Adaptation of cultured decapod crustaceans to changing salinities: Physiological responses, molecular mechanisms and disease implications
Yousuf Dar Jaffer, Irfan Ahmad Bhat, Ishfaq Nazir Mir, Raja Aadil Hussain Bhat and M. Junaid Sidiq contributed equally to this study.
Abstract
In recent years, the production of economically important crustaceans, and decapods in inland saline areas has increased considerably. The osmoregulatory capacity of these decapods renders them culturable in wide salinity ranges, contributing to a global industry valued at billions of dollars. Therefore, gaining insights into the fundamental mechanisms that drive the adaptive capacity of crustaceans to thrive in diverse salinity ranges is essential. This comprehensive review paper unveils the pivotal adaptations of decapods that allow them to flourish in diverse salinities, ranging from freshwater to saline waters. This article discusses the molecular mechanisms of osmoregulation in decapod crustaceans with more emphasis on Litopenaeus vannammei. Moreover, the importance of maintaining an ideal osmotic balance for efficient digestion and nutrient absorption in L. vannamei is discussed. Furthermore, the effect of salinity on disease resistance in these species is explored, highlighting the need for effective disease management in aquaculture. Overall, this review explores the multifaceted factors influencing decapod crustaceans' adaptation to shifting salinities and also emphasizes the ongoing need for continued research in this domain.
1 INTRODUCTION
Crustaceans started conquering freshwaters some 600 million years ago (Mya) and are the most diverse aquatic invertebrates.1 The order Decapoda is the largest order of crustaceans and comprises more than 14,750 extant species occupying marine and freshwater habitats.2-4 The evolutionary osmoregulatory competence developed by decapod crustaceans has virtually allowed them to survive all the available niches across the globe.5 Apart from marine, estuarine and freshwater counterparts, decapod shrimps also have desert-living and arboreal counterparts.5 The most common clades of decapod crustaceans were derived from marine ancestors.6 The story of some shrimp-like taxa conquering brackish and fresh waters started in the Carboniferous period about 350 Mya.7 The freshwater trichodactylid and potamoid and mangrove grapsid crabs are believed to have invaded between 70 and ≈87 Mya, and the caridean shrimps about ≈70 Mya.8, 9 The crustaceans require optimal biochemical and physiological adaptations to survive in less saline ecosystems which have been achieved by developing different molecular, physiological, ecological and behavioural mechanisms.4, 8, 10 The production of decapod shrimps has increased in recent times through aquaculture and diversification of fishing practices, contributing millions of tons to aquatic seafood production worldwide every year. Decapod shrimps, mainly contributed by Penaeids are considered the most valuable internationally traded commodity in aquaculture.11 In the year 2018 alone, crustacean production reached 9.4 million tonnes (69.3 billion US$), in which L. vannammei alone accounted for 51.7% of the total production.12
It has been more than a decade since the last systematic review conducted by Roy et al.,13 which focused on the cultural aspects of the shrimp in inland saline waters. Other important reviews that discussed osmoregulation and its evolution, gene and growth performance in crustaceans were also published 10 years ago.5, 14, 15 Since then, the literature has rapidly expanded, and important studies and developments have been added on this topic that need to be accounted for. Currently, there is a lack of consolidated information on the regulation of gene expression involved in osmoregulation, even though the genetic mechanisms involved have been studied in detail. Additionally, there is a need for further exploration into the effects of changes in salinity on the nutritional aspects, digestion and overall health of shrimps. These topics require comprehensive discussions to enhance our understanding of them. The main objective of this review is to examine the impact of alterations in salinity levels on crucial physiological indicators, such as growth, nutritional status, digestion and disease resistance in crustaceans. Furthermore, this review aims to explore our current understanding of the molecular mechanisms involved in osmoregulation, which is essential for maintaining physiological balance in crustaceans under changing salinity conditions. The information compiled in the present review could be a valuable resource for understanding the various physiological adaptations that may occur in economically significant decapod species when they are raised in varying salinity environments, including freshwater.
2 FROM SALTWATER TO FRESHWATER: SUCCESSFUL ADAPTATION OF DECAPODS
2.1 Adaptation in different environments
The success of decapods in freshwaters is related to their osmoregulation capacity, which is the osmotic (and ionic) homeostasis within a cell or organism relative to the ionic property of the surrounding environment. This process, termed osmoregulation involves a series of mechanisms where different epithelial tissues are utilized. Anisosmotic Extracellular Regulation (AER), the term used by various researchers including Florkin and their associates16-19 (Figure 1), involves salt absorption or secretion of salt through epithelia such as gill and antennal glands aided by low cuticle permeabilities.5, 16, 20 Eventually, in freshwaters, high osmotic gradient is maintained, while in brackish waters, stable haemolymph osmolalities are kept.20 Among freshwater animals, decapods are superior osmoregulators, maintaining 400–500 mOsm/kg H2O of haemolymph osmolality despite water osmolalities of approximately ~15 mOsm/kg H2O.18 The osmoregulation mechanism is assisted by decreased epithelial osmotic and ionic permeability, primarily by production of dilute urine and active branchial salt absorption.5, 18, 21, 22
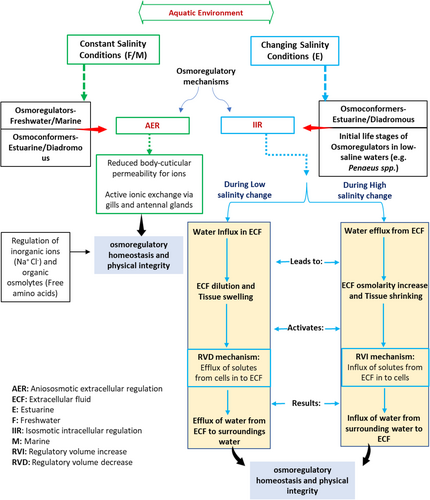
However, Freire et al.6 believe that in conditions of changing external salinity, such as in estuarine/brackishwater habitats, efficient cell volume regulation mechanisms (IIR = isosmotic intracellular regulation) proposed first by Florkin23 are also essential and have a longer evolutionary significance than osmoregulatory (AER) mechanisms (Figure 1). Under such mechanisms, during the medium salinity reduction, the extracellular fluid (ECF) is diluted which induces the influx of water and the tissue cells swell. This is compensated by the process of regulatory volume decrease (RVD), in which the efflux of solutes (inorganic and organic) from the cells to the ECF occurs leading to water efflux. On the other hand, in high saline conditions, ECF osmolality increases which induces the efflux of water and ultimately leads to cell shrinkage. This is complemented by the regulatory volume increase (RVI) mechanism in which an influx of solutes (inorganic and organic) from ECF towards the cell and ultimately water inflow occurs, restoring cell volume24, 25 (Figure 1).
2.2 IIR mechanisms and evolutionary significance
The IIR mechanisms are also considered a prerequisite in the initial stages of development in low-saline waters.21 Over time, the extracellular mechanisms have evolved, leading to less pressure on the IIR strategies and decreased IIR capacities.21, 26 In crustaceans exhibiting varying degrees of osmoregulatory/osmoconforming behaviour, euryhalinity is the synergism of AER and IIR mechanisms.18, 23, 27 Thus, the osmoregulators (freshwater species) are in less demand of IIR mechanisms than euryhaline conformers who rely entirely on IIR mechanisms.17, 18 Nevertheless, freshwater species like Macrobrachium potiuna display the highest capacity of IIR and AER among other palaemonid shrimps of the genus Macrobrachium.10, 28 Whereas, the estuarine species, Palaemon pandaliformis although being a powerful osmoregulator AER displays a low capacity of IIR.10 It follows that both AER and IIR mechanisms work in tandem to maintain osmo-ionic homeostasis (or allostasis) to different degrees, depending on the species' niche and evolutionary history.5, 28-30
2.3 Haemolymph's role and osmoregulation
In crustaceans, haemolymphs serves as an intermediary between cells/tissues and the ambient environment, significantly influencing IIR. The haemolymph osmolality is primarily determined by the inorganic ions usually sodium and chloride; however, free amino acids (FAA) synthesized or formed by the breakdown of haemolymph/muscle proteins are also believed to serve osmoeffectors in several crustacean species.31-33 FAA like glycine, proline and alanine play a significant role in osmoregulation during exposure to different salinity environments as found in Penaeus stylirostris, P. japonicus, Paleomon elegans and crayfish Procambarus clarkii.31, 34-36 Salinity has a strong relationship with haemolymph osmolality and the latter has been found to decrease in several penaeids with a drop in salinity.37 For instance, the Pacific white shrimp (L. vannamei) exhibited varied haemolymph osmolality depending on the stage and salinity, reflecting its adaptive mechanisms. Additionally, organic osmolytes like free amino acids significantly contribute to the RVD mechanism in decapods. Interestingly, hyper-/hypo-regulators like L. vannamei rely less on cell volume regulation than euryhaline osmoconformers.
