Dynamic autonomic nervous system states arise during emotions and manifest in basal physiology
Abstract
The outflow of the autonomic nervous system (ANS) is continuous and dynamic, but its functional organization is not well understood. Whether ANS patterns accompany emotions, or arise in basal physiology, remain unsettled questions in the field. Here, we searched for brief ANS patterns amidst continuous, multichannel physiological recordings in 45 healthy older adults. Participants completed an emotional reactivity task in which they viewed video clips that elicited a target emotion (awe, sadness, amusement, disgust, or nurturant love); each video clip was preceded by a pre-trial baseline period and followed by a post-trial recovery period. Participants also sat quietly for a separate 2-min resting period to assess basal physiology. Using principal components analysis and unsupervised clustering algorithms to reduce the second-by-second physiological data during the emotional reactivity task, we uncovered five ANS states. Each ANS state was characterized by a unique constellation of patterned physiological changes that differentiated among the trials of the emotional reactivity task. These ANS states emerged and dissipated over time, with each instance lasting several seconds on average. ANS states with similar structures were also detectable in the resting period but were intermittent and of smaller magnitude. Our results offer new insights into the functional organization of the ANS. By assembling short-lived, patterned changes, the ANS is equipped to generate a wide range of physiological states that accompany emotions and that contribute to the architecture of basal physiology.
1 INTRODUCTION
For over a century, one of the most contentious areas in emotion research has been the role of the autonomic nervous system (ANS). A direct thoroughfare that connects brain to body, and body to brain (Levenson, 2014), the ANS (together with the somatic nervous system) supports emotion generation and homeostasis via organized sympathetic and parasympathetic nervous system pathways (Craig, 2002; Critchley & Harrison, 2013). While some maintain the ANS lacks precision (Barrett, 2006a; Russell, 2003), turning on and off in an undifferentiated fashion during affective states (Cannon, 1927), others assert its contribution is more refined, orchestrating unique physiological patterns across the body that distinguish among emotions (Damasio & Carvalho, 2013; James, 1884; Keltner & Cordaro, 2017; Levenson, 2014). A better understanding of the functional organization of the ANS (i.e., how the ANS might produce predictable yet dynamic physiological changes throughout the periphery) will help to resolve ongoing disagreements about ANS patterning during emotions (Barrett, 2017; Levenson, 2014; Russell & Barrett, 1999).
1.1 ANS patterns and emotions
According to functionalist theories, emotions are brief (on the order of seconds), multisystem processes that are accompanied by patterned suites of ANS and motor changes that shape feelings and motivate actions (Adolphs, 2017; Cosmides & Tooby, 2000; Dolan, 2002; Ekman, 1992; Gross, 2015; Keltner et al., 2019; Levenson, 2014). In this view, emotions serve adaptive functions and reflect time-tested reactions to survival-relevant cues (Adolphs, 2017; Cosmides & Tooby, 2000; Dolan, 2002; Ekman, 1992; Gross, 2015; Keltner et al., 2019; Levenson, 2014). Each emotion, therefore, is thought to be characterized by a unique ANS pattern that differentiates it from other emotions and that encourages certain types of thoughts, feelings, and actions. Whereas negative emotions can be important for physical safety, positive emotions often play central roles in social relationships (Fredrickson, 1998; Levenson, 2003). For example, disgust is a negative emotion that helps to keep individuals safe from potential contaminants, and sadness signals the value of something that is lost. In the family of positive emotions, nurturant love fosters enduring interpersonal bonds through feelings of concern, amusement promotes social connection through play and humor, and awe inspires attention to the collective by feeling part of something larger than oneself (Griskevicius et al., 2010).
Most prior laboratory-based investigations that have searched for emotion-specific ANS patterns have reduced complex physiological cascades into static “snapshots” using change scores. Change scores reflect the difference between the mean level in a physiological channel during an emotion-inducing task and the mean level during a baseline period and are a straightforward and efficient way of quantifying ANS reactivity. Numerous studies that have used change scores have found dissociable ANS patterns for certain negative and positive emotions (Christie & Friedman, 2004; Ekman et al., 1983; Kragel & LaBar, 2013; Levenson et al., 1990; McGinley & Friedman, 2017; Rainville et al., 2006; Shiota et al., 2011; Stephens et al., 2010). Multivariate approaches, which look for ANS patterns using change scores across numerous physiological channels at once, were also successful in distinguishing among various emotions (Kreibig, 2010; Kragel & LaBar, 2013; Kragel & LaBar, 2014a).
Meta-analyses of studies using change scores, however, have failed to find robust emotion-specific ANS patterns (Behnke et al., 2022; Quigley & Barrett, 2014; Russell, 2003; Siegel et al., 2018). Functionalist theorists often conclude that methodological differences account for the inconsistent ANS patterns across studies of emotional reactivity. For example, studies vary in which emotions they elicited and of what intensity, which physiological measures were collected and from which body part (Mauss et al., 2005), and which type of stimuli were used (i.e., pictures, film clips, music, guided imagery, or autobiographical recall) (McGinley & Friedman, 2017; Siedlecka & Denson, 2019). By averaging physiological activity over an entire trial, change scores also treat emotions as if they were sustained, rather than short-lived, responses by reducing an ANS signal's rich temporal dynamics into a single measure. An alternative explanation for the inconsistent findings across studies, however, is that emotions are not characterized by ANS patterns but rather are accompanied by inherently variable physiological changes that differ across people and contexts. According to constructionist theory, the ANS activities that arise during emotions are highly flexible, unfolding in different ways in each instance of emotion, and largely lacking a predictable structure (Barrett, 2017; Siegel et al., 2018). New analytic approaches that search for brief, dynamic ANS patterns have the potential to determine whether stereotyped (i.e., non-random) physiological changes emerge during emotions when examined on shorter timescales.
1.2 Functional organization of basal physiology
Emotions are processes that unfold over time, but even at rest, the outflow of the ANS is continuous and dynamic. An ongoing exchange between sympathetic and parasympathetic pathways shapes physiological activity to meet the ever-changing demands of the internal and external milieus (Berntson et al., 2017). At rest, maintenance of regular rhythms is a mainstay of homeostasis, but unexplained variability in basal physiology adds complexity to an otherwise steady state (Goldberger et al., 2001; Jacono & Dick, 2011).
