The impact of transcutaneous vagus nerve stimulation on anterior cingulate cortex activity in a cognitive control task
Özde Sönmez and Elfriede Holstein shared first author.
Abstract
Transcutaneous vagus nerve stimulation (tVNS) offers a non-invasive method to enhance noradrenergic neurotransmission in the human brain, thereby increasing cognitive control. Here, we investigate if changes in cognitive control induced by tVNS are mediated through locus coeruleus-induced modifications of neural activity in the anterior cingulate cortex. Young healthy participants engaged in a simple cognitive control task focusing on response inhibition and a more complex task that involved both response inhibition and working memory, inside a magnetic resonance imaging scanner. The tasks were executed using a randomized within-subject design, with participants undergoing auricular tVNS and sham stimulation in separate sessions. tVNS significantly changed performance in the simple control task reflected in a greater propensity to respond. Furthermore, we observed a significant increase in neural activity in the anterior cingulate cortex during the simple cognitive control task under tVNS. Functional connectivity analyses revealed positive coupling between neural activity in the locus coeruleus and anterior cingulate cortex, however, this was not modulated by tVNS. The findings suggest that non-invasive stimulation of the vagus nerve can modulate neural activity in the anterior cingulate cortex. While these neural effects suggest an impact of tVNS in a key region involved in conflict monitoring and cognitive control, the behavioral effects are more indicative of a shift in response bias rather than enhanced cognitive control.
1 INTRODUCTION
There has been an increased interest in understanding how the loss of noradrenergic neurotransmission impacts neurodegenerative disorders like Parkinson's or Alzheimer's disease. Noradrenergic neurons, which mainly originate in the locus coeruleus (LC), are critical for executive functions, including working memory, response inhibition, and cognitive flexibility, which are often affected in the aforementioned disorders (Holland et al., 2021). Hence, stimulation of noradrenergic neurotransmission could be a potential therapeutic target for improving executive functions in Parkinson's or Alzheimer's disease.
Transcutaneous vagus nerve stimulation (tVNS) is a non-invasive form of electrically stimulating the vagus nerve through electrodes placed on either the ear or the neck (Bonaz et al., 2018; Henry, 2002; Raedt et al., 2011). The stimulation activates the LC, via the nucleus of the solitary tract, leading to an increase in noradrenaline levels (Colzato & Beste, 2020; Frangos et al., 2015). This increase in noradrenaline has been suggested as one key factor mediating the therapeutic effects of both invasive and non-invasive vagus nerve stimulation (Butt et al., 2020; Yakunina et al., 2017).
Prior studies have shown that non-invasive stimulation of the vagus nerve facilitates a variety of cognitive functions in healthy subjects and patient samples (Colzato & Beste, 2020). In healthy individuals, the largest effect is found for executive functions (Ridgewell et al., 2021). For example, tVNS can be implemented to improve executive functions (Borges et al., 2020; Fischer et al., 2018; Keute et al., 2020; Pihlaja et al., 2020; Vonck et al., 2014), including, but not limited to, inhibition control (Borges et al., 2019; Pihlaja et al., 2020) and cognitive flexibility (Borges et al., 2019; Colzato & Beste, 2020; Vonck et al., 2014). Additionally, tVNS can enhance associative memory performance, facilitate response selection (Colzato & Beste, 2020; Pihlaja et al., 2020), adaptation to conflict, cognitive control (Fischer et al., 2018; Pihlaja et al., 2020), and reward learning (Kühnel et al., 2020).
Cognitive control plays a paramount role in overriding prepotent responses, ignoring goal-irrelevant stimuli and attention shifting (Pihlaja et al., 2020). Neurochemically, it depends on ascending monoaminergic and cholinergic systems with several overlapping, yet distinct functions. Given their widespread cortical projections and diverse receptor subtypes, these systems can broadly and precisely transfer information. They operate on different timescales, enabling the rapid adaptation of behavior as well as the stabilization of behavior over longer periods. While classically dopaminergic stimulation has been related to increases in working memory (Wang et al., 2004) and noradrenergic stimulation with noradrenaline reuptake inhibitors to response inhibition (Chamberlain et al., 2006), recent evidence also indicates a role of α2 receptors in prefrontally mediated working memory processes (Chamberlain & Robbins, 2013; Cools & Arnsten, 2022). In an electroencephalography (EEG) study, Fischer et al., 2018, found that tVNS impacted both behavioral and electrophysiological measures of cognitive control. They theorized that changes in conflict adaptation are predominantly modulated by the vagus nerve-LC (VN-LC) pathway. The dorsal part of the anterior cingulate cortex (ACC) is a prime conflict processing region (Aston-Jones & Cohen, 2005; Krebs et al., 2013) and has been proposed to constitute the core of a network involved in cognitive control (Cole & Schneider, 2007). The ACC has a strong reciprocal functional and structural connectivity with the LC (Chandler & Waterhouse, 2012; Gompf et al., 2010; Joshi & Gold, 2022). The association between the LC and the ACC with respect to cognitive control is believed to be of pronounced importance (Aston-Jones & Cohen, 2005). For example, response conflicts are detected by the ACC which then signals the prefrontal cortex to recruit cognitive control to adjust successive behavior (Fischer et al., 2018). Using functional magnetic resonance imaging (fMRI), Krebs et al. (2013) found parallel involvement of the ACC and LC during semantic interference processing, suggesting that conflict-related changes in the ACC are associated with activity changes in the LC. Therefore, it is reasonable to assume that tVNS-induced changes in cognitive control may be mediated via LC-induced activity changes in the ACC.
