Localization of CD9 Molecule on Bull Spermatozoa: Its Involvement in the Sperm–Egg Interaction
Contents
Tetraspanin CD9 is one of the egg membrane proteins known to be essential in fertilization process. The presence and localization of CD9 molecule in spermatozoa and its possible function in reproduction are still unclear. In our study, we describe the localization of CD9 on bull spermatozoa. In the immunofluorescence assay, the positive signal has been observed in the high proportion of sperm cells as a fine grains either on the apical part or through the entire anterior region of sperm head. CD9 recognized by monoclonal antibody IVA-50 was detected on freshly ejaculated (83.4 ± 3.7%) and frozen-thawed (84.3 ± 2.3%) sperm. The same reaction pattern was observed on sperm capacitated for 1 h, 2 h, 3 h and 4 h (83.6 ± 2.0%; 84.0 ± 1.5%; 85.7 ± 0.8%; 77.5 ± 10.8%). The presence of CD9 exclusively on plasma membrane of the bovine sperm has been detected by Western blot analysis of the protein fractions after the discontinuous sucrose gradient fractionation of the bull sperm. Moreover, probable role of the sperm CD9 molecule in fertilization process of cattle has been suggested as sperm treatment with anti-CD9 antibody significantly reduced (by 25%, p ≤ 0.001) the number of fertilized oocytes compared to control group in fertilization assay in vitro.
Introduction
Mammalian fertilization is a very complex process, depending on many events, including interaction between gametes mediated by multiple proteins on their surface. Successful binding of spermatozoa to zona pellucida followed by fusion is an essential step to zygote formation. Tetraspanin CD9 is one of the molecules which involvement in fertilization process is permanently considered. The essential role of the oocyte CD9 in gamete fusion was demonstrated in mice more than 10 years ago by the inability of CD9 knockout mouse oocytes to fuse with sperm (Kaji et al. 2000; Le Naour et al. 2000; Miyado et al. 2000) and by the ability of anti-CD9 antibodies to inhibit this fusion (Chen et al. 1999; Le Naour et al. 2000; Miller et al. 2000; Takahashi et al. 2001). Reduction of sperm binding and fusion after oocyte treatment with anti-CD9 antibodies was also reported in pig (Li et al. 2004) and cattle (Zhou et al. 2009). The presence of CD9 on mice sperm was described by Rubinstein et al. (2006) and Barraud-Lange et al. (2007). They hypothesized that CD9 tetraspanin was transferred with several other proteins from oocyte to the fertilizing spermatozoa via trogocytosis process. Miyado et al. (2008) supported this hypothesis, but they have suggested that this transfer is supported by exosome-like vesicles, released from the oolemma into the perivitelline space. Later, the coexistence of both ways of membrane transfer was confirmed by Barraud-Lange et al. (2012). Contrary to Chen et al. (1999) and Li et al. (2004), the expression of CD9 on mouse spermatozoa was reported by Ito et al. (2010) and confirmed by Barraud-Lange et al. (2012). Beside rodents, the presence of CD9 was reported on boar sperm by Kaewmala et al. (2011) and in the protein extracts of the bovine sperm (Cupperová et al. 2014). Caballero et al. (2013) proposed the transfer of CD9-positive microvesicles to bovine spermatozoa during the epididymal maturation.
Although the role of CD9 tetraspanin has been widely studied and many experimental data and hypotheses are available, the exact role of this molecule in the sperm–egg interaction is still unclear. In terms of its involvement in the fertilization process, the oocyte plasma membrane CD9 has been studied in more detail, but CD9 expressed on spermatozoa cannot be omitted from the consideration. Moreover, in fertilization process, the differences on physiological level (genital tract, gametes) as well as on molecular level exist between various species, so using other models than rodents in the study of CD9 role could help to improve the interpretation of fertilization process in its complexity.
The aim of present work was to study the involvement of bovine CD9 molecule in reproduction. Our work has been aimed at determination of the precise localization of CD9 on ejaculated bovine spermatozoa as well as at monitoring CD9 dynamics during sperm capacitation. Moreover, the potential role of the sperm CD9 molecule in fertilization process in cattle has been examined under in vitro conditions.
Material and Methods
All chemical reagents were obtained from Sigma-Aldrich (St. Louis, MO, USA) unless otherwise noted.
