Effects of Understory Planting Patterns on the Accumulation of Secondary Metabolites in Panax quinquefolius L. and Their Possible Mechanisms
Funding: National Key Research and Development Project, Grant/Award Number: 2023YFC3503802; Key Technology Research and Development Program of Shandong, Grant/Award Number: 2022TZXD0036; Construction Project for Sustainable Utilization of Valuable Traditional Chinese Medicine Resources, Grant/Award Number: 2060302; Natural Science Foundation of Shandong Province, Grant/Award Number: ZR2022MH101; ZR2024MH253.
ABSTRACT
Understory planting patterns significantly influence the accumulation of secondary metabolites in the roots of Panax quinquefolius L. However, research on the mechanisms underlying these effects remains limited. Two treatments, field planting and understory planting, were established in this study. Liquid Chromatography–Tandem Mass Spectrometry (LC–MS/MS) was utilized to determine the differences in root secondary metabolites of P. quinquefolius across these two planting modes. Additionally, the differences in the bacterial and fungal compositions of rhizosphere soils were analyzed using 16S rRNA and ITS sequencing. Untargeted metabolomics was utilized to evaluate variations in soil metabolites within the rhizosphere. Observations of rhizosphere arbuscular mycorrhizal fungi (AMF) colonization were conducted with a compound light microscope. The relationships between root secondary metabolites, rhizosphere microorganisms, and differential soil metabolites were assessed using Procrustes Analysis and Mantel-test correlation analysis. The results indicated that the content of ginsenosides of the understory planting pattern P. quinquefolius increased. Additionally, there was an observed increase in rhizosphere microbial diversity and community complexity, as well as enhanced arbuscular mycorrhizal fungi (AMF) colonization. The relative abundance of microorganisms showed significant correlations with the content of plant secondary metabolites. Notably, the contents of rhizosphere soil metabolites columbin and glycyl-L-leucine increased, while ginsenosides and rutin levels decreased, exhibiting significant correlations with ginsenoside content in the roots of P. quinquefolius. This paper explores the mechanisms underlying the quality formation of P. quinquefolius in understory planting, focusing on rhizosphere microecology and soil metabolism, and establishes a foundation for developing ecological understory planting practices.
1 Introduction
American ginseng, derived from the dried root of Panax quinquefolius L., has been utilized in Traditional Chinese Medicine (TCM) for over 300 years (Committee 2020). Characterized by its coolness, sweetness, and slight bitterness, it is believed to tonify qi, nourish yin, clear heat, and generate body fluids according to TCM principles (Committee 2020; Yuan et al. 2010). Modern research has established that American ginseng is abundant in ginsenosides, polysaccharides, volatile oils, and other active compounds, which exhibit anti-tumor, anti-fatigue, and immune-enhancing properties (Yang et al. 2020, 2023). In recent years, the utilization of American ginseng in medicinal and health care products has surged, with China emerging as one of the leading producers of P. quinquefolius, yielding over 8000 tons annually. The mainstream cultivation method for P. quinquefolius is field cultivation, a type of monoculture that involves utilizing shade nets to provide necessary cover. According to the Chinese Pharmacopoeia, only roots that are 4 years old or older are deemed suitable for use in Chinese medicine. However, the long-term implementation of field cultivation has resulted in the degradation of soil quality, negatively impacting the overall quality of American ginseng. Therefore, it is crucial to explore and establish sustainable planting patterns to improve the cultivation practices of P. quinquefolius.
P. quinquefolius is native to the mixed coniferous and broadleaf forests of Canada and the United States. The low light penetration, the covering of deciduous leaves, the presence of overgrown weeds, and the specific soil conditions collectively create an optimal “home” environment for P. quinquefolius. Literature indicates that ginseng cultivated in such environments demonstrates superior quality (Sheban et al. 2021). This suggests that the home environment plays a critical role in the development of American ginseng's quality. To establish a rational planting pattern, we simulated a home-like environment and cultivated P. quinquefolius within a forest setting, enabling the species to thrive in conditions that resemble its natural habitat. It has been shown that forest-grown ginseng contains higher concentrations of ginsenosides, polysaccharides, polyphenols, proteins, and other active compounds compared to garden-grown ginseng (Wang et al. 2021). However, currently the effects of a forest-like environment on the accumulation of active substances in P. quinquefolius and the underlying mechanisms remain unexplored in existing literature. Understanding the influence of understory environments on the quality of P. quinquefolius and elucidating the associated mechanisms will provide a foundation for developing a rational and ecological planting pattern.
The rhizosphere microecology serves as a second genome for plants and is a crucial factor in determining the quality of medicinal plants (Bai et al. 2022). Recent studies have indicated that understory planting methods can significantly alter the rhizosphere microecology of plants. For instance, planting Amorphophallus konjac under forest conditions increased the relative abundance of rhizosphere Actinobacteria and Nitrospirae, as well as enhanced alpha bacterial diversity (Ma et al. 2024). Similarly, underscoring the benefits of forest environments, Gastrodia elata grown in such settings exhibited a reduction in the abundance of soil pathogenic microorganisms (Xie et al. 2024). Previous research by our team has established a close relationship between the rhizosphere microorganisms of P. quinquefolius and its metabolic processes (Duan et al. 2024). For example, the presence of Acidobacteriota, Chloroflexi, and Myxococcota in the rhizosphere has been shown to influence the production of secondary metabolites. Notably, the active component (20R) ginsenoside Rg3 was positively correlated with various bacteria, particularly Acidobacteriota. Furthermore, arbuscular mycorrhizal fungi (AMF), which belong to the Glomeromycota clade, can infect plant roots and establish symbiotic relationships with plants. Our research has demonstrated that colonization by AMF promotes the synthesis of ginsenosides (Ran et al. 2023). Consequently, it is evident that alterations in rhizosphere microecology may serve as a significant mechanism underlying the enhanced quality of understory-cultivated P. quinquefolius.