Additionally, in contrast to indirect outcome measures of LC activation, fMRI provides objective data by measuring blood flow changes associated with neuronal activity that can gauge LC activity with reasonable spatial precision (Ludwig et al., 2021). A few prior fMRI studies investigated the effects of non-invasive tVNS in healthy volunteers and confirmed the activation of the nucleus of the solitary tract, the LC, as well as a wide variety of cortical regions (Liu et al., 2020). For example, Badran and colleagues found increased blood oxygenation level-dependent (BOLD) activity in the anterior and mid-cingulate cortex, the caudate, the operculum, and the cerebellum when comparing tVNS with sham stimulation during rest (Badran et al., 2018). However, as those studies were performed without tasks, it is unclear whether such tVNS-induced changes contribute to the observed beneficial effects on executive function. Furthermore, none of these studies have addressed whether electrical stimulation affects the quality of MR images. In the present study, the quality of the MRI data was checked a priori.
The present study was performed to investigate whether tVNS-induced changes in cognitive control are mediated via LC-induced activity changes in the ACC. TVNS, or respective sham stimulation, was applied inside an MR scanner while subjects performed either a simple cognitive control task tapping into response inhibition, or a complex task tapping into two aspects of cognitive control—response inhibition and working memory. The response inhibition paradigm was chosen because this executive function is most closely linked to the noradrenergic system (Chamberlain et al., 2006; Chamberlain & Robbins, 2013). The working memory component was included based on meta-analytic findings indicating that tVNS improves response inhibition, particularly as task complexity increases (Ridgewell et al., 2021). Additionally, working memory is modulated by pharmacological interventions targeting noradrenergic neurotransmission (Cools & Arnsten, 2022). We hypothesized that tVNS would modulate neural activity in the ACC and LC-ACC connectivity.
2 METHODS
2.1 Participants
The required sample size was determined by an a priori power analysis using G*Power (Faul et al., 2007) based on a literature review on the cognitive effects of tVNS by Colzato & Beste, 2020. Assuming a moderate effect size (Cohen's d = 0.5) resulted in an estimated sample size of 34 for detecting a difference between means in a within-subjects design with a power of 0.8 (α = .05). To account for possible drop-outs and outliers, we recruited 47 participants. A total of 38 participants met the following criteria: healthy, right handed, aged between 18 and 40, normal or corrected vision, and no medication (except for contraceptives). Participants were without contraindications for MRI examinations, neurological and psychiatric disorders, or pre-existing cardiac illness. All participants gave written informed consent prior to the experiment and the experiment was approved by the ethics committee of the Medical Faculty of the University of Oldenburg (ethics approval number 2021-079).
Three participants dropped out of the study after the first session and were excluded from all further analyses, leaving a sample of 35 participants (male/female 17/18, mean age = 25.42 ± 3.43). For behavioral analyses and task-based analyses, one further participant had to be excluded because button presses were not recorded due to a hardware problem. Additionally, three participants performed below chance level and were removed. For the task-based analyses of fMRI data, two further data sets had to be excluded due to excessive head movements during task performance, leaving a total of 29 data sets. For cohesion of the results, these data sets with motion problems were also excluded from the behavioral analysis. For the resting-state analysis, in addition to the three dropouts, two participants were excluded due to excessive movement during rest. This resulted in a total sample size of 33 for the resting-state analysis.
2.2 Transcutaneous auricular vagus nerve stimulation
tVNS was applied in a single-blind within-subject design. The order of active and sham conditions was randomized and counterbalanced across participants with at least 3 days in between the two sessions. The tVNS device we used (Cerbomed; NEMOS®, Erlangen, Germany) is CE certified for depression, anxiety, and chronic pain (Farmer et al., 2021). The device delivers an electrical stimulation at 25 Hz with a rhythmic square pulse (Yuan & Silberstein, 2016) with a pulse width of 200–300 μs and an on–off cycle of 30 s. Stimulation intensity can be adjusted individually.
Prior to applying the tVNS device, the stimulation area was cleaned using alcohol. Then, a conductive gel was applied to ensure good conduction. For the active (tVNS) condition, the tVNS device was fitted to the cymba conchae (Figure 1a) of the left external ear via an earpiece containing two titanium electrodes connected to a battery-operated stimulator. For the sham stimulation, the electrodes were applied upside down to the earlobe (Figure 1b), which is considered to be free of vagal innervation (Peuker & Filler, 2002). To ensure that the device stayed in place during the experiment, the device was fixed onto the ear using medical tape.