2.4 Species-specific adaptations
The penaeid species exhibit hyper- and hypo-osmotic regulation with salinities below and above isosmotic values respectively, as demonstrated by numerous studies.38, 39 The isosmotic point of Penaeidae varies from 671.3 mOsm/kg (21.1 ppt) in the case of L. vannamei40 to 824 mOsm/kg (26.8 ppt) in the case of L. setiferus.41 L. vannamei has been shown to maintain a narrow range of haemolymph osmolality in various salinity levels (1, 5, 7, 15 and 25 ppt).40
On the other hand, the Palaemonid shrimps, originally of marine origin, have successfully invaded freshwaters via independent events.8, 42 The freshwater prawns and shrimps of the Macrobrachium genus hyperosmoregulate in freshwater or low salinities, and become hypoconformers at higher salinities (except M. equidens and M. olfersii).43 Their larval development adaptations and migration principles emphasize their habitat preferences and survival strategies.5, 8, 44
2.5 Energetic consideration and ion transport
The ability to actively transport (uptake) essential ions from dilute waters, especially freshwaters, requires more energy due to the existence of a large ionic concentration gradient.45 The survival and growth in these water are predominantly determined by the energetically driven ion-motive ATPase enzymes, such as Na+/K+-ATPase and V-type H+-ATPase.46, 47 It suggests that the presence of salt makes it energetically cost-effective for certain species to complete the larval cycle in brackish waters. In such a medium the larvae maintain the hydro-mineral balance between the external environment and the haemolymph, which promotes its growth and survival. It also defines the migration principle towards upstream and downstream in most of the Macrobrachium species in search of brackish waters during the spawning period for hatching.8 Additionally, migration patterns in search of optimal salinity waters during spawning emphasize the importance of salinity for their life cycles.
Overall, decapods have demonstrated exceptional success in freshwater habitats because of their excellent osmoregulation abilities. To maintain ionic homeostasis, many epithelial tissues, including gill and antennal glands, are used in osmoregulation, which combines isosmotic intracellular regulation (IIR) and anisosmotic extracellular regulation (AER) mechanisms. The IIR mechanisms are more significant in euryhaline conformers that live in brackish environments, whereas AER mechanisms are more common in freshwater species. Yet, depending on the species' habitat and evolutionary history, both AER and IIR processes cooperate to maintain osmo-ionic homeostasis to varying degrees. The IIR is primarily affected by inorganic ions and free amino acids, with the haemolymph serving as an intermediary medium between the cells and the environment (Figure 1).
3 MOLECULAR MECHANISMS CONTROLLING OSMOREGULATION
The capacity of crustaceans for osmoregulation is essential for their ability to adapt to environmental changes, and this is determined by the difference between the osmolarity of their haemolymph serum and the osmolarity of the surrounding medium.48, 49 Like other biological processes, osmoregulation is regulated by molecular events, and the responsible genes can either work independently or in collaboration to bring about the necessary changes. The genomic data collected from several decapods suggested that in the osmoregulatory process, almost 32 genes are involved50 with Na+/K+-ATPase (NKA) being the master gene, followed by Carbonic anhydrase (CA), Na+/H+-exchanger (NHE), Na+/K+/2Cl− cotransporter (NKCC) and V-type proton (H+)-ATPase (VAT) (Table 1).50 In the context of salt homeostasis in fishes, three key ion transporters—NKA, NKCC and the cystic fibrosis transmembrane conductance regulator (CFTR) channel, NHE, Rhcg and Rhbg (Rhesus glycoproteins) play significant roles.51 The significance of the osmoregulatory genes is supported by their high mRNA expression levels in decapods reared under different salinities.52-54 This part of the article covers the genes that play a crucial role in regulating osmoregulation in crustaceans, specifically decapods. It discusses the structure of these genes and how they are expressed and regulated when the local environmental conditions change.
| Gene/transcript | Family and isoforms | Main residing organs | Relation with salinity | Functions | References |
|---|---|---|---|---|---|
NKA Na+/K+-ATPase |
P-type ATPase family – |
Epithelia of osmoregulatory organs | Upregulates against all salinity change | Master gene in osmoregulation; High species and environment-related specificity; Works as sodium pump; Involved in: osmoregulation, pH maintenance, gaseous exchange and ecdysis. |
Moshtaghi et al.,54 Jorgensen et al.62 and Ziegler75 |
CA Carbonic Anhydrase |
Zn metalloenzyme CAg, CAc, Cab |
Gills, hepatopancreas and muscle | Significant role in low salinities in estuarine conditions | Generates H+ + HCO3 from CO2 and counter-transport them with Na+/H+ and Cl−/HCO3− ions; Involved in osmoregulation and pH maintenance in gills; Aids in coping salinity related stress. |
Mitchell and Henry79 and Ali et al.87 |
NKCC Na+/K+/2Cl− cotransporter |
Cation-chloride cotransporter (CCC) family NKCC1 and NKCC2 |
Exocrine glands (NKCC1); kidney (NKCC2) | Upregulates on exposure to low salinity in marine waters | Transports Na+, K+ and Cl−simultaneously and maintains electroneutrality; Resorb the ions from kidney into haemolymph; Branchial uptake of ions during ecdysis; Symporter in salt uptake for maintaining hypertonicity of haemolymph |
Gamba,90 Havird et al.67 and Maraschi et al.97 |
NHE Na+/H+-exchanger |
Solute carrier (SLC)-9A family – |
Brush border cells of hepatopancreas | Upregulates due to the factors that change pH, including salinity | Transports Na+ in exchange for H+; Maintains: ionic balance, pH homeostasis, cell volume regulation | Counillon and Pouysségur99 and Ge et al.80 |
VTA or HAT V-type proton (H+)- ATPase |
Ion-motive ATPases – |
Plasma, gills and branchiostegites | Downregulates against all salinity changes | Acidify intracellular organelles; Boosts secondary ion transport across whole epithelia by proton delivery |
Beyenbach104 and Havird et al.93 |
3.1 Na+/K+-ATPase
NKA was first discovered in the nerve cells of shore crab (Carcinus maenas) by Jens Christian Skou (1957) who described the influence of Na+ and K+ on the ATP hydrolytic activity for which he was awarded the Nobel prize in 1997. It is simply a sodium pump that plays an important role in the energy-demanding process like osmolyte transportation, acid–base regulation, nitrogenous waste removal and gas exchange in gills. The main role of this enzyme is to activate the ion exchange between the internal body and surrounding water to maintain the osmotic balance in both vertebrates and invertebrates.52, 53
NKA enzyme belongs to P-type ATPase family of genes and structurally it has a catalytic α-subunit comprising about 1000 amino acids (binding sites for Na+and K+ and ATP) and an auxiliary β subunit (55–60 kDa) which regulates ion binding at the α subunit and acts as a chaperone.55-57 The γ-subunit of enzyme in crustacean NKA was also found by Silva et al. (2012). Molecular cloning of α-subunit of NKA from three crustacean species viz, brine shrimp (Artemia franciscana), American lobster (Homarus americanus) and the blue crab (Callinectes sapidus) helped to decode its primary structure58-60 and results revealed that the subunit is highly conserved across the species.
NKA is found in the membrane of epithelial cells in organs responsible for osmoregulation which pumps out 3 Na+ ions in blood\haemolymph and pumps in 2 K+ ions into the cell at the expense of one ATP molecule.61, 62 In numerous studies on both invertebrates and vertebrates, the expression of NKA is regulated during the change of salinities.61, 63-66 In low salinities, the expression of NKA transcript is high which leads to the assimilation of more sodium from the surrounding medium.61 When the animals were transferred from freshwater to higher salinity, the expression of NKA was increased.67 Other studies supporting the facts are that the NKA enzyme has a higher affinity towards Na+ in the case of freshwater crustacea while this property is reversed in marine osmoconformers (reviewed by Lucu and Towle61). A study revealed that in the larval stages (zoea V and decapodid) at both 5 and 25 ppt salinities, the expression of NKA-α was higher in M. pantanalense, an inland species, compared to M. amazonicum, an estuarine species. However, this difference in NKA-α expression was not observed in the juvenile stage of these species.68
Immunohistochemical analysis of the gill in some palaemonids (M. rosenbergii, M. olfersi, M. amazonicum and M. acanthurus) revealed that the NKA is mostly located on lamellar septum cells of gills.47 In the giant prawn, M rosenbergii, all the individual gills were observed to have a similar NAK activity suggesting their joint importance in establishing the osmotic balance.69 In general, the difference is only noticed in the crabs, making their gills unique in all invertebrates to respond in the osmoregulatory process.