Soil metabolism refers to the processes of material exchange and energy transformation that occur between soil organisms—including plant roots, soil animals, and microorganisms—and the soil environment, primarily through respiration. Devi et al. (2017) have reported that forest soil ecosystems significantly influence soil biometabolites. These soil metabolites are intimately connected to plant metabolic processes. Gfeller et al. (2023) demonstrated that soil metabolites can affect the growth and metabolism of winter wheat, highlighting their critical role in plant development. Additionally, Cheng et al. (2022) established a correlation between soil metabolites and the accumulation of plant secondary metabolites. Compared to field soil, the soil metabolites in understory environments undergo alterations that may affect the secondary metabolite profiles of P. quinquefolius. Consequently, it is essential to explore these effects in depth to understand the implications of soil metabolic changes on the quality and medicinal properties of this species.
In this article, we collected the roots and rhizosphere soil of P. quinquefolius under two cultivation methods: field planting and forest planting. Our study accomplished the following objectives: (i) we employed an untargeted metabolomics approach to compare the differences in secondary metabolites of P. quinquefolius between the two planting methods; (ii) we utilized 16S and ITS rRNA sequencing to compare the soil microbial communities in the rhizosphere; (iii) we applied untargeted metabolomics to analyze the differences in soil metabolites within the rhizosphere; and (iv) we examined correlations between plant differential secondary metabolites and soil microorganisms, as well as soil metabolites. This research elucidates the potential mechanisms influencing quality formation in P. quinquefolius through the lens of rhizosphere microorganisms and soil metabolism. This study seeks to establish an appropriate understory cultivation pattern to enhance the quality of P. quinquefolius while also providing valuable data support.
2 Materials and Methods
2.1 Experimental Design and Sample Collection
The experimental setup was conducted in the primary production area of P. quinquefolius in Wendeng, China (122°11′23″ E, 37°09′32″ N). The study incorporated both farmland and understory conditions, with P. quinquefolius cultivated at the field planting base in Zhangjiachan Town and the understory planting base in Shuangdingshan, also within Zhangjiachan Town. This production area is characterized by a continental monsoon climate, featuring four distinct seasons. The average annual temperature is 11.9°C, with an average precipitation of 767.8 mm, 2364.0 h of sunshine per year, and a frost-free period lasting approximately 195 days.
In July 2020, P. quinquefolius seeds were uniformly sown in both the farmland and understory planting bases. For the farmland cultivation, sandy loam soil previously used for wheat production was selected, and seed beds were prepared following the methodology described by Duan et al. (2024). Shade nets were subsequently installed to provide necessary protection. Understory planting of P. quinquefolius occurs beneath natural broadleaf forests, including tree species such as Cupressus funebris, Castanea mollissima, and Broussonetia papyrifera, which provide natural shading with their leaves. Sandy loam soil with a pH ranging from 5.5 to 6.8, characterized by high humus content, or alternatively live loess, was utilized. During planting, a seed density of 3.5 to 5 g per square meter was maintained to ensure uniformity. Weeds were allowed to grow naturally within the planting areas.
In July 2024, whole plant samples and rhizosphere soil samples were collected from each experimental area, with samples from the farmland and forest sites designated as control (T1) and treatment (T2), respectively. Rhizosphere soil was collected using a sterile spade at a depth of 20 cm, ensuring a distance of 2 mm from the roots of each plant. Larger clumps of soil were gently shaken off, while fine soil particles adhered to the roots and were carefully brushed away using a sterile brush. This fine soil was then sieved through a 2 mm mesh and collected in sterile self-wrapping bags. Soil samples were obtained from at least five P. quinquefolius roots and were immediately placed on dry ice for transport back to the laboratory, where they were stored at −80°C for subsequent soil metabolism and microbiome analyses. Each plant sample was divided into two parts: one portion was flash-frozen with liquid nitrogen and stored at −80°C for plant metabolic analyses, while the other portion was kept on dry ice and swiftly transported to the laboratory for the determination of ginsenoside contents and arbuscular mycorrhizal fungi (AMF) infestation rates.
2.2 16S and ITS rRNA Sequence Analysis of P. quinquefolius Rhizosphere Soil
Genomic DNA was extracted from 0.5 g of freeze-dried soil samples utilizing the CTAB method (Chen et al. 2024). The purity and concentration of the extracted DNA were assessed through agarose gel electrophoresis. A measured volume of DNA was transferred to a centrifuge tube and diluted to a final concentration of 1 ng μL−1 using sterile water. The amplification of the 16S rRNA and ITS genes was conducted via polymerase chain reaction (PCR) with specific primers. The 16S V4 region was amplified using the forward primer (5′-GTGCCAGCMGCCGCGGTAA-3′) and the reverse primer (5′-GGACTACHVGGGTWTCTAAT-3′). The ITS1-1F region was amplified using the forward primer (5′-CTTGGTCATTTAGAGGAAGTAA-3′) and the reverse primer (5′-GCTGCGTTCTTCATCGATGC-3′). Subsequently, a library was constructed utilizing the TruSeq DNA PCR-Free Sample Preparation Kit (Beijing Novogene Biotechnology Co. Ltd), and quantification was performed using both Qubit and quantitative PCR (q-PCR). The qualified libraries were then sequenced on the Illumina NovaSeq 6000 platform at Beijing Novogene Biotechnology Co. Ltd. The resulting Amplicon Sequence Variants (ASVs) were generated, and species classification annotations were assigned accordingly. To assess the complexity and stability of the microbial community composition, alpha and beta diversity metrics were calculated using the QIIME2 software, facilitating a comparison of differences between the samples.