Functionalist and constructionist theories have different perspectives about the significance of basal physiology, but little is known about its functional organization. Functionalist theories propose that, at rest, the ANS lacks a predictable organization yet creates a complex physiological backdrop on which emotions unfold. In this view, emotions are considered programs in the nervous system that activate when needed (Cosmides & Tooby, 2000), coordinating disparate ANS and motor systems that may not be coordinated in basal physiology. According to constructionist theory, in contrast, ongoing bodily states are more than just a physiological backdrop and instead play a central role in emotions and affective experience. In this perspective, emotions are created as people use knowledge to make meaning of the internal condition of the body in a given context (Barrett et al., 2007; Jackson et al., 2019). The physiological milieu, therefore, is thought to shape baseline feelings (e.g., also referred to as “mood” or “core affect”) that traverse the dimensions of affective space yet are not tethered to predictable ANS patterns (Russell & Barrett, 1999). Studies that investigate whether basal physiology has a functional organization are lacking but could help to forge new links between the phasic and tonic activities of the ANS.
1.3 Goals of the present study
Here, we leveraged the rich temporal dynamics of multichannel physiological recordings to examine the functional organization of the ANS during an emotional reactivity task and an undirected resting period. We obtained a broad array of continuous respiratory, cardiovascular, and electrodermal measures that have been utilized in numerous prior studies to assess a variety of sympathetic and parasympathetic nervous system activities (Sturm et al., 2018). During the emotional reactivity task, participants viewed video clips (Sturm et al., 2021), which are ideal for evoking and measuring ANS changes over time (Dixon & Gross, 2021; Gross, 2015; Scherer, 2009). We induced positive and negative emotions (awe, sadness, amusement, disgust, or nurturant love) that have survival relevance (Campos et al., 2013; Ekman et al., 1983; Fredrickson, 1998; Keltner & Haidt, 2003; Levenson, 2014; Shiota, 2021; Shiota et al., 2011, 2014, 2017; Sturm et al., 2020) and can be reliably evoked with videos and images in a laboratory setting (Gross & Levenson, 1995; McGinley & Friedman, 2017; Rottenberg et al., 2007; Shiota et al., 2011; Siedlecka & Denson, 2019). As prior studies have found evidence that each of these emotions is associated with a distinct physiological profile (Ekman et al., 1983; Kreibig, 2010; Shiota et al., 2011), elicitation of these emotions provided an appropriate testing ground for novel analytic approaches aiming to uncover ANS patterns. Prior to the emotional reactivity task, participants sat through a two-minute resting period to assess basal physiology. In line with functionalist perspectives, we expected brief ANS patterns would emerge from the second-by-second physiological recordings and distinguish among the trials of the emotional reactivity task. We next explored whether ANS patterns reminiscent of those that arose during the emotional reactivity task were also present in the resting period and, thus, suggest a previously unrecognized functional organization of basal physiology.
2 METHOD
2.1 Participants
Fifty-nine healthy adults were recruited from the Hillblom Aging Network, a longitudinal cohort followed by the University of California, San Francisco (UCSF) Memory and Aging Center. Participants provided informed consent prior to participation, and the study was approved by the UCSF Human Research Protection Program. Participants underwent a comprehensive multidisciplinary evaluation that included assessment of their neurological, neuropsychological, and daily functioning as well as a neuroimaging assessment. All participants were cognitively normal and free of current or previous neurological conditions and psychiatric disorders. All participants had a Clinical Dementia Rating Scale score of 0 (range 0–3), which indicates intact daily functioning (Morris, 1997), and a Mini-Mental State Examination score of 28 or higher (range 0–30), which indicates normal mental status (Folstein et al., 1975). The Geriatric Depression Scale (Yesavage et al., 1982), which assesses mood symptoms over the past 2 weeks, was used to screen for depressive symptoms. Race and ethnicity, handedness, and years of education were reported by the participants. At the time of the emotional assessment (see Procedure section), no participants were taking medications that might affect ANS physiology (i.e., stimulants, acetylcholinesterase inhibitors, beta-blockers, or psychotropics). Participants with more than 20% missing data in any physiological channel across the emotional reactivity task were excluded from our analyses; 14 out of 59 participants were excluded based on this criterion, which resulted in a final sample of 45 participants. The demographic and cognitive data for the final sample are presented in Table 1.
| Mean (SD) | |
|---|---|
| Sample size | 45 |
| Age (years) | 73.6 (4.2) |
| Sex: Male/Female | 13/32 |
| Handedness: Right/Left | 42/3 |
| Education (Years) | 17.7 (1.8) |
| Race (Asian/Black/White) | 1/1/43 |
| Ethnicity (Hispanic, Latino) | 1 |
| Mini-mental state examination (/30) | 29.3 (1.0) |
| Clinical dementia rate scale total (/3) | 0.0 (0.0) |
| Benson figure 10-min recall (/17)*** | 10.8 (3.4) |
| Modified trails (correct lines per minute) ** | 38.2 (14.4) |
| Modified trails errors*** | 0.3 (0.7) |
| Phonemic fluency (# correct in 60 s)** | 16.0 (3.9) |
| Semantic fluency (# correct in 60 s) | 21.8 (3.9) |
| Design fluency correct (# correct in 60 s)** | 11.6 (4.6) |
| Digits backward** | 5.2 (2.1) |
| Benson figure copy (/17)** | 14.9 (3.3) |
| Modified Boston naming test correct (/15)** | 13.7 (4.6) |
| Geriatric depression scale (/30)**** | 2.2 (2.7) |
- Note: For questionnaires, the highest attainable score is shown in the parentheses. SD = standard deviation. **completed by 43 out of 45 participants; *** completed by 42 out of 45 participants; **** completed by 31 out of 45 participants.
2.2 Procedure
Participants completed a laboratory-based assessment of emotions at the UCSF Center for Psychophysiology and Behavior. Participants were seated in a comfortable chair in a well-lit experiment room. Sensors were applied to obtain continuous measures of physiological activity (Figure 1a). Participants were recorded with a semi-obscured remotely controlled video camera throughout the testing session; behavior from the videos was not analyzed in the present study. Participants completed a battery of tasks designed to assess emotional reactivity, empathy, emotion regulation, and resting ANS physiology. Only data from the emotional reactivity task and resting period were included in our analyses.
