Mitochondrial characteristics in oocytes of the domestic cat (Felis catus) after in vitro maturation and vitrification
Abstract
Contents
The objective of this study was to evaluate mitochondria in immature and in vitro-matured domestic cat oocytes and to assess for the first time the effect of vitrification on mitochondrial traits. Mitochondrial distribution and aggregation were assessed using confocal microscopy after staining with the fluorescent dye—MitoTracker® Red CMXRos. Only cells at the germinal vesicle and the metaphase II stages of nuclear development, representing immature and mature oocytes, respectively, were included in our study. Our study shows that 80% of immature and 100% of mature oocytes exhibit a peripheral pattern of mitochondria distribution, indicating that, in contrast to the situation in other species, the mitochondria of cat oocytes are not dispersed throughout the cell after in vitro maturation but instead maintain a strong affinity for the oocyte periphery near the membrane. However, a loss of aggregation was observed during in vitro maturation—78% of immature oocytes showed homogeneous granulation versus only 18% of mature oocytes (p < .001). The increased intensity of MitoTracker® Red CMXRos staining after in vitro maturation (p < .05) may be tentatively attributed to an increase in mitochondrial activity but could likewise reflect a concomitant appearance of sulphhydryl groups in cytoplasm (known to be targeted by the dye). Mitochondrial distribution did not change upon vitrification; however, dye intensity decreased (p < .05) and mitochondrial aggregation was intensified in both immature and mature vitrified cat oocytes.
1 INTRODUCTION
Before fertilization the oocyte must undergo two processes: nuclear and cytoplasmic maturation. The nuclear maturation refers to the ability of an oocyte to complete meiosis (Otoi, Murakami, Ooka, Karja, & Suzuki, 2001). The cytoplasmic maturation of mammalian oocytes is a complex process involving relocation and conformational changes of numerous organelles, namely mitochondrial relocalization, aggregation and changes in activity (Fulka, First, & Moor, 1998).
Morphological features of oocytes, such as number of cumulus cell layers, oocyte shape, as well as colour (different tones between black and gray) and homogeneity of cytoplasm are first indications for their quality (Wood & Wildt, 1997). However, the assessment of these morphological characteristics is not sufficient to accurately predict the developmental competence of an oocyte.
Luvoni, Pellizzari, and Battocchio (1997) showed that after cryopreservation, feline GV oocytes of normal morphology were unable to resume meiosis, what supports the hypothesis that morphology is not a sufficient predictor of oocyte quality. A more comprehensive way of evaluating oocyte quality is to assess the condition of subcellular structures, such as the nucleus, cytoskeleton and mitochondria (Curcio et al., 2014; Lei, Guo, Liu, Tan, & Li, 2014; Mikołajewska, Müller, Niżański, & Jewgenow, 2012; Turathum, Saikhun, Sangsuwan, & Kitiyanant, 2010).
Mitochondria play important roles in cellular energy metabolism in oocytes (De los Reyes et al., 2011). Adenosine triphosphate (ATP) is produced in oocytes by oxidative phosphorylation and is necessary for several functions including maintenance of cellular homeostasis (Brevini, Vassena, Francisci, & Gandolfi, 2005), meiosis (De los Reyes et al., 2011), regulation of apoptosis (Torner et al., 2004) and calcium signalling (Dumollard et al., 2004). Several studies conducted in different species, including humans (Van Blerkom, 2011), cattle (Stojkovic et al., 2001), pigs (Sun et al., 2001; Torner et al., 2004) horses (Torner et al., 2007) and dogs (De los Reyes et al., 2011), revealed that mitochondrial distribution and changes in their activity are valid markers of oocyte maturation competence. Furthermore, mitochondrial aggregation appears to be essential for controlling intracellular pH, protein function and cytoskeleton organization (De los Reyes et al., 2011).
In different mammalian species, changes in mitochondrial traits after vitrification have been shown. Most authors highlights a decrease in activity of mitochondria upon vitrification (Chen, Han, Liu, Wang, & Huang, 2012; Lei et al., 2014; Nazmara, Salehnia, & HosseinKhani, 2014; Shi et al., 2007; Zander-Fox, Cashman, & Lane, 2013), but changes in distribution (Lei et al., 2014) and morphology of mitochondria were also observed (Turathum et al., 2010) in mouse and dog oocytes, respectively.