2.3 Metabolomic Analysis
The roots of the plants were washed, chopped, and ground into a powder using liquid nitrogen. A total of 0.1 g of the treated plant and soil samples (six replicates per group) were weighed and subsequently frozen in liquid nitrogen. The metabolite analyses of roots and soil were conducted through untargeted metabolomics at Novogene Biotechnology Co. Ltd. Ultra-high performance liquid chromatography tandem mass spectrometry (UHPLC–MS/MS) analyses were performed using a Vanquish UHPLC system (Thermo Fisher) coupled with an Orbitrap Q Exactive TMHF-X mass spectrometer. The solvent gradient consisted of 0.1% formic acid aqueous solution (referred to as A) and acetonitrile (referred to as B), following the gradient profile: 0–2 min, 99%–80% A; 2–3 min, 80%–50% A; 3–7 min, 50%–20% A; 7–7.5 min, 20%–1% A; 7.5–9 min, 1% A; 9–9.1 min, 1%–99% A; and 9.1–10 min, 99% A. The column temperature was maintained at 40°C with a flow rate of 0.2 mL min−1. The raw data files (.raw) generated from mass spectrometry were processed using Compound Discoverer 3.3 (CD 3.3) software. Molecular formulas for metabolite identification were predicted based on molecular ion peaks and fragment ions, and matched against high-resolution secondary spectral databases, including mzCloud (https://www.mzCloud.org/), mzVault, and the MassList primary database. Further annotation of metabolites was performed using the KEGG database (http://www.kegg.jp/kegg/compound/). Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were conducted using the MetaX software. The criteria for differential metabolite screening were set to include a Variable Importance in the Projection (VIP) score > 1, a p-value < 0.05, and a fold change (FC) > 1.5 or < 0.667.
2.4 Analysis of Ginsenoside Content by LC–MS/MS
The extraction and determination of ginsenosides were conducted in accordance with the Chinese Pharmacopoeia, with some modifications (Ran et al. 2021b). A precise weight of 0.5 g of ginseng powder was sieved through a 200-mesh filter, and 70% methanol was added to a 25 mL volumetric flask. The mixture was subjected to ultrasonication at 50°C for 50 min, then allowed to stand overnight. Following this, the solution was extracted and filtered, and the methanol was evaporated using a rotary evaporator at 45°C. Subsequently, 5 mL of 70% methanol was added to dissolve the residue, and the extract was filtered through a 0.22 μm micropore membrane into a sample vial. For the analysis, an HPLC-20A system (Shimadzu) was employed, utilizing a YMC-Pack ODA-A column (250 mm × 4.6 mm, 5 μm) with a mobile phase composed of acetonitrile (A) and 0.1% phosphoric acid in water (B). The gradient elution program was as follows: 0–25 min, 19%–20% A; 25–60 min, 20%–40% A; 60–90 min, 40%–55% A; and 90–100 min, 55%–60% A. The flow rate was maintained at 1.0 mL/min−1, the column temperature was set to 40°C, and the injection volume was 20 μL. The peak areas for ginsenosides Rg1, Re, Rb1, Rh1, Ro, Rb2, Rb3, and Rd were recorded at 203 nm. Standards for ginsenosides were procured from Chengdu Mansit Biotechnology Co. Ltd., and quantitative analysis was performed utilizing the standard curve method.
2.5 Analysis of the Infestation Rate and Intensity of the Rhizosphere of P. quinquefolius
2.6 Statistical Analyses
Statistical analysis was conducted using R (version 4.1.0). Partial least squares discriminant analysis (PLS-DA) and principal component analysis (PCA) were employed to identify differential metabolites present in roots and rhizosphere soils, with a significance threshold set at variable importance in projection (VIP) scores greater than 1.0. Comparisons between distinct cultivation patterns were performed using one-way analysis of variance (ANOVA) with SPSS 26.0 (IBM Inc.). Graphical representations were created with Origin 2021 (Origin Lab) and GraphPad Prism version 9.5 to elucidate differences in metabolite content across cultivation patterns. Pearson correlation analysis was utilized to assess the relationships between genera and metabolites. Correlation heatmaps and network diagrams were generated using the Corrplot software package for R (version 4.1.0). Additionally, Zi–Pi plots were created using the ggClusterNet software package in R (version 4.1.0) to identify key microbial nodes, with thresholds set at Zi > 2.5 and Pi > 0.62. Tukey's t-test was applied to evaluate the separation between different cultivation patterns, and differences were deemed statistically significant at p < 0.05.
3 Results and Analysis
3.1 Effects of Understory Patterns on the Accumulation of Secondary Metabolites in the Roots of P. quinquefolius
Samples of P. quinquefolius were analyzed using metabolomics on the LC–MS/MS platform, resulting in the detection of a total of 1390 metabolites within the plant samples. Partial Least Squares Discriminant Analysis (PLS-DA) was conducted to examine the differences between the two treatment groups following quality control (Figure 1A). The analysis revealed a distinct separation, indicating significant metabolic differences between roots of field-grown and forest-grown P. quinquefolius. Specifically, 351 differential metabolites (DMs) were identified in the roots of P. quinquefolius cultivated in the forest compared to those grown in the field (Figure 1B). Among these, 180 metabolites were significantly up-regulated, including Plumbagin, γ-Glutamylglutamine, and Bergenin, while 171 metabolites were significantly down-regulated. These findings suggest that the understory planting pattern influences the metabolite profile of P. quinquefolius roots. Furthermore, the Kyoto Encyclopedia of Genes and Genomes (KEGG) pathway analysis identified the top 20 pathways exhibiting significant changes in plant metabolism (Figure 1C). Compared to field cultivation, forest planting notably enriched pathways related to glycerophospholipid metabolism, linoleic acid metabolism, arachidonic acid metabolism, and flavonoid biosynthesis in P. quinquefolius.

Ginsenoside constituents serve as indicators of the physiological activities and biological functions of P. quinquefolius. Screening for ginsenosides, the principal active compounds, among the total DMs revealed that ginsenoside Rd., R-Notoginsenoside R2, ginsenoside Rg2, and ginsenoside Rb1 were highly expressed under T2 conditions (Figure 1D). The ginsenoside content in P. quinquefolius samples was analyzed using High-Performance Liquid Chromatography (HPLC; Figure 1E). Notably, the concentrations of ginsenosides Re, Rb1, Rh1, Ro, Rb2, and Rb3 were significantly higher (p < 0.05) in the roots of forest-cultivated P. quinquefolius compared to those grown in the field, with increases of 11.8%, 62.9%, 61.7%, 45.5%, 160%, and 72.4%, respectively. In contrast, the content of ginsenoside Rg1 was significantly lower in forest-grown plants. Furthermore, total ginsenosides content in the roots of forest-grown P. quinquefolius was significantly elevated by 41.9% (p < 0.05) compared to their field counterparts (Figure 1F). These findings demonstrate that the contents of ginsenoside Rd., ginsenoside Rg2, and ginsenoside Rb1 were enhanced in understory-cultivated P. quinquefolius.
