Melatonin Mediates Methylglyoxal Homeostasis and Autophagy During Seed Germination Under Polyethylene Glycol-Induced Drought Stress in Upland Cotton
Funding: This work was supported by the University Grants Commission (UGC), New Delhi, for providing the Junior Research Fellowship [UGC NET, December 2021 and June 2022 (merged session), NTA Ref. No. 220510093898, dated 5th November 2022] and the University of Hyderabad for the UGC-BBL Fellowship for carrying out the doctoral research work. P.G. gratefully acknowledges the financial support received from the University of Hyderabad-Institute of Eminence Research Project (UoH-IoE-RC3-21-041), dated 13th December 2021) for carrying out the research work. The infrastructural facilities established with the support of UGC-SAP-DRS-1 (Level-1, Phase-1), DST-FIST-Level-II (Phase-2), DBT-BUILDER, and DST-PURSE programs have been utilized for the research work, which is gratefully acknowledged.
ABSTRACT
Methylglyoxal (MGO), a toxic byproduct of glycolysis, acts as a signaling molecule at low levels, but its overaccumulation during drought stress disrupts redox balance and accelerates cell death in plants. Contrarily, melatonin maintains redox balance, particularly during stress. The redox status and MGO levels might differ in drought-sensitive and drought-tolerant varieties, so shall the melatonin's effect. This present study evaluated the effect of melatonin priming on MGO detoxification and autophagy during seed germination under polyethylene glycol (PEG)-induced drought stress in drought-sensitive (L-799) and drought-tolerant (Suraj) varieties of upland cotton. Melatonin priming increased endogenous melatonin content, reduced MGO accumulation and advanced glycation end-products (AGEs), and downregulated the expression of MGO biosynthesis genes in L-799 under stress. The expression and activities of glyoxalases and nonglyoxalases were upregulated, showing melatonin's effectiveness in MGO detoxification. Additionally, melatonin priming upregulated TPI1, PGK5, and PK1 expressions and downregulated HK3 expression, allowing better conversion of glucose to pyruvate, leading to reduced MGO in L-799. The downregulated expression of necrosis-related genes with reduced cell death in L-799 shows the potential of melatonin priming in maintaining cell viability under stress. Furthermore, the upregulated expression of SnRK1.1 and SnRK2.6 genes and the KIN10 protein levels confirmed improved autophagy in melatonin-primed L-799 under stress, as evidenced by enhanced autophagy markers (ATGs, MDC-stained bodies, lipidated-ATG8). Despite lowered ABA, melatonin-mediated MGO homeostasis likely activated MAPK6, thus inducing autophagy independent of ABA in stressed plants. Conversely, Suraj seedlings showed a limited response to melatonin priming under stress possibly owing to its inherent stress tolerance and higher endogenous melatonin. Overall, this study illustrates melatonin's role in regulating MGO homeostasis and autophagy under drought stress in cotton.
1 Introduction
Seed germination is a pivotal phase of the plant's life cycle that signifies the transition of a quiescent seed into a metabolically active state, leading to the emergence of seedlings (El-Maarouf-Bouteau 2022). A healthy germination process ensures the production of uniform and robust seedlings through efficient resource utilization and increasing photosynthetic potential that results in higher yields (Ali and Elozeiri 2017). Drought is a major abiotic stress factor that adversely affects the seed germination process and consequently diminishes the growth of the seedlings and their establishment (Martínez-Ballesta et al. 2020; Maksimović et al. 2021). Water unavailability disrupts the fundamental imbibition process during germination, impeding the enzymatic systems essential for nutrient mobilization (Bove et al. 2002). Additionally, drought stress causes intricate osmotic adaptations within seeds, including the accumulation of stress-responsive osmoregulators (Pamuru et al. 2021). It also disrupts hormonal signaling and redox homeostasis and inhibits the cell elongation processes required for radicle emergence (Pamuru et al. 2021). Therefore, it is essential to improve seedling growth and plant development under drought stress to ensure crop productivity even under water-limited conditions.
Autophagy, one among several physiological processes, modulates drought stress and ensures the survivability of the plants (Tang and Bassham 2021). This self-degradation involves the sequestration and degradation of nonfunctional cellular components, such as damaged organelles and proteins, within specialized vesicles called autophagosomes (Su et al. 2020; Wang et al. 2021). The controlled breakdown and recycling of these components provide energy and essential building blocks for cell survival and growth (Yang et al. 2019). Drought conditions were reported to enhance the autophagy process by improving the expression of autophagy-related genes (ATGs) and autophagosome formation. Additionally, atg-mutants showed hypersensitivity to drought (Liu et al. 2009). Seed autophagy is a finely orchestrated process that ensures the efficient mobilization of stored reserves, providing energy and nutrients essential for germination and vigorous seedling development (Iglesias-Fernández and Vicente-Carbajosa 2022; Wang et al. 2022).
Methylglyoxal (MGO) is a highly reactive, toxic compound formed as a byproduct of glycolysis and other metabolic pathways, both enzymatically and nonenzymatically, in plants (Allaman et al. 2015). It is produced spontaneously via the removal of a phosphoryl group by β-elimination from the 1,2-enediolate of the triose sugars dihydroxyacetone phosphate (DHAP) and glyceraldehyde-3-phosphate (G3P; Hoque et al. 2012; Hossain et al. 2015). As a dicarbonyl compound, MGO causes cellular damage by interacting with proteins, lipids, and deoxyribonucleic acid (DNA). Its toxicity is owing to its highly reactive nature, resulting in the generation of reactive oxygen species (ROS) and the formation of advanced glycation end products (AGEs), which inhibit the germination and growth of plants (Hoque et al. 2012). Despite being toxic, recent research has highlighted the hormetic response of MGO as it functions as a signaling molecule at lower intracellular levels by modulating various physiological processes in plants (Li 2016). Park et al. (2020) reported that MGO is a potent inducer of apoptosis, whereas autophagy imparts a protective role against MGO-induced cell death. Similarly, Lee et al. (2020) and Kim et al. (2020) highlighted that MGO regulates both autophagy and apoptosis within animal cellular systems. However, the precise interplay and connection between these intricately intertwined pathways remain to be comprehensively elucidated, particularly within the realm of plant systems.