The NKA activity in crustacea is influenced by physicochemical factors like the ionic composition of the medium, pH, temperature and some inhibitors. The pH value ranges from 7.0 to 7.7 for the optimum activity of the enzyme.70, 71 The optimum temperature for the maximum activity ranges from 37°C to 40°C in freshwater and brackishwater crustacea with some exceptions like in Callinectes sapidus and Orconectes limosus the values are 23°C and 27°C, respectively.70, 72, 73 The phospholipid bilayer's composition and fluidity are believed to modulate NAK activity. As far as inhibitors of NKA activity are concerned, orthovanadate an organic compound and ouabain have been found to completely inhibit this enzyme's function.74
In sternal epithelial cells, it was found that the activity of the enzyme increases in the premolt and intramolt stages of crustacea. It suggests that the ATPase of the NKA helps in Na+ and K+ exchange during the mineralization process of the cuticle when ecdysis is happening.75 The increased absorption of water during ecdysis dilutes the haemolymph ions and to protect the dilutions, the NKA is activated which re-establishes the concentrations of Na+, K+ and Cl−.76
3.2 Carbonic anhydrase
CA is a Zn metalloenzyme that catalyses the rapid inter-conversion of CO2 and HCO3−.77 The reaction is like CO2 + H2O⇌ H+ + HCO3−, where H+ + HCO3− serves as counter-transporter for Na+/H+ (NH4+) exchangers and Cl−/HCO3− cotransporter.78 The CA plays a key role in the salt transport mechanism in the species at low salinities, especially in estuarine species.79 In crustacea, the major role of this enzyme is to regulate the branchial ion transport.5, 30 Knockdown of CA in crustacea downregulated other important osmoregulatory genes suggesting its role in acid–base balance.80 For the catalytic function of CA, there is a binding site for Zn, threonine-99 loop, the substrate binding pocket and the mechanism for proton shuttling.81-83 The tissue distribution studies of CA in crustacea disclosed its presence in gills, hepatopancreas and muscle while low expression was detected in the heart, haemocytes and intestine brachial chamber.80, 84-88
There are three isoforms of CA: glycosyl–phosphatidylinositol-linked carbonic anhydrase (CAg), which becomes active in high salinity conditions; cytoplasmic carbonic anhydrase (CAc), which functions in low salinity environments; and beta carbonic anhydrase (CAb), for which information is currently limited.86 The available literature on the isoforms reveals that CAg is localized on the outer membrane of cells and accelerates the mobilization of haemolymph HCO3 to molecular CO2 and helps in the excretion of the latter through the gills.89 Similarly, CAc is in the cytoplasm and helps in counter exchange H+ and HCO3− ions for the Na+/H+ and Cl−/HCO3− in gills.89 CAc is the main isoform of CA that plays a role in the pH changes as well as in maintaining the systematic acid balance in crustaceans.80 Ridge tail white prawn Exopaiaemon carinicauda, white shrimp L. vannamei and freshwater crayfish, Cherax quadricarinatus, exposed to changing pH and saline-alkaline stress showed upregulation of CAc and CAg mRNA expression levels in gills while CAb transcript level remained unchanged, suggesting the role of former two in coping with the stress conditions in crustacea.80, 86, 87
In conclusion, CA plays a vital role in acid–base balance and osmoregulation, especially in high-saline environments. Besides this, it constitutes one of the primary components of osmotic regulation and is highly expressed in the organs that play a role in osmotic and ionic regulation. The function of CAb isoform in crustacea is unavailable so to elucidate its role in osmoregulation it becomes an interesting area of research.
3.3 Na+/K+/2Cl− cotransporter
NKCC is a membrane transport protein that belongs to the cation-chloride cotransporter (CCC) family of proteins and helps in osmoregulation by transporting Na+, K+ and Cl− across cell membranes simultaneously.90 It sometimes acts as a symporter and maintains electroneutrality by transporting two positively charged ions in parallel with two negatively charged ions, that is, Na+ and K+ with 2 Cl−.89 In vertebrates, NKCC manifests in two isoforms: NKCC1 which facilitates the trans-epithelial secretion of chloride ions and modulates the excitability of certain neurons. On the other hand, NKCC2 plays a crucial role in the renal system by contributing to the reabsorption of salt in the kidneys.91-93 Basal expression reports of NKCC exhibited its presence in all the tissues with the highest in the posterior gills.94
In weak hyper-osmoregulators, NKCC plays a crucial role in apical NaCl absorption. Neohelice granulata exposed to varying degrees of salt water (dilute or concentrated) resulted in an increase in the mRNA expression of NKCC.92 Most of the studies in crustacea determine that NKCC is upregulated when exposed to low salinity.92, 93, 95 Similarly, M. australiense, a freshwater prawn challenged with increased salinity resulted in an increase in the mRNA expression of NKCC.54 Available reports confirmed that NKCC has an osmoregulatory function in M rosenbergii, M. australiense and M. koombooloomba.52-54, 96 The mRNA expression of NKCC was upregulated in the moult cycle, especially during the postmolt stage suggesting the involvement of this transcript in branchial ion uptake during ecdysis that correlates with the role of NKCC in the gills in osmoregulation. The expression of the NKCC gene in palaemonid shrimps is positively correlated with the over-regulation of Cl− in the haemolymph, indicating a potential role of the NKCC symporter in salt uptake. Additionally, the synthesis of gill NKCC protein is associated with the hyper-regulation of haemolymph osmolality, further supporting this hypothesis.97
In general, NKCC transcript is highly expressed in the gills of crustacea and plays a significant role in maintaining osmoregulation in high-saline environments.
3.4 Na+/H+-exchanger
NHE belongs to superfamily cation/proton antiporters (CPA) and is a member of the solute carrier (SLC) 9A family that transports Na+ in exchange for H+ (1:1 stoichiometry) during the regulation of ionic balance, pH homeostasis, cell volume regulation and other osmoregulatory processes.98-100 Since its first isolation in the rat kidney, 9 isoforms (1–9) of the genes have been identified in higher animals reviewed by Donowitz et al.101 All the isoforms of NHE have a similar topological structure containing N- terminus 11–13 transmembrane domains for ion exchange and have a regulatory region on C-terminus. In invertebrates, the NHE has been studied in brush border cells of hepatopancreas and results reveal that a different type of electrogenic exchange protein is found which displays a transport stoichiometry of 2Na+/1H+.102 At physiological pHi (intracellular pH) in cells, NHE basal activity is very low but when pHi is low, the activity of NHE activity increases sharply for acidified pHi adjustment by promptly extruding protons in exchange for extracellular Na+.103 In, P. vannamei, the knockdown of NHE resulted in reduced haemolymph pH when exposed to low-pH challenge, suggesting its role in maintaining the acid\base homeostasis in crustacea.103 Supporting this data, in ridge tail white prawn, Exopalaemon carinicauda, the knockdown of CAc resulted in the downregulation of NHE and finally the mortality of the species reared under saline–alkaline stress.80 A study revealed that in the larval stages (zoea V and decapodid) of M. pantanalense and M. amazonicum at both 5 and 25 ppt salinities, the expression of NHE3 was higher at 5 ppt.68 In general, the role of NHE was deciphered by different approaches and the evidence supports that it maintains the extracellular (circulating) and intracellular pH regulation in crustacea.
3.5 V-type proton (H+)-ATPase
VAT or HAT are membrane-associated, highly conserved enzymes that acidify intracellular organelles and boost secondary ion transport across whole epithelia by proton delivery from the cytoplasm to the extracellular medium and in the plasma membrane, it pumps protons in many tissue types.104 Initially, it was isolated from the intracellular vacuoles and later in the plasma membranes where it performs its leading role.105 In M. amazonicum, the subcellular localization by immunofluorescence demonstrated its presence in the gills and in the branchiostegites.106 VAT is formed of two domains V0 and V1 that, in turn, have many subunits, namely six subunits (a, d, c, c′, c″ and e) for V0 and eight (A–H) for V1 which are arranged differently.107, 108 In the V1 domain, A and B subunits have catalytic sites for ATP hydrolysis which is used to carry H+ across the membrane, whereas V1 has the function of proton translocation.109-112 In freshwater crustacea, that are hyper-osmotic, VTA plays an important role in maintaining osmoregulation.95, 113
The VAT mRNA expression studies in crustacea have been mostly performed by exposing them to varying pH and the transcript level was found to be upregulated when invertebrates were treated with low salinity or low pH.67, 114 A negative correlation between pH and VTA has been reported, in which at low pH VTA is upregulated and vice versa.114 Individuals transferred from the optimum to varying (higher and lower) salinities, resulting in the downregulation of VTA.93 When M. amazonicum was exposed to a salinity level of 21%, there was a rapid increase in VAT mRNA expression within the first hour. However, this expression sharply declined to a negligible level after a period of 10 days.46, 115 So, VAT is the main enzyme that maintains osmoregulation and is implemented during pH and salinity changes in crustacea.
3.6 Other important genes
Besides the master genes involved in osmoregulation in crustacea, lots of minor genes also play a key role in maintaining homeostasis in changing environments. Alkaline phosphatase is involved in ion binding; precipitation of ions and metabolic process during osmoregulation.53 Arginin kinase, which is involved in the production of ATP in invertebrates through hydrolysis, is doubled when some crustacea are transferred from high salinity to low salinity, suggesting its role in the osmoregulation. Aquaporin, a key player in the regulation of water channels, is a crucial component among genes responsible for osmoregulation. Its significance is particularly notable in the maintenance of homeostasis in freshwater environments.54 Similarly, some other genes include Crustacean cardiovascular peptide (involved in body fluid regulation, stress tolerance), Bestrophin (maintains cellular water homeostasis), ATP-binding cassette 12 (ABC12, engaged in osmotic stress regulation), Calreticulin (Ca2+ homeostasis), Mitogen-activated protein kinase 38 and Mitochondrial carrier protein (osmotic stress response) that have been studied to play an active role in the osmoregulation.52, 53, 96 The minor genes involved in osmoregulation are discussed properly in a review by Rahi et al.50
In general, the genomic basis for osmoregulation in crustacea particularly in decapods has been studied well and still, there is a scope to use powerful tools like sequencing technologies, transcriptomics and proteomics to elucidate the exact mechanism the genes play in maintaining the homeostasis. To enhance our understanding of the genomic mechanisms involved in osmoregulation among crustaceans, it is imperative to expand the scope of sequencing beyond model species and incorporate a broader range of non-model crustacean species.