Antibodies
A hybridoma cell lines producing mAbs (monoclonal antibodies) directed against the bovine CD9 molecule were obtained after immunization of BALB/c mice with bovine platelets using the standard hybridoma production protocol (Dušinský et al. 1988). The specificity of CD9 mAb (IVA-50) used in this study was verified on the III International Workshop of Leukocyte Antigens of Ruminants in Davis (USA) 1997 and by Tomášková et al. (1999). Supernatant used in IVF assays was without sodium azide (to avoid its harmful effect during in vitro cultivation). Purified IVA-50 and IGg2a were obtained from Exbio (Prague, Czech Republic), and polyclonal rabbit anti-CD9 antibody (ABIN741015) from antibodies-online GmbH (Aachen, Germany). Secondary antibodies, anti-mouse and anti-rabbit IgG-FITC conjugate were supplied by Vector laboratories (Burlingame, CA, USA).
Sperm and oocytes
Fresh ejaculates and frozen-thawed sperm obtained from Slovak Breeding Services, Inc. (Nitra, Slovakia) were used for all experiments. Donors of sperm were Holstein bulls (age 4–6 years, average body condition score 3.5). Freshly ejaculated spermatozoa were separated from seminal plasma via centrifugation at 600 × g for 10 min and resuspended in TL medium for sperm cell capacitation (TL medium) (Minitube, Čeľadice, Slovakia). The frozen sperm thawed from pellets were diluted in phosphate-buffered saline (PBS) for immunostaining or in TL medium for capacitation and in vitro fertilization (IVF) assay. Cumulus–oocyte complexes (COC) were aspirated from ovaries obtained at a slaughterhouse and transported to the laboratory in physiological saline at 37°C. Cumulus–oocyte complexes with intact compact cumulus and homogenous ooplasm were collected under a stereomicroscope Olympus SD30.
Sperm capacitation
Sperm were resuspended to the final concentration of 108/ml in TL medium and incubated for 30 min or 4 h (frozen-thawed or freshly ejaculated) at 39°C with 5% CO2 in humidified atmosphere. The capacitation state of sperm was assessed by the chlortetracycline (CTC) fluorescence assays, described previously by Fraser et al. (1995). Briefly, after the capacitation process, sperm suspensions were centrifuged, the TL medium was removed, and sperm were resuspended in phosphate-buffered saline (PBS) and mixed with equal volume (45 μl/45 μl) of CTC solution (750 μmol/l CTC in 130 mmol/l NaCl, 5 mmol/l cysteine, 20 mmol/l Tris-HCl, pH 7.8) and incubated for 30 min. Cells were then fixed by 8 μl of 12.5% paraformaldehyde in 0.5 mol/l Tris-HCl (pH 7.4). After incubation, sperm suspension was placed on a glass slide, smeared, mounted with Vectashield-DAPI (Vector Laboratories) and overlaid by a cover slip. To avoid the evaporation and CTC fading, slides were kept in a wet chamber until the evaluation was carried out. Samples were evaluated under a Leica DM5500 B epifluorescence microscope at ×400 magnification. Fluorescence images were recorded using a Leica DFC340 FX digital camera and processed using Leica Advanced Fluorescence software (Mikro s.r.o., Prague, Czech Republic).
Sperm fractionation
Fractionation was made according Somanath and Gandhi (2004). Ten microlitre of fresh ejaculate was diluted 1 : 2 with Krebs Ringer Bicarbonate medium (devoid of BSA, containing 2.37 mmol/l fructose). Fifteen microlitre of diluted semen was layered on 20 ml of 1.3 mol/l sucrose/0.9% NaCl and centrifuged for 30 min at 2000 × g at 4°C (Hettich 420R). The sperm pellets resuspended in 108 ml of 0.15 mol/l NaCl/5 mmol/l HEPES pH 7.0 were layered on 1.3 mol/l sucrose/0.9% NaCl (six separate conical centrifuge tubes with 18 ml sperm suspension on 20 ml sucrose) and centrifuged for 20 min at 34 000 × g at 4°C (Beckman SW32 Ti rotor). The final pellets were resuspended in 20 ml 0.15 mol/l NaCl/5 mmol/l HEPES pH 7.0 with protease inhibitors and sonicated 10 × 10 s intervals (Soniprep 150, 30 Amplitude microns power). Membranes were separated from homogenate (3 ml) by discontinuous sucrose gradient consisting of 4 ml 1.75 mol/l sucrose and 4 ml 1.3 mol/l sucrose and centrifugation for 3 h at 95 000 × g at 0–5°C (Beckman SW 40 Ti rotor). The band of material at the interface between the sample and 1.3 mol/l sucrose was the plasma membrane fraction; the band at the 1.3 mol/l/1.75 mol/l sucrose interface was acrosomal membrane fraction. The inner acrosomal membranes and equatorial segments remained closely associated with the sperm heads in the pellets. The final materials were diluted with PBS and pelleted by centrifugation for 30 min at 120 000 × g (Beckman SW 40 Ti rotor), solubilized with 1% (v/v) Triton X-100 for 1 h at 4°C. Protein concentrations were determined by BCA assay kit (Pierce, Rockford, IL, USA) and microplate spectrophotometer (Multiskan GO, Thermo Scientific, Waltman, MA, USA).