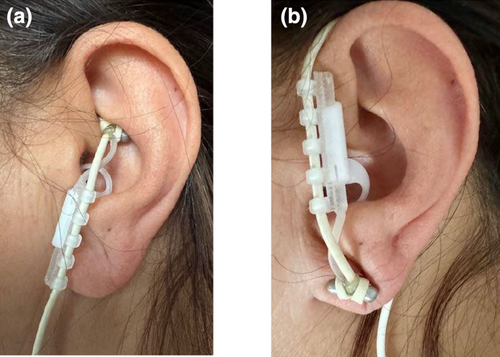
The stimulation intensity was adjusted for each participant prior to scanning. The amplitude was increased until the participant reported an “uncomfortable but not painful” sensation that could be maintained for the duration of the experiment. Stimulation ranged from 1.0 to 4.0 (M = 2.5 ± 0.8) mA for the tVNS and from 1.6 to 5.0 (M = 3.2 ± 1.0) mA for the sham stimulation. Stimulation was applied via an MR-conditional cable that was passed through a waveguide into the scanner. The stimulation began with the onset of each task or rest, with the predetermined and personal stimulation intensity and lasted for the duration of the task or rest. To ensure that participants could barely differentiate between the active and sham conditions, the stimulator was activated in both scenarios. The subjective effects of active and sham stimulation were assessed by asking participants to rate the discomfort of the stimulation on a scale from 0 to 4 after the experiment. Additionally, they were asked whether they perceived the stimulation throughout the entire scanning session (yes/no).
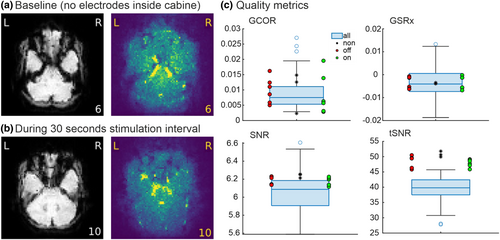
To exclude that tVNS induced artifacts or increased noise in the MR images, we (i) evaluated the effects of the tVNS device and stimulation in a pilot scanning session in a single volunteer and (ii) compared these effects with the collected data of the study. Evaluations were done visually and objectively with the MRIQC toolbox (Esteban et al., 2017). For the pilot scanning session, three 30 s on-and-off cycles of stimulation were run under three different conditions, with the same MR protocol as described in MRI data acquisition. The first condition was the baseline condition without any stimulation electrodes inside the MR room. In the second condition, the electrodes were placed in the MR room and attached to the ear, but stimulation was not switched on. In the last condition, the stimulation was switched on and off. Visually, there were no artifacts or signal losses evident in the functional data related to the usage of the electrodes or the stimulation. The MRIQC analysis provides evidence that all important parameters (i.e., signal-to-noise ratio (SNR), temporal SNR (tSNR), and ghost-to-signal ratio (GSR)) are in the same range across the three conditions (Figure 2). Furthermore, the collected data of all participants were also in the same range. Thus, we conclude that the stimulation of the vagus nerve did not affect the quality of MRI data.
2.3 Procedure
Each session began with practicing the tasks outside the scanner. Then, the tVNS electrodes were applied to the left ear with the respective position, and the current strength (mA) of the device was adjusted. Inside the MR scanner, the session started with a short practice (∼5 min) followed by a resting-state scan (∼5 min). Afterward, the two tasks (the simple and the complex cognitive control tasks) were performed, each lasting 7 and a half minutes, including a 30 s pause in the middle. In addition, on the 1st day, a structural image (T1-weighted image) was acquired (∼6 min).
2.4 Experimental task
To investigate the effects of tVNS on cognitive control, we used a go/no-go task that targets one aspect of cognitive control, namely response inhibition (Georgiou & Essau, 2011). We focused on response inhibition given prior evidence that it is modulated by tVNS (Keute et al., 2020; Pihlaja et al., 2020) as well as pharmacological stimulation targeting the noradrenergic system (Chamberlain et al., 2006). The go/no-go task was either used alone (simple cognitive control task) or was combined with an n-back task (complex cognitive control task) targeting an additional function, working memory, and increasing demands on cognitive control given prior evidence that tVNS effects are strongest when response inhibition is measured under difficult task conditions (Ridgewell et al., 2021).
2.4.1 Simple cognitive control task
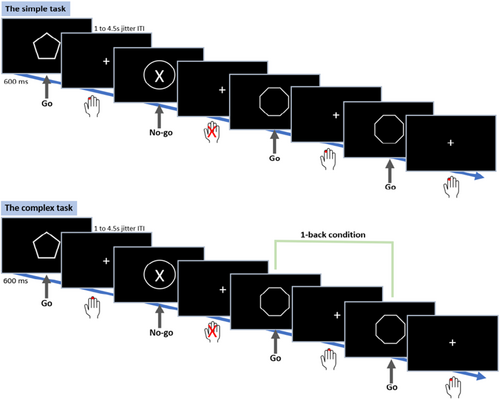
We used an adjusted version of the go/no-go task from the Human Connectome Project (HCP) known as the conditioned approach response inhibition task (CARIT; Winter & Sheridan, 2014). Eight different white-outlined geometrical shapes were presented on a black background. The six go shapes consisted of plaques (rectangles with rounded corners), trapezoids, pentagons, hexagons, octagons, and parallelograms. The two no-go shapes were squares and circles. Go and no-go stimuli were presented for 600 ms and the fixation jitters (inter-trial intervals) varied across trials: 1.0 s (26 trials), 1.5 s (34 trials), 2.5 s (28 trials), and 4.5 s (4 trials). A total of 184 trials were presented, 92 per run, with a pause in between. The percentage of no-go trials was randomized, whereby approximately 25% of all trials were no-go trials across all participants for both tasks. The participants were instructed to press the response button with their right index finger as soon as they saw a go stimulus (i.e., encouraging speed), and to withhold their response for no-go stimuli (Figure 3).