3.2 Effects of Understory Patterns on the Diversity and Structural Composition of Microbial Communities in the Rhizosphere of P. quinquefolius
Rhizosphere soil samples of P. quinquefolius were analyzed using high-throughput sequencing technology. After quality control, we obtained 35,182,750 valid tags from 16S rRNA gene sequencing and 22,952,351 valid tags from the ITS gene sequencing. Following DADA2 noise reduction and clustering, a total of 8586 bacterial amplicon sequencing variants (ASVs) and 877 fungal ASVs were retained. The dilution curves (Rarefaction Curves) for bacterial (Figure S1a) and fungal (Figure S1b) genes leveled off, indicating sufficient sequencing depth. Additionally, the diversity and richness of bacterial and fungal communities in the samples were assessed using the α-diversity index (Table 1). Compared to the field conditions, the α-diversity indices of soil microorganisms in the rhizosphere of P. quinquefolius under forest conditions showed significant differences. Specifically, the Chao1 values and Observed_features indices for both bacteria and fungi were significantly higher (p < 0.05), while the Shannon index for understory fungi increased significantly (p < 0.05) by 38.89% compared to the field. These findings suggest that understory planting enhances the α-diversity indices of microbial communities in the rhizosphere of P. quinquefolius, particularly in terms of the Chao1 value and Observed_features index.
| Treatments | Node | Edge | Average degree | Average clustering coefficient | Average path distance | Modularization |
|---|---|---|---|---|---|---|
| Field-grown | 148 | 230 | 1.554 | 0.248 | 1.261 | 1.850 |
| Forest-grown | 105 | 341 | 3.248 | 0.384 | 1.359 | 1.376 |
Differences in the rhizosphere soil microbial communities of P. quinquefolius between the two sample groups were analyzed using principal coordinate analysis (PCA) and non-metric multidimensional scaling (NMDS) based on weighted UniFrac distances (Figure S2). The results indicated a notable separation of bacterial communities (Figure S2a,c), suggesting that understory planting significantly influenced the composition of the rhizosphere bacterial community. The top 10 bacterial phyla associated with P. quinquefolius are illustrated in Figure 2A. Dominant phyla included Proteobacteria, Acidobacteriota, Bacteroidota, and Verrucomicrobiota, which collectively accounted for more than 70% of all sequences. Understory planting resulted in significant increases in the relative abundance of the understory bacterial phyla Crenarchaeota, Cyanobacteria, Gemmatimonadota, and Acidobacteriota by 94.14%, 58.53%, 40.46%, and 55.84%, respectively (p < 0.05), compared to field samples. Conversely, the relative abundances of Verrucomicrobiota, Bacteroidota, Actinobacteriota, and Proteobacteria decreased significantly (p < 0.05). At the genus level (Figure 2B), several understory bacterial genera, including WD2101_soil_group, Candidatus_Koribacter, ADurb.Bin063-1, Subgroup_13, Candidatus_Solibacter, Subgroup_2, Nitrospira, Bryobacter, Pedosphaeraceae, SC-1-84, Ellin6067, and Candidatus Nitrosotalea, exhibited significantly higher relative abundances (p < 0.05). The dominant fungal phyla identified were Ascomycota and Basidiomycota (Figure 2C). The relative abundance of the understory fungal phylum Glomeromycota was significantly higher than that observed in the field samples (p < 0.05). In contrast, the relative abundance of Rozellomycota, Basidiomycota, and Mortierellomycota was significantly lower (p < 0.05). At the genus level, the relative abundances of the understory fungal genera (Figure 2D) Plenodomus, Plectosphaerella, Oehlia, and Hypocreales_gen_Incertae_sedis were significantly higher (p < 0.05). The distribution maps of the microbiome and the linear discriminant analysis (LDA) values for bacterial biomarkers revealed significant differences at both the phylum and genus levels (Figure S3a,c). A total of 33 biomarkers were identified through LDA and effect size analysis for bacteria, while 12 biomarkers were identified for fungi (Figure S3b,d). Overall, Figure 2 illustrates that understory planting enhanced the α-diversity indices of rhizosphere microorganisms in P. quinquefolius and increased the relative abundance of dominant bacteria such as Crenarchaeota, Acidobacteriota, and Glomeromycota.

3.3 Effects of Understory Patterns on the Interactions and Network Complexity of Microorganisms in the Rhizosphere of P. quinquefolius
Pearson correlation heatmaps illustrating the relationships between dominant bacteria and fungi at the phylum and genus levels, using a significance threshold of p < 0.05 (Figure 3). At the phylum level (Figure 3A), Rozellomycota exhibited a significant positive correlation with Actinobacteriota and Verrucomicrobiota, while demonstrating a significant negative correlation with Chloroflexi, Gemmatimonadota, and Crenarchaeota. At the genus level (Figure 3B), Cephalotrichum was significantly positively correlated with Acidibacter, Granulicella, Pedobacter, Pseudomonas, Rhodanobacter, and Chthoniobacter, whereas it was significantly negatively correlated with Candidatus_Nitrosotalea. Similarly, Candolleomyces showed a significant positive correlation with Acidibacter, Granulicella, Candidatus_Udaeobacter, and Rhodanobacter, while it was significantly negatively correlated with Candidatus_Nitrosotalea. Furthermore, Leptodophora displayed a significant positive correlation with both Acidibacter and Granulicella. These results indicate that understory planting not only altered the structure of the rhizosphere microbial community but also likely modified the interactions among microorganisms.