Melatonin is well known for its role in regulating sleep and circadian rhythms in animals and has emerged as a significant player in plant biology, influencing various physiological processes during stress conditions (Pan et al. 2023). Several reports have demonstrated that it combats abiotic stresses by regulating redox homeostasis and improving photosynthetic attributes. Li et al. (2018) reported that melatonin enhances maize's heat tolerance by detoxifying MGO, helping to maintain osmotic balance. Melatonin in combination with salicylic acid and gibberellic acid was shown to scavenge excess MGO by improving the glyoxalase (GLY) system in wheat and tomato, thus mitigating the effects of salinity stress (Siddiqui et al. 2020; Talaat and Todorova 2022). Melatonin is also known to have a cleaving effect on crosslinks in AGEs, thus contributing to their degradation (Takabe et al. 2016) and also prevents AGE-induced apoptosis via stimulation of the autophagy process (Jin et al. 2018).
Cotton (Gossypium hirsutum L.) is a globally important cash crop that yields fiber and oil, and plays an important role in India's economic development. The significance of cotton extends beyond economics, encompassing cultural, social, and industrial domains (Zahid et al. 2021). However, the germination and yield potential of this “white gold” is negatively affected by adverse environmental conditions, especially drought, which is reported to reduce the germination rate up to 27.75% (Bai et al. 2020) and can decrease the yield up to 67% (Zafar et al. 2023). Drought disintegrates cellular harmony by accelerating the production of several toxic compounds such as methylglyoxal. Despite reports highlighting its potential to inhibit germination (Hoque et al. 2012), there have been studies stating that MGO enhances seed germination (Majláth et al. 2021). Therefore, the influence of drought-induced MGO production on the germination process is still obscure. Although melatonin is already reported to improve seed germination under drought conditions (Bai et al. 2020), its regulatory effect on intracellular MGO homeostasis is yet to be explored. Additionally, drought-induced autophagy helps sustain the survivability of plants (Liu et al. 2009), and therefore whether drought-induced MGO production and its homeostasis by melatonin can influence autophagy is still a question. Hence, the present research was aimed at investigating the role of melatonin in the modulation of intracellular MGO and autophagy induction in drought-sensitive (L-799) and drought-tolerant (Suraj) varieties of upland cotton at germinating stage under polyethylene glycol (PEG) induced drought stress. To the best of our knowledge, this is the first study to show that melatonin priming mediated MGO homeostasis activate autophagy independent of ABA by elevating expression of MAPK in the L-799 variety of upland cotton.
2 Materials and Methods
2.1 Seed Materials and Priming
Seeds of two upland cotton varieties, viz., L-799 (drought-sensitive) and Suraj (drought-tolerant) were obtained from the Regional Agricultural Research Station, Guntur, Acharya N. G. Ranga Agricultural University (ANGRAU), Andhra Pradesh, India, and the Central Institute for Cotton Research (CICR), Maharashtra, India, respectively. Seeds were surface sterilized with the fungicide bavistin at 3% (w/v) for 5 min and 70% (v/v) ethanol for 3 min, followed by washing them with autoclaved double-distilled water. The seeds of the two varieties were primed with different concentrations (5, 10, 25, 50, and 100 μM) of melatonin (Sigma-Aldrich) by imbibing them for 24 h in the dark. Seeds imbibed in deionized water served as controls. The seeds were then dried for 1 h at room temperature (RT) in the dark for back-drying and were evenly placed on moist sterile germination paper in germination boxes for 2 days in the dark at RT. On the basis of the initial experiments using different concentrations (10%, 15%, 20%, and 25% w/v) of polyethylene glycol (PEG-6000), 18% PEG treatment for 4 days was selected for the study as it was optimal for providing drought stress, whereas 20% PEG resulted in the death of seedlings within 3 days and 15% PEG induced delayed stress responses in the L-799 variety. The two-day-old imbibed seeds were treated with drought stress by providing 18% (w/v) PEG dissolved in Hoagland solution and were allowed to grow in a culture room at 25°C ± 2°C, 65% ± 2% relative humidity under a 16:8 h light and dark photoperiod for another 4 days. All experiments were conducted with six-day-old seedlings of L-799 (drought-sensitive) and Suraj (drought-tolerant) varieties, with four treatments used for each variety that include control (C), PEG-treated (P), melatonin-primed control (M), and melatonin-primed PEG-treated (MP). Thus, each experiment consisted of eight treatments (samples), with three biological replicates used in each treatment. All experiments were repeated three times at different times.
2.2 Measurement of RWC, ROS, Lipid Peroxidation, and Electrolyte Leakage in Control and Stressed Seedlings With and Without Melatonin Priming
2.3 Protein Extraction and Enzymatic Assays of GLY I, II, and III
Crude protein of experimental seedlings was isolated to determine enzyme activities. Protein was extracted from fresh seedlings (200 mg) with an extraction buffer containing 100 mM sodium phosphate buffer (pH −7.0), 50% glycerol, 16 mM magnesium sulfate (MgSO4), 0.2 mM phenylmethylsulfonyl fluoride (PMSF), and 0.2% (w/v) polyvinylpolypyrrolidone (PVPP). The extracted proteins were quantified employing the Lowry method (Lowry et al. 1951) and used in enzyme assays.