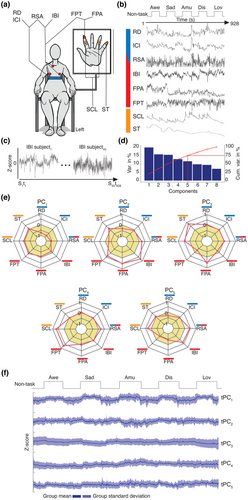
2.2.1 Tasks
Emotional reactivity task
Participants completed five “emotion trials” in which they viewed emotionally evocative video clips that were approximately 90 s in length (range: 88–104 s). Each video clip was selected to elicit awe, sadness, amusement, disgust, or nurturant love, respectively. Piloting in an independent sample (n = 14, 8 females, age range 27–31 years; see Table S3) and our prior work have shown these video clips elicit the target emotions (Sturm et al., 2021). The awe video was from Planet Earth and showed landscapes and vistas, the sad video was from 21 Grams and showed a mother in a hospital receiving bad news about her family, the nurturant love film clip was from Babies and showed infants and toddlers crawling and playing with animals, the disgust film clip was from YouTube.com and showed ear wax being removed from an ear, and the amusement film clip was from YouTube.com and showed a baby laughing while watching someone rip up paper. We chose to elicit these negative and positive emotions because they can be reliably evoked in a laboratory setting with images and videos (Gross & Levenson, 1995; McGinley & Friedman, 2017; Rottenberg et al., 2007; Shiota et al., 2011; Siedlecka & Denson, 2019), activate various components of the sympathetic and parasympathetic nervous systems, and have been associated with distinct ANS profiles in prior research (Ekman et al., 1983; Kreibig, 2010; Shiota et al., 2011).
Each video clip was preceded by a 61-s pre-trial baseline in which participants were asked to try to clear their mind and followed by a 31-s post-trial recovery period in which participants viewed a black “X” on a white screen (herein, referred to together as “non-task” periods; Figure 1b). This task design allowed participants to alternate between periods of resting fixation and periods of positive and negative emotion elicitation. After viewing each video clip, participants answered questions about the video and their emotional experience. All questions were presented visually on the computer monitor and via prerecorded audio recordings that they heard through speakers.
Resting period
Prior to the emotional reactivity task, participants sat quietly through a 2-min resting period in which they watched a black “X” on a white screen. They were instructed to try to clear their mind during the trial.
2.2.2 Measures
Self-report measures
To ensure that participants had paid attention during they task, they were asked, “What happened in this movie?” after viewing each video clip and selected their answer from multiple choice. Participants also reported their experience of anger, sadness, disgust, fear, awe/amazement, love/affection, amusement/happiness, excitement/enthusiasm, embarrassment, pride, and surprise while watching each video clip on a three-point scale (0 = none, 1 = a little, 2 = a lot).
Physiological recordings
Continuous measures of physiological activity were obtained with Biopac MP150 bioamplifiers and a computer equipped with AcqKnowledge software (v4.4, https://www.biopac.com/) (Sturm et al., 2018): (I) Inter-beat interval (IBI): Electrodes were placed in a bipolar configuration on opposite sides of the participant's chest; the heart rate was calculated as the number of R waves from the electrocardiogram per minute. (II) Inter-cycle breath interval (ICI): A pneumatic bellows-based respiration transducer was stretched around the thoracic region, and the respiration rate was measured as the number of inspirations per minute. (III) Respiration depth (RD): The point of the maximum inspiration minus the point of maximum expiration was determined from respiratory tracing. (IV) Finger pulse amplitude (FPA): A photoplethysmograph recorded the amplitude of blood volume in the finger using a photocell taped to the distal phalanx of the index finger of the nondominant hand. (V) Finger pulse transit time (FPT): The time interval in milliseconds was measured between the R wave of the electrocardiogram and the upstroke of the peripheral pulse at the finger site, recorded from the distal phalanx of the index finger of the nondominant hand. (VI) Skin temperature (ST): A thermistor attached to the distal phalanx of the little finger of the nondominant hand recorded temperature in degrees Fahrenheit. (VII) Skin conductance level (SCL): SCL, a measure of sympathetic activity (Critchley, 2002), was measured through a constant-voltage device passing a small voltage between Ag/AgCl Silver 8 mm electrodes (using an electrolyte of sodium chloride) attached to the palmar surface of the middle phalanges of the ring and index fingers of the non-dominant hand. (VIII) Respiratory sinus arrhythmia (RSA): RSA, a measure of parasympathetic activity (Bernston et al., 1997), was calculated with the peak-valley approach as the difference in msec between the shortest inter-beat interval during inspiration and the longest inter-beat interval during expiration.
Physiological data were processed using a custom pipeline scripted in AcqKnowledge. Briefly, algorithms identified and marked the signature components of each waveform, and these marks were then visually inspected for errors and noise. Outliers in the data were considered more or less than three standard deviations from the mean level during the trial; these periods were interpolated if their duration was three seconds or less and deleted if their duration was greater than three seconds. Second-by-second averages for each channel were then exported for use in subsequent analyses.
2.3 Statistical analyses
R (https://www.r-project.org/) and MATLAB2020a (https://www.mathworks.com/products/matlab.html) were used for the analyses.
2.3.1 Emotional reactivity task
Self-report measures
Correct responses to the questions regarding each video clip's content were scored as 1, and incorrect responses were scored as 0. These scores were used to determine whether there were any trials in which a participant obtained a score of 0 and, hence, did not understand or recall the content of the video. For the emotional experience questions, we conducted analyses of variance to determine whether there was a main effect of emotion trial on each type of emotional experience.
Physiological recordings
Incidental missing values were replaced through spline interpolation using the imputeTS package in R. Between 0.2% (IBI) and 5.5% (RSA) of missing data across the whole sample was replaced through the interpolation procedure (Figure S1). The time series of the ANS channels were then z-scored within each participant using the scale function in R (Figure 1c), which ensured that individual differences in ANS dynamics were comparable across participants and measures.
Principal component analysis and time series of the principal components
For each ANS channel, the standardized time series were then concatenated across the sample (Figure 1c). We then conducted a principal component analysis (Hotelling, 1933), a dimensionality reduction technique that uncovers latent modes of covariation, using the factoextra package in R. The first five principal components (PCs) were selected for further analysis. Each PC was characterized by a set of eigenvector loadings that reflected the contribution of the ANS signals to each PC. The time series of each PC (tPC) was calculated by multiplying the standardized second-by-second data in each ANS channel by its PC loadings and then summing the weighted time series of the individual channels. We repeated this approach for each of the PCs, which yielded five tPCs with distinct temporal dynamics. This temporal PCA approach has been widely used in neurogaming and electrophysiological studies (Calhoun et al., 2001; Kato et al., 2015; Shine et al., 2019; Smith et al., 2012), since it enables to decompose each ANS signal in its distinct constituents, each characterized by distinct patterns of co-fluctuation. The tPCs reflected the continuous fluctuations in each PC's magnitude across the emotional reactivity task.