4 CHANGING SALINITIES AND THEIR EFFECT ON THE PHYSIOLOGY OF P. VANNEMMEI
L. vannemmei has quickly become the preferred choice for aquaculture farmers around the world, making it one of the fastest-growing segments in the industry.116 L. vannamei has become the dominant species in farmed shrimp production, accounting for an impressive 77% of total global output, as noted by César Molina et al.117 This is largely due to its outstanding traits such as rapid growth, high survival rate and most important: remarkable tolerance to varying salinity levels. L. vannemmei is an ideal species to investigate the effects of salinity gradients on physiology, owing to its broad range of salinity tolerance levels.118 So, this review primarily examines the impact of various salinity gradients on the growth, nutritional adaptability, digestion and disease resistance of P. vannamei.
4.1 Combined effect of the temperature and salinity on growth of P. vannamei
While the average water temperature in most of the shrimp farms varies from 15°C to 32°C, the best survival temperature for the growth of L. vannamei juveniles and adults is about 20–30°C and 27°C, respectively.119, 120 Kır et al.121 found that L. vannamei has a remarkable ability to survive in a broad range of temperatures, ranging from 7.2°C to 41.9°C (CTmin − CTmax: 7.2–41.9°C) and suggested a temperature range of 25–30°C for effective production. The exposure of shrimp to lower water temperatures induces oxidative stress, DNA damage, lipid peroxidation, changes in osmolality, arrests growth, or even causes death.122-124 The reduction in water temperature was seen to lower the osmoregulatory capacity of P. stylirostris both at low and high salinity and the effect was more pronounced in sub-adults than juveniles.125 During the cold stress (13°C for 24 h), L. vannamei exhibited significant alterations in 30 proteins (up- and down-regulated) studied.126 The physiological response of L. vannamei to temperature fluctuation (28–13°C) in low-salinity water was studied by Wang et al.127 It was found that the number and volume of β-cells in hepatopancreas tubules significantly increased. The lipids and protein in plasma responded more rapidly to temperature fluctuation, while the contents of glucose remained stable while lowering the temperature from 28°C to 13°C. It was concluded that the increase of β-cells facilitates gluconeogenesis to synthesize glucose from protein and lipid, by which shrimps maintain glucose demand under cold stress. The protein and lipid were observed to be the main energy source of L. vannamei during temperature fluctuation.127 To understand further the mechanism of homeostasis during temperature fluctuation, Wang et al.128 studied the intestinal transcriptome profiles of L. vannamei reared at 5 ppt. Metabolic inhibition was found as a strategy to cope with cold stress and lipid metabolism served as an important factor during temperature fluctuation. Also, cold stress caused histological damage to the shrimp intestine, which was self-repaired after the temperature was reversed. The sudden exposure of L. vannamei reared at 5 ppt and pH 8.0 to low temperature (from 23 ± 2°C to 12 ± 2°C) induced oxidative stress, DNA damage, lipid peroxidation and changes in osmolality within 3 h.122 The oxygen consumption in juveniles of L. vannamei was more stable at 25°C and 30°C for 13 and 25 ppt salinity in all size groups.129 In L. vannamei subjected to continuous salinities, the isosmotic point increased from 717 to 823 mmol/kg as a function of temperature. The best growth was achieved under these testing conditions with the T–S combination of 32°C and 28 ppt.130 Overall, it has been found that L. vannamei can self-regulate the changes in temperature to a certain extent. However, with a sudden increase in water temperature, there is a significant effect on survival, behavioural responses and immunological parameters.127, 131
In crustaceans, there is an overproduction of reactive oxygen species (respiratory burst) and a sharp reduction in total haemocyte count at low temperatures.132, 133 In conclusion, water temperature plays a crucial role in the survival and growth of L. vannamei in shrimp farms. The ideal water temperature for juveniles and adults is around 20–30°C and 27°C, respectively. The study of homeostasis mechanisms during temperature fluctuation can lead to a better understanding of how to improve the shrimp farming industry's management practices.
4.2 Salinity and nutritional importance
Nutrients are the components or part of food/feed which provide nourishment and are required for the body's functions like maintenance, development, growth and reproduction. It can also be defined as the component of food, the excess or deficiency of which may produce a malfunction of the body. Nutrients play numerous roles and are essential for the shrimp, like any other animals, to stay alive, active and healthy to grow. These are the building blocks of all the tissues of the animals and are involved in all the chemical reactions that facilitate life processes. Dietary requirements can be established for protein and amino acids, lipids and fatty acids, carbohydrates, vitamins and minerals. The salinity gradients have a profound impact on the nutritional requirements of the penaeid shrimps. The comprehensive description of the nutritional requirements of (P. vannamei) till 2015 was illustrated in the review of Li et al.134 In this section, the detailed compilation of most of the studies published after 2015 on various aspects of nutritional requirements or interventions at different salinity gradients or salinity stresses will be summarized (Table 2).
| Nutrient | Salinity (ppt) | Requirement (% in feed) | Critical findings | References | |
|---|---|---|---|---|---|
| Dietary Protein | 2.0 | 35% | Improved growth, performance and nutritional efficiency | Gao et al.139 | |
| 8.0 | 32% | Talukdar et al.136 | |||
| 15.0 | 39.3%–39.7% | Jana et al.137 | |||
| 30.0 | 45.93% | Sui et al.138 | |||
| 60.0 | 46.73% | ||||
| 30.0 | 30% | Lee and Lee140 | |||
| Methionine | 30–33 | 0.91% | 0.55 g | Lin et al.142 | |
| 0.67% | 4.18 g | ||||
| 0.66% | 9.77 g | ||||
| Tryptophan | 0.5–0.8 | 0.365%–0.395% | Jin et al.143 | ||
| Phenylalanine | 0.5–1.2 | 1.58% | |||
| Dietary lipid | 28.0 | 15.4% | Xie et al.147 | ||
| 6–7 | 10%–12% | Improved growth; high immune and antioxidative activity | Zhang et al.148 | ||
| EFA | 25.0 | 1.2% | Improved growth; Reduced the stress due to hypo- and hyper-salinity | Jannathulla et al.149 | |
| n-3 HUFA | 26.5–28 | 0.89% | Good growth and reduction of body saturated fat content; Increase of EPA and DHA in the body; Prevention of salinity stress | Yang et al.150 | |
| 26.5–28 | 0.9% | Subadults | Improved growth and body composition, especially that of n-3 HUFA | Zhang et al.152 | |
| 0.51% | Adults | ||||
| Phospholipids | All levels | 3–5% | Phospholipid content is not influenced by salinity | Li et al.134 | |
| Dietary carbohydrate | 3.0 | 15–20% | Good growth; Alleviation of ammonia stress at low salinity | Wang et al.160 | |
| Dietary vitamins: Myoinositol | 26.0 | 2.705 g/kg | Good growth and antioxidative metabolism | Chen et al.165 | |
| 6.0 | 4.89 g/kg | Improved resistance to low salinity stress | |||
| Choline | 17.4 | 5.421 g/kg | Good growth, nutritional efficiency and wellbeing | Shi et al.166 | |
| Vitamin D3 | 10–15 | 6366 IU/kg | Improved growth and well-being at low salinity | Wen et al.167 | |
| Vitamin E and Vitamin C | 17–20 | 1.0 and 0.3 g/kg | Improved growth and survival; Reduced oxidative stress due to low salinity; An interactive effect, along with dietary lipids | Ebadi et al.168 | |
| Potassium (K+) and Magnesium (Mg2+) ions | 10.0 | 5 and 150 mg/kg | Mitigated physiological stress due to low salinity; Better growth and survival at various salinities | Jahan et al.170 | |
| Magnesium (Mg+): Calcium (Ca+) | 8.4–8.8 | 3:1 ratio in culture water | Aruna and Felix172 | ||
| Magnesium citrate (containing 11.23% elemental magnesium) | 10 | 2.86 g kg−1 | Panmei et al.223 | ||
| Magnesium (Mg2+) | 2 | 2.60–3.46 g/kg | Cheng et al.224 | ||
| Phosphate (PO43−) | 2 | 0.93% (0.5% Ca2+) | Cheng et al.225 | ||
| Potassium (K+) | 4 | 1.48% | Liu et al.226 | ||
| Potassium (K+) | 4 | 1% | Roy et al.227 | ||
| Copper (Cu2+) | 30 | 16–32 mg kg−1 | Davis et al.228 | ||
4.2.1 Proteins and amino acids
The requirement of dietary protein and amino acids of commercially important shrimps varies depending on the water quality parameters, especially the salinity.135 Salinity is assumed to be a highly energy-consuming stressor; hence percentage of salinity always needs to be considered before formulating the feed for shrimps. Talukdar et al.136 conducted an 8-week feeding trial to evaluate the effect of graded levels of dietary proteins on growth and physio-metabolic responses of L. vannamei juveniles reared in inland saline waters of 8 ppt salinity. It was observed from the study that the dietary optimal level supporting the maximum growth of L. vannamei at 8 ppt salinity inland saline water is 32%. The same level of dietary protein was found to be beneficial for improving the health status of the shrimps. However, when the optimal dietary level was examined in juvenile L. vannamei reared in inland saline water of 15 ppt salinity, it was suggested that 39.3%–39.7% promoted the weight gain, specific growth rate and feed conversion ratio in a significant manner.137 However, previously Sui et al.,138 mentioned that the estimated optimal dietary protein levels in white leg shrimp were 45.93% and 46.74% at salinities of 30 and 60 ppt, respectively. Furthermore, in low salinity (2 ppt) waters, as the inclusion level of dietary protein is increased, the growth responses were also improved until a point where the trend decreased significantly.139 In this study, a 35% inclusion level of dietary protein has been found to be optimum for significantly increasing the growth and activity of digestive enzymes. Moreover, at a higher salinity level of 30 ppt, after conducting three feeding trials it was concluded that the growth rate was significantly higher at 30% protein inclusion in the diets of L. vannamei juveniles.140 It is important to consider the effects of salinity when embarking on shrimp farming, particularly in relation to the amount of protein included in their diet. This consideration is crucial in ensuring successful shrimp cultivation practices. However, in addition to the inclusion level, the source of protein also impacts the response of growth in shrimps. Gil-Núñez et al.141 reported that the optimum protein levels for diets formulated with ingredients like soybean meal and fish meal were 34.8% and 29.3%, respectively, which infers that not only the level of dietary protein, but the amino acid profile is also has an enormous influence on the growth and physiological functions of shrimps.