2.4.2 Complex cognitive control task
To increase demands on cognitive control, a 1-back working memory condition was added as a secondary condition to the go/no-go task. Again, 184 trials were presented (92 per run) with approximately 25% no-go trials, whereby two of the shapes making up the go stimuli were removed to increase 1-back conditions in the trials. Participants were tasked with pressing a response key with their right index finger when two go stimuli in a row were the same, and with their right middle finger when two subsequent go stimuli were different. Similar to the simple task version, participants were further encouraged to respond as fast as possible and instructed to withhold their response for no-go stimuli (Figure 3).
Tasks were presented using Presentation© (Version 21.1, Presentation, 2019). Task order (simple vs. complex task) was randomized and counterbalanced across participants.
2.5 MRI data acquisition
We used a 3T MRI machine (MAGNETOM Prisma, Siemens Erlangen Germany) with a 20-channel head coil. Resting-state and task-based data were acquired using a 2D multiband T2*-weighted gradient echo planar imaging EPI sequence with BOLD contrast, from the Center for Magnetic Resonance Research (CMRR), University of Minnesota (Feinberg et al., 2010; Moeller et al., 2010; Xu et al., 2013) (TR = 850 ms, TE = 30 ms, flip angle = 62°, distance factor = 0%, slice thickness = 2.5 mm, field of view = 192 × 192 mm, matrix = 76 × 76, voxel size = 2.5 × 2.5 × 2.5 mm3, 48 slices, multiband factor = 4). Anatomical images were acquired using magnetization-prepared rapid gradient echo (MPRAGE) sequences (224 slices in the sagittal plane, TR = 2000 ms, TE = 2.07 ms, slice thickness = 0.75 mm, flip angle = 9 degrees, 50% distance factor).
2.6 MRI data analysis
MATLAB (version 2019b) and the Statistical Parametric Mapping (SPM)© toolbox (SPM12; Penny et al., 2006) were used for all data analyses.
2.6.1 Preprocessing
The following preprocessing steps were taken for both the task fMRI and the resting-state analyses. Time series were realigned to the first image using a least squares approach and rigid body transformations (six parameters). The images were co-registered twice. During the first co-registration, the mean functional image was used as the reference image and the structural image was the source image. During the second co-registration, the single subject T1 image from the SPM12 canonical folder (Penny et al., 2006) was entered as the reference image, the structural image as the source image, and the realigned functional images as other images. Next, the images were segmented into gray and white matter using forward deformations. Normalization of the functional and anatomical images to MNI space was achieved using the normalization parameters obtained during segmentation. For the functional images, forward deformation and trilinear interpolation were used. For the anatomical images, forward deformation and fourth-degree spline interpolation were used. Participants with excessive movement (total motion >3 mm) were precluded from the task-based analysis. For the resting-state data, participants with excessive motion (total motion >3 mm or scan-to-scan motion >1) were excluded from the analysis.
2.6.2 Task-based general linear model analysis
Task-based images were additionally spatially smoothed (8 mm Gaussian filter). Statistical analysis was conducted using a mixed-effects model. At the single-subject level (“first-level”), three regressors were built: “Hit” go trials, “Correct Rejection” no-go trials, and errors (“Miss” go and “False Alarm” no-go combined). These regressors are convolutions of a stick function with a canonical synthetic hemodynamic response function time locked to the onsets of the stimulus. The six motion regressors from realignment were included to account for head movement. In addition, to remove the influence of remaining scan-to-scan motion larger than 1 mm, an additional scan nulling regressor according to Lemieux et al. (2007) was used. Data were high-pass filtered at 1/128 Hz to account for non-physiological slow drifts in the measured signal and modeled for temporal autocorrelations across scans with an AR(1) model. The general linear model was used to calculate regression coefficients (betas) for each regressor at each measured voxel in the brain and linear contrasts between these betas. To identify neural correlates of cognitive control, we computed for each participant the contrast of interest, no-go>go, which was subsequently entered into the second-level group analyses. To investigate the effects of tVNS on cognitive control during the simple and complex cognitive control task, we used paired sample t tests comparing brain activity under the tVNS and the sham stimulation for the contrast no-go>go. Statistical inference was based on cluster size. We used a cluster identification threshold of p < .001; activation clusters are reported as statistically significant for p < .05 corrected for family-wise errors (FWE). Mean beta values were extracted as a function of trial type (no-go/go) and stimulation condition (tVNS/sham).