Analysis of variance and multinomial logistic regression
To run analyses that were more comparable with prior studies of emotional reactivity, we first reduced the tPCs during each non-task period and each emotion trial into static averages and used analyses of variance and multinomial logistic regression analyses to examine whether the mean tPC amplitudes differed among the emotion trials. For each participant, we computed the average magnitude of each tPC during the non-task periods and each emotion trial. We conducted five analyses of variance (one for each tPC) with post hoc Bonferroni-corrected t-tests (p < .05) to assess whether the average tPC magnitudes differed across the emotion trials (aov and t.test packages in R). To assess whether each emotion trial was characterized by a distinct pattern of tPC activity, we performed multinomial logistic regression analyses (Engel, 1988) using the R packages nnet and caret. In model1, we tested whether the average tPCs from each emotion trial predicted the trial in which they were acquired (i.e., non-task periods averaged across trials as well as the awe, sadness, amusement, disgust, or nurturant love trials). In model2, we omitted the non-task periods and included only the data from the emotion trials, which allowed us to conduct a more stringent test of whether the mean tPCs could differentiate among the emotion trials alone. A 10-fold cross-validation approach was used to derive a confusion matrix for each model, which reflected the correct classification of each emotion trial and provided measures of sensitivity, specificity, and accuracy.
Low-dimensional manifold
Next, we preserved the second-by-second fluctuations of the tPCs and examined the group-averaged activity in each tPC during the non-task periods and emotion trials. To investigate the trajectory of the tPCs within a low-dimensional embedding space (Shine et al., 2019), we created a topological manifold (Kato et al., 2015). Manifolds facilitate the exploration of dynamic systems and have been used to uncover neural mechanisms of complex human and non-human animal behaviors (Kato et al., 2015; Shine et al., 2019). To compute representative trajectories for the tPCs, we created two separate matrices in which the columns stored the second-by-second data for each tPC from the non-task periods or the emotion trials. Given that the trials in the emotional reactivity task differed in length, we used linear interpolation (fillmissing function in MATLAB R2020a) to derive time series of the same length, matched to the duration of the longest trial. In each matrix, we averaged across the columns to compute the mean time series of each tPC across the non-task periods and the emotion trials and then plotted the trajectories into the embedding space. We also created another pair of matrices in which we removed the non-task periods to inspect the temporal dynamics of the tPCs during the emotion trials alone.
K-means clustering and ANS state analyses
We performed k-means clustering (kmeans function in MATLAB R2020a), an approach that considers each time point independently and is agnostic to the temporal order of the data (Lloyd, 1982), with 10,000 iterations and 10 replications on the group-averaged tPCs from the emotion trials, which yielded clusters of dynamic ANS activity. The optimal number of clusters, or ANS states, was confirmed with a silhouette analysis. Each time point (i.e., each second of the time series data) was assigned to the nearest cluster-centroid—the arithmetic mean of all of the time points that belong to that cluster—using Euclidean distance. A cross-tabular confusion matrix was used to investigate the percentage of seconds of each emotion trial that were assigned to each ANS state. The classperf function in MATLAB R2020a was used to derive measures of classification sensitivity and specificity.
We next examined whether the ANS states found in the tPC data averaged across the sample were also evident in individual participants. We identified the cluster-centroid of each ANS state, which is considered its prototype, and assigned every time point in the tPCs of the individual participants to the closest ANS state based on Euclidean distance. We used each participant's resultant ANS state occupancy time series to compute the percentage of time points from each emotion trial that were assigned to each ANS state, a measure of ANS state fractional occupancy. We then conducted a two-way analysis of variance (p < .05) to determine whether there were differences in ANS state fractional occupancies across the emotion trials (aov package in R). We next conducted multiple linear regressions to investigate whether age, sex, or handedness were associated with time spent in a trial-predominant ANS state. We also used a two-way analysis of variance with a random intercept for participant nested in the factor signal (p < .05) to compare the amplitude of the physiological signals across the ANS states (lmer package in R). We then used the ANS state occupancy time series to quantify the number of times participants transitioned into the predominant ANS state in each emotion trial and the average duration of each state (i.e., dwell time), in seconds.
We performed additional control analyses in which we conducted k-means clustering on the tPCs from the emotion trials in individual participants. The ANS states that were identified in individual participants were then aligned to those found at the group level based on the Euclidean distance between the individually generated cluster-centroids and the group-level cluster-centroids (pdist function in MATLAB R2020a). We again used a two-way analysis of variance to determine whether there were differences in ANS state fractional occupancies across the emotion trials (aov package in R).
Resting period
Principal component analysis and time series of the principal components
To preprocess the ANS time series from the 2-min resting period, we used the same procedures as described above for the emotional reactivity task. We then conducted a principal component analysis on the standardized ANS time series data from the resting period, concatenated across participants. Computing separate principal component analyses on two distinct data sets can yield similar components that have a different sign or order of eigenvector loadings. To identify correspondent PCs across the emotional reactivity task and the resting period, we used cosine distance (Dc)—which is better suited than other distance measures to compare the magnitudes of two independent, short vectors—to compare the eigenvector loadings of individual PCs. We reordered the PCs from the resting period in such a way to maximize the similarity with the eigenvector loadings of PCs derived from the emotional reactivity task (pdist function in MATLAB R2020a). This process also involved iteratively assessing the similarity between the eigenvector loadings from the emotional reactivity task decomposition and the inverse of the eigenvector loadings derived from the resting period decomposition. After identifying the most similar PCs, we vectorized the eigenvector loading matrices and used Pearson's correlation coefficients (p < .05; corr function in MATLAB R2020a) to examine the correspondence between the PCs derived from the resting period and those from the emotional reactivity task. We then generated tPCs for the resting period by using the rearranged eigenvector loadings derived from the principal component analysis of the resting period ANS time series.