To further investigate how understory cultivation influences the interaction patterns within the microbial community of the rhizosphere soil of P. quinquefolius, we assessed the topological properties of the rhizosphere microbial community and mapped the corresponding network (Figure 3C,D, Table 2). The overall topological characterization revealed significant differences in microbial network indices between field and understory soils (Table 2). Specifically, the number of edges, average degree, average clustering coefficient, and average path distance of microbial networks in the understory soils were notably higher than those in the field soils. This suggests that understory planting enhances the complexity of microbial networks in rhizosphere soils. Within this context, Oehlia diaphana emerged as a key node in the understory microbial community, as indicated by network connectivity analysis (Figure 3E,F). This species plays a pivotal role in recruiting plant growth-promoting rhizobacteria (PGPR), including Subgroup_2, Nitrospira, and Bryobacter.
| Treatments | Bacteria | Fungi | ||||
|---|---|---|---|---|---|---|
| Chao1 | Observed_features | Shannon | Chao1 | Observed_features | Shannon | |
| Field-grown | 2136.43 ± 197.39b | 2102.33 ± 187.38b | 9.53 ± 0.10a | 457.12 ± 3.47b | 460 ± 3.21b | 3.78 ± 0.11b |
| Forest-grown | 2867.76 ± 90.23a | 2851.33 ± 91.21a | 10.08 ± 0.20a | 560.12 ± 10.02a | 563 ± 1.27a | 5.25 ± 0.59a |
PICRUSt2 analysis was employed to predict the functional profiles of soil bacteria associated with P. quinquefolius using the KEGG Orthology (KO) database (Figure S4a). The functional profiles of bacteria in field soil are primarily related to environmental information processing, cellular processes, and human diseases, whereas the functional profiles of bacteria in forest soil were more closely associated with metabolism, genetic information processing, and organic systems. The functional classification of fungi was conducted using FUNGuild analysis (Figure S4b). The ecological functional groups of rhizosphere soil fungi associated with P. quinquefolius in the field predominantly consisted of endophytic fungi, endophytic phytopathogens, and phytopathogens. In contrast, the understory fungal functional groups were primarily composed of phytopathogens, tufted mycorrhizae, and zoopathogenic fungi. These findings demonstrate that understory planting enhanced the topological properties of soil microbial networks such as the number of edges and average abundance, increased network complexity, and altered the predominant functional types of microorganisms.
3.4 Correlation Analysis of Soil Microorganisms and Secondary Metabolites in the Roots of P. quinquefolius
The consistency of 351 differential metabolites (DMs) in the roots of P. quinquefolius with 189 genera of root-associated differentially abundant bacteria (DEBs) and 21 genera of differentially abundant fungi (DEFs) was assessed using Procrustes Analysis. This analysis aimed to determine whether changes in the DMs were correlated with DEBs and/or DEFs (Figure 4). The small M2 values (p < 0.05) observed between DMs and DEBs (Figure 4A) for both P. quinquefolius and rhizosphere samples indicated a high degree of concordance. In contrast, the M2 value between DMs and DEFs (Figure S5a) was significantly larger (p > 0.05), suggesting that DEFs may have a lesser impact on those metabolites. The redundancy analysis (RDA) using the top 10 bacterial phyla from canonical data and 351 DMs from P. quinquefolius identified the bacterial phyla that play a key role (Figure 4B). The DMs of P. quinquefolius collected from the field were significantly associated with the bacterial phyla Actinobacteriota (p = 0.015) and Verrucomicrobiota (p = 0.033). Additionally, Chloroflexi (p = 0.005), Acidobacteriota (p = 0.006), and Gemmatimonadota (p = 0.009) contributed to the variation in DMs observed in understory-planted P. quinquefolius. In contrast, only Glomeromycota, a group of AMF within the phylum Fungi (Figure S5b), influenced the changes in DMs of forest-grown P. quinquefolius.
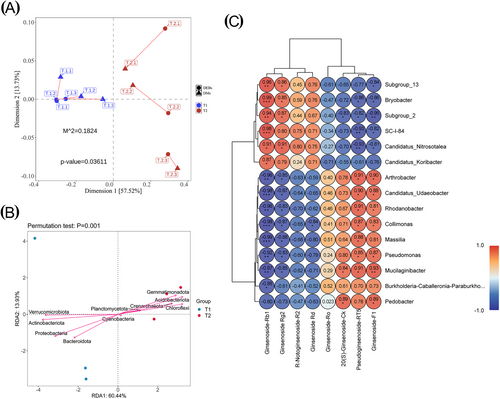
We further analyzed the ginsenoside analogs in the DMs of P. quinquefolius in relation to 15 prominent genera of differentially abundant bacteria (DEBs; Figure 4C). The results indicated that ginsenoside-Rb1 and ginsenoside-Rg2 exhibited a significant positive correlation with Subgroup_13, Bryobacter, Subgroup_2, SC-I-84, Candidatus_Nitrosotalea, and Candidatus_Koribacter. Conversely, they showed significant negative correlations with Collimonas, Candidatus_Udaeobacter, Arthrobacter, Rhodanobacter, Massilia, Pseudomonas, and Mucilaginibacter (p < 0.05). In contrast, pseudoginsenoside-RT5 and ginsenoside-F1 exhibited opposite relationships with these DEBs genera compared to ginsenoside-Rb1 and ginsenoside-Rg2. These findings suggest that understory bacteria, including Chloroflexi, Acidobacteriota, and Gemmatimonadota, as well as the fungi Glomeromycota, may influence the accumulation of ginsenosides in P. quinquefolius.
3.5 Effects of Understory Planting Patterns on Mycorrhizal Colonization of P. quinquefolius
Previously, we established that the phylum Glomeromycota plays a crucial role in shaping the DMs in forest-cultivated P. quinquefolius (Figure S5b). AMF categorized within the Glomeromycota subgroup are widely distributed in nature and constitute a crucial component of rhizosphere microecology, with the potential to regulate the synthesis of plant secondary metabolites. We assessed the potential enhancement of P. quinquefolius quality through different planting patterns by determining the rate of mycorrhizal colonization. Observations revealed distinct mycelial and vesicular structures stained blue–black with trichothecene blue in the root system of P. quinquefolius, indicating successful mycorrhizal symbiosis, particularly in the understory planting configuration (Figure 5A,B). Understory planting of P. quinquefolius resulted in a dense mycelium network and an increased production of sub-spherical or elliptical vesicles. Compared to traditional field planting, the rates of mycorrhizal colonization and infestation intensity in the understory planting of P. quinquefolius experienced significant increases of 140% and 201.88% (p < 0.05), respectively (Figure 5C). These findings indicate that the rates and intensities of mycorrhizal colonization in forest planting are significantly higher than those observed in field planting.
