performed on its greater curvature to obtain two hemiovaries. Each hemiovary was sectioned into four symmetrical fragments, totalling eight fragments for each entire ovary, which were located distant or near to the ovulatory fossa. The fragments related to a smaller ovarian curvature were defined as being near to the ovulatory fossa (NOF1 + NOF2; Figure 2), and the fragments that were located mostly on the greater ovarian curvature were defined as being far from the ovulatory fossa (FOF3 + FOF4; Figure 2).
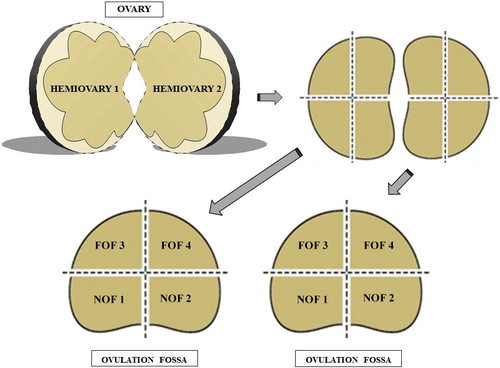
2.2 Histological processing
The fragments of both experiments were immediately fixed in Carnoy for 12 hr (Bufalo et al., 2016), kept in 70% ethanol for 24 hr and then dehydrated in ethanol, subjected to diafinization in xylene, embedded in paraffin and cut sequentially at a thickness of 5 μm for mounting blades. The staining was performed using periodic Schiff's acid and haematoxylin according to Gomes et al. (2015). The histological slides were observed under an optical microscope at magnifications of 20 × and 40 × .
2.3 Follicles classification
Follicles were classified according to the stages of development as follows: (i) primordial follicles (an oocyte surrounded by a layer of flattened granular cells); (ii) developing follicles, comprising primary follicles (an oocyte surrounded by a single layer of granulosa cuboid cells); and secondary follicles (an oocyte surrounded by more than one complete layer of granulosa cuboid cells; Gomes et al., 2015).
Follicles were also evaluated as either intact or degenerate according to oocyte morphology and granulosa cells. Follicles classified as morphologically intact had intact oocytes and granulosa cells arranged in discrete layers without pyknotic nuclei. Degenerate follicles were defined as follicles containing an oocyte with pyknotic nuclei and/or oocytes surrounded by disorganized granulosa cells detached from the membrane and with a retracted cytoplasm (Andrade, Seneda, Alfieri, Frederico, & Figueiredo, 2012; Gomes et al., 2015; Haag et al., 2013).
2.4 Statistical analysis
The proportions of primordial and developing follicles for each region (C1; C2; C3; NOF1 + NOF2; FOF3 + FOF4) were compared. Each variable was analysed for normal distribution using the Kolmogorov–Smirnov test. The statistical model was non-parametric, using Fisher's exact test to compare the primordial follicle percentages and development between the ovarian regions, as well as the follicular integrity. The level of significance was p ≤ .05. All statistical analyses were performed using Minitab® 16.1.1 statistical software.
3 RESULTS
3.1 Experiment 1
In this investigation, 900 slides were examined, containing 2,700 histological sections. Follicles were found only in 12.4% (355/2,700) of the assessed histological sections. A total of 1,130 follicles were found, being 1,054 (93.3%) primordial, and 76 (6.7%) were undergoing development. Considering integrity, 93.71% of the total was morphologically intact.
There were differences (p < .05) in the presence of pre-antral follicles in the inner (41.6%; 470/1130), middle (32.4%; 366/1130) and outermost (26%; 294/1130) regions of the equine ovary. The innermost area showed a higher percentage of pre-antral follicles than the other two regions (p < .05; Table 1). The proportions of primordial follicles found in each region were equivalent, but a significant difference was found when a comparison was made between primordial and developing follicles about follicular integrity. The inner region showed more degenerated follicles than the middle and outermost regions (p < .05; Table 1).
| Ovarian Region | Total % (n) | Morphologically Normal Follicles | Degenerated Follicles | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Primordial % (n) | Developing % (n) | Primordial % (n) | Developing % (n) | ||||||
| Inner (C1) | 41.6a (470) | 88.0a,A | 414 | 3.2a,C | 15 | 7.1a,B | 33 | 1.7a,C | 8 |
| Middle (C2) | 32.4b (366) | 89.1a,A | 326 | 6.0a,B | 22 | 2.2b,C | 8 | 2.7a,C | 10 |
| Outer (C3) | 26.0c (294) | 90.5a,A | 266 | 5.4a,B | 16 | 2.4b,BC | 7 | 1.7a,C | 5 |
| Total | 100 (1130) | 89.1 | 1006 | 4.7 | 53 | 4.2 | 48 | 2.0 | 23 |
- Values followed by small letters (a, b, c) and inside the same column differ statistically (p < .05) between the ovarian regions (inner, middle and outer).
- Values followed by capital letters superscript (A, B, C) and inside the same line differ statistically (p < .05) between the proportion of primordial and development follicles.
3.2 Experiment 2
In this investigation, 800 slides were examined, containing 2,400 histological sections. The follicles were present in only 10.3% (247 of 2,400) of the assessed fragments. A total of 938 follicles were identified, and 894 (95.30%) were primordial follicles, 44 (4.7%) were undergoing development and 96.05% of the total were morphologically normal.