ANS state extraction
To determine the degree to which the ANS states from the resting period resembled those from the emotional reactivity task, we computed the Euclidean distance between each time point in the tPCs from the resting period and the cluster-centroids of the ANS states from the emotion trials. Time points close to a cluster-centroid (less than one standard deviation from the mean distance of every resting period time point to any cluster-centroid) were assigned to the nearest ANS state. As above, we then computed the ANS state fractional occupancy and dwell time for each participant. We used a one-way analysis of variance, with Bonferroni-corrected post hoc t-tests (p < .05; aov package in R) to compare the ANS state fractional occupancies of participants. We again used cosine distance to compare the mean magnitude of the tPCs in each ANS state during the resting period with those during the emotional reactivity task (pdist function in MATLAB R2020a). We then performed a three-way analysis of variance (p < .05) to assess whether the mean magnitudes of the tPCs in each ANS state differed between the resting period and the emotional reactivity task (aov package in R). For each ANS state, the tPC magnitudes from the resting period and the emotional reactivity task were compared with two-sample t-tests (p < .05, Bonferroni-corrected for a total of 25 pairwise comparisons; t.test package in R).
3 RESULTS
3.1 Emotional reactivity task
3.1.1 Self-report measures
All participants attended to the video clips (Table S1) and reported experiencing the target emotions (Table S2). Whereas participants tended to report experiencing the target emotion (i.e., disgust or sadness) for the negative emotion trials, they often endorsed the target emotion (i.e., awe, amusement, or nurturant love) as well as other types of positive emotional experience for the positive emotion trials, consistent with prior studies (Shiota et al., 2011).
3.1.2 PC1 temporal dynamics separated the non-task periods from the emotion trials
The z-scored ANS activity time series (Figure S2) were concatenated across participants, and principal component analysis was used to extract five PCs. Each PC explained at least 10% of the total variance, and, together, they explained 75% (see Figure 1d and Figure S3). The PCs lacked one-to-one mappings with the cardiovascular, respiratory, and dermal signals and, thus, suggested a more complex ANS organization during the emotional reactivity task. Activity in some channels, however, loaded more strongly on certain PCs than on others: respiratory activity (i.e., respiration depth and inter-cycle interval) loaded strongly on PC1; cardiovascular activity (i.e., finger pulse amplitude and inter-beat interval) on PC2; dermal activity (i.e., skin temperature and skin conductance level) on PC3; cardiovascular and dermal activity (i.e., finger pulse transit time and skin conductance level) on PC4; and inter-beat interval and skin conductance level on PC5 (Figure 1e).
The five tPCs had distinct temporal dynamics when examined on a second-by-second basis (Figure 1f). When we reduced the tPCs during each non-task period and each emotion trial into static averages, analyses of variance and multinomial logistic regression analyses confirmed that the mean tPC amplitudes differed among the emotion trials (Figure S4a–g). Consistent with prior studies, these results suggested there were some reliable differences in mean ANS activity among distinct emotions (Figure S4) (Ekman et al., 1983; Kreibig, 2010; Shiota et al., 2011). Although our models reached above-chance classification probabilities, we expected more robust ANS differences among the emotion trials may be embedded within the temporal dynamics of the tPCs.
In our next analyses, we preserved the second-by-second fluctuations of the tPCs to investigate whether they offered additional insights into the architecture of ANS patterning. Examination of the group-averaged tPCs suggested that activity in tPC1 aligned with the task structure and showed comparable increases during all the emotion trials (Figure 2a). The magnitude of tPC1 was more negative during the non-task periods (i.e., reflecting slower, deeper respiration and slower heart rate) and more positive during the emotion trial (i.e., reflecting faster, shallower respiration and faster heart rate; Figure S5). The other tPCs, however, did not show a similar time course but instead exhibited more complex fluctuations, especially during the emotion trials (Figure 2c–f).
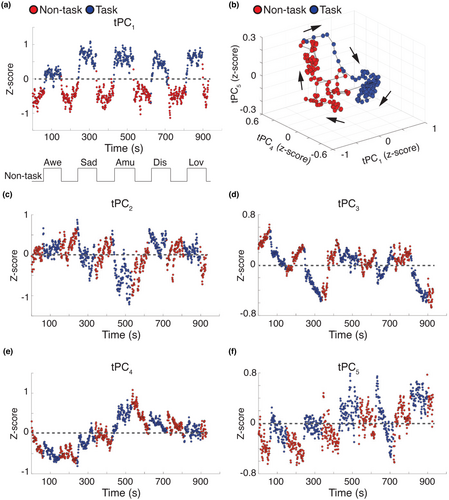
When we modeled the temporal trajectories of the five tPCs during the non-task periods and the emotion trials in a low-dimensional manifold, the overall trajectory of ANS activity in the low-dimensional manifold continued to separate the non-task periods from the emotion trials (Figure 2b), which confirmed physiological responsivity during video viewing (Kato et al., 2015; Shine et al., 2019). Consistent with theories that emphasize arousal as a central dimension of emotions (Russell, 2003; Siegel et al., 2018), some ANS changes (e.g., respiration depth) differentiated the emotion trials from the non-task periods but did not distinguish among the emotion trials themselves.
3.1.3 Specific ANS states were predominant during the emotion trials across the group
We next removed the pre-trial baseline and post-trial recovery periods to inspect the temporal dynamics of the tPCs during the emotion trials alone, a more rigorous search for ANS patterning. We plotted the low-dimensional tPCs space and labeled each time point by the emotion trial in which it was acquired. This plot revealed five dynamic ANS patterns, each aligning with a different emotion trial (Figure 3a). We next applied unsupervised k-means clustering to the tPCs to confirm the presence of distinct ANS patterns during the emotion trials. This technique also uncovered five clusters or “ANS states” in the tPCs, a solution that was further supported by a silhouette analysis (Figure S6). When plotted in the low-dimensional space (Figure 3b), the spatial topography of these ANS states largely mirrored that which emerged when the tPC time points were instead labeled with their attendant emotion trial. Indeed, there was a predominant ANS state for each emotion trial (Figure 3c) that had high levels of sensitivity (0.88) and specificity (1.00). In the awe trial, 100% of the time points aligned with ANS State 1; in the sadness trial, 92% aligned with ANS State 2; in the amusement trial, 78% aligned with ANS State 3; in the disgust trial, 67% aligned with ANS State 4 and 33% with ANS State 5; and in the nurturant love trial, 87% aligned with ANS State 5. We found identical ANS states when we included frame-by-frame summary measures of image brightness, image contrast, and auditory signals as additional covariates in our analyses, which suggested differences in the audiovisual properties of the emotion videos that did not account for the physiological differences we found across trials (Figure S7).