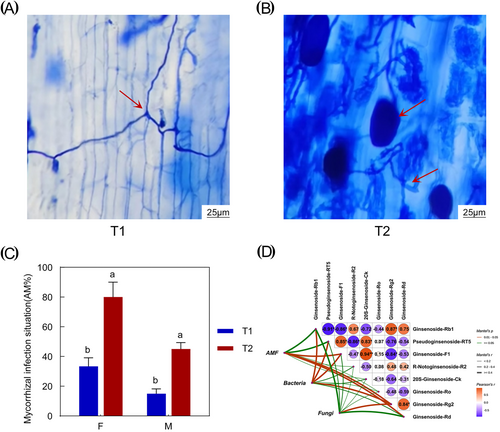
We further analyzed the effects of soil bacteria, fungi, and AMF on the secondary metabolism of P. quinquefolius (Figure 5D). The results indicated significant correlations (p < 0.05) between the synthesis of ginsenosides in P. quinquefolius and the infestation of soil bacteria, fungi, and AMF. Notably, the synthesis of ginsenoside Rg2 exhibited a highly significant positive correlation (p < 0.01) with the presence of soil bacteria, fungi, and AMF. Additionally, soil bacteria showed a highly significant correlation (p < 0.01) with the synthesis of ginsenoside Rb1, pseudoginsenoside RT5, and ginsenoside F1. Furthermore, AMF infestation was also highly significantly correlated (p < 0.01) with the synthesis of ginsenoside Rd. These findings suggest that understory cultivation enhances rhizosphere AMF colonization, which in turn significantly influences the synthesis of secondary metabolites in P. quinquefolius.
3.6 Effects of Understory Patterns on the Rhizosphere Soil Metabolites of P. quinquefolius
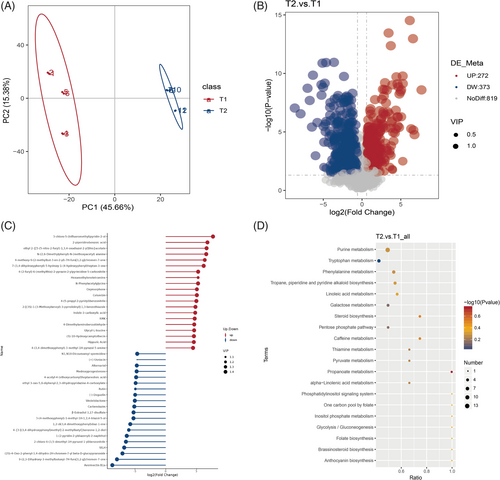
Rhizosphere soil samples of P. quinquefolius were analyzed using metabolomics on the LC–MS/MS platform to assess the impact of understory planting on soil metabolites. In total, 1464 metabolites were identified, comprising 876 detected in positive ion mode and 588 in negative ion mode. Principal component analysis (PCA; Figure 6A) revealed a distinct separation between the T1 and T2 groups, indicating a significant difference in rhizosphere soil metabolites between field and forest plantations of P. quinquefolius. The comparative analysis identified 645 differential metabolites (DMs; Figure 6B), out of which 272 were significantly upregulated and 373 were downregulated in the rhizosphere soil of understory plantings. Notably, the forest soil exhibited an increase in metabolites such as hexamethylene tetramine, columbin, glycyl-L-leucine, and hippuric acid (Figure 6C), which are associated with plant growth and stress tolerance. Conversely, allelochemicals, including rhamnose, demonstrated a declining trend. In addition, 11 ginsenosides from the soil DMs were screened and subjected to cluster analysis, revealing a significant reduction in ginsenoside content within the soil of forest ginseng (Figure S6). The KEGG pathway analysis highlighted the top 20 enriched pathways of soil metabolism (Figure 6D). Compared to field conditions, forest planting significantly enhanced pathways related to purine metabolism, phenylalanine metabolism, alkaloid biosynthesis, and linoleic acid metabolism. These results indicate substantial changes in rhizosphere soil metabolites and metabolic pathways associated with understory planting.
3.7 Correlation Analysis of Soil Metabolites and Root Secondary Metabolites of P. quinquefolius
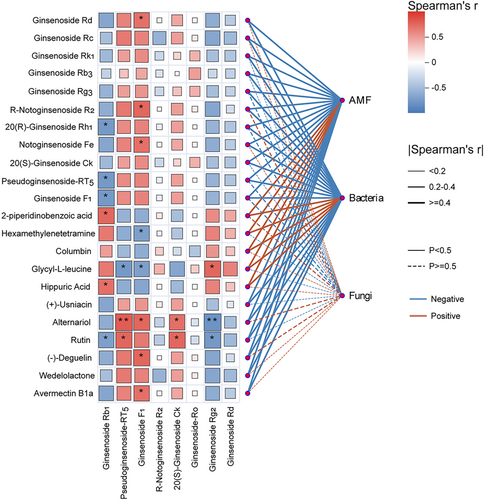
A mantel test correlation analysis was performed between typical soil DMs and plant secondary metabolites, specifically ginsenosides (Figure 7), revealing a significant correlation. Notably, the soil metabolites 20(R)-ginsenoside Rh1, pseudoginsenoside-RT5, ginsenoside F1, and rutin exhibited a significant negative correlation with the root secondary metabolite ginsenoside Rb1. Additionally, soil metabolites alternariol and rutin showed a significant positive correlation with pseudoginsenoside-RT5, ginsenoside F1, and 20(S)-ginsenoside Ck, while demonstrating a significant negative correlation with ginsenoside Rg2 in the roots of P. quinquefolius. Furthermore, soil metabolites were found to be significantly correlated with plant-derived extracellular bacteria (DEBs; Figure S7). Notably, rutin exhibited a significant negative association with SC-I-84, Candidatus _Nitrosotalea, Subgroup_13, and Candidatus_Koribacter. These findings suggest that soil metabolites are intricately linked to the accumulation of ginsenoside constituents in plants.