Electrophoresis and Western blotting
Membrane protein extracts were separated by 12% SDS-PAGE (10 μg/well) under non-reducing conditions and proteins were transferred to nitrocellulose membranes by semidry electroblotting. Membranes were blocked with 5% low fat milk and immunoblotted with IVA-50 followed by goat anti-mouse IgG conjugated with alkaline phosphatase, and bands were visualized using NBT/BCIP (4-nitro-blue tetrazolium chloride/5-bromo-4-chloro-3-indolyl-phosphate) solution (MP, Biomedicals, LLC, Solon, OH, USA) to detect CD9 molecule; or with biotinylated WGA followed by streptavidin-HRP conjugate (Pierce, Rockford, IL, USA) and chemiluminescence analysis to detect glycoproteins from plasma membrane.
Sperm immunolabelling
Two different procedures were used to identify CD9 in sperm cells. The suspension of freshly ejaculated or frozen-thawed sperm (1.106/ml) was treated for 45 min in TL medium containing mAb IVA-50 or ABIN741015 (polyclonal rabbit anti-CD9 antibody), (10 μg/200 μl sperm suspension) in the humidified atmosphere of 5% CO2 at 39°C in Eppendorf tube. After washing, the sperm suspension was smeared on slides and fixed by cold acetone–methanol (1 : 1) wet fixation, dried and after washing incubated with FITC-conjugated anti-mouse IgG (1 : 300 in PBS) 30 min at room temperature in the dark. In the second procedure, the sperm smears were prepared by acetone-methanol (1 : 1) wet fixation and incubated overnight with mAb IVA-50 or polyclonal CD9 antibody (1 : 100) in humidified chamber at 4°C. Control immunofluorescence study was performed with mouse IgG2a or rabbit sera. Anti-mouse or anti-rabbit IgG-FITC conjugates were used as secondary antibodies. Nuclear DNA of both CD9-reactive and non-reactive sperm was stained with Vectashield-DAPI. Acrosomal status of sperm was evaluated by Peanut agglutinin-TRITC (PNA-TRITC, Vector Laboratories) conjugate staining protocol. PNA staining is a suitable and useful instrument for acrosomal integrity evaluation of bovine sperm as we published previously (Jankovičová et al. 2011). Particularly, PNA binds exclusively the carbohydrate sequence Gal-β (1-3)-GalNAc of the outer acrosomal membrane proteins of buffalo spermatozoa (Kitiyanant et al. 2002). Immunostaining was evaluated under Leica DM5500 B epifluorescence microscope at ×400 magnifications. The fluorescence images were recorded using a Leica DFC340 FX digital camera and processed using Leica Advanced Fluorescence software.
In vitro fertilization
The cumulus–oocyte complexes aspirated from ovaries were maturated in TCM/199 medium [4.5 ml TCM-199 with 10% foetal calf serum, 50 μl sodium pyruvate (27.5 mg/ml physiological saline), 10 μl PMSG (5 UI/ml), 10 μl hCG (5 UI/ml), 5 μl gentamicin (40 mg/ml) for 20–24 h at 39°C in a humidified atmosphere with 5% CO2 and then the cumulus cells were removed by 5 min vibrations]. Frozen spermatozoa thawed in TL medium were resuspended to concentration 1 × 106/100 μl and treated for 45 min with TL medium containing IVA-50 (2 μg/10 μl DMEM without sodium azide) or DMEM (10 μl) as a control, washed in TL and resuspended with IVF medium (Minitube, Celadice, Slovakia) (2 × 106/100 μl). The matured oocytes (25) and sperm (0.5 × 106) were co-incubated at 39°C with 5% CO2 for 24 h. After fertilization, sperm cells were removed from the presumptive zygotes by 1 min vibrations. Embryos were incubated in ISM1 culture medium (East Port, Prague, Czech Republic) at 39°C with 5% CO2 for 24 h, washed, fixed with 4% paraformaldehyde, mounted on slides with Vectashield-DAPI and evaluated under a Leica DM5500 B epifluorescence microscope.