2.6.3 Functional connectivity analyses
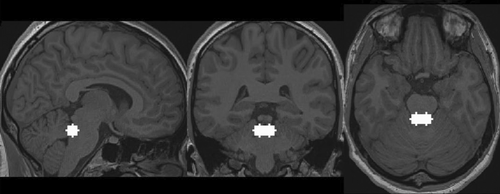
To investigate whether the coupling between the LC and the ACC during task and resting state was modulated by tVNS, ROI-to-ROI analyses of functional connectivity were conducted. For the LC, an ROI made up of two overlapping 6 mm spheres was generated based on the coordinates (left LC: −3.2, −38.2, −24.8; right LC: 5.4, −37.9, −24.7; Figure 4) from Astafiev et al. (2010). The LC spheres also corresponded with the location reported by Keren et al. (2009), Sasaki et al. (2006), and German et al. (1988). The LC spheres were then compared to the probabilistic 7 T-based human LC atlas from Ye et al. (2021) to ensure that the sphere encompassed the LC. Afterward, a visual inspection of the seed region was conducted, whereby the LC mask was overlaid on normalized functional and structural images of the participants. Additionally, visual inspection revealed that the fourth ventricle was not part of the LC spheres. The ACC ROI was generated using WFU PickAtlas's Human Atlas (Maldjian et al., 2003) and encompassed Brodman's area 32.
For the analysis, the preprocessed (but not smoothed) functional data time series were pruned from physiological noise, such as cardiac and respiratory activity, and motion artifacts using nuisance regression with regressors accounting for head movement (six realignment parameters) and signal fluctuations in white matter and cerebrospinal fluid. To account for spurious correlations related to the co-activation of LC and ACC during task performance, we additionally regressed out task activation using the regressors built for the task-based analysis which were convolved with the hemodynamic response. The cleaned signal time courses were averaged (within each ROI) and bandpass was filtered between 0.008 and 0.09 Hz.
Single-subject Fisher z-transformed Pearson's correlation coefficients between the average ROI time series' were calculated for tVNS and sham stimulation separately for each task and resting state. Paired t tests were conducted to determine whether these correlation coefficients were significantly different from one another on the group level.
Given that the LC is a small brain region, we additionally investigated the magnitude and the reliability of the correlation. For this purpose, the LC time series was periodically shifted across all columns using the MATLAB function “cxcorr” (Levine, 2022), and the correlations between the shifted LC time series and the ACC seed were conducted anew. Averaged z-transformed circular correlation coefficients were then plotted against the coefficient of the original correlation. Circular cross-correlation can be used to determine whether the LC-ACC correlation is a consequence of noise as shifting the LC time series should cause the correlation between the LC and the ACC to collapse.
2.7 Behavioral data analysis
Behavioral data were prepared in MATLAB© (2019b, The MathWorks Inc., 2019) and analyzed using RStudio© (Version 1.3.1093; R Core Team, 2019). Hits, misses, false alarms, and correct rejections were extracted for each subject in each task (simple/complex) and condition (tVNS/sham). The percentage of hits was calculated with the following formula: Hits % = (Hitgo/(Hitgo + Missgo)) × 100. The percentage of correct rejections was calculated with the following formula: Correct rejections % = (Correct rejectionno-go/(Correct rejectionno-go + False alarmno-go)) × 100. Task data of subjects who performed below chance level were excluded (chance level: 56%; based on permutation tests for 184 trials with a cut-off p value of .05). Additionally, sensitivity (d′) and response bias (c) were calculated using the R package “psycho” (Makowski, 2018), whereby extreme values were adjusted according to Hautus, 1995. Sensitivity is a measure of how well “go” trials are discriminated from “no-go” trials and was calculated as the z value of hit rate minus false alarm rate (d′ = z(hit) – z(false alarm)). Response bias, in the context of the go/no-go task, reflects a subject's tendency to either more likely respond or refrain from responding and was calculated as the negative average of the z scores corresponding to the hit rate and the false alarm rate, where the z scores are derived using the inverse cumulative normal distribution function (c = −0.5 × (z(hit) + z(false alarm))). Wilcoxon signed-rank tests were used to compare percentages of hits, correct rejections, d′, c, and response times under tVNS and sham conditions in the simple and complex cognitive control task due to the non-normal distribution of the data. Note that there were no session effects for either task.
3 RESULTS
3.1 Behavioral data and subjective reports
The behavioral results for the simple and complex tasks can be found in Table 1.
| Simple task | Complex task | |||
|---|---|---|---|---|
| Sham | tVNS | Sham | tVNS | |
| Hit % | 96.89 (6.84) | 98.6 (4.79) | 88.4 (11.33) | 91.54 (6.92) |
| Miss % | 3.11 (6.84) | 1.4 (4.79) | 11.6 (11.33) | 8.46 (6.92) |
| False alarm % | 11.56 (10.71) | 13.21 (13.5) | 6.58 (9.58) | 4.82 (5.22) |
| Correct rejection % | 88.44 (10.71) | 86.79 (13.5) | 93.42 (9.58) | 95.18 (5.22) |
| Sensitivity (d′) | 3.56 (0.94) | 3.71 (0.69) | 2.98 (0.86) | 3.14 (0.69) |
| Response bias (c) | −0.46 (0.37) | −0.63 (0.39) | 0.15 (0.32) | 0.12 (0.19) |
| RTs “go” trials in ms | 510 (30) | 500 (30) | 700 (50) | 670 (40) |
- Note: N = 29.