The GLY I (EC 4.4.1.5) and GLY II (EC 3.1.2.6) enzyme activities were determined by measuring the generation and utilization of S-lactoylglutathione (SLG; Singla-Pareek et al. 2003; Sahoo et al. 2021). To determine GLY I activity, the reaction mixture containing 100 mM sodium phosphate buffer, pH −7.5, 3.5 mM MGO, 1.7 mM glutathione (GSH) and 16 mM MgSO4 was incubated in the dark for 7 min, which forms the intermediate substrate hemithioacetal. After incubation, 25 μg of crude protein extract was added to 1 mL of the reaction mixture, and the absorbance was taken at 240 nm within a timescale that showed a linear increase in the absorbance. The molar extinction coefficient of the generated SLG was 3370 M−1 cm−1. Enzyme activity was calculated accordingly and expressed as μmol min−1 mg−1 protein. To determine GLY II activity, 25 μg of crude protein was added to buffer containing 50 mM 3-(N-morpholino) propanesulfonic acid (MOPS, pH −7.2), and 0.6 mM SLG, and the decrease in absorbance was measured at 240 nm. The molar extinction coefficient of generated D-lactate was 3.1 mM−1 cm−1 and expressed in μmol min−1 mg−1 protein.
GLY III (EC 4.2.1.130) enzyme activity was measured by the colorimetric method proposed by Ghosh et al. (2022) with slight modifications. Total protein of 20 μg was added to 10 mM sodium phosphate buffer (pH −7.4) and 1 μM of MGO and incubated for 15 min at 45°C. Then, 2,4-dinitrophenylhydrazine (DNPH) solution was added, and the sample was incubated for another 15 min at RT, and the absorbance was measured at 550 nm with a UV–Vis (ultraviolet–visible) spectrophotometer (Shimadzu). The buffer with MGO and DNPH solution devoid of protein was used as a control. Specific activity was calculated by subtracting the final MGO content from the initial one and expressed in nmol min−1 mg−1 of protein.
2.4 Determination of Glutathione Pool
Total glutathione was measured according to the method described by Sahoo et al. (2017). The seedlings were macerated, homogenized in potassium phosphate buffer (0.5 M, pH 7.5) and centrifuged at 10,000 g for 15 min at 4°C. The resulting supernatant (1 mL) was added to 100 μL of 2-nitrobenzoic acid (DTNB, 10 mM), 200 μL of bovine serum albumin (BSA, 10 mM), and 100 μL of nicotinamide adenine dinucleotide (NADH, 0.5 mM) and incubated at 37°C for 15 min. The absorbance was taken at 412 nm and expressed as μmol g−1 fresh weight (FW). For the determination of oxidized glutathione (GSSG), the GSH was masked by adding 2-vinylpyridine to the supernatant and incubated for 1 h at 25°C. The extract (100 μL) was combined with 600 μL reaction buffer (100 mM potassium phosphate buffer and 5 mM EDTA, pH 7.5), 100 μL of glutathione reductase (GR, 20 U ml−1), and 100 μL of DNTB (10 mM). The reaction was initiated by adding 100 μL of nicotinamide adenine dinucleotide phosphate (NADPH, 2.5 mM), and the rate of absorption was measured at 412 nm. The results are expressed as nmol g−1 FW. The reduced glutathione (GSH) content was determined by subtracting GSSG from total glutathione.
2.5 Estimation of MGO Content
Methylglyoxal was estimated as described previously by Ghosh et al. (2014). Approximately 300 mg of seedlings was macerated with liquid nitrogen, homogenized in 2.5 mL perchloric acid (0.5 M), and centrifuged after 5 min at 12,000 g for 10 min at 4°C. The resulting supernatant, mixed with charcoal (10 mg ml−1) for decolorization, was neutralized with 1 M sodium hydrogen phosphate (Na2HPO4). For quantification, a reaction mixture was prepared using 250 μL of 7.2 mM 1,2-di-aminobenzene, 100 μL of 5 M perchloric acid, 10 μL of 100 mM sodium azide, and 650 μL of neutralized supernatant. After incubation at RT for 3 h, the absorbance was taken at 336 nm. A standard curve was plotted using various concentrations of MGO (0, 5, 10, 25, 50, and 100 μM), and the content was expressed as μmol g−1 FW.
2.6 Quantification of Glucose Content
Total sugars were extracted from seedlings following the method of Giannoccaro et al. (2006) and detected using reverse phase high-performance liquid chromatography (HPLC; LC-20 AD Shimadzu). Approximately 100 mg of seedlings were ground in 1 mL of milliQ water in a 1:10 (w/v) ratio for 15 min in a rotospin. After centrifugation at 12,000 g for 10 min at RT, 500 μL of the clear supernatant was mixed with 1.5 mL of 95% acetonitrile for 30 min in the rotospin. The resulting supernatant was collected and evaporated in a dry bath at 95°C. The residue was re-dissolved in 1 mL of milliQ water and filtered through a 0.22-μm filter paper using syringe filters. Different sugars were separated isocratically through reverse phase HPLC using an amino (NH2) column with acetonitrile and water (70:30) as the mobile phase. The flow rate was set to 1 mL min−1, and the absorbance was detected at 190 nm (UV) using a photodiode array (PDA) detector. The glucose peak was identified by spiking with standards, and concentrations were calculated using an external standard calibration method (Sreeharsha et al. 2018).