Statistical analysis
The percentage of IVA-50-reactive fresh ejaculated sperm was compared to group of IVA-50-reactive frozen-thawed sperm as well as sperm capacitated for 1 h, 2 h, 3 h and 4 h. Significance of differences was evaluated by Student's t-test using the statistical software Sigma Plot 11.0 (Systat Software Inc., San Jose, CA, USA). For all specimens, staining patterns were quantified by scoring a minimum of 200 sperm cells for each assay. Each experiment was repeated at least three times, and percentage scores were calculated. Significance of differences in the fertilization rate of oocytes was analysed by a Mann–Whitney Rank Sum test (Sigma Plot 11.0.). In each experiment, the group of oocytes fertilized by sperm pre-treated by mAb IVA-50 was compared to control group of sperm (incubated in DMEM only). Experiments were repeated four times, and percentage scores were calculated.
Results
Indirect immunofluorescence, CTC and PNA assays
The immunofluorescence assay showed that mAb IVA-50 and polyclonal anti-CD9 antibody stained high proportion of sperm cells (75–85%). The positive immunofluorescent signal appeared as a fine grains either on the apical part or through the entire anterior region of the sperm head (Fig. 1); small part of sperm with no staining or staining limited to the equatorial region has been considered as sperm passing a ‘false’ acrosome reaction; spermatozoa with completely lost or damaged acrosome.
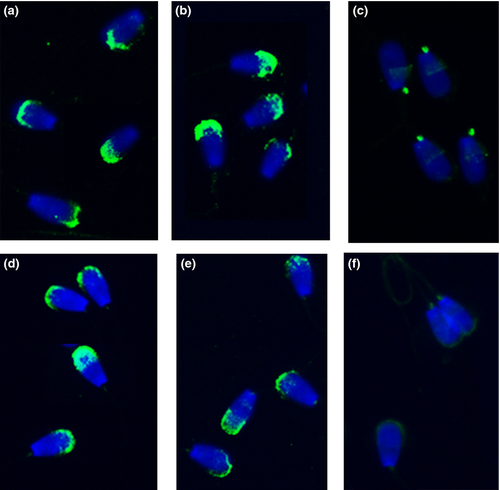
The fraction of IVA-50-positive cells in freshly ejaculated spermatozoa and frozen-thawed (capacitated-like) spermatozoa represented 83.4% and 84.3%, respectively. Capacitation process (1–3 h) did not extensively change the portion of IVA-50-reactive spermatozoa. A small decrease in the number of reactive sperm has been observed only in the group of sperm capacitated for 4 h (77.5%), probably due to the increase in the portion of sperm with detached acrosomes. However, no significant difference between the staining of mAb IVA-50 on freshly ejaculated compared to frozen-thawed as well as capacitated bovine sperm has been noticed. The sperm smears analysed for CD9 presence were evaluated also for acrosomal status of sperm using PNA-TRITC labelling. PNA binding was observed in 80–81% of freshly ejaculated sperm and sperm during the capacitation process. When the capacitation has been completed (after 4 h), more than 75% sperm showed an intact acrosome. PNA-binding tests were performed also with frozen-thawed (capacitated-like) sperm, and comparable results were obtained (83.2% of sperm was PNA stained). IVA-50 and polyclonal antibody recognized CD9 molecule only on sperm with undetached acrosome (Fig. 2). We can conclude that CD9 staining is consistent with PNA lectin labelling as the quantified staining patterns of anti-CD9 antibodies and PNA lectin did not differ significantly. Typical reaction pattern of IVA-50, described above, has been preserved during the capacitation process and stayed uniform on freshly ejaculated or frozen-thawed sperm. All results are summarized in the Table 1. The same results were obtained using polyclonal antibody against bovine CD9 molecule (data not shown).