The percentage of hits was high in both the simple and the complex cognitive control tasks (Table 1). The Wilcoxon signed-rank test revealed a significantly higher percentage of hits under tVNS compared to sham in the simple cognitive control task, with a small effect size (V = 31, p = .033, d = .29). In the complex cognitive control task, an increase in the percentage of hits under tVNS was observed only at a trend level (V = 139, p = .092).
The percentage of correct rejections was high in both the simple and the complex cognitive control tasks (Table 1). The Wilcoxon signed-rank test revealed no significant difference in the percentage of correct rejections between the sham and tVNS conditions neither in the simple (V = 258, p = .215) nor in the complex cognitive control task (V = 103, p = .184).
To assess the difference in performance quality between the sham stimulation and tVNS, we examined d′ and c. There was no significant difference in d′ scores between sham stimulation and tVNS in the simple cognitive control task (V = 174, p = .353) nor the complex cognitive task (V = 151, p = .154). In other words, the ability to discriminate between go and no-go trials was not changed by tVNS.
With respect to c, the Wilcoxon signed-rank test revealed a significant difference in c between sham stimulation and tVNS in the simple cognitive control task with a small effect size (V = 346, p = .007, r = .25), with a significantly more liberal response bias in the tVNS condition. There was no significant difference in c between the sham stimulation and tVNS in the complex cognitive task (V = 243, p = .589).
Wilcoxon signed-rank tests were conducted to compare the differences between the reaction times in go trials under the sham and tVNS conditions for both of the tasks. Reaction times for go trials were not significantly different between the sham and tVNS conditions in the simple cognitive control task (Vsimple = 168, psimple = .425). A trend toward faster reaction times under tVNS was observed for the complex cognitive control task (Vcomplex = 139, pcomplex = .090).
To assess whether participants could distinguish between the sham and tVNS conditions, we examined their subjective reports of discomfort and perception of the stimulation. A Wilcoxon signed-rank test revealed no significant difference in participants' discomfort between the sham (median = 0) and tVNS (median = 1) stimulations (V = 27.5, p = .593). An exact McNemar's test determined that there was no statistically significant difference in participants' perception of stimulation between the sham and tVNS conditions (N = 18, p = 1.00).
3.2 Task-based analyses
To investigate whether tVNS-induced changes in cognitive control are mediated via LC-induced activity changes in the ACC, we compared neural activity in “no-go” as compared to “go” trials under tVNS and sham stimulation in the simple and complex cognitive control tasks. In a whole brain analysis, for this interaction effect, we found higher BOLD activity in a large, bilateral cluster with a peak in the right ACC ([x = 14; y = 44; z = 4, Z = 4.27), p < .001 FWE corr, cluster size 1136 voxels] under the tVNS condition (Figure 5a) in the simple task. The beta estimates illustrate the higher BOLD activity in the no-go trials after tVNS as compared to sham stimulation (Figure 5b). There were no brain areas showing increased activity after sham as compared to tVNS. We also found no evidence for a modulation of BOLD activity in ACC in the complex task.
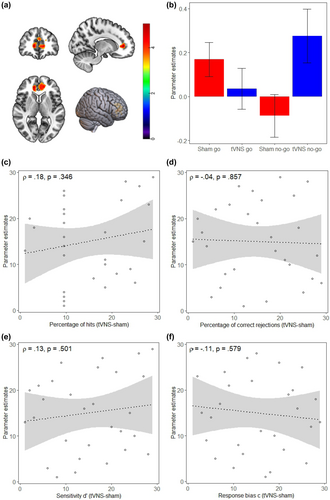
To further investigate the relationship between behavioral and neural effects of tVNS in the simple cognitive control task, we examined how these activity changes related to tVNS-induced accuracy improvements on an individual level. There was no significant relationship between individual behavioral benefits and changes in neural activity for either hits or correct rejections in the simple cognitive control task (Figure 5c,d), nor was there a significant relationship between behavioral benefits and neural activity for d′ or c (Figure 5e,f).
3.3 Functional connectivity analyses
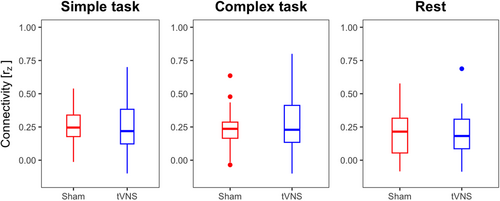
To investigate whether tVNS modulates LC-ACC connectivity during task and resting state, Fisher z-transformed Pearson's correlation coefficients between the average LC and ACC time series' were calculated for each stimulation condition. We found positive coupling between LC and ACC for both tasks and tVNS as well as for sham stimulation (Figure 6). Paired t test, however, revealed no significant differences between stimulation conditions (simple task: t(28) = −0.354, p = .726; complex task: t(28) = 0.981, p = .335; rest: t(32) = −0.554, p = .584). This suggests that functional connectivity between the LC and ACC did not change under tVNS.