2.7 Determination of Pyruvate Content
The pyruvate content was quantified by homogenizing 500 mg of seedlings in 1 mL water, which was allowed to stand for 10 min at RT, and centrifuged at 10,000 g for 5 min. To the resulting supernatant, 1 mL of 0.25 g l−1 DNPH in 1 M hydrochloric acid (HCl) was added and placed in a water bath at 37°C for 10 min, and 1 mL of 1.5 M sodium hydroxide (NaOH) was added. Then, 1 mL of 1.5 M NaOH was added, and absorbance was measured at 515 nm. Standards were prepared by adding 25–200 μL of 1 mM sodium pyruvate and reducing the amount of water in the assay accordingly (Anthon and Barrett 2003).
2.8 Quantification of Endogenous Melatonin Content
Seedling samples were weighed, sliced into small (3–5 mm) pieces, and immersed in chloroform vials, followed by overnight shaking at 4°C. The seedling pieces were removed, and the solvent in the vial was evaporated under nitrogen (N2) gas at 4°C. The resulting residue was dissolved in acetonitrile, filtered through a 0.2 μm polyvinylidene difluoride (PVDF) membrane filter, and used for HPLC analysis. A Shimadzu HPLC system equipped with a C18 column (Phenomenex KINETEX, 250 mm × 4.6 mm) was used to determine the melatonin content. The mobile phase used was a 50:50 mixture of water and acetonitrile with a flow rate of 1 mL min−1, and detection was performed at 280 nm using UV. The melatonin levels were quantified using pure melatonin (Sigma-Aldrich) as a standard, following the method described by Arnao and Hernández-Ruiz (2014).
2.9 Determination of Antiglycation Activity
where Fs and Fb represent the fluorescence intensities of the sample and blank, respectively.
2.10 Measurement of the Loss of Cell Membrane Integrity
Evans blue, an anionic molecule, selectively permeates only ruptured plasma membranes. To quantitatively assess dye absorption per unit dry weight of plant samples, the seedlings were immersed in a 0.5% (w/v) Evans blue solution and incubated for 15 min at RT under vacuum. Subsequently, the samples were transferred to a destaining solution and incubated at 60°C for 30 min to 1 h. The absorption of the supernatant was measured at 600 nm using the destaining solution as a blank, and the pellet was dried at 60°C overnight, and the weight was determined. The A600 values were normalized to dry weight (Minina et al. 2013).
2.11 Quantification of ATP
The adenosine triphosphate (ATP) content in the seedlings was measured using the protocol described by Zhang et al. (2020). Approximately 10 mg of leaf tissue was powdered in liquid N2, followed by homogenization in 100 μL of 0.1 M HCl. The homogenate was then combined with 820 μL of buffer solution (1 M citric acid and 1 M di-sodium hydrogen phosphate) along with 80 μL of chloroacetaldehyde. The reaction mixture was incubated for 10 min at 80°C and subsequently centrifuged (12,000 g, 10 min). The resulting supernatant was filtered through 0.25 μm filter paper. The filtrate was then subjected to HPLC analysis, and adenosine 5′-triphosphate disodium salt hydrate (Sigma) was used as the standard.
2.12 Determination of Autophagosomes and Plant Cell Viability
Monodansylcadaverine (MDC) staining was used to detect autophagy following the protocol of Contento et al. (2005). Seedlings were incubated in 0.05 mM MDC (Sigma-Aldrich) in phosphate-buffered saline (PBS) for 10 min, followed by three washes with PBS at RT. Seedlings were imaged using a Leica SP8 laser scanning confocal microscope (NLO 710, Carl Zeiss) with a 4′,6-diamidino-2-phenylindole (DAPI)-specific filter. The excitation and emission wavelengths for MDC were 345 and 455 nm, respectively. Fluorescein diacetate (FDA) staining was used to detect cell viability. The seedlings were stained with 5 μg ml−1 (FDA; Sigma) for 5 min and were washed three times using sterile water (Guan et al. 2019). Fluorescence signals were visualized on a confocal microscope with excitation and emission wavelengths of 488 nm and 525 nm. The quantitative measurement of fluorescence intensity was carried out using ImageJ.
2.13 Total Protein Extraction and Immunoblot Analysis
The seedlings were macerated in liquid N2 and homogenized in a buffer containing 50 mM Tris–HCl (pH -8.0), 150 mM sodium chloride (NaCl), 1 mM phenyl-methanesulfonyl fluoride, and 10 mM iodoacetamide (Chung et al. 2009) and protease inhibitor cocktail. For immunoblot analysis of ATG8, 15% of SDS-PAGE gel was prepared with 6 M urea (Wang et al. 2019) and for MAPK6 (Mitogen Activated Protein Kinase 6) and KIN10 (Snf1-Related Kinase homolog 10), 10% of SDS-PAGE gels were prepared. Following electrophoresis, the protein-containing SDS-PAGE gels were transferred to a nitrocellulose membrane. ATG8 and ATG8-PE levels were determined using an anti-ATG8 antibody (AS14 2769, Agrisera) in 1:500 dilution, anti-MPK6 (AS12 2633, Agrisera) in 1:250, and anti-SnRK1 alpha1 (AS214581, Agrisera) in 1:500. Histone H3 was used as a loading control. Thus, for determining the His-H3 level, anti-histone-H3 (AS10710, Agrisera) was used as primary antibody in 1:2000 dilution. An antirabbit antibody conjugated to horseradish peroxidase (HRP) was used as the secondary antibody.
2.14 Quantification of ABA
The ABA content was quantified using Liquid Chromatography–Mass Spectrometry/Mass Spectrometry (LC–MS/MS). Samples were prepared according to the method outlined by Pan et al. (2010). The analysis was conducted on an Agilent Quadrupole Time of Flight (Q-TOF) LC/MS 6520 series system (Agilent Technologies) equipped with a ZORBAX RX-C18 column (4.6 × 150 mm, 5 μm, Agilent Technologies) at 24°C, as described in the report of Shreya et al. (2022). The mass detection range was set between 100 and 2000 m z−1.