| Sperm samples | Capacitated sperm (%) Mean ± SEM | IVA-50 reactivity (%) Mean ± SEM | PNA reactivity (%) Mean ± SEM |
|---|---|---|---|
| Freshly ejaculated | 33.5 ± 4.8 | 83.4 ± 3.7 | 87.0 ± 3.6 |
| Frozen-thawed | 77.3 ± 9.5 | 84.3 ± 2.3 | 83.2 ± 1.5 |
| Fresh 1 h capacitated | 30.8 ± 6.2 | 83.6 ± 2.0 | 81.3 ± 3.0 |
| Fresh 2 h capacitated | 55.4 ± 10.5 | 84.0 ± 1.5 | 81.3 ± 0.8 |
| Fresh 3 h capacitated | 92.0 ± 5.7 | 85.7 ± 0.8 | 80.3 ± 1.8 |
| Fresh 4 h capacitated | 60.3 ± 10.0 | 77.5 ± 10.8 | 75.3 ± 10.6 |
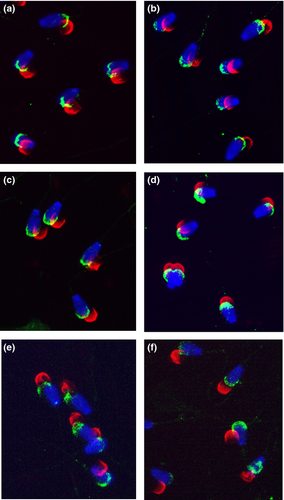
These results indicate that CD9 is localized in the plasma membrane of bovine spermatozoa.
Fractionation of sperm
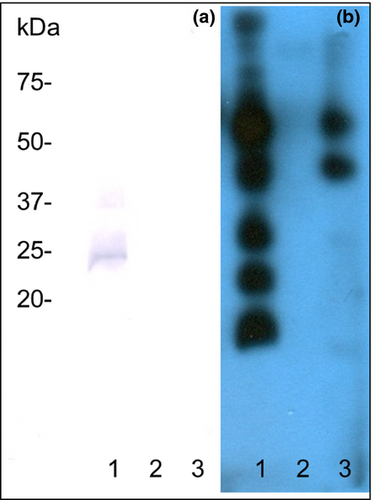
To verify the localization of CD9 in sperm plasma membrane, bovine sperm cells were fractionated and individual fractions analysed for CD9 presence by immunoblotting with mAb IVA-50. As shown in Fig. 3a, CD9 was detected exclusively in the plasma membrane fraction. The identity of this fraction was confirmed by staining with biotinylated WGA lectin (Fig. 3b).
IVF
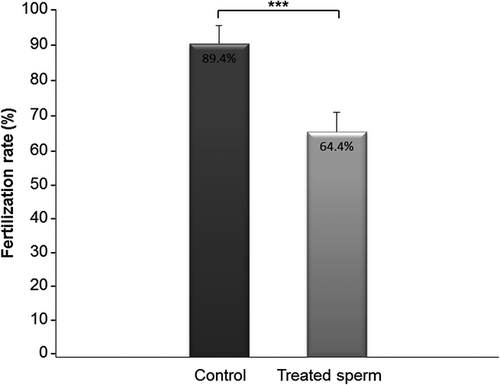
After confirming the localization of CD9 to plasma membrane of capacitated bovine sperm, we focused on the study of the possible role of sperm CD9 molecule in the fertilization process in vitro. To verify the interaction of CD9 with monoclonal and polyclonal antibodies, suspension of frozen-thawed sperm (non-fixed, non-permeabilized) was subjected to an indirect immunofluorescence assay after IVA-50 or ABIN741015 treatment. More than 55% of sperm reacted already after 45 min incubation with both antibodies. In IVF assay, the sperm treatment with IVA-50 (azide-free hybridoma supernatant) significantly decreased the number of fertilized oocytes (64.4%) compared to control group (the sperm suspension treated with azide-free DMEM) (89.4%) (Mann–Whitney, p ≤ 0.001; n = 239) (Fig. 4 and Table 2). Similar results were obtained when fresh ejaculates were used in the identical IVF experiment (data not shown).
| Experiment | 1 | 2 | 3 | 4 | ||||
|---|---|---|---|---|---|---|---|---|
| Control | Treatment | Control | Treatment | Control | Treatment | Control | Treatment | |
| Number of oocytes | 38 | 47 | 29 | 27 | 27 | 26 | 20 | 25 |
| Maturation rate (%) | 82.86 | 69.86 | 76.92 | 75.00 | ||||
| Fertilization rate (%) | 96.55 | 69.23*** | 89.47 | 66.67*** | 85.00 | 56.52*** | 86.67 | 65.00*** |
- (Mann–Whitney Rank Sum test, ***p ≤ 0.001, n = 239).