Because of the negative membrane potential, mitochondria accumulate lipophilic cations and several fluorescent probes have been developed to monitor mitochondrial function in living cells (Buravkov, Pogodina, & Buravkova, 2014; Cottet-Rousselle, Ronot, Leverve, & Mayol, 2011; Johnson, Walsh, Bockus, & Chen, 1981; Niżański, Partyka, & Rijsselaere, 2012; Smiley et al., 1991). However, fluorescence emitted by these conventional mitochondrial markers is often susceptible to photobleaching during microscopic examination and the marker exhibit photoinduced toxicity (Chen, 1989). Furthermore, the mitochondrial membrane potential, which is required for marker accumulation and fluorescence, is lost when cells become fixed. For this study, we applied MitoTracker® Red CMXRos, a dye which has been developed as a non-toxic indicator of relative changes in mitochondrial membrane potential, and which can be used in living cells (Cottet-Rousselle et al., 2011; Pendergrass, Wolf, & Poot, 2004). Additionally, this dye contains a thiol-reactive group that allows for the preparation of stable specimens by fixation (Poot et al., 1996). However, recent findings on covalent MitoTracker® Red CMXRos binding to sulphhydryl groups of proteins and lipids (Buravkov et al., 2014) require a careful interpretation of fluorescence intensity data in terms of mitochondrial potential. Mitochondrial properties in immature and mature feline oocytes have not been thoroughly studied. The two existing reports only address mitochondria distribution in fresh domestic cat oocytes but revealed contradictory results (González, Gómez, Pope, & Brandt, 2012; Martins, Fernandes, Minto, Landim-Alvarenga, & Lopes, 2009). Whereas one study did not reveal mitochondria redistribution upon maturation (González et al., 2012) which has been shown in oocytes of many mammalian species (Stojkovic et al., 2001; Sun et al., 2001; Torner et al., 2007; Van Blerkom, 2011), in the second study, such a redistribution of mitochondria was found in oocytes from adult queens (Martins et al., 2009). The aim of this study was to verify mitochondrial distribution and aggregation, but also to characterize intensity of the fluorescent dye in cat oocytes before and after in vitro maturation. We also investigated how these mitochondrial traits changed in response to vitrification. A better understanding of basic physiological events in cat oocytes could help improve the success and implementation of ART in wild felids.
2 MATERIALS AND METHODS
Unless otherwise stated, all chemicals were purchased from Sigma-Aldrich (St. Louis, MO, USA).
2.1 Experimental design
The study consisted of two experiments. In the first experiment, 49 immature (GV) and 45 mature (MII) fresh oocytes were analysed. In the second experiment, vitrified-warmed oocytes were analyzed—35 immature and 33 mature oocytes. All experimental groups of oocytes were stained (i) with MitoTracker® Red CMXRos to evaluate the mitochondrial characteristics and (ii) with Hoechst 33342 to evaluate the nuclear maturation stage.
2.2 Oocyte collection and in vitro maturation (IVM)
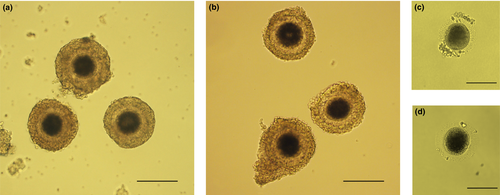
Ovaries from healthy adult domestic cats were acquired by routine ovariohysterectomy performed at local veterinary clinics and were treated as described previously (Hribal et al., 2014; Mikołajewska et al., 2012). Ovaries were either inactive, intermediate or follicular stage, but ovaries with large follicles or CL were discarded. Cumulus oocyte complexes (COCs) were obtained after slicing ovaries with a scalpel blade in a washing medium (WM) consisting of the following: medium 199 containing Earle salts, 3 mg/ml bovine serum albumin (BSA), 0.1 mg/ml cysteine, 1.4 mg/ml HEPES, 0.25 mg/ml sodium pyruvate, 0.6 mg/ml sodium lactate, 0.15 mg/ml l-glutamine and 0.055 mg/ml gentamicin. Only oocytes with dark, homogenous cytoplasm, surrounded by several layers of compacted cumulus cells were selected for the experiments (Figure 1a). To proceed with in vitro maturation (IVM), COCs were placed into 400 μl of maturation medium under 400 μl mineral oil. The maturation medium consisted of WM added with 0.02 IU/ml each of follicle-stimulating hormone (FSH) and luteinizing hormone (LH) obtained from porcine pituitary glands. Maturation was conducted at 38.5°C in the presence of 5% CO2 in a humidified atmosphere for 24 hr.