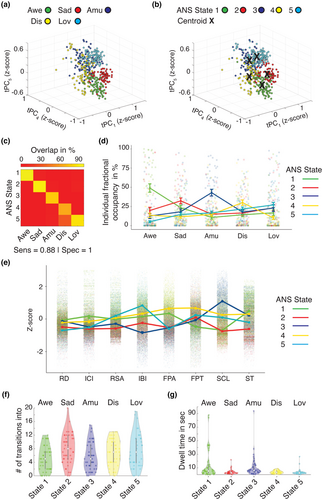
3.1.4 Trial-predominant ANS states were present at the individual level
As the ANS states were derived from the time series data averaged across the sample, we next examined the degree to which these states were evident in individual participants. A two-way analysis of variance comparing the fractional occupancy scores across the emotion trials found a main effect of ANS state, F(4,1100) = 5.1, p < .0005, and an interaction between ANS state and emotion trial, F(16,1100) = 13.2, p < .0005. This analysis indicated that, as found at the group level, individual participants most often occupied ANS State 1 during the awe trial (group average = 43%), ANS State 2 during the sadness trial (group average = 30%), ANS State 3 during the amusement trial (group average = 45%), ANS State 4 during the disgust trial (group average = 28%), and, to a lesser extent, ANS State 5 during the nurturant love trial (group average = 24%; Figure 3d, p < .05 Bonferroni-corrected t-tests). Time spent in each of these ANS states was not associated with age, sex, or handedness (Table S4) or with the intensity of participants' self-reported experience of the associated trial's target emotion (Figure S8). As an additional test, we conducted k-means clustering on the tPCs; here we limited each analysis to an individual participant's data. By computing the Euclidean distance between the cluster-centroids in each participant and those derived at the group level, we again assigned each second of the tPCs to the corresponding ANS state. Like the results conducted across the sample, these analyses indicated that a single ANS state was predominant during each emotion trial (Figure S9). Each of the ANS states was characterized by a unique physiological profile that included distinct constellations of activity in all of the channels (Figure 3e and Table S5).
To investigate whether each emotion trial's predominant ANS state was short-lived or long-lasting, we quantified the number of times participants transitioned into that state during each trial. On average, participants transitioned 5.0 times into ANS State 1 during the awe trial; 8.0 times into ANS State 2 during the sadness trial; 5.7 times into ANS State 3 during the amusement trial; 6.8 times into ANS State 4 during the disgust trial; and 7.4 times into ANS State 5 during the nurturant love trial (Figure 3f). We next computed the median duration, in seconds, of these states each time they emerged. These analyses revealed the median duration, or dwell time, was 5.3 s for ANS State 1 during the awe trial; 2.7 s for ANS State 2 during the sadness trial; 6.0 s for ANS State 3 during the amusement trial; 3.5 s for ANS State 4 during the disgust trial; and 2.5 s for ANS State 5 during the nurturant love trial (Figure 3g).
3.2 Trial-predominant ANS states were detectable in basal physiology
Our results suggested the ANS assembles brief, dynamic physiological patterns that distinguish among emotions. To investigate whether trial-predominant ANS patterns were detectable within resting physiology, we next performed a principal component analysis on the standardized ANS time series data, concatenated across the sample, from a 2-min resting period that preceded the emotional reactivity task. This analysis found PCs with loadings that were similar to those found during the emotional reactivity task (Figure 4a and Figure S10).
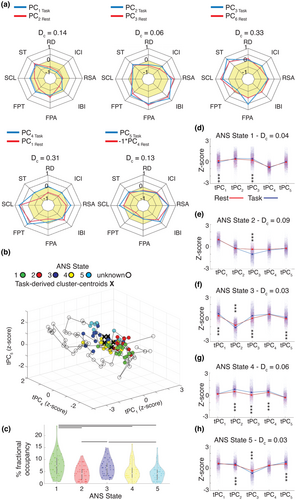
As before, we next generated the corresponding tPCs and computed the Euclidean distance from each time point from the resting period to the cluster-centroids from the emotion trials. Here, these distances reflected the similarity between each second of the resting period tPCs and the trial-predominant ANS states. We assigned time points near a cluster-centroid to the closest ANS state; time points that did not align with one of the cluster-centroids (mean across participants = 72%) remained unassigned (Figure 4B, Figures S11 and S12). Participants spent 7%–57% of the resting period in an ANS state that resembled those from the emotional reactivity task (Figure 4c and Figure S11). A one-way analysis of variance, F(4,220) = 8.1, p < .05, with Bonferroni-corrected pairwise t-tests found that, on average, participants spent more time in ANS State 1 (7.8%) and ANS State 3 (6.5%) than in ANS State 2 (4.3%), ANS State 4 (5.1%), or ANS State 5 (4.2%; Figure 4c). When considering each ANS state's associated emotion trial, these findings suggested that, at rest, participants spent more time in ANS states emblematic of awe and amusement than in those of sadness, disgust, or nurturant love. In contrast to the emotional reactivity task, where the ANS states lasted several seconds on average before dissipating, here their presence was intermittent and brief, with a median duration of 1.7 s for ANS State 1; 1.3 s for ANS State 2; 1.6 s for ANS State 3; 1.5 s for ANS State 4; and 1.5 s for ANS State 5 (Figure S11c).
The presence of ANS states at rest that resembled those from the emotional reactivity task suggested an organization to basal physiological outflow that has previously gone unrecognized. We conducted additional tests to evaluate the degree of similarity between the ANS states uncovered at rest and those found during the emotion trials. Measures of cosine distance (Dc), in which lower values indicate higher similarity, confirmed that the ANS states derived from the resting period had tPC profiles that were similar to those from the emotion trials (Dc State 1 = 0.04, State 2 = 0.09, State 3 = 0.03, State 4 = 0.06, State 5 = 0.03, Figure 4d–h). A three-way analysis of variance with Bonferroni-corrected pairwise t-tests (p < .05) comparing the tPC magnitudes of the ANS states from the resting period with those from the emotion trials also found a main effect of condition (resting period versus emotion trials), F(1,112,875) = 2.6, p < .05 (Figure 4d–h). Taken together, these results indicated that the ANS states from the resting period had a similar composition to those from the emotion trials but, on average, were of a lower magnitude.