Discussion
The presence of CD9 has been confirmed on gametes and tissues of various mammals as mouse, rat, pig and cattle, including the man. Although its function has been studied by various approaches, obtained results differ notably. Moreover, the oocyte CD9 has been studied preferentially, so it is difficult to hypothesize about the precise role of CD9 on sperm. The presence of CD9 was reported on mouse and rat spermatogonia (Kanatsu-Shinohara et al. 2004; Kierszenbaum et al. 2006; Rubinstein et al. 2006; Barraud-Lange et al. 2007). An appearance of CD9 during sperm development and its localization on mouse sperm was studied by Ito et al. (2010). Besides the rodents, the presence of CD9 was reported on boar sperm by Kaewmala et al. (2011) and in Western blot of bovine sperm extract by Cupperová et al. (2014).
Regarding cattle, no data were available so far concerning CD9 on ejaculated bull sperm, although Caballero et al. (2013) observed the transfer of CD9-positive microvesicles to bovine epididymal sperm. In our analyses, positive fluorescent signal was detected by mAb IVA-50 and polyclonal anti-CD9 antibody in acrosomal region of freshly ejaculated as well as frozen-thawed bovine sperm. Simultaneously, acrosomal status was assessed by PNA lectin. PNA labelled the outer acrosomal membrane of intact sperm acrosomes or sperm in the process of degenerative acrosome loos (called ‘false’ acrosome reaction) but still having a part of acrosome. IVA-50 recognized the bovine CD9 antigen on more than 80% of evaluated sperm in all samples of freshly ejaculated and frozen-thawed sperm and the staining did not differ significantly from PNA labelling. Compared to pig, Kaewmala et al. (2011) also detected CD9 molecule in the acrosomal region and acrosomal membrane of spermatozoa within the sections of boar testis and epididymis. On the contrary, Li et al. (2004) could not detect CD9 on ejaculated boar sperm frozen in liquid nitrogen. This inability to detect CD9 on boar sperm has been explained by storage in liquid nitrogen; no freezing sensitivity of bovine sperm CD9 was observed in our experiments. Moreover, the reaction pattern has not been influenced by membrane permeabilization, and CD9 has been detected on the intact as well as membrane-permeabilized bovine spermatozoa.
It is difficult to distinguish the exact antigen localization within the acrosome region because sperm head is covered by three membranous complexes. Intact sperm are covered with plasma and outer acrosomal membranes and finally, the inner acrosomal membrane which is closely associated with sperm head. Moreover, the acrosomal content localized between the outer and inner acrosomal membrane is another place of protein occurrence. To precisely localize the CD9 molecule on the bull sperm, we performed fractionation of bovine sperm yielding purified plasma membranes and acrosomal membranes. In our experiments, CD9 molecule was present exclusively in the protein extract of sperm plasma membrane fraction. Surface localization of CD9 is in agreement with Caballero et al. (2013) who observed the transfer of CD9-positive microvesicles (30–120 nm) to membrane surface of epididymal bovine spermatozoa, preferentially incorporated into the sperm plasma membrane in the acrosomal region and mid-piece.