2.15 RNA Isolation, cDNA Synthesis, and Quantitative Real-Time PCR
Total ribonucleic acid (RNA) was isolated using the cetyltrimethylammonium bromide (CTAB)-ammonium acetate method (Zhao et al. 2012). RNA quality was assessed through gel electrophoresis and nanodrop 2000 UV–Vis spectrophotometer (Thermo Scientific). Primers targeted for the genes were synthesized using GenScript1 (Supplementary table 1). Complementary deoxyribonucleic acid (cDNA) was synthesized from the total ribonucleic acid (RNA) using primescript 1st strand synthesis Kit (Takara Bio Inc.), and Real-time polymerase chain reaction (PCR) was performed on mastercycler realplex (Eppendorf). Actin4 was used as an internal control, and the relative fold-change of RNA expression was estimated using the 2−ΔΔCT method (Livak and Schmittgen 2001).
2.16 Statistical Analysis
The data represent the mean values of three independent treatments, with three replicates per treatment in each experiment. These data were subjected to one-way analysis of variance (ANOVA) using SigmaPlot (12.0 version) software. The error bars depicted in the graph represent the standard error (± SE) of the mean values. To evaluate the significance of differences between treatments, Duncan's multiple range test (DMRT) was performed at a significance level (p ≤ 0.05). All the graphs were made using PRISM8, and Figure 9 was done using the BioRender software.
3 Results
3.1 Effect of Melatonin on Seedling Phenotype and RWC Under Drought Stress
The effects of melatonin priming on the growth of the seedlings varied in drought-distinguished varieties depending on the concentrations used. The seeds of L-799 primed with 25 μM melatonin showcased improved growth under PEG-induced drought stress, surpassing other concentrations. This concentration also resulted in the highest radicle length among all the primed control seedlings (Figure 1A and Figure S1A). Contrarily, in Suraj, seeds primed with melatonin at all applied concentrations did not cause distinct phenotypic changes in the seedlings compared to unprimed seedlings under stress (Figure 1A and Figure S1B). Therefore, the 25 μM concentration of melatonin was chosen for comparative studies in both varieties.
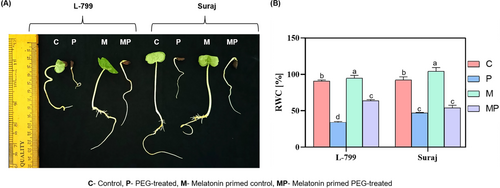
The relative water content (RWC), an indicator of cellular turgor pressure, reflects the plant's water status and capacity to maintain turgor. Melatonin-primed L-799 exhibited significantly higher RWC (63.52%) compared to unprimed seedlings during drought stress. Conversely, Suraj showcased no significant difference in RWC between primed and unprimed seedlings under stress (Figure 1B).
3.2 Influence of Exogenous Melatonin Priming on Endogenous Melatonin Content
Exogenous melatonin priming is reported to increase the endogenous melatonin content in cotton under drought stress (Supriya et al. 2022). In the seedlings of unprimed stressed L-799, endogenous melatonin decreased by 1.11 times compared to the controls, whereas in primed seedlings it increased by 4.87 times compared to unprimed seedlings under stress. Intriguingly, unprimed Suraj seedlings under stress exhibited 2.31 times higher melatonin content than the controls. On the other hand, primed Suraj showed a decrease in melatonin by 1.11 times compared to unprimed seedlings under stress (Figure 2A).

The expression of the melatonin biosynthesis gene serotonin N-acetyl transferase 2 (SNAT2) was downregulated by 17.54-fold in unprimed stressed seedlings compared to controls. However, priming caused significant upregulation (98.59-fold) compared to unprimed seedlings in L-799 under stress. Contrastingly, in Suraj, although unprimed stressed seedlings exhibited significantly enhanced expression of SNAT2 (10.94-fold) compared to controls, the primed seedlings showed a 7.10-fold reduced expression compared to unprimed seedlings under stress conditions. Furthermore, the expression levels of the phytomelatonin receptor 1 (PMTR1) were notably increased in both L-799 (1.91-fold) and Suraj (1.07-fold) in melatonin-primed seedlings compared to respective unprimed seedlings under drought stress (Figure 2B, Figure S2A,B).
3.3 Role of Melatonin on Intracellular ROS Accumulation, Lipid Peroxidation, and Electrolyte Leakage Under Drought Stress
During stress conditions, there was an overaccumulation of O2− and H2O2 in unprimed plants as reflected by intense NBT (Figure 3A) and DAB staining (Figure 3B). However, priming effectively reduced ROS accumulation compared to unprimed plants in both varieties. The intracellular H2O2 decreased by 25.31% and 26.69% in primed seedlings compared to respective unprimed seedlings under stress in L-799 and Suraj varieties, respectively (Figure 3C).
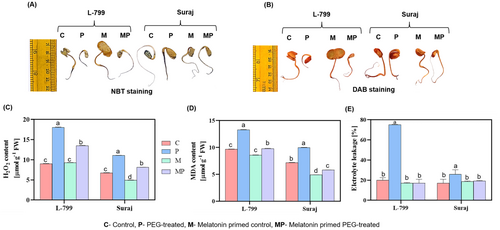
In unprimed stressed seedlings, MDA levels rose by 37.43% in L-799 and 38.38% in Suraj. Priming mitigated this effect, reducing MDA by 26.33% and 41.50%, respectively (Figure 3D). Electrolyte leakage, a marker of membrane damage, was significantly elevated in unprimed stressed seedlings by 3.76 times in L-799 and 1.51 times in Suraj, compared to controls. Melatonin priming reduced EL by 4.41 times in L-799 and 1.3 times in Suraj under stress conditions (Figure 3E).