2.3 Oocyte vitrification and warming
Oocytes were vitrified to prove whether the mitochondria pattern reflects impaired oocytes quality after thawing. All vitrification manipulations were performed at room temperature. Immature oocytes or oocytes subjected to IVM were washed in WM and vitrified as follows: groups of 2–4 oocytes were incubated for 3 min in 5% ethylene glycol (EG) and 5% dimethyl sulphoxide (DMSO) in Dulbecco's phosphate-buffered saline (DPBS), followed by a 3-min incubation in 10% EG and 10% DMSO in DPBS. Finally, oocytes were transferred into vitrification solution (VS: 20% EG, 20% DMSO, 20% FCS and 1.5 M trehalose in DPBS with 10% Ficoll PM-70) and immediately placed on cryoloops (plastic inoculating loop) in a minimum volume (<0.5 μl) of VS. Within 30 s, the oocytes were plunged into liquid nitrogen. After at least 1 day of storage, vitrified oocytes were warmed by plunging the cryoloop into warming solution (WS: 1.5 M trehalose in DPBS, 20% FCS and 10% Ficoll PM-70) for 30 s. Subsequently, oocytes were serially incubated for five-min intervals in WS diluted 1:2 (vol:vol) in DPBS, WS diluted 1:4 (vol:vol) in DPBS and then in DPBS. All warming manipulations were performed in prewarmed (38.5°C) solutions on a 38°C heating stage. Oocytes were transferred to WM and were maintained at 38.5°C in the presence of 5% CO2 for 15 min before analysis.
2.4 Mitochondria and DNA staining
All evaluated oocytes were denuded before staining using The STRIPPER® (Origio, Malov, Denmark). Groups of five oocytes were incubated for 30 min in prewarmed (38.5°C) MitoTracker® Red CMXRos DPBS solution (0.2 μM/ml) in a 5% CO2 incubator at 38.5°C to stain the active mitochondria. After staining, oocytes were fixed in 2% formaldehyde solution for 15 min. Subsequently, oocytes were incubated in Hoechst 33342 DPBS solution (2 μg/ml) for 10 min to stain the DNA. Fixation and Hoechst 33342 staining procedures were performed at room temperature. After every staining or fixing procedure, oocytes were washed three times in DPBS supplemented with 5% BSA. Oocytes were mounted on poly-L-lysine-treated slides, sealed under coverslips with Vaseline® (Unilever, London, UK) and stored for a maximum of 48 hr in a humid dark chamber at room temperature until microscopic analysis. Nuclear configurations were assessed using an epifluorescent microscope (Leica DMLB, Wetzlar, Germany), and oocytes nuclear configuration was classified based on the description proposed by De los Reyes et al. (2011). Chromatin configurations were classified as follows: immature or germinal vesicle (GV), when the vesicle was clearly visible; resumption of meiosis or germinal vesicle break down (GVBD), when the chromatin was dispersed and initiating condensation; first metaphase (MI), when bivalents (pairs of chromosomes) become arranged on the metaphase plate and are attached to the meiotic spindle; and mature or second metaphase (MII), manifested by the presence of chromosomes in the second metaphase plate, with the first polar body (PB) extruded. Because the objective of the study was to demonstrate the differences in mitochondrial parameters between oocytes in the immature (GV) and the mature (MII) stage, oocytes presenting other nuclear configurations (MI or GVBD) or oocytes with signs of degeneration or damage were excluded from our analysis. For mitochondrial evaluation, samples were observed at 200× magnification using an Olympus FluoView1000 Laser Scanning Confocal Microscope (Olympus, Tokyo, Japan) equipped with a helium-neon laser (543 nm). Each oocyte was observed along the z-axis based on 10 serial confocal planes with a 5-μm step size beginning from the equatorial plane and advancing towards the polar plane. The equatorial and polar planes were examined directly.