4 Discussion
4.1 Understory Planting Patterns Promote the Enhancement of Secondary Metabolites in P. quinquefolius
The contents of the secondary metabolites ginsenoside Rd., R-notoginsenoside R2, ginsenoside Rg2, and ginsenoside Rb1 were significantly higher in P. quinquefolius cultivated under understory conditions compared to traditional field cultivation. These secondary metabolites are closely associated with the clinical applications and overall quality of P. quinquefolius (Kim et al. 2013; Liu et al. 2023). Additionally, Lan et al. (2023) reported that the understory cultivation of Panax ginseng enhances its quality. These findings indicate that “returning home” is feasible. It is also conducive to the improvement of the quality of P. quinquefolius medicinal materials. In addition, the understory planting mode does not occupy farmland and reduces problems such as continuous cropping obstacles (Bao et al. 2020). Utilizing understory land can potentially increase farmers' income. Consequently, understory planting is anticipated to be an effective strategy for the ecological cultivation of P. quinquefolius.
4.2 Soil Microbial Diversity and Community Complexity Are Important Mechanisms That Influence Secondary Metabolism
Understory cultivation, compared to traditional field methods, enhances the accumulation of beneficial bacteria and increases the complexity of the rhizosphere soil microbial community. At the individual level, this cultivation method may enrich a diverse population of beneficial microbes, including key species such as Oehlia diaphana, which encompasses beneficial groups like the WD2101_soil_group (plant growth promoters; Wongkiew et al. 2021), Nitrospira (involved in nitrogen cycling; Siczek et al. 2020), and Bryobacter (associated with carbon cycling; Hu et al. 2022). These taxa contribute to the colonization of the rhizosphere and foster microbial interactions. Research has indicated that Oehlia diaphana, a newly characterized genus of rhizosphere fungi within the phylum Glomeromycota, plays a crucial role in plant nutrition and symbiotic relationships, showing potential applications in the regulation of microbial communities (Corazon-Guivin et al. 2023). At the community level, the microbial network within the understory rhizosphere exhibits an increase in the number of connections (edges), average degree, and average clustering coefficient, suggesting heightened diversity and complexity among the dominant flora. Studies have shown that high-diversity microbial communities typically display greater ecological stability and functional efficiency, facilitating nutrient acquisition for plants and enhancing stress resistance, thereby improving overall quality (Xiao et al. 2024). Moreover, the synthesis and accumulation of secondary metabolites in medicinal plants are closely linked to the rhizosphere microbial community (Yuan et al. 2022). Thus, the complexity of rhizosphere microbial communities in understory P. quinquefolius is considered a critical factor in determining the quality of this medicinal plant.
The diversity and structural composition of soil microorganisms in the rhizosphere increased under the forest cultivation model. Specifically, the relative abundance of the soil bacterial phyla Crenarchaeota, Cyanobacteria, Gemmatimonadota, and Acidobacteriota was significantly higher in the understory compared to conventional field settings. Previous research has indicated that these phyla are pivotal in the nitrogen and carbon cycles, thereby contributing essential nutrients for plant growth (Gonçalves et al. 2024). At the genus level, there was a notable increase in the relative abundance of SC-1-84, Candidatus_Nitrosotalea, and Hypocreales_gen_Incertae_sedis. Studies have shown that SC-1-84 enhances plant disease resistance against pathogens such as Neofusicoccum parvum (Leal et al. 2021). Meanwhile, Candidatus_Nitrosotalea facilitates nitrogen cycling and utilization through soil ammonia oxidation (Lehtovirta-Morley et al. 2016), and Hypocreales_gen_Incertae_sedis promotes plant growth while effectively controlling pests and diseases (Kepler et al. 2017). These microbial changes collectively support the growth and development of P. quinquefolius and enhance its resistance to stress. In terms of fungi, the rhizosphere exhibited an enrichment of the fungal phylum Glomeromycota, which includes AMF that form symbiotic relationships with plant roots. Research has shown that AMF plays a crucial role in promoting plant growth and enhancing stress tolerance (Volpe et al. 2023). Our previous studies indicated that the inoculation of P. quinquefolius with AMF significantly promoted growth and increased ginsenoside accumulation (Ran et al. 2021a). Furthermore, experiments on AMF colonization under different planting environments revealed a significant increase in both the colonization rate and intensity of AMF in the rhizosphere of P. quinquefolius cultivated in forests, resulting in a robust symbiotic association with the plant. Additionally, procrustes analysis showed that Chloroflexi, Acidobacteriota, Gemmatimonadota, and Glomeromycota significantly affected the changes in DMs of understory-cultivated P. quinquefolius roots, which may be key microorganisms promoting ginsenoside accumulation. The interactions within the soil microbial community and their effects on plant health and quality under forest cultivation conditions have been highlighted in studies such as those by Ma et al. (2024), which reported on the beneficial effects of the microbial community on plant resistance to pests and diseases and overall quality improvement. Correlation analyses further indicated that the content of active components in P. quinquefolius, such as ginsenoside Rg2, is significantly associated with soil bacterial taxa (e.g., SC-1-84, Candidatus_Nitrosotalea), fungal diversity, and AMF colonization. This suggests that the rhizosphere microbial diversity of understory P. quinquefolius is a critical factor in determining the quality of this medicinal plant.