3.3 Post hoc power analysis
We conducted a post hoc power analysis, based on established methods (Lenth, 2007), based on the two-way analysis of variance of the individual fractional occupancy scores across the emotion trials. We entered the following parameters, F(4,1100) = 5.1, where four was the degrees of freedom for the five emotion trials, 1100 was the degrees of freedom for the number of time points across the trials, and 5.1 was the F value from the two-way analysis of variance of the individual fractional occupancy scores across the emotion trials. For a sample size of 45 and an alpha level of 0.05, the power of this analysis was 0.95, which suggests we had high power to detect the effects of interest in our study.
4 DISCUSSION
The present study expands current models of the functional architecture of the ANS and helps to elucidate its role in emotion generation. We took a novel approach to the dimensionality reduction of multichannel ANS recordings and, by harnessing the moment-to-moment fluctuations in physiological outflow across the sample, uncovered five ANS states during an emotional reactivity task. Each ANS state was comprised of a unique set of physiological patterns that distinguished among the emotion trials. In individual participants, the duration of each ANS state was brief, lasting for several seconds before dissipating or changing in structure. Even the predominant ANS states in each trial were fluid, coming and going and allowing other states to unfold while participants watched the emotion-inducing videos. Remarkably, ANS states with a similar intrinsic functional organization were also present in basal physiology, though their presence was intermittent and their magnitude, less intense.
4.1 Dynamic ANS states distinguished among the emotion trials
Consistent with functionalist theories, our results suggest emotions are accompanied by ANS patterns—coordinated bursts of physiological activity that are the products of organized sympathetic and parasympathetic nervous system pathways (Adolphs, 2017; Ekman, 1992; Gross, 2015). Unlike prior studies that used change scores to quantify differences between ANS activity during an emotion-inducing task and a pre-trial baseline, thereby reducing the rich temporal dynamics of physiological activity into a single measure, we leveraged the second-by-second variability in the tPCs to search for ANS patterns during the emotion trials. The five ANS states that distinguished among the emotion trials were not evident in the time series of any one PC alone, but emerged when we incorporated multiple tPCs in the unsupervised clustering algorithms. When each tPC was examined on its own, only activity in tPC1 aligned with the task structure, increasing during the emotion trials and decreasing during the non-task periods, a pattern that may reflect general elevations in arousal during emotions versus rest (Russell, 1980, 2003). The other tPCs, however, exhibited more complex fluctuations that were less yoked to trial onset and offset.
Our analytic approach allowed ANS patterns to emerge as short-lived, rather than sustained, configurations at any time (and during any trial) of the emotional reactivity task. Each video clip was selected to elicit a target emotion, but even within a single video, ongoing changes in lighting, music, and dialogue as well as variation in characters' facial expressions, voices, and movements might elicit different emotions (or emotions of different intensities) as plots unfold, tensions mount, and resolutions surface. Consistent with the dynamic nature of the video clips, the ANS states that we detected were fleeting, lasting 2 to 6 s on average, and dynamic, assembling and disassembling multiple times per emotion trial. The ANS states could have emerged at any point during the emotional reactivity task, but analyses conducted across the group and in individual participants indicated that each ANS state arose more often in one emotion trial than in others. These results suggest that predictable, non-random physiological changes arose across the participants as they viewed the same set of evocative stimuli. As we took a novel analytic approach to examining ANS changes during emotions, it is difficult to make direct comparisons between the physiological activities that characterized each ANS state in the present study and those found in prior investigations. Our results, however, are largely consistent with previous research that has investigated the ANS specificity of different emotions. ANS State 3 in our study, for example, was predominant during the amusement trial and was characterized primarily by faster heart rate (shorter inter-beat interval), faster respiration rate (shorter inter-cycle interval), higher skin conductance level, and lower respiratory sinus arrhythmia. Apart from respiratory sinus arrhythmia, all of the other channels responded in ways that were consistent with those found in most previous studies using change scores (Table S5). While there is some variability between the physiological patterns that were predominant during each emotion trial in our study and those detected in prior research, additional studies are needed to determine whether some of these differences reflect the duration over which physiological changes were measured or the varying content in the stimuli themselves.
The goal of the present study was not to define each emotion's hallmark ANS pattern, and it seems improbable that we discovered the singular physiological fingerprint of the target emotions. The ANS states that differentiated among the emotion trials here likely represent one possible manifestation—rather than the only manifestation—of the ANS changes that arise during each emotion. As emotions (Cowen & Keltner, 2021; Siegel et al., 2018), different stimuli may elicit related yet distinct variations of the ANS patterns that we found with this set of video clips. Other video clips that elicit amusement, for example, may evoke ANS states that vary somewhat in their composition but that resemble each other more than ANS states that arise during video clips that elicit sadness. The physiological changes that arise in response to different emotion-inducing video clips are likely meaningful and reflect the dynamic content of the stimuli and the ongoing appraisals of the viewer (Barrett, 2006b; Siegel et al., 2018). As the same ANS states emerged across participants in the present study, however, our results suggest the physiological patterns that unfold in response to a specific set of evocative video clips are not random. Although additional studies are needed to determine whether ANS states that resemble those we detected in our study also emerge in response to different video clips or different types of affective stimuli, our results suggest hardwired ANS patterns emerge across individuals who are viewing the same emotion-inducing stimuli.
4.2 Dynamic ANS states were present in basal physiology
The intrinsic organization of basal physiology is not well understood, but functionalist and constructionist theories have differing views about its role in emotions and emotional experience. According to functionalist theories, emotions disrupt homeostasis and organize activities across the ANS (and between the ANS and other systems) that may be uncoordinated at rest (Cosmides & Tooby, 2000; Levenson, 2003, 2014). In this view, basal physiology is the backdrop on which emotions unfold that may lack its own functional organization. According to constructionist theory, in contrast, basal physiology plays a central role in shaping ongoing affective experience (Russell, 2003), but whether it has an intrinsic architecture that influences these subjective feelings is unknown.