Methionine is considered as the limiting amino acid, especially in plant-based ingredients as protein sources in the diets of shrimps. An optimal dietary methionine level of 0.91% and 0.67% was estimated in L. vannamei in the size groups of 0.55 ± 0.01 and 4.18 ± 0.05 g, respectively at salinity ranges of 30–33 ppt.142 However, the increase in the size of shrimp results in reduced dietary methionine levels. Hence, in the size group of 9.77 ± 0.08 g, the optimal dietary level was observed to be 0.66% at 30–33 ppt.142 Tryptophan is also an important essential amino acid that improves the growth and health status of shrimps. Pacific white shrimp when reared in low salinity water (0.5–0.8 ppt), the optimal dietary tryptophan requirement was found to be 0.365%–0.395% tryptophan of dry diet, which corresponds to 8.90–9.63 g/kg of dietary protein.143 Like other essential amino acids, the requirement of phenylalanine is also important to be satisfied for the proper growth and survival of animals including shrimps. In the low salinity waters of range 0.5–1.2 ppt, the requirement of 1.58% dietary level of phenylalanine, corresponding to 3.86% of dietary protein was determined to be optimum for the proper growth of L. vannamei juveniles.144 Therefore, it is always recommended to conduct studies on amino acid requirements at different salinity levels. Hence, there is ample scope to furnish knowledge not just about the inclusion level of dietary proteins but the quality of protein as well in the commercially important penaeid species, especially P. vannamei.
4.2.2 Lipids and fatty acids
In contrast to finfishes, penaeid shrimps are not efficient enough to utilize dietary lipids due to the absence of bile salts which are necessary for their absorption. However, the recommended level of lipids in commercial feeds ranges from 6% to 7% as reported in the previous study.145, 146 Nonetheless, the highest weight gain was observed at 15.4% in post-larval L. vannamei shrimp at 28 ppt salinity.147 According to study conducted by Zhang et al.,148 a 12% dietary lipid level for 30 days and a 10% lipid level for 60 days were found optimal for the growth of P. vannamei. Therefore, it can be concluded that the dietary optimal lipid levels of shrimps may be complex that depend on several factors, among which salinity and lipid sources are the important ones that cannot be ignored.
Jannathulla et al.149 evaluated the effect of different dietary lipid levels on the growth performance of L. vannamei reared in three salinity levels. In the study, it was recommended that a combination of 25 ppt salinity and 6% lipid level at 12 g/kg of dietary essential fatty acids are ideal for significantly improving the growth performance of white leg shrimp. However, the amelioration of salinity stress could be achieved by increasing the level of lipid/EFA in the diet and a better tolerance was reported at hyposaline (2 ppt) in contrast to hypersaline (50 ppt) conditions.149 Yang et al.150 investigated the effect of n − 3 high-unsaturated fatty acid (n-3HUFA) levels on growth performance, fatty acid composition and antioxidant enzyme activities of juvenile P. vannamei. It was reported that the dietary supplementation of n-3HUFA at optimal levels (0.89%) leads to improved growth performances and significantly reduces the content of saturated fatty acids (SFAs) in hepatopancreas and tail muscle. However, the eicosapentaenoic acid (EPA) and docosahexaenoic acid (DHA) contents were increased significantly in hepatopancreas and tail muscle at optimal n-3HUFA dietary supplementation. Furthermore, n-3HUFA at optimal levels can promote immune responses of juvenile L. vannamei and can protect them from salinity stresses.150 The improved survival rate against the salinity stress could partially be inferred by the modification of the gill fatty acid composition along with the larger area of gills that leads to increased osmoregulatory capacity and Na+/K+-ATPase activity.151 Similarly, the optimal dietary n-3HUFA level was concluded to be 0.9% and 0.51% for subadult and adult respectively, which improved the growth performances in the salinity ranges of 26.5–28.0 ppt.152
In addition to the lipid and EFA levels, the lipid source also influences the growth and survival of shrimps reared in low-salinity waters. Previously, three diets containing coconut oil, fish oil, or an equal mixture of both differing in fatty acid profiles were fed to white leg shrimp at two different salinity levels (3 and 30 ppt) and it was observed that mixed oil-based diet significantly increased the growth rate and survival.153 This study suggests that the mixed oil diet may have promoted efficient growth in the subjects by enhancing their osmoregulation ability through the presence of highly unsaturated fatty acids (HUFAs), while also conserving energy by sparing amino acids. Additionally, the diet provided an adequate energy supply through the consumption of SFAs. According to Chen et al.,153 shrimp farming can be successful at a salinity level as low as 3 ppt if an appropriate combination of lipid sources is used, even though the optimal salinity for shrimp farming is generally considered to be 30 ppt. The rearing of L. vannamei juveniles at low salinity (3 ppt) equally increased the ability to convert alpha-linolenic acid into DHA compared to the shrimps cultured in high salinity.154 However, the fish oil-free diets when supplemented with DHA resulted in improved growth performance of L. vannamei juveniles at 35.8 ± 0.65 ppt salinity.155 In contrast, the inclusion of arachidonic acid showed a negative impact on the overall performance of L. vannamei juveniles.156 In addition to EFA, phospholipids and cholesterol are also vital components of biological membranes regulating the membrane permeability and the function of enzymes and moulting hormones. Phospholipid requirement was reported to be in the range of 3%–5% regardless of the salinity in the juvenile of L. vannamei.134
4.2.3 Carbohydrates
Carbohydrates are cheap sources of energy that can spare dietary protein for energy. However, shrimps do have a limited ability to utilize dietary carbohydrates compared to other animals like poultry and mammals.157 The carbohydrate requirement of crustaceans has been observed to vary in the ranges of 20%–30% of the total dietary intake, regardless of the carbohydrate sources.158 But it has been found at extreme salinity levels, the demand for energy increases in shrimps. To fulfil the demand, carbohydrates were a good immediate source of energy.159 Wang et al.160 reported that growth performance was improved with a 15%–20% inclusion of carbohydrates in the diet. In this study, the dietary carbohydrate provides extra energy for osmoregulation and growth besides alleviating the adverse impacts of ammonia stress at low salinity (3 ppt) in L. vannamei. In situations of stress including the one due to salinity extremities, protein is important for repairing and maintaining the tissues. However, high protein in situations like salinity stress is not recommended because the feeds could be costly and may affect the water quality. However, as mentioned previously carbohydrates act as the immediate source of cheap energy, hence their inclusion can spare the protein for energy. The statement is justified by Wang et al.,161 that reported protein sparing effect of carbohydrates by feeding 34:19 as the protein-to-carbohydrate ratio at low salinity. In this ratio, the growth was found to be optimum, and the ammonia stress was also alleviated.