Given the null findings, we performed an additional circular correlation coefficient analysis to demonstrate that functional connectivity analyses can be reliably performed in a small brain region like the LC. This analysis revealed that the correlation coefficients of shifted LC and ACC time series are distributed around 0 (distributions are depicted in Figure S1). Hence, the observed LC-ACC correlation is unlikely due to noise.
4 DISCUSSION
We investigated the effects of tVNS on cognitive control and neural activity in the LC and ACC. Participants completed two cognitive control tasks and underwent a resting-state fMRI scan during two separate sessions: one with tVNS and one with sham stimulation. Overall, our findings suggest that tVNS increases activity in the ACC in a cognitive control task. Behaviorally, we observed changes that were more indicative of a shift in response bias rather than enhanced conflict resolution or inhibition control. Our study is the first human neuroimaging study to illuminate the role of neural activity and functional connectivity in the LC and ACC during tVNS in both task and rest conditions.
4.1 Behavioral effects of tVNS
Our behavioral results show a significantly higher hit rate and a more liberal response bias in the simple cognitive control task under tVNS, as well as a trend for faster reaction times in the complex task.
While the observed increase in hit rate would align with findings of improved accuracy in a more complex go/no-go task after tVNS (Keute et al., 2020) or the meta-analytically confirmed view that tVNS can specifically enhance executive functions (Ridgewell et al., 2021), our additional analysis within the framework of signal detection theory provides evidence for a change in decision-making strategy. More liberal response biases have been previously observed in both humans and animals in go/no-go tasks when the proportion of go stimuli is high and the required responses are unambiguous (Bedi et al., 2023; Narasimhan et al., 2023). In our study, we observed a liberal response bias in the simple cognitive control task, which was further increased under tVNS. Given the higher frequency of go compared to no-go trials and the task instruction to respond quickly, this shift in bias may be interpreted as optimizing performance by aligning with task demands. In this context, a liberal bias could be advantageous in a task prioritizing speed, where the cost of slow responses to go trials might outweigh occasional false alarms.
Surprisingly, adding a working memory component to the go/no-go task did not result in larger tVNS effects (apart from a trend for faster reaction times). This contrasts with prior observations that tVNS enhances response selection processes under conditions of high selection demands (Jongkees et al., 2018) or in conditions where response inhibition requires working memory processes (Beste et al., 2016). Several non-invasive tVNS studies (Beste et al., 2016; Burger et al., 2016; Konjusha et al., 2023; Pihlaja et al., 2020) have demonstrated improvements in working memory. A recent study by Konjusha et al. (2023) showed that tVNS primarily impacts the gate closing mechanisms of working memory, thereby promoting stabilization and protection from interference.
It is important to note, however, that the overall evidence for the beneficial effects of tVNS remains mixed (Ridgewell et al., 2021) and may depend on stimulation parameters, the specific tasks employed, or the participants' prior experience (Konjusha et al., 2022). Borges et al. (2020) investigated tVNS's impact on four tasks assessing inhibitory control and cognitive flexibility and found performance improvements exclusively within a set-shifting paradigm. Another study on dual-task performance reported only small and transient effects on cognitive control (Sommer et al., 2023). The pattern of our behavioral effects with increased hit rate and larger negative response bias suggests that participants had a greater propensity to respond rather than to inhibit. Whether the numeric decrease in false alarms and increase in correct rejections in the complex cognitive control task may hint toward additional effects when inhibition requires working memory needs to be investigated in future studies with a higher number of no-go trials.
4.2 Neural effects of tVNS
Our neuroimaging data provide evidence that tVNS modulates ACC activity in a cognitive control task. The ACC is believed to contribute to the generation of theta oscillations in response to cognitive conflicts (Cavanagh & Frank, 2014). In macaques, it has been demonstrated that essential aspects of cognitive control are encoded by rhythmic theta-band activity within neuronal circuits in the ACC (Womelsdorf et al., 2010). This suggests that increased mid-frontal theta activity could be a neural mechanism through which tVNS enhances cognitive control. Supporting this, Keute et al. (2020) observed increased mid-frontal theta activity during go–stop conflicts in a complex go/no-go task. Furthermore, a recent study by (Wienke et al., 2023) on phasic tVNS stimulation during an emotional Stroop task found stimulation-induced increases in mid-frontal theta and alpha power, providing additional evidence of tVNS's neural effects in the medial prefrontal cortex. The increased ACC activity we observed with functional neuroimaging during the simple cognitive control task (i.e., go/no-go task) thus complements these prior EEG studies.