Considering that MitoTracker® Red CMXRos accumulates preferentially in active mitochondria, mitochondrial distribution was assessed based on the localization of red fluorescence (Cottet-Rousselle et al., 2011; De los Reyes et al., 2011). The mitochondrial distribution was evaluated in the equatorial plane according to Brevini et al. (2005). Oocytes presented one of the following patterns: (i) peripheral—mitochondria only in the periphery of the oocyte, (ii) homogeneous—mitochondria distributed throughout the entire oocyte and (iii) heterogeneous—mitochondria distributed heterogeneously (Figure 2a–c).

Mitochondrial aggregation was evaluated in the polar plane according to De los Reyes et al. (2011). Mitochondrial aggregation was categorized into two groups: (i) no aggregation—finely distributed without aggregation, (ii) homogeneous granular—with large granules (approximately 5 μm in diameter) distributed throughout the cytoplasm (Figure 2d,e).
According to methods described by Torner et al. (2004, 2007), we measured the intensity of MitoTracker® Red CMXRos fluorescence (arbitrary units) using FV1000 ASW software (Olympus Deutschland GmbH, Hamburg, Germany) in the polar plane. Microscope adjustments, filters, offset and gain remained constant throughout the experiments. The area of measurement was adapted to the size of each oocyte, and the average intensity of every oocyte was used for statistical analysis.
2.5 Statistical analysis
Mitochondrial distribution and aggregation patterns were verified and evaluated using a chi-square test. Mitochondrial fluorescence intensity readouts were compared using a one-way analysis of variance (one-way ANOVA) followed by Tukey's post hoc test (Statistica for Windows; StatSoft Inc., Tulsa, OK, USA). Probabilities <.05 were considered statistically significant.
3 RESULTS
3.1 Oocyte collection and in vitro maturation
Of the COCs (n = 132) collected for the first experiment, 52 were evaluated before IVM. Ninety-four per cent (49/52) were included in the experiment as GV stage oocytes. Eighty COCs were matured in vitro for 24 hr, and 56% (45/80) reached the MII stage.
For the second experiment, 165 COCs were collected. Seventy oocytes were vitrified before IVM. After warming, 56% (39/70) of oocytes were morphologically normal, and no signs of cryoinjuries, such as zona pellucida ruptures, oocyte membrane damage or cytoplasm irregularities, were observed (Figure 1b). Thirty-five oocytes were included in the second experiment as GV stage oocytes. Ninety-five oocytes were matured in vitro for 24 hr, 58% (55/95) of which reached the MII stage (Figure 1c). After vitrification and warming, 60% (33/55) of the oocytes retained a normal physiological appearance and were included in the experiment (Figure 1d).
Altogether, in two experiments, 162 oocytes were analysed.
3.2 Distribution and aggregation of active mitochondria
In the first experiment, most immature (80%) and all mature (100%) oocytes showed a peripheral mitochondrial distribution pattern in the equatorial plane (Table 1).
| Oocyte group | Peripheral, % (n) | Homogeneous, % (n) | Heterogeneous, % (n) |
|---|---|---|---|
| Fresh immature | 80 (39/49)a | 16 (8/49)a | 4 (2/49)ab |
| Fresh mature | 100 (45/45)a | 0b | 0a |
| Vitrified immature | 80 (28/35)a | 6 (2/35)ab | 14 (5/35)b |
| Vitrified mature | 94 (31/33)a | 3 (1/33)ab | 3 (1/33)ab |
- Different superscript letters (a, b) within columns represent significant differences within groups (p > .05).
In the polar plane, where the degree of mitochondrial aggregation is visible, 78% of immature oocytes showed a homogeneous granular pattern with large granules (~5 μm in diameter) distributed throughout the cytoplasm. In contrast, in the group of mature oocytes, finely distributed mitochondria without aggregation were present in 82% of the cells (Table 2).
| Oocytegroup | No aggregation, % (n) | Homogeneousgranular, % (n) |
|---|---|---|
| Fresh immature | 22 (11/49)a | 78 (38/49)a |
| Fresh mature | 82 (37/45)b | 18 (8/45)b |
| Vitrified immature | 20 (7/35)a | 80 (28/35)a |
| Vitrified mature | 27 (9/33)a | 73 (24/33)a |
- Different superscript letters (a, b) within columns represent significant differences within groups (p < .001).
In the second experiment, vitrification did not influence mitochondrial distribution, but changes in mitochondrial aggregation (p < .05) after cryopreservation of mature oocytes were observed (Table 2). The most common pattern in all cryopreserved oocytes was a homogeneous granular pattern with large mitochondrial aggregations distributed throughout the cytoplasm, which was observed in 80% and 73% of immature and mature oocytes, respectively (Table 2).