The carbohydrate utilization efficiency depends on their nature and complexity. Complex carbohydrates are better utilized and release energy slowly after digestion. Simple carbohydrates such as glucose are directly absorbed by the energy source. From the practical viewpoint, the dietary inclusion of simple sugars is not recommended due to their prohibitive cost and immediate absorption without releasing the energy in a slow manner. However, salinity gradients beyond the optimal ranges, result in stress, which can be compensated for by providing simple sugars like glucose which is an immediate energy source. Wang et al.158 observed that complex carbohydrate sources were not ideal for L. vannamei in low salinity (3.0 psu) waters. It was reported that glucose-fed groups displayed better growth performances than complex carbohydrates like potato starch due to greater energy demand in stressful conditions like salinity extremes. Similarly, Qiao et al.162 observed the best growth performances in sucrose and glucose-fed groups of L. vannamei than in the complex carbohydrates. During hypo-osmotic stress, the facilitative glucose transporter 1 (GLUT1) has been found to be more active in transporting glucose in shrimps to meet the energy demand.163 Furthermore, to combat the low salinity stress, carbohydrates in the form of prebiotics such as inulin could be supplemented in diets up to the levels of 0.4%.164
4.2.4 Vitamins
Vitamins are important for several biological functions not limited to growth, health and reproduction. These organic compounds need to be incorporated into diets in minor proportions. Like finfish, vitamins are essential for shrimps. Vitamins are the compounds that regulate the metabolism of nutrients and hence, it is always recommended to supplement them in the shrimp diets for efficient performance. The graded levels of quasi-vitamin have been supplemented in the diets of L. vannamei to investigate the effects on antioxidative functions and salinity stress tolerance. It was estimated that 2705 mg/kg of Myoinositol was required for glutathione peroxidase activity of L. vannamei.165 Furthermore, the inclusion level of 4.89 g/kg diet also improved the salinity stress tolerance of L. vannamei when salinity was decreased from 26 ppt to 6 ppt.165 On the other hand, dietary choline was also reported to improve the antioxidant activity and growth performance in white leg shrimp at 17.4 ± 2.7 ppt salinity.166 Vitamin D3 is a lipid-soluble pro-hormone that is important for protecting skeletal integrity along with maintaining the homeostasis of calcium and phosphate. Diets with graded levels of Vitamin D3 were fed to L. vannamei and based on the whole-body ash content 6366 IU/kg was required at low salinity (10–15 ppt) rearing conditions.167 Vitamins also display the interaction effects on growth performance, feed utilization and antioxidant activity. For instance, vitamin E and vitamin C have been observed to show an interactive effect at 1.0 g/kg and 0.3 g/kg, respectively, with a dietary lipid level of 7% and resulted in better growth performance and antioxidant activity of L. vannamei and therefore the tolerance to salinity stress.168
4.2.5 Minerals
Minerals are important for skeletal development, osmoregulation and several other physiological functions. Shrimps are widely cultured in the coastal regions with salinities ranging from 15 to 40 ppt. In these coastal regions, the shrimps obtain minerals from both the aquatic environment and the feed. Nowadays, shrimp farming, particularly P. vannamei, is being practised in areas with lower to moderate salinity levels ranging from 0 to 10 ppt. This is because this shrimp has a broader range of salinity tolerance. However, the ionic compositions of waters with different salinity levels vary, which means that the shrimp require mineral supplements for optimal growth, survival and to cope with salinity stress. For instance, low-salinity waters have been reported to contain low concentrations of potassium and magnesium ions.169 Hence, the requirement for certain minerals increases in low-salinity waters. Jahan et al.170 reported enhanced growth of white leg shrimp in inland ground saline waters of low salinity when fortified with minerals like K+ and Mg2+ at the levels of 5 and 150 mg/kg, respectively. Similarly, several studies report the improved performances of the shrimps when supplemented with minerals in low saline waters.134 The ionic profile is variable in waters of different salinities and the actual mineral requirement is difficult to evaluate due to the variability in the ionic composition of waters. The improper ratios of ions in water result in the osmotic stress of shrimps which hampers their growth and survival. The ratio of ions such as Na:K and Mg:Ca was revealed to be preferably around 28:1 and 3.4:1 respectively.171 Similarly, other studies also reported that for better growth and survival the Mg:Ca ratio needs to be maintained at 3:1.172 Therefore, it is always recommended to evaluate the ionic composition of ambient water before supplementing the minerals for better growth, survival and other physiological functions.
4.3 Effect of salinity on digestive physiology
The digestive physiology of certain economically significant crustaceans is influenced by the changing salinity levels in their surrounding culture water.173 The deviation of the optimum salinity levels (20–25 ppt)134 for shrimp rearing alters the normal physiological homeostasis leading to osmoregulatory-dependent pressure in cells, thus increasing the body energy demand (Figure 2).88 Likewise, it also triggers the activity of substrate-specific digestive enzyme activities in the hepatopancreas of L. vannamei.137 Previously, authors have demonstrated that salinity has a variable impact on hepatopancreatic digestive enzymes, osmoregulation, growth metrics, immune responses and survival of shrimp.139, 174-176
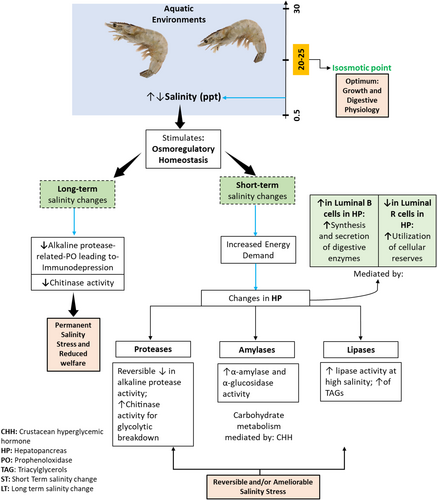
Li et al.175 conducted a study on P. vannamei, investigating the impact of different salinity levels (3, 17 and 32 ppt) on the activities of digestive enzymes. A significantly higher trypsin and amylase activity was observed at 3 ppt salinity followed by 32 ppt. However, lipase-specific activity did not vary significantly despite variations in salinity levels. The surge in digestive enzyme activities may be due to an increase in the number of hepatopancreatic luminal B cells (blasenzellen cells) at both extreme salinities. It facilitates the synthesis and secretion of digestive enzymes to compensate for extra energy demand through osmoregulation via gill. Similarly, a decrease in the abundance of luminal R cells (restzellen cells) at 3 ppt indicates the utilization of cell reserves to cope with low salinity conditions. In contrast to this, the activity of the digestive and antioxidant enzymes decreased at very high salinities, particularly above 50 ppt salinity176 Alkaline proteases such as trypsin and chymotrypsin are the key digestive enzymes of protein metabolism in shrimp.136 Apart from this, they also modulate the immune system by triggering prophenoloxidase defence system.177, 178 Synthesis and production of these enzymes depends on the mRNA and protein synthesis.179 In a study, Gao et al.139 assessed the impact of varying salinity levels (2, 10, 20 and 30 ppt) on metabolic-related gene expression of P. vannamei. The hepatopancreatic trypsin and chymotrypsin mRNA expression significantly downregulated with the decreasing salinity levels and the lowest mRNA expression was observed at 2 ppt salinity. The study concludes that the rate of synthesis of these digestive enzymes was inhibited at low salinity leading to stress, ultimately poor growth and immunodepression. Gao et al.174 evaluated the effect of short-term (24 h) and long-term salinity stress (56 days, 2 ppt as low saline water and 30 ppt as natural seawater) on hepatopancreatic digestive-related genes of P. vannamei. Relative mRNA expressions of trypsin and chymotrypsin 1 and hepatopancreatic genes were unchanged in short-term salinity stress but decreased by 1.33- and 1.54-fold in long-term salinity stress at low salinity. The findings specify that these genes support short-term stress recovery in shrimp, but long-term salinity stress leads to immunodepression and poor digestive performance and growth of shrimp. However, mRNA expression of the chitinase gene increased 3.07-fold at short-term salinity stress but downregulated by 1.45-fold at long-term low salinity stress conditions. This observation indicates that the activity of this enzyme increased to digest more food to compensate for the hypo-osmotic stress-mediated metabolic energy demand via activation of the glycolytic pathway180 and immuno-protective efforts of P. vannamei. But long-term hypo-osmotic stress leads to damage of the hepatopancreas that resulted in immunodepression, abnormalities in digestive physiology and growth retardation of shrimp. Gaxiola et al.173 studied the factorial effects of salinity levels (15 and 40 ppt), dietary carbohydrate (2.3% and 42.3%) and moult cycle of L. vannamei and found that the specific activity of carbohydrases (α-amylase, hexokinase, α-glucosidase and hexokinase IV) were significantly altered in both the rearing salinity conditions. At low salinity, α-amylase activity was highest when fed with low and high levels of dietary carbohydrates (salinity and moult stages interaction) whereas highest α-glucosidase activity was found in low carbohydrate-fed group (salinity and dietary carbohydrate interaction). The findings are particularly associated with the hormonal regulation of growing stages over synthesis and production of digestive enzymes concomitant with the digestion of carbohydrates for metabolic energy needs at low salinity levels. However, hexokinase and hexokinase IV activity were found highest in high carbohydrate-fed groups (salinity and carbohydrate levels interaction). Elevated activities of hexokinase and hexokinase IV are linked with higher need of glycogen and energy for faster moulting processes and physiological adaptations for growth regulated by crustacean hyperglycemic hormone (CHH).181, 182 Chen et al.183 described higher hepatopancreatic lipid metabolic enzyme activities viz., fatty acid synthase (FAS), hormone-sensitive lipase (HSL), Δ5 fatty acid desaturase (Δ5 FAD), Δ6 fatty acid desaturase (Δ6 FAD), adipose triacylglycerol lipase (ATGL) and elongase of very long chain fatty acids 6 (ELOVL 6) at high salinity (30 ppt) as compared to very low salinity (3 ppt). This function is mainly attributed to the higher degradation of fatty acids to produce triacylglycerols which are further transported to gill via lipoproteins to satiate the higher energy demand for salinity-dependent osmoregulatory stress. At low salinity, temperature also impairs the plasma protein metabolic enzymes and ALT (alanine aminotransferase) activities in P. vannamei. A decrease in temperature from 28°C to 13°C at low saline conditions (5 ppt) gradually decreases the plasma ALT activities and ruptured B cells were detected in the dilated lumen of hepatopancreas indicating severe damage of the organ as well as the reduced digestive capacity of shrimp L. vannamei.127 However, the restoration of hepatopancreatic B cells occurred during the temperature reoccurrence process (13–28°C).