Regarding the regional location of ovarian pre-antral follicles, it was concluded that the regions close to the ovulatory fossa (NOF1 + NOF2; 58.7%; 551/938) presented higher follicular concentrations about the distant regions of the ovulatory fossa (FOF3 + FOF; 41.3%, 387/938, p < .05; Table 2). The ratio of the percentages of primordial and developing follicles found in each region was equivalent, although there was a difference when comparing the proportions of the integrities of primordial and developing follicles. The FOF region showed similar proportions of primordial degenerate and developing follicles to those of the NOF region (p > .05; Table 2).
| Ovarian region | Total % (n) | Morphologically Normal Follicles | Degenerated Follicles | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Primordial % (n) | Developing % (n) | Primordial % (n) | Developing % (n) | ||||||
| Near of OF (NOF) | 58.7a 551/938 | 91.8a,A | 506/551 | 4.0a,B | 22/551 | 2.6a,BC | 14/551 | 1.6a,C | 9/551 |
| Foreign of OF (FOF) | 41.3b 387/938 | 93.8a,A | 363/387 | 2.6a,B | 10/387 | 2.8a,B | 11/387 | 0.8a,B | 3/387 |
| Total | 100 938/938 | 92.6A | 869/938 | 3.4B | 32/938 | 2.7B | 25/938 | 1.3B | 12/938 |
- Values followed by small letters (a, b, c) and inside the same column differ statistically (p < .05) between the ovarian regions (inner, middle and outer).
- Values followed by capital letters superscript (A, B, C) and inside the same line differ statistically (p < .05) between the proportion of primordial and development follicles.
Clusters of follicles were observed in the ovarian tissue, defined as a number greater than two pre-antral follicles very close to each other. These clusters contain only primordial follicles, but many of them showed the first changes of transition to primary follicles (Figure 3).
4 DISCUSSION
Our study demonstrated that equine pre-antral follicles are not equally distributed in the ovarian parenchyma. Also, the follicular integrity may be different according to the regions of the ovary.
The ovarian development is quite peculiar for equine females (Szlachta & Tischner, 2008). In the first year of life, the foal ovary gradually takes the shape of a bean (Ono et al., 2016). This is due to the regression of cell masses in the stroma according to the cessation of hormonal influence. There is a faster growth of ovarian poles to form the ovulatory fossa. The germinal epithelium is concentrated in the area of the ovulatory fossa, as this is the only region of the ovarian surface in which ovulation can occur (Budras, Sack, & Röck, 2008). For adult mares, a detailed distribution of ovarian follicles was unknown, due to the lack of material for study.
Comparing to other farm animals, mare ovaries are not so easily available for scientific purposes, because slaughterhouses for horses are not so common as those for cattle (Gomes et al., 2015). Also, procedures such as the ovarian biopsy (Haag et al., 2013) will not fit the study of follicular distribution as the collected fragments have a little representation of the total mass of the ovary. Thus, there are few studies that cover the follicular distribution in mares. A recent study estimated the follicular density in mare ovaries via fragments collected from an ovarian biopsy procedure (Alves et al., 2016). However, this investigation did not determine the full distribution of follicles, as only small fragments were analysed.
Our study used whole ovaries to obtain precise results for the distribution of ovarian follicles in mares. The ovarian fragmentation in established areas is a reliable alternative to obtaining a precise identification of follicular location (Stansfield et al., 2011). In our work, two strategies of ovarian fragmentation were used, each one with several layers. It was possible to create a new model of ovarian study, covering all regions with specific characteristics, for example the ovulatory area, the inner portion and others.
Distinct patterns between the regions of the equine ovary were identified. The middle, external and area far from the ovulatory fossa had lower amounts of pre-antral follicles than the internal area and region next to the ovulatory fossa. Considering the follicular integrity, the middle and outer regions (with less concentration of follicles) demonstrated higher proportions of intact follicles. It is not clear why it happens. Perhaps some paracrine factors may be involved, besides aspects related to possible differences in the blood distribution in the ovarian parenchyma, like suggested by Alves et al. (2016). Our study did not cover such aspects, but it is known that hormones, growth factors and gases can impact follicular degeneration (Araujo et al., 2014).
The heterogeneous distribution of pre-antral follicles presented in this study brings a novelty on the matter, adding new data on the knowledge previously established (Ginther, 1992). Our results can contribute to a better understanding of the equine ovaries, which have peculiar characteristics, like their large size (González et al., 2015) and the large diameter of antral follicles (Evans 2003).
In our study, clusters of primordial follicles were observed in the fragments belonging to the region near the ovulatory fossa of the equine ovary. This corroborates with findings from Leonel, Bento-Silva, Costa e Silva, and Zúccari (2015), who reported the presence of follicles distributed in small clusters in the equine ovarian tissue. It is not clear if those clusters are keeping this organization during the follicular growth. However, as only primordial follicles were found in such kind of group, that clusters perhaps could dissociate when follicles start activation.
In recent researches related to in vitro culture of equine follicles, it was observed as difficult to find them included in ovarian fragments, even when these fragments were removed in the inner portion of the equine ovary (Alves et al., 2015; Gomes et al., 2015). The equine species has a low follicular density (Driancourt et al., 1982), and many times, the fragments do not present any follicle, as observed in our study. Other domestic species have much more follicles (bovine—Erickson, 1966; ovine—Land, 1970; caprine—Lucci et al., 1999), besides the feasibility of getting ovaries in slaughterhouses. Therefore, our data herein presented will help the scientific community with data useful for other assisted reproductive techniques that use equine follicles, for example ovarian tissue transplantation, cryopreservation, oocyte maturation and others.
5 CONCLUSION
This study suggests new concepts for the location of ovarian follicles in equine species, contributing to the success of reproductive biotechnologies as follicular in vitro culture. A greater presence of pre-antral follicles in the internal and next to ovulatory fossa regions was found, and an increased presence of intact follicles in the middle and outer regions was also observed.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
SMG, CBS, AGL and IB collected the ovaries and processed all samples. LAB and FB performed the analysis and first corrections. MMS was the coordinator of all steps, prepared the final version and the submission.