3.3 Intensity of mitochondrial staining
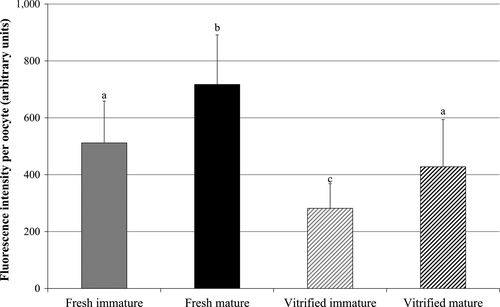
The fluorescence intensity, within a single oocyte was significantly higher in the in vitro matured oocytes relative to the immature oocytes in both fresh and vitrified oocytes (p < .05). Average intensity of fluorescence in vitrified oocytes was significantly lower (p < .05) than in fresh oocytes, in both immature and mature oocytes groups (Figure 3).
4 DISCUSSION
In this study, we investigated (i) whether the distribution and aggregation of mitochondria as well as the fluorescence intensity of the mitochondrial dye MitoTracker® Red CMXRos in domestic cat oocytes changes upon in vitro maturation and (ii) whether these mitochondria characteristics are influenced by vitrification of oocytes.
Mammalian species employ different strategies of mitochondrial distribution during oocyte maturation. In humans, bovines, swine and equines, most immature oocytes show peripheral mitochondrial localization (Stojkovic et al., 2001; Sun et al., 2001; Torner et al., 2007; Van Blerkom, 2011). During cytoplasmic maturation, the mitochondria migrate centrally and eventually become evenly distributed throughout the cytoplasm. In the rhesus monkey, cynomolgus monkey and hamster, oocyte mitochondria appear evenly distributed throughout the cytoplasm in immature oocytes, and no distributional changes occur during maturation (Bavister & Squirrell, 2000; Suzuki, Satoh, & Toyokawa, 2005; Yin, Duffy, & Gosden, 2006).
Using transmission electron microscopy (TEM), Martins et al. (2009) showed that changes in mitochondrial distribution in cat oocytes are dependent on the maturation stage and the degree of sexual maturity. They observed that mitochondria were distributed in the periphery of oocytes collected from prepubertal queens, both before and after 24 hr of in vitro maturation. In contrast, in oocyte obtained from adult cat mitochondria after 24 hr of in vitro maturation were evenly distributed. It was shown that mitochondrial distribution depends on the age of the female and sexual maturity of peripubertal female (Pawlak et al., 2012). In our study, all the ovaries originated from adult females. Furthermore, the results of Martins et al. (2009) should be considered cautiously as Liu et al. (2010) proposed that because of the size of a mammalian oocyte, TEM methodology is limited in determining the complete distribution and geometrical features of the mitochondria. In another study on cat oocyte mitochondrial distribution, González et al. (2012) examined mitochondria in immature and in in vitro- and in vivo-matured cat oocytes. The authors did not observe a clear pattern in the relocalization of active mitochondria during oocyte maturation, as 84% of immature, 87% of in vitro-matured and 71% of in vivo-matured oocytes showed peripheral mitochondrial localization. In the present study, similar to González et al. (2012), 80% of immature oocytes showed peripheral mitochondria localization. Mitochondria of all in vitro matured fresh oocytes were peripherally located.
The functional significance of mitochondrial localization to the oocyte periphery remains unknown. Some authors have suggested that peripheral localization of mitochondria in the GV stage oocyte is related to the high-energy requirement of the cortex of the oocyte. GV stage oocytes are supported by cumulus cells, which are connected to the oolemma by gap junctions to supply nutritional resources (Liu et al., 2010; Sun et al., 2001). This process, which is involved in intercellular cooperation, could result in high-energy demand explaining the mitochondrial localization in immature oocytes. When compared to cattle or pigs, success of feline oocyte IVM remains lower. We suspect that IVM conditions, which do not fully mimic in vivo conditions, could also cause inadequate changes in mitochondrial distribution. However, as described above, results from in vivo-matured oocytes presented by González et al. (2012) confirm that the peripheral location of mitochondria is a normal pattern of domestic cat oocytes. Additional studies that directly compare mitochondria arrangement in oocytes after maturation in vitro and in vivo may explain whether our results and those reported by González et al. (2012) are specific for cat species or whether these results are due to the suboptimal in vitro conditions used.