4.4 Salinity changes: immune response and impact on disease outcome
Beyond the baseline metabolic rates needed for sustaining life, additional energy is expended on activities such as locomotion, digesting food, building new tissues, reproducing and responding to stress or disease. Growth can be hindered or even reversed when these demands increase either due to heightened stress or disease. The energy required for osmoregulation is considered a part of the baseline maintenance component.184 Different species or groups of animals exhibit various strategies for osmoregulation. Salinity stress negatively affects immune parameters in crustaceans and can increase the virulence of pathogens in hosts, leading to increased mortality. Rapid fluctuations in salinity levels can have detrimental effects on the osmoregulation, physiology and immune responses of decapod crustaceans.185 Several studies have investigated the effects of salinity on the immune responses of different crustacean species (Figure 3). Luqing and Lingxu186 observed a gradual decrease in the bacteriolytic and antibacterial activity of the haemocytes of Fenneropenaeus chinensis and L. vannamei in response to a rapid reduction in salinity from 30 to 15 ppt over a 10 h period. However, the phenoloxidase activity of these shrimps increased over the same period. Vargas-Albores et al.187 demonstrated that prophenoloxidase activity increased directly with salinity in Farfantepenaeus californiensis reared at salinity levels between 28 and 44 ppt. Similarly, several other reports have indicated a decrease in haemocyte count and an increase in phenoloxidase activity in response to changes in salinity in Marsupenaeus japonicus188 and Portunus trituberculatus.189
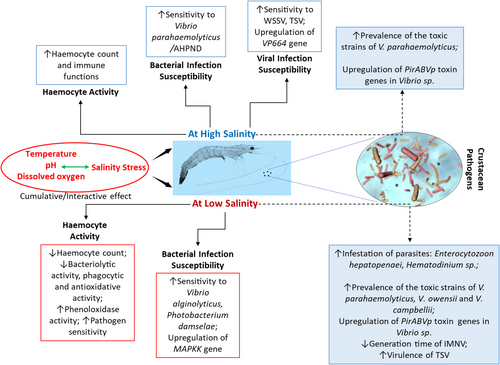
The impact of salinity on haemocyte numbers in crustaceans has been studied by several researchers. Cheng and Chen190 observed an increase in haemocyte numbers in M. rosenbergii when salinity levels changed from 0 to 5, 10, or 15 ppt over a period of 7 days. On the other hand, Perazzolo et al.191 observed that the total heamocyte count (THC) Farfantepenaeus paulensis gradually declined when salinity was decreased from 34 to 22 ppt or 13 ppt over 3 days, before stabilizing between 7 and 28 days. Le Moullac and Haffner132 observed that F. paulensis reared in 34 ppt salinity had a significantly higher THC than those reared in 22 and 13 ppt. Similarly, giant freshwater prawn M. rosenbergii reared in 15 ppt had a significantly higher THC compared to the prawns reared in freshwater.190 From these findings, it can be inferred that salinity levels have a significant impact on haemocyte count in crustaceans. Overall, decreasing salinity levels may lead to a decline in haemocyte count, indicating potential suppression of immune response in crustaceans. Conversely, higher salinity levels may promote an increase in haemocyte count, suggesting enhanced immune response. These findings highlight the importance of salinity as a crucial environmental factor influencing the immune responses of crustaceans, which may have implications for their health, survival and overall immune defence against pathogens. The neuroendocrine-immunoregulatory network has also been found to play a principal role in adapting to salinity changes in crustaceans. Zhao et al.192 proposed that the network serves as the main centre for sensing stress and initiating an immune response in L. vannamei. Furthermore, several studies have reported that salinity stress can cause a decrease in haemocyte count and enzyme activity related to disease resistance, while an increase in sensitivity to pathogens in crustaceans.132, 187, 193 Lu-Qing et al.194 have suggested that the haemocyte count of shrimp decreases under low salinity due to an increase in haemolymph volume. As a result, the total haemocyte count appears to decrease in relation to the haemolymph volume. This may explain why there is no significant change in the immune enzyme activity per unit protein in the haemolymph with decreasing salinity.
Overall, salinity stress can have significant effects on the immune responses of various crustacean species, including changes in haemocyte count, bacteriolytic and antibacterial activity and phenoloxidase activity. These findings suggest that the impact of salinity stress on crustacean immune responses is complex and may depend on various factors, including species, salinity levels and exposure duration.
4.4.1 Effect of salinity changes in shellfish diseases
Salinity is an important factor in the development and transmission of diseases in crustaceans. It can affect the prevalence, severity and transmission of microbial infections in aquatic organisms. In this section, we are going to highlight the role of salinity in the transmission of microbial and parasitic infections in crustaceans, particularly decapods.
4.4.2 Complex relationship between salinity and viral diseases
Several studies have shown that salinity variations can disrupt the physiology of crustaceans to cope with the viral infections. Ramos-Carreño et al.195 conducted a study to investigate the susceptibility of L. vannamei to WSSV in different salinities. The authors found that L. vannamei exhibited lower susceptibility to WSSV in intermediate salinities of 15 and 28 ppt compared to the seawater salinity of 34 ppt. The finding corroborates with previous studies conducted on related species.196-198 The authors have also demonstrated that L. vannamei was highly susceptible to WSSV under extreme salinities of 5 and 54 ppt, resulting in increased mortality rates compared to those exposed to intermediate salinities of 15 and 28 ppt. Moreover, exposure to extreme salinities resulted in the upregulation of VP664 transcript levels, with the highest levels observed after 24 h post-infection (hpi).195 These findings indicate that extreme salinities can exacerbate the pathogenicity of WSSV in P. vannamei, and that intermediate salinities may be a potential strategy for reducing its impact on shrimp aquaculture.
In addition, several other researchers have reported that salinity can increase the susceptibility of shrimp to WSSV infection, and may even trigger outbreaks of the disease.194, 196, 197 Gao et al.198 suggest that higher salinities (≥ 30 ppt) may be more favourable for WSSV proliferation than lower salinities (≤15 ppt). However, the authors did not observe any significant differences in WSSV replication between different levels of salinity, indicating that changes in salinity may not be the only decisive factor affecting WSSV replication. Therefore, the impact of salinity on WSSV infection may be complex, and other factors such as temperature, pH and dissolved oxygen levels should also be taken into consideration when managing shrimp aquaculture to prevent WSSV outbreaks. Furthermore, Xue et al.199 conducted a study to investigate the effect of salinity and total ammonia concentration on the susceptibility of Chinese shrimp to WSSV infection. The authors found that the levels of WSSV infection and shrimp mortality were significantly lower in the lower salinity group (20 ppt) compared to the higher salinity groups (30 and 40 ppt). Interestingly, the transfer of shrimp to lower temperatures and lower salinity was observed to enhance their resistance to WSSV infection. These findings suggest that salinity is an important factor affecting the susceptibility of shrimp to WSSV infection, and that the management of salinity levels could be an effective strategy for mitigating the impact of WSSV on shrimp aquaculture.
In general, Tendencia et al.200 suggested that salinity fluctuation in shrimp culture systems is often positively correlated with temperature and pH. This correlation can make it difficult to isolate the specific effect of salinity fluctuation on WSSV outbreaks and may hinder its accurate representation in studies. Therefore, it is important to consider and control the potential confounding effects of other environmental factors when investigating the impact of salinity on WSSV infection in shrimp aquaculture.
As an example, abrupt changes in temperature and salinity resulting from heavy rainfall in Mexico have been found to increase the viral loads in shrimp, leading to an alarming mortality rate of 80%.201 Such fluctuations in salinity and temperature have been observed to weaken the shrimp immune system, making them more susceptible to viral replication. This, in turn, leads to a significant increase in viral loads, thereby compromising the health and survival of the shrimp. Additionally, low salinity levels and a low hardness of pond water are stress factors that can exacerbate the shrimp vulnerability to Vibrio bacteria, which is responsible for causing WSSV disease. These stress factors further compromise the immune system of shrimps, leaving them even more susceptible to infections and diseases. Another virus, Taura syndrome virus (TSV) has the ability to remain viable for as long as 10 days in water with a salinity level of 5–10 ppt and can infect Pacific white shrimp, resulting in significant mortalities.202 The study suggests that appropriate measures need to be taken to limit the exposure of shrimp to TSV, particularly in environments with a salinity level of 5–10 ppt, where the virus can remain infectious for an extended period.
In a study conducted by Vieira-Girão et al.,203 quantitative real-time PCR was used to investigate the relationship between salinity levels and the replication of viruses in shrimp. The results of the study indicated that low salinity levels have a positive effect on the replication and proliferation of infectious myonecrosis virus (IMNV), The generation time of IMNV decreased from 57.4 min at the optimal salinity level of 35 ppt to 25.2 min at a stressing concentration of 5 ppt. Similarly, another study demonstrated that a decrease in salinity level is positively related to the reduction in the generation time of persistent infectious hypodermal and haematopoietic necrosis virus (IHHNV), which commonly co-infects shrimp in farm ponds.203 The studies suggest that low salinity levels can facilitate the replication and spread of these viruses, which can have detrimental effects on the health and survival of shrimp in aquaculture facilities.