4.3 The Composition and Content of Rhizosphere Soil Metabolites Is Another Mechanism That Influences Secondary Metabolism
In terms of soil metabolic processes related to rhizosphere microecology, our findings indicated that the metabolites in understory plantation soils were significantly altered compared to those in conventional fields, resulting in the identification of a total of 645 DMs. Specifically, in forest soils, metabolites such as 2-piperidinobenzoic acid, columbin, glycyl-L-leucine, and hippuric acid were found to be elevated. Research has shown that columbin exhibits various biological activities, including anti-inflammatory properties and the ability to regulate plant growth (Pacheco et al. 2009). Moreover, the concentration of columbin within the rhizosphere's fungal symbiotic system was significantly and positively correlated with the length of AMF hyphae, suggesting that columbin may indirectly influence plant growth and metabolism by promoting the growth and distribution of rhizosphere AMF (Meng et al. 2023). Glycyl-L-leucine is proposed to serve as a nitrogen source for plants and may enhance root biomass in Arabidopsis thaliana (Muratore et al. 2021). We hypothesized that the increase in these soil DMs contributes to the accumulation of secondary metabolites in the roots of P. quinquefolius. Correlation analyses revealed that the contents of 2-piperidinobenzoic acid and hippuric acid were significantly and positively correlated with the accumulation of ginsenoside Rb1 in the roots of P. quinquefolius. Additionally, glycyl-L-leucine showed a significant positive correlation with the levels of ginsenoside Rg2. These results suggest that understory cultivation may directly or indirectly enhance the synthesis of ginsenosides in the roots of P. quinquefolius by increasing the content of these specific soil DMs.
Conversely, the concentrations of ginsenosides and rutin in the understory soil were found to be lower. Wang et al. (2023) reported that ginsenosides act as allelopathic autotoxic substances in P. quinquefolius, inhibiting photosynthesis and impairing plant growth. In addition, Li et al. (2013) discovered that when ginseng plants experience abiotic stress, including allelopathic toxicity, there is a differential expression of ginsenoside biosynthetic genes. The results of this study indicate that the low levels of ginsenosides and rutin in the understory soil, in conjunction with the high concentrations of secondary metabolites in the roots of P. quinquefolius, suggest an influence of soil metabolism on plant secondary metabolism. Correlation analyses revealed a significant negative correlation between the soil contents of ginsenosides and rutin with most plant secondary metabolites and microorganisms. Likhanov et al. (2021) reported that rutin, as an allelochemical, inhibits the activity of PGPR at high concentrations, thereby reducing the soil nutrient conversion efficiency. Furthermore, Goodwin and Hsiang (2024) found that ginsenoside components released from ginseng root secretions could enhance the growth of certain bacterial populations by providing sugars as nutrients through bacterial glycoside hydrolases. Soil fungi such as Cladosporium xylophilum are capable of efficiently transforming ginsenosides Rb1, Rg1, and Rc into smaller ginsenosides (Li et al. 2022). This suggests that understory rhizosphere microorganisms may decompose soil ginsenosides to obtain essential nutrients for their growth. Additionally, the metabolic pathways related to purine metabolism, phenylalanine metabolism, and alkaloid biosynthesis in understory soils were significantly enriched. Research has shown that purines, as key metabolites in soybeans, can attract beneficial Pseudomonas species that help resist salt stress (Zheng et al. 2024). The enrichment of purine metabolism may enhance stress resistance in P. quinquefolius. Overall, these findings suggest that understory soil metabolites may positively influence plant growth and metabolism through their effects on microbial activities. An optimal soil environment appears to recruit beneficial microbial communities that promote plant metabolism. This study solely compares the rhizosphere soil microorganisms and soil metabolism of P. quinquefolius grown in forest understories and fields, and correlates these findings with differential secondary metabolites. Therefore, further studies investigating the impact of various factors, including root exudates and soil physical and chemical properties, on the secondary metabolites of P. quinquefolius are necessary to provide a theoretical basis for its quality formation and ecological cultivation.
5 Conclusions
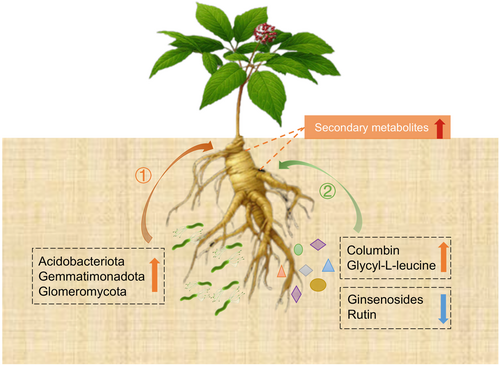
This study demonstrated that the understory cultivation method significantly promoted ginsenoside accumulation in the roots of P. quinquefolius and a potential mechanism was identified (Figure 8). First, from the perspective of rhizosphere microorganisms, understory planting reshaped the microbial community in the rhizosphere of P. quinquefolius. The relative abundances of Acidobacteriota, Gemmatimonadota, and Glomeromycota have increased, promoting the formation of a symbiotic system between AMF and root systems. This has led to an increase in the diversity and complexity of the microbial network, playing a crucial role in the synthesis of secondary metabolites in P. quinquefolius. Second, from the standpoint of rhizosphere soil metabolism, the concentrations of metabolites such as columbin and glycyl-L-leucine increased in the understory soil, thereby enhancing root biomass and stress resistance. In contrast, the levels of ginsenosides and rutin were found to decrease, which likely mitigated the allelopathic autotoxicity of P. quinquefolius and promoted the normal expression of genes involved in ginsenoside synthesis. In this study, we explored the mechanisms by which understory planting influences the accumulation of secondary metabolites in P. quinquefolius from the perspective of rhizosphere microecology. This research lays the groundwork for establishing an ecological planting model in understory environments.
Author Contributions
Y.W. conducted the majority of the experiments and authored the manuscript. Z.L. and R.W. contributed assistance during the experimental process. H.Y. offered suggestions for the manuscript. L.F., L.G., and J.Z. conceptualized and designed the research, guided the experiments, and revised the manuscript. All authors have read and approved the final version of the manuscript.
Acknowledgments
This work was financially supported by the National Key Research and Development Project (2023YFC3503802), the Key Technology Research and Development Program of Shandong (2022TZXD0036), the Construction Project for Sustainable Utilization of Valuable Traditional Chinese Medicine Resources (2060302) and the Natural Science Foundation of Shandong Province (ZR2022MH101, ZR2024MH253).
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
The data that supports the findings of this study are available in the Supporting Information of this article.