Noradrenergic neurons originating from the LC are vital for executive functions, including working memory, response inhibition, and cognitive flexibility (Holland et al., 2021). A recent neuroimaging study in healthy elderly volunteers provides evidence that the integrity of the LC is related to the efficiency of response inhibition (Tomassini et al., 2022). Several lines of evidence indicate a strong connection between LC and ACC in solving such cognitive control processes. Firstly, significant reciprocal functional and structural connectivity exists between these two brain regions (Chandler & Waterhouse, 2012; Gompf et al., 2010; Joshi & Gold, 2022). Our functional connectivity analyses corroborate this connectivity between the ACC and LC in humans during both rest and task conditions. Notably, we did not observe any differences in functional connectivity strength during rest compared to the two task conditions requiring cognitive control. This finding is surprising given that LC activity is primarily observed during arousal and attention (Berridge & Waterhouse, 2003; Sara & Bouret, 2012) and that recent animal studies indicate context-dependent correlations between LC and ACC activity (Joshi & Gold, 2022). Another unexpected finding was the absence of differences in connectivity strength during active or sham stimulation. This contrasts with a recent tVNS study in mice that showed increased LC connectivity with the rest of the brain after stimulation, in the absence of general stimulation-induced activity differences as measured with c-fos (Brambilla-Pisoni et al., 2022). So, do we need to revise the idea that activation of the LC and subsequent release of noradrenaline is the major effect in non-invasive human tVNS? Konjusha et al. (2023) investigated the effects of tVNS in a working memory paradigm and found that only one working memory mechanism, the stabilization of information, was affected by tVNS. This effect was reflected by an increase in alpha activity in the EEG. The authors argue that GABAergic mechanisms, rather than catecholaminergic mechanisms, may play a more important role in the effects of tVNS. While changes in the GABAergic system have been observed after successful long-term vagus nerve stimulation in epilepsy patients (Marrosu et al., 2003), we believe these effects are secondary to the activation of ascending neuromodulatory systems induced by vagus nerve stimulation. Anatomically, information from the vagus nerve reaches the nucleus of the solitary tract, a relay station that given its afferents and efferents can integrate cognitive and interoceptive information (Holt, 2022). The major efferent projections of the nucleus of the solitary tract reach the extended amygdala, hypothalamus, and the ascending neuromodulatory systems in LC (noradrenaline), ventral tegmental area (dopamine), and raphe nuclei (serotonin). Cholinergic basal forebrain projections are targeted via the LC. Using calcium imaging in mice, Collins et al. (2021) provided evidence that invasive vagus nerve stimulation results in the activation of ascending cholinergic and noradrenergic projections, leading to robust and widespread cortical activation. Hence, future human MRI studies with on–off auricular tVNS stimulation designs, combined with fine-grained structural delineations of these small nuclei, are needed to uncover the mechanisms of auricular tVNS in the human brain.
Although we did not find evidence for increased LC-ACC connectivity under tVNS, we believe that fMRI is a valuable tool for delineating the neural pathways through which tVNS exerts its effects on cognitive control. We provide evidence that the prefrontal ACC may be one region in this pathway. Additionally, we thoroughly evaluated whether the electrical stimulation at the ear lobe induced any artifacts or increased noise in MR images and provided evidence that online stimulation can be applied in an MRI setting without compromising data quality. Future studies should employ fMRI methods optimized for brainstem imaging and neuromelanin-sensitive sequences to enable delineation of the LC based on the participant's anatomy. Like many other studies, we here used available anatomic maps rather than individual anatomy, which is less precise. Nevertheless, our additional circular correlation coefficient analysis suggests that the correlations found were reliable, and correlation coefficients of the LC were in a similar range as those reported with optimized methods (Mazancieux et al., 2023).
5 CONCLUSION
Our study provides evidence that tVNS can increase neural activity in the ACC in a cognitive control task. We were, however, not able to provide evidence that LC activation contributes to such changes nor that these changes are related to previously observed enhancements of cognitive control. Given our findings of tVNS-induced changes in decision-making strategies, we suggest that future behavioral tVNS studies include assessments of decision-making across various cognitive functions.
AUTHOR CONTRIBUTIONS
Özde Sönmez: Data curation; formal analysis; investigation; visualization; writing – original draft. Elfriede Holstein: Data curation; formal analysis; investigation; visualization; writing – original draft. Sebastian Puschmann: Formal analysis; methodology; supervision; writing – review and editing. Tina Schmitt: Formal analysis; methodology; visualization; writing – review and editing. Karsten Witt: Methodology; resources. Christiane M. Thiel: Conceptualization; funding acquisition; supervision; writing – original draft.
ACKNOWLEDGMENTS
This work was supported by the Research Training Group (RTG) 2783, funded by the German Research Foundation (DFG)—Project ID 456732630. Scanning was supported by the Neuroimaging Unit of the Carl von Ossietzky Universität Oldenburg funded by grants from the German Research Foundation (3T MRI INST 184/152-1 FUGG). The CMRR sequence was kindly provided by the University of Minnesota Center for Magnetic Resonance Research. We thank Gülsen Yanc, Katharina Grote, and Ute von Varel for assistance with MRI scanning.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflicts of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in OSF at http://doi.org/10.17605/OSF.IO/H5JCD.