In the present study, no changes in the mitochondrial distribution between fresh and cryopreserved oocytes were observed for immature or mature oocytes. This lack of change indicates that mitochondrial distribution is not subject to change during vitrification and thus likely does not explain cryopreservation-related limitations of oocyte competence. Notably, we observed a change in the degree of mitochondrial aggregation between immature and mature oocytes. In 78% of GV stage oocytes, the mitochondria formed large granules (~5 μm in diameter), whereas 82% of MII stage oocytes did not show mitochondrial aggregation.
Unlike our results, studies performed on oocytes of other species, such as horses (Torner et al., 2007), cattle (Stojkovic et al., 2001) and pigs (Sun et al., 2001), showed an increase in mitochondrial aggregation during the in vitro maturation process. According to Bavister and Squirrell (2000), a moderate degree of mitochondrial aggregation is normal, which could be important for oocyte maturation and eventually embryonic development. For instance, mitochondrial aggregation plays a role in modulating the local free calcium concentration, which also mediates oocyte activation (Sun et al., 2001). Disorders in mitochondrial aggregation increase cell permeability to external calcium. The increase in intracellular free calcium in turn activates hydrolytic enzymes, reduces energy production and ultimately results in cell death (Berridge, Bootman, & Lipp, 1998). A study on dog oocytes by De los Reyes et al. (2011) showed small mitochondrial granulations throughout the entire ooplasm in most immature oocytes and large granulations in most mature oocytes. According to De los Reyes et al. (2011), some mitochondrial aggregation appears to be essential for controlling intracellular pH, protein function and cytoskeleton organization.
The reports mentioned above suggest that the aggregation increase is a normal phenomenon, and the formation of aggregates is associated with a significant increase in mitochondrial activity (Egerszegi et al., 2010). In cat oocytes, aggregation occurs only in the GV stage, which is unlike other species. Nevertheless, when aggregations are dissolved during the maturation process, fluorescence intensity of the mitochondrial dye increases and is higher in MII oocytes (Figure 3). The enigmatic role of mitochondrial aggregation in GV stage oocytes requires elucidation, but results of the present study, which are consistent with results obtained by De los Reyes et al. in dogs (2011), suggest that even in immature oocytes, mitochondrial aggregation is a physiologically normal event in some species.
We observed an increase in mitochondrial aggregation in in vitro-matured oocytes after vitrification. In a study performed by Noto et al. (1993), mitochondrial aggregation was observed in non-surviving blastomeres of cryopreserved human embryos, which could suggest that such a large increase in mitochondrial aggregation could be a sign of poor viability in oocytes. Although changes in mitochondrial aggregation that appear after vitrification may have been induced by the cryopreservation process, mitochondrial aggregation may not necessarily lead to lower oocyte competence. Indeed, some ultrastructural changes in oocytes or embryos that appear after cryopreservation are reversible (Dalcin et al., 2013; Yin et al., 2006). Based on our results, we hypothesize that changes in mitochondrial aggregation patterns could help explain the decrease in cat oocyte competence observed after vitrification; however, more studies on this subject are required.
According to Egerszegi et al. (2010), photometric measurement of the fluorescence intensity of mitochondria-specific dyes provides valuable information with respect to the number of mitochondria and their metabolic activity. In some species, such as humans, no changes in mitochondrial activity during oocyte maturation were noted (Wilding et al., 2001), whereas in most other studied species, such as pigs, bovines, rodents and horses, mitochondrial activity per oocyte peaked shortly before ovulation (Brevini et al., 2005; Rho et al., 2002; Suzuki et al., 2005; Torner et al., 2004, 2007). A high energy/ATP demand for the resumption of meiosis (spindle formation, chromosome condensation, polar body expulsion) and fertilization initiated in the MII stage could explain the increased mitochondrial activity (Cui et al., 2009; Suzuki et al., 2005). Investigations of El Shourbagy, Spikings, Freitas, and St John (2006) and Reader, Cox, Stanton, and Juengel (2014) showed that in pigs and sheep, respectively, competent, mature oocytes had significantly more mtDNA content than immature oocytes suggesting also an increase in the number of mitochondria. Luminescence-based measurement of intracellular ATP from mouse oocytes has shown that mitochondrial oxidative phosphorylation is the major source of ATP synthesis in the unfertilized oocyte and that the number of mitochondria correlates with the capacity for ATP generation (Dumollard et al., 2004; Sun et al., 2001). Studies on bovine and human oocytes showed a correlation between the level of mitochondrial respiration and the rate of embryo development (Stojkovic et al., 2001; Wilding et al., 2001). A study on equine oocytes confirmed that higher metabolic activity of mitochondria correlates with higher developmental competence (the ability to develop into an embryo) of oocytes after IVM (Torner et al., 2007). The removal of oocytes from the follicular environment could require higher metabolic activity and a concomitant increase in ATP content after IVM (Stojkovic et al., 2001).