In summary, it is crucial to carefully monitor the salinity and temperature levels in shrimp ponds, particularly during heavy rainfall, to prevent abrupt fluctuations that can have detrimental effects on the health and survival of the shrimp. Maintaining suitable environmental conditions can help to enhance the shrimp immune system and reduce their susceptibility to viral infections and diseases. This can be achieved through the implementation of effective quarantine protocols, improvements in pond water quality and biosecurity measures. Further research is necessary to better understand the mechanisms underlying the relationship between salinity levels and virus replication in shrimp, which could help inform the development of more effective preventative measures in the future.
4.4.3 How salinity affects bacterial diseases in shellfish
Salinity affects bacterial diseases in shellfish by influencing the growth, survival and virulence of bacterial pathogens. Wang and Chen204 investigated the immune response and susceptibility of L. vannamei to Vibrio alginolyticus at varying salinity levels. The results revealed that shrimp transferred from 25 ppt salinity to low salinity levels of 5 and 15 ppt exhibited lower immunity and decreased resistance against V. alginolyticus infection, indicating a negative impact of salinity reduction on shrimp immune response and disease resistance. In a similar study, the impact of salinity on the immune response and susceptibility of P. monodon to Photobacterium damselae subsp. damselae was investigated by Wang and Chen.205 The findings indicated that P. monodon exposed to low salinity levels of 5 and 15 ppt or high salinity level of 35 ppt after being transferred from a salinity level of 25 ppt, exhibited low immunity and high susceptibility against P. damselae infection. This suggests that salinity levels play a significant role in influencing the immune response and susceptibility of P. monodon to bacterial infection.205 Furthermore, a recent study by Fan et al.206 isolated and characterized a MAPKK gene from P. monodon, which was found to be involved in the innate immune mechanism as well as its adaptation to low-salinity environments. This further highlights the complex interplay between the immune response and salinity levels in P. monodon.
Sanathkumar et al.207 detected the presence of V. parahemolyticus in low-saline groundwater, along with other Vibrio species. Their findings strongly suggest that the ongoing mortality events in L. vannamei were likely caused by a co-infection involving V. parahemolyticus and WSSV, underscoring the potential of bacterial infections to exacerbate the severity of viral diseases in aquatic animals in low saline waters.207 According to the study conducted by Bauer et al.208 the population of Vibrio bacteria in shrimp recirculating aquaculture systems (RAS) is greatly influenced by water salinity levels. The study found that at lower salinity levels of 15 ppt, a large number of potentially pathogenic Vibrio species, including V. parahaemolyticus, V. owensii and V. campbellii, were present. These species are known to produce PirA and PirB toxins, which are responsible for causing Acute Hepatopancreatic Necrosis Disease (AHPND) in shrimp. On the other hand, at higher salinity level of 30 ppt, these bacteria were not detected. Furthermore, the study did not detect pirAB toxins in any of the isolates, but several other pathogenicity factors were present, especially in the V. parahaemolyticus isolates. Therefore, it can be concluded that a decrease in water salinity may lead to a shift in the Vibrio population towards pathogenic species, which could increase the risk of disease outbreaks in shrimp in RAS. Despite the absence of pirAB toxins in most of the Vibrio isolates in the low-salinity, it is important to note that V. owensii was detected at a salinity level of 15 ppt. Since V. owensii is capable of carrying pirAB toxins, a decrease in water salinity may indeed increase the risk of infection in shrimp by V. owensii. Therefore, it is crucial to maintain appropriate water salinity levels in RAS-based culture to prevent the proliferation of potentially pathogenic Vibrio species, and to minimize the risk of disease outbreaks in shrimps. On the contrary, Soto-Rodriguez et al.209 observed higher expression of PirABVp toxin in Vibrio sp., resulting in a high cumulative mortality in L. vannamei when maintained under high and lower saline conditions.209 Further experiments are necessary to determine the precise effect of salinity on pirABVp expression. Nonetheless, these findings support the notion that the expression of the toxin is salinity-dependent, as previously suggested by Soto-Rodriguez et al.210
On the other hand, a study has found that AHPND is more lethal to shrimp in high-salinity environments than low-salinity ones. Shrimp maintained at 20 ppt showed the highest survival rate (78.70%) among the infected group, while those maintained at 28 ppt exhibited significantly lower survival rates (54.89%).211 Uninfected shrimp did not show significant differences in mortality rates at different salinities. The increased salinity levels cause stress on shrimp and lead to the expression of virulent genes in V. parahaemolyticus.212 Environmental factors, including salinity, have been found to have an impact on both the production of toxins and the survival of shrimp. However, further research is required to fully understand the relationship between salinity, the expression of pirABVp genes and the pathogenesis of AHPND. Scientific investigations have also provided compelling evidence that changes in salinity levels can have significant impacts on the phagocytic activities of crustaceans. Cheng et al.213 reported significantly lower phagocytic activity and clearance efficiency of M. rosenbergii against the pathogen Lactococcus garvieae in freshwater as compared to 5% and 15% salinity levels.
4.4.4 Role in parasitic infections
Salinity is considered as critical environmental factors that affect the growth and survival of both shellfish and their parasites. Salinity can influence the osmotic balance, metabolism and immune response of the shellfish,134, 214 which, in turn, can affect their susceptibility to parasitic infections.215 Moreover, salinity can affect the life cycle, development and infectivity of the parasites.216, 217 Therefore, understanding the impact of salinity on parasitic infections in shellfish is crucial for improving the management practices and control of shellfish diseases. The impact of salinity on parasitic infections can vary depending on the type of parasite and the shellfish species. In general, it is observed that high salinity can increase the prevalence and intensity of parasitic infections in shellfish, while low salinity can reduce it.218, 219
In P. monodon, the Enterocytozoon hepatopenaei (EHP) infection can occur at a salinity as low as 2 ppt; however, the prevalence and the severity of the EHP infection are higher at a salinity of 30 ppt.220 Similarly, Hematodinium sp., a dinoflagellate parasite, has been found to infect blue crabs (Callinectes sapidus) in estuaries along the Atlantic coast of the United States. However, reports of infections in blue crabs have been found limited to areas with a salinity level greater than 11 ppt.221 Further experiments involving in vitro cultures have shown that the infective stages of the parasite (dinospores) quickly lose their ability to infect blue crabs when exposed to salinities below 15 ppt.221 This explains the lack of natural infections in blue crabs at low salinities, as the parasite appears incapable of transmission under such conditions. Likewise, Pung et al.222 found that shrimp infected with Microphallus turgidus metacercarial cysts were present at all collection sites, with Palaemonetes pugio having the highest prevalence and density of these parasites in areas with salinity levels of at least 20 ppt.
Overall, the studies suggest that salinity can significantly influence the prevalence, intensity and infectivity of parasitic infections in shellfish. However, the exact mechanisms underlying these effects are not well understood and may vary between parasite–host systems. Further research is needed to fully elucidate the role of salinity in the epidemiology of parasitic diseases in shellfish. It can be stated that the impact of salinity on parasitic infections in shellfish is complex and depends on various factors, such as the type of parasite, the shellfish species and the duration and magnitude of exposure to different salinity levels. Understanding how salinity affects microbial and parasitic infections in shellfish can help to develop effective management and control strategies for shellfish diseases.
5 CONCLUSION
In conclusion, the ability of crustaceans to adapt to different salinity levels is crucial for their survival in changing aquatic environments. The molecular mechanisms controlling osmoregulation in crustaceans are complex and involve various proteins and genes that respond to the salinity changes in aquatic habitats. The salinity impacts the growth and well-being of crustaceans and ultimately has bearing on the quality and quantity of the harvest. Also, salinity directly influences nutritional efficiency and can be among the key parameters for determining the dietary demands of crustaceans. The osmotic balance in crustaceans also dictates the functionality of their digestive systems, and the long-term salinity changes can reduce digestion efficiency. Moreover, understanding the interplay between salinity, pathogens and immune functions is regarded as crucial for effective disease management in crustacean aquaculture.
Overall, the review provides insights into a range of factors that affect the adaptation of decapod crustacea to changing water salinities and highlights the need for continued research in this area. The information might help to ensure sustainable aquaculture development and the conservation of important aquatic species, like crustaceans. The review also provides valuable insights for researchers engaged in rearing economically important decapods, especially in the context of changing salinities such as RAS and inland saline waters. By highlighting the challenges that may arise in this process, the information might help researchers to anticipate and overcome potential obstacles. Additionally, it also sheds light on the salinity levels that are conducive to the optimal growth and development of decapods, particularly for P. vannemei, which can aid researchers and culturists in making informed decisions regarding their rearing practice.
AUTHOR CONTRIBUTIONS
Raja Aadil Hussain Bhat: Conceptualization; writing – original draft; writing – review and editing; validation; methodology; investigation. Yousuf Dar Jaffer: Conceptualization; validation; methodology; writing – review and editing; writing – original draft. Irfan Ahmad Bhat: Investigation; conceptualization; validation; writing – review and editing; writing – original draft. Ishfaq Nazir Mir: Conceptualization; writing – original draft; writing – review and editing; methodology; investigation. M. Junaid Sidiq: Writing – original draft; writing – review and editing; investigation. Prasanta Jana: Writing – original draft; writing – review and editing.
ACKNOWLEDGEMENTS
Jaffer Yousuf Dar was supported by Netaji Subhas-ICAR International Fellowships (NS-ICAR 2019 IFs) from the Indian Council of Agricultural Research, India.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this article.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing not applicable to this article as no datasets were generated or analysed during the current study.