3.4 Effect of Melatonin on Intracellular MGO Levels
The MGO content was significantly increased (9.39 times) in L-799 unprimed stressed seedlings compared to controls, whereas melatonin priming reduced it by 7.23 times. In Suraj, stress led to a 2.6 times decrease in MGO, and priming caused no notable difference compared to the unprimed conditions (Figure 4A).
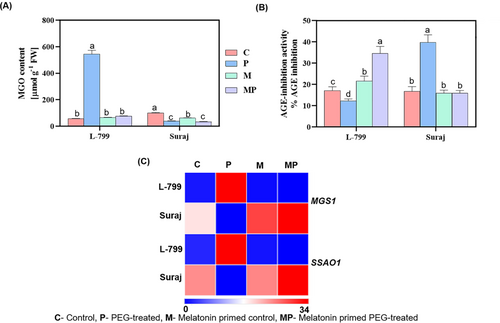
MGO is known to form AGEs in the cell. The AGE inhibition (%) activity decreased 1.4 times in unprimed stressed seedlings, whereas priming elevated the activity by 2.83 times compared to unprimed in L-799 under stress. Interestingly, unprimed stressed Suraj exhibited 2.36 times enhanced inhibition activity compared to controls, whereas priming decreased it 2.48 times compared to unprimed seedlings under stress (Figure 4B).
Methylglyoxal synthase (MGS) and semicarbazide-sensitive amine oxidase (SSAO) genes are involved in MGO biosynthesis. The transcript levels of both MGS1 and SSAO1 increased significantly by 33.89- and 18.20-fold in unprimed stressed seedlings, respectively, compared to controls in L-799. However, priming significantly alleviated the expressions by 178.36- and 67.40-fold compared to the unprimed seedlings under stress conditions. In contrast, unprimed Suraj showed downregulation of MGS1 (2.50-fold) and SSAO1 (2.85-fold), whereas priming increased their expression by 5.12- and 4.08-fold compared to unprimed seedlings under stress, respectively (Figure 4C, Figure S2C,D).
3.5 Effect of Melatonin Priming on Glyoxalase and Nonglyoxalase Systems
Glyoxalase I, II, and III are enzymes involved in the MGO detoxification pathway. The activities of these enzymes decreased by 1.12, 4.64, and 5.03 times in unprimed stressed seedlings compared to controls in L-799. However, priming increased these enzyme activities by 1.70, 6.64, and 22.33 times compared to unprimed seedlings under stress. In Suraj, glyoxalase activities increased 2.58, 1.77, and 2.67 times in unprimed seedlings but declined upon priming by 1.30, 1.47, and 2.59 times under stress, indicating a contrasting response for detoxification (Figure 5A–C). Transcript levels of GLYI-1, GLYII-2, and GLYIII-DJ-1 followed similar trends, being downregulated in unprimed stressed L-799 and upregulated upon priming compared to unprimed seedlings under stress, whereas it was vice versa in Suraj (Figure 5E and Figure S3A–C).

Lactate dehydrogenase (LDH), methylglyoxal dehydrogenase (MGD), and methylglyoxal reductase (MGR) are genes of the nonglyoxalase system involved in MGO scavenging. The expression of these genes was downregulated by 12.50, 25, and 2 folds in unprimed stressed seedlings but was upregulated (84.37-, 77.75-, and 1.80-fold, respectively) in primed seedlings compared to unprimed in L-799 under stress. In contrast, Suraj displayed an upregulated expression of 10.09-, 9.19-, and 1.63-fold in unprimed stress compared to controls, respectively. Although priming upregulated the expression of MGR1 (1.13-fold), the expression of LDH5 (2.29-fold) and MGD1 (3.34-fold) was downregulated significantly compared to unprimed seedlings under stress (Figure 5E and Figure S3D,F).
3.6 Effect of Melatonin on Glutathione Levels
Glutathione serves as a fundamental antioxidant, and the GSH/GSSG ratio plays a crucial role in maintaining the redox homeostasis of plants under stress. Melatonin priming elevated the GSH levels (3.03 times), GSH/GSSG ratio (3.31 times), and total glutathione (1.42 times) in L-799 seedlings compared to unprimed seedlings under stress. Contrarily, Suraj showed no notable changes in these attributes in primed and unprimed seedlings under stress. However, priming reduced GSSG levels slightly by 1.05 times in L-799 and 1.10 times in Suraj under stress (Figure 5D and Figure S4A–C). Furthermore, priming elevated GR1 expression by 241.76-fold in L-799 and 1.16-fold in Suraj, compared to respective unprimed seedlings under stress (Figure 5E and Figure S4D).
3.7 Effect of Melatonin on Glycolysis and Related Enzymes Under Stress
In primed L-799 seedlings, the expression of glycolysis-related genes showed distinct changes, with a 3.46-fold decrease in hexokinase 3 (HK3) and a 2.7-fold increase in triosephosphate isomerase (TPI1) expression compared to unprimed seedlings under stress. Unlike L-799, unprimed Suraj displayed a 1.52-fold decrease in HK3 and a substantial increase (12.11-fold) in the expression of TPI1 compared to primed seedlings under stress. Priming also upregulated the expression of phosphoglycerate kinase 5 (PGK5) and pyruvate kinase 1 (PK1) by 9.68-fold and 31.33-fold in L-799, whereas it downregulated them by 2.98-fold and 1.21-fold, respectively, in Suraj compared to unprimed seedlings under stress conditions (Figure 6A and Figure S5A–D).