So far, no reports on mitochondrial activity in cat oocytes are available. We determined the average fluorescence intensity of MitoTracker® Red CMXRos in the polar plane of each oocyte and observed a significant increase during the in vitro maturation (Figure 3). However, according to the dye's covalent binding to sulphhydryl groups, it may only tentatively be considered as potential sensitive mitochondrial dye, and we cannot discern whether the activity or the number of mitochondria amplified after IVM. Furthermore, it needs to be mentioned that Buravkov et al. (2014) also demonstrated that the dye with the advantage to be stable after fixation not only binds sulphhydryl groups in mitochondria, but also in the cytoplasm of cells. Interestingly, it is reported that the content of free cytoplasmic sulphhydryl groups, particularly of glutathione, increases upon oocyte maturation in different species which is dependent on the presence of cumulus cells and precursor molecules (bovine: de Matos, Furnus, & Moses, 1997; horse: Luciano, Goudet, Perazzoli, Lahuec, & Gérard, 2006). We therefore suggest that the increase in dye intensity upon maturation may not solely be caused by an increase in activity or number of mitochondria but also by additional sulphhydryl sources in cytoplasm which is supported by the partly observed diffuse staining pattern (Figure 2d), particularly in the fresh mature oocytes.
In the present study, a significant decrease in fluorescence intensity of oocytes subjected to vitrification was observed (Figure 3). Our findings are consistent with results of studies on the oocytes of other species (Chen et al., 2012; Lei et al., 2014; Nazmara et al., 2014; Zander-Fox et al., 2013). Zander-Fox et al. (2013) suggested that the decrease in mitochondrial activity may be due to osmotic stress within the oocyte caused by exposure to high level of cryoprotectants which are used in vitrification procedure to prevent ice crystal formation. Other authors suggested that non-physiological temperatures, phase transition, pH instability and osmotic pressure change during dehydration–rehydration might also be factors affecting the function or number of mitochondria (Chen et al., 2012; Lei et al., 2014; Nazmara et al., 2014; Shi et al., 2007). Taking together, we hypothesize that the fluorescence intensity of MitoTracker® Red CMXRos affected by vitrification may reflect a decreasing meiotic and developmental competence of oocyte.
In conclusion, it is known that mitochondria generate the energy required for critical periods of oocyte development. Their distribution, aggregation and activity are related to the level of cell metabolism, proliferation and differentiation. Our study indicates that the predominantly peripheral arrangement of mitochondria in immature cat oocytes is also clearly observed in all mature oocytes. The level of mitochondrial aggregation decreases during in vitro maturation. The fixable dye MitoTracker Red CMXRos is clearly suited to analyse these morphological mitochondrial properties. It remains to be investigated whether the observed maturation-related increase in intensity reflects an increase in activity or number of mitochondria or a change in available sulphhydryl groups in cytoplasm as well. Vitrification procedure did not exert an influence on mitochondrial localization but induced mitochondrial aggregation and decreased dye intensity.
ACKNOWLEDGEMENTS
We thank the veterinary clinic of the Berlin Animal Shelter for collecting and providing cat ovary samples.
CONFLICT OF INTEREST
None of the authors have any conflict of interest to declare.
AUTHOR CONTRIBUTIONS
In the submitted article, all co-authors contributed in the experimental design and manuscript writing. Dr. Natalia Sowińska was responsible for performing the experiments. Dr. Karin Muller contributed in fluorescence analysis of oocytes. Prof. Dr. Katarina Jewgenow and Prof. Dr. Wojciech Niżański contribute in project planning and project funding.