Our results suggest that, within the relative quiescence of basal physiology, there are fleeting ANS patterns that resemble those seen during emotions. The magnitude of the ANS changes that occurred during the resting period was less intense than of the ANS states that occurred during the emotional reactivity task, and their presence was intermittent, with many seconds of the resting period not aligning with an ANS state seen during video-viewing. Many unanswered questions remain regarding the significance of these ANS states in basal physiology. For example, whether these transient ANS states reflect “flickers” of emotions that color momentary, or even more enduring, subjective experiences or suggest a “readiness” property of the ANS that prepares the organism to react to self-relevant stimuli in predictable ways are questions that warrant future research. Additional studies are also needed to determine whether the presence of emotion-relevant ANS patterns in basal physiology relates to other domains including subjective experience, facial behavior, and neural activity.
By forging links between physiological activity during an emotional reactivity task and basal physiology, the present study offers new insights into the functional organization of the ANS. Our findings in the ANS parallel results from studies of the central nervous system (Deco et al., 2008; Fox et al., 2005; Lurie et al., 2020; Smith et al., 2009). Neuroimaging studies have shown that task-free (“resting state”) functional connectivity patterns mirror task-based neural activation patterns, a finding that highlights the robust functional organization of brain networks across participants and conditions (Fox et al., 2005; Smith et al., 2009). Here, we found the ANS patterns that arose during the emotional reactivity task were also detectable in a task-free resting period that assessed basal physiology. Our results suggest that the ANS, like the brain, generates similar activity patterns across task-based and task-free contexts. The ANS patterns that we found during emotions and rest are likely the products of distributed brain systems (Kragel et al., 2016; Kragel & LaBar, 2014b; Lettieri et al., 2019), and how the brain and the ANS interact over time during emotion generation will be an important question for future studies.
4.3 Limitations
There are important limitations to our study that should be considered. First, we used short video clips, presented in the same order, to elicit five target emotions and associated ANS changes. Our analytic approach was agnostic to the order of the video clip presentation. Although the tPCs included in the analyses did not appear to drift or to exhibit tonic changes over time, we cannot rule out the possibility that order effects or other factors related to the study design (e.g., participant fatigue across the task) influenced our findings. The ANS states, therefore, could emerge at any time—during any trial—of the emotional reactivity task, regardless of which emotion that trial was intended to elicit. Despite this flexibility, the ANS states that we detected still clustered in certain emotion trials more than others. Future studies that search for ANS patterns in response to emotion-inducing stimuli that are presented in a randomized order will be needed to address this question in more detail, however.
Second, our sample was comprised of healthy older adults, most of whom were highly educated and self-identified as non-Hispanic White. To rule out the presence of an underlying neurodegenerative disease or psychiatric illness, all participants underwent extensive neurological, neuropsychological, functional, and neuroimaging assessments and were determined to be neurologically and psychiatrically healthy by a multidisciplinary diagnostic team. While it is possible that healthy older adults may be a powerful population in whom to investigate emotional reactivity because they have had a lifetime to develop and refine their emotional responses, future studies that investigate ANS states in younger and more diverse populations will be needed to determine whether there are any age- or ethnicity-related differences in physiological patterning during emotions.
Third, we did not find associations between the amount of time participants spent in the ANS states and their self-reported emotional experience. Our experiential data were based on a three-point scale, and participants only reported on their overall experience of specific emotions at the end of each trial. These limitations likely hindered our ability to find associations between ANS activity and subjective feelings. How basal ANS physiology contributes to mood or core affect is unknown (Russell, 2003), but studies that obtain more fine-grained measures of subjective experience will be needed to map the complex associations between underlying ANS states and self-reported feelings. As there are individual differences in how people use language to express their feelings (Barrett et al., 2007; Jackson et al., 2019), it is likely that verbal emotion labels will not exhibit a one-to-one mapping with an underlying ANS state, an important topic for future investigation. Consistent with prior work (Shiota et al., 2011), participants in the present study were more selective in their endorsement of the target negative emotions than of the target positive emotions during the emotional reactivity task. Whether these reporting differences reflect greater differentiation among ANS states that accompany negative feelings and greater overlap among the ANS states that accompany positive feelings is unknown but could be explored in additional research.
5 CONCLUSIONS
From the spontaneous firing rates of neurons (Deco et al., 2008) to the intrinsic connectivity of distributed brain networks (Lurie et al., 2020), the dynamic properties of physiological systems are critical for understanding complex functions. The ANS is also dynamic, yet its functional organization is poorly understood. The present study expands current models of the functional architecture of ANS physiology. Our work offers novel insights into how the ANS produces brief physiological patterns during emotions and rest and highlights the ways in which the ANS creates both stability and flexibility in its continuous outflow.
AUTHOR CONTRIBUTIONS
Lorenzo Pasquini: Investigation; methodology; visualization; writing – original draft; writing – review and editing. Fatemeh Noohi: Methodology; writing – original draft; writing – review and editing. Christina R. Veziris: Data curation; writing – original draft; writing – review and editing. Eena L. Kosik: Data curation; writing – original draft; writing – review and editing. Sarah R. Holley: Data curation; writing – original draft; writing – review and editing. Alex Lee: Methodology; writing – original draft; writing – review and editing. Jesse A. Brown: Methodology; writing – original draft; writing – review and editing. Ashlin R. K. Roy: Data curation; writing – original draft; writing – review and editing. Tiffany E. Chow: Investigation; writing – original draft; writing – review and editing. Isabel Allen: Investigation; writing – original draft; writing – review and editing. Howard J. Rosen: Supervision; writing – original draft; writing – review and editing. Joel H. Kramer: Supervision; writing – original draft; writing – review and editing. Bruce L. Miller: Supervision; writing – original draft; writing – review and editing. Manish Saggar: Methodology; supervision; writing – original draft; writing – review and editing. William W. Seeley: Investigation; supervision; writing – original draft; writing – review and editing. Virginia E. Sturm: Funding acquisition; investigation; supervision; writing – original draft; writing – review and editing.
ACKNOWLEDGMENTS
We thank the participants for their contributions to our research. We thank Edoardo Pasquini for his contribution as a scientific illustrator.
FUNDING INFORMATION
This work was supported by the Global Brain Health Institute, Hillblom Healthy Aging Network, John Douglas French Alzheimer's Foundation, and NIH grants K99-AG065457 to LP and R01AG052496 and R01AG057204 to VES.
CONFLICT OF INTEREST
The authors report no conflict of interests.
Open Research
DATA AVAILABILITY STATEMENT
Data, code, and materials used in the analyses are publicly available on GitHub (https://github.com/lollopasquini/Dynamic_ANS).