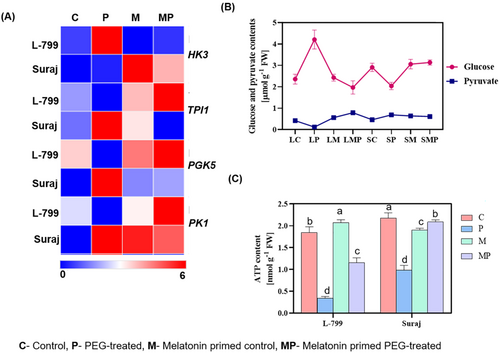
The intracellular glucose content was elevated (1.78 times) in unprimed stressed L-799, whereas it declined by 2.13 times upon priming, suggesting enhanced glycolytic turnover. In Suraj, stress reduced glucose content (1.42 times) and priming also lowered it (1.53 times) compared to the control (Figure 6B, Figure S5E). The content of pyruvate, a key glycolysis product, dropped 3.5 times in unprimed L-799, but priming increased it 6.58 times under stress. However, in Suraj, stress elevated pyruvate levels by 1.50 times compared to control, but priming caused a marginal decline (1.13 times) compared to unprimed seedlings under stress (Figure 6B, Figure S5E,F).
3.8 Effect of Melatonin on ATP Levels
Stress conditions are known to reduce the intracellular ATP content. In this study, stress conditions decreased the intracellular ATP level in unprimed seedlings of both L-799 (5.44 times) and Suraj (2.21 times) compared to respective controls. However, priming improved the level significantly by 3.40 times in L-799 and 2.15 times in Suraj varieties compared to respective unprimed seedlings under stress (Figure 6C).
3.9 Regulation of Autophagy Under Drought Stress by Melatonin
Melatonin is known to regulate autophagy to sustain the growth and survivability of plants under stress conditions. Lesser MDC-stained bodies (autophagosomes) with notably downregulated expressions of ATG8c (3.70-fold), ATG3 (3.70-fold), ATG5 (4-fold), and ATG18a (3.70-fold) in unprimed stressed compared to controls in L-799 indicate reduced autophagosome formation. Priming reversed the situation, as evident from increased autophagosome structure (MDC-stained structure) with upregulated expression of ATG8c (12.55-fold), ATG3 (12.81-fold), ATG5 (9.16-fold), and ATG18a (12.37-fold) in primed seedlings compared to unprimed seedlings under stress. Unlike L-799, the transcript levels of the autophagy-related genes were significantly higher in unprimed stressed seedlings compared to primed seedlings of Suraj. However, no marked difference was observed in MDC-stained bodies in primed and unprimed stressed Suraj seedlings (Figure 7A,B and Figure S6A–D). The immunoblot analysis of ATG8 revealed two distinct bands between 15 KDa and 10 KDa, corresponding to free ATG8 and lipidated ATG8 (ATG8-PE). In L-799, unprimed stressed seedlings showed faint ATG8-PE bands, whereas primed seedlings showed intensified bands. In contrast, Suraj exhibited higher ATG8-PE in unprimed than primed seedlings under stress (Figure 7C). Constitutively stressed 1 (COST1) negative regulator of autophagy showcased downregulated expression by 2.22 and 2.50 folds in unprimed seedlings of both L-799 and Suraj compared to respective controls under stress. Interestingly, priming caused a differential effect on COST1 expression under drought stress in these varieties as the expression decreased (3.75-fold) in L-799 but was elevated (4.94-fold) compared to respective unprimed seedlings (Figure 7B and Figure S7B).
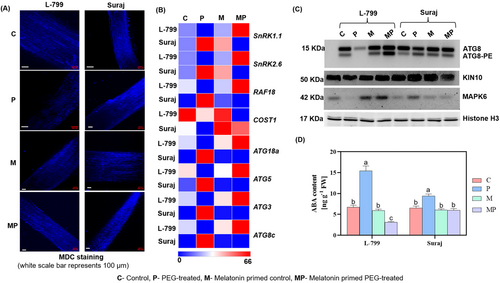
ABA, a known regulator of both drought response and autophagy, increased in unprimed stressed seedlings of both varieties (2.30-fold in L-799, 1.44-fold in Suraj) compared to respective controls. Melatonin priming decreased the intracellular ABA content by 4.92 and 1.57 times compared to unprimed seedlings under stress in both L-799 and Suraj, respectively (Figure 7D).
Sucrose nonfermenting1-related kinase (SnRKs) acts as a positive regulator of autophagy. The expressions of SnRK2.6 (6.60-fold) and SnRK1.1 (6.73-fold) were downregulated, along with the reduced intensity of KIN10 protein in unprimed stressed L-799 compared to controls. Priming enhanced the levels of both transcripts (38.80-fold and 39.46-fold, respectively) and the protein intensity of KIN10 compared to unprimed seedlings of L-799 under stress. The unprimed seedlings of Suraj displayed significantly upregulated expression of SnRK2.6 (4.17-fold), SnRK1.1 (4.17-fold) and an intensified band of KIN10 protein compared to primed seedlings under stress (Figure 7B,C and Figure S6E,F).
MAP kinase (MAPK), another important cellular signaling molecule, can regulate several molecular pathways. Upon priming, L-799 exhibited significantly higher expression of Rapidly Accelerated Fibrosarcoma 18 (RAF18; 196-fold), a B4 MAPK and MAPK6 protein level compared to unprimed seedlings under stress conditions. Whereas Suraj under stress exhibited higher levels of RAF18 transcripts (19.28-fold) and MAPK6 protein in unprimed, as compared to primed seedlings (Figure 7B,C and Figure S7A).