Prior to the successful fertilization, mammal spermatozoa undergo the capacitation process in female genital tract. On the molecular level, the capacitation process is accompanied by rearrangement of sperm surface proteins acquired from seminal plasma as well as epididymal secretion, but no data are available regarding the dynamics of CD9 molecule during capacitation in cattle. It is well known that process of cryopreservation influences the sperm plasma membranes permeability and changes its protein composition. Moreover, frozen-thawed bovine spermatozoa were referred to as capacitated or able to capacitate very easily (in 30 min) (Bailey and Buhr 1994; Zhao and Buhr 1995). To inspect the pattern of CD9 during the capacitation process, we therefore analysed the freshly ejaculated, frozen-thawed (capacitated-like) sperm and also the sperm during the capacitation in vitro using anti-CD9 antibodies. The chlortetracycline (CTC) fluorescence analyses, based on assessment of Ca-related changes during capacitation state of spermatozoa, were applied to detect the portion of capacitated sperm. When frozen-thawed (capacitated-like) as well as freshly ejaculated sperm capacitated for 4 h were analysed, comparable results were observed. Taken all together, the capacitation process did not change the pattern of CD9 molecule on freshly ejaculated and frozen-thawed sperm. In all tested samples, the IVA-50 reactivity exceeded 77%. When CD9 study has been carried out in mice, only 10% of capacitated or freshly recovered cauda epididymal sperm has been stained and fluorescent signal appeared mainly as a thin line in the acrosomal region (Barraud-Lange et al. 2012).
Despite the fact, that CD9 molecule is highly conserved in mammals, species-dependent differences in gamete antigens organization are obvious and distinct mechanisms of involvement of CD9 in the fertilization process are possible.
Based on facts that CD9 is present on the bull spermatozoa before the contact with oocyte and localization of sperm CD9 does not change during the capacitation, we supposed that CD9 molecule ‘is queueing’ for the right moment to participate in some of the subsequent events in the fertilization process. Therefore, we were interested whether the CD9 molecule could play a role in sperm–egg interaction. This hypothesis was confirmed in IVF assay, when sperm pre-treatment with IVA-50 resulted in 25% reduction (p ≤ 0.001) of fertilization rate. From previous experiments, it was apparent, that the number of sperm bound to zona pellucida of oocytes after sperm pre-treatment was also reduced by half (J. Antalíková, J. Jankovičová, M. Simon, P. Cupperová, K. Michalková, Ľ. Horovská,unpublished results). It should be noted that this is the first study of sperm CD9 involvement in IVF assay as oocytes were usually affected by antibody treatment in similar experiments in cattle (Zhou et al. 2009), pig (Li et al. 2004) and mice (Chen et al. 1999; Le Naour et al. 2000; Miller et al. 2000; Takahashi et al. 2001). In mice, it has been documented that CD9-deficient sperm did not lose fertilizing potential when mated with wild type mice (Kaji et al. 2000; Le Naour et al. 2000; Miyado et al. 2008), whereas CD9−/− females were infertile (Miyado et al. 2000). Previously, it has been discussed that infertility of CD9-/- mouse females could be reversed by oocyte membrane material including CD9 (Kaji et al. 2000; Miyado et al. 2008), but these suggestions have not been confirmed by others (Gupta et al. 2009; Barraud-Lange et al. 2012). Moreover, Barraud-Lange et al. (2012) confirmed the observation of Ito et al. (2010) that mouse spermatozoa express CD9 antigen itself. A hypothesis about the crucial role of CD9 during boar sperm development and especially within the maturation of sperm in epididymis has been proposed by Kaewmala et al. (2011). On the other hand, Ito et al. (2010) suggested that CD9 on spermatozoa in mice could serve as a reorganizer of sperm membrane proteins involved with CD9 in tetraspanin web or can act in secondary attachment of sperm to zona pellucida interacting with other inner acrosomal proteins. Eventually, sperm–egg fusion could be facilitated via the homophilic interaction between sperm CD9 and egg CD9 under physiological conditions. From our results, it is obvious that bovine sperm CD9 is not essential for sperm–egg binding and fusion as the mAb IVA-50 did not cause total inhibition of fertilization. However, even if sperm bovine CD9 is not fusogenic itself, CD9 expressed on sperm plasma membrane probably plays a supporting role in some events during gamete interaction. Finally, we suppose that function of sperm CD9 in fertilization process is most likely related to the formation of multimolecular complexes consistent with its tetraspanin character.
Acknowledgements
This work was supported by grants VEGA 2/0006/12 and APVV/0137/10. The authors are thankful to Dr. Ivan Hapala for English correction and Zuzana Nádaždyová for skilful technical assistance.
Conflict of interest
None of the authors have any conflict of interest to declare.
Author contributions
Petra Cupperová and Jana Antalíková designed the study. Jana Antalíková, Jana Jankovičová and Michal Simon analysed data and drafted article. Petra Cupperová, Jana Antalíková, Katarína Michalková and Ľubica Horovská performed the experiments and analysed the data. All authors have read and approved the final manuscript.