3.10 Role of Melatonin on Cell Viability and Cell Death
Water loss during drought stress, coupled with increased osmotic stress, compromises cell membrane integrity. Under drought stress, primed L-799 and Suraj seedlings displayed lower intensity of Evans blue staining, indicating high cell viability compared to unprimed seedlings (Figure 8A). Genes such as bcl-2-associated athanogene 2 (BAG2), BAG6, metacaspase 9 (MC9), and suppressor of gamma response 1 (SOG1) play pivotal roles in cell death. Under stress, unprimed L-799 seedlings exhibited elevated expression of BAG2 (1185.81-fold), BAG6 (11.37-fold), MC9 (10.38-fold), and SOG1 (66.33-fold) genes compared to the controls. Primed seedlings of L-799 showcased reduced transcript levels, corresponding to BAG2 (919.23-fold), BAG6 (6.38-fold), MC9 (5.16-fold), and SOG1 (7.52-fold), compared to unprimed seedlings under stress. In the Suraj variety, although unprimed stressed seedlings exhibited higher expressions of BAG6 (2.01-fold) and MC9 (1.65-fold) compared to controls, their expressions were lower in comparison to L-799. However, no significant change was observed in BAG2 and SOG1 expression in unprimed and primed seedlings of Suraj under drought stress conditions (Figure 8B and Figure S7C–F).
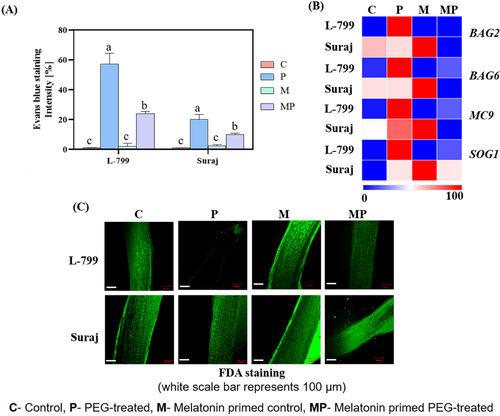
Cell viability, assessed using FDA staining, distinguishes live and dead cells by detecting intercellular esterase activity. In L-799, primed seedlings displayed higher staining (indicating more viable cells) than unprimed seedlings with lower staining (indicating less viable cells) under stress. In Suraj, both primed and unprimed seedlings showed higher staining intensity under stress (Figure 8C).
4 Discussion
MGO is spontaneously produced and overaccumulates during drought and other abiotic stresses and thus poses a threat to cellular health (Askari-Khorasgani and Pessarakli 2019). Several molecules have emerged as priming agents to mitigate the detrimental impact of drought stress (Paparella et al. 2015). Melatonin is one such molecule, which has been reported to improve germination and seedling growth under stress conditions (Ahmad et al. 2021; Awan et al. 2023). In the present study, melatonin priming at 25 μM was effective in improving the seedling phenotypes of L-799 as well as RWC, whereas no significant differences were noticed in primed Suraj as compared to unprimed seedlings under stress (Figure 1B). The differences in endogenous melatonin content in drought-distinguished varieties could be a reason for the differential responses to melatonin priming under PEG-induced drought stress conditions, as suggested by Supriya et al. (2022).
4.1 Melatonin Priming Raised Endogenous Melatonin Levels and Regulated Intracellular ROS and AGEs Under PEG-Induced Drought Stress
Intracellular melatonin plays a crucial role in mitigating the stress-induced detrimental effects in plants (Zhang et al. 2014), whereas exogenous application is reported to improve the endogenous content, especially under drought stress conditions (Chen et al. 2020; Guo et al. 2022; Supriya et al. 2022). The PMTR1-mediated signaling is known to regulate seed germination and seedling growth (Yin et al. 2022), leaf senescence (Bai et al. 2022), stomatal closure, and circadian stomatal rhythm via MAPK (Lee and Back 2016; Yang et al. 2021) under osmotic and drought stress (Wang et al. 2021). In L-799, elevated melatonin levels correlated with upregulated expression of the PMTR1 and SNAT2 genes in primed seedlings compared to unprimed seedlings under stress, suggesting a potential role of exogenous priming in elevating endogenous content that might contribute to improved stress tolerance. Elevated endogenous melatonin content, PMTR1 and SNAT2 expression, coupled with better stress tolerance in unprimed conditions compared to controls in Suraj, can be explained by its inherent drought-tolerant nature. Contrastingly, despite elevated PMTR1, insignificant changes in melatonin content and a decrease in SNAT2 expression were observed in primed Suraj compared to unprimed seedlings under stress. This discrepancy explains that exogenous priming primarily enhances PMTR1 expression, whereas the elevated melatonin content might trigger feedback inhibition of the SNAT gene, thereby regulating endogenous melatonin content in primed Suraj (Figure 2A,B, Figure S2A,B), as reported earlier by Shreya et al. (2022).
Drought disrupts cellular redox potential by elevating the ROS production, ultimately disordering cellular antioxidant harmony, and increasing lipid peroxidation and EL (Cruz de Carvalho 2008). Moreover, drought also disrupts glycolytic flux, leading to the accumulation of triose phosphate intermediates, which are converted to MGO (Hossen et al. 2022). Furthermore, Hussain et al. (2018) and Hasan et al. (2020) reported that drought-induced MGO production triggers ROS accumulation through AGEs formation and can also be another reason for higher production of H2O2, MDA, and EL, leading to increased cellular damage in both varieties under stress. Melatonin attenuates the ROS generation and AGEs formation and thus exhibits antiaging properties (Sehirli et al. 2021). Decreased AGE inhibition potential under drought compared to controls in L-799 signifies the increase in AGEs formation and consequential harmful impact of the stress. However, priming-mediated enhanced AGE inhibition activity in L-799 suggests a protective effect of melatonin against AGEs formation, similar to the result of Takabe et al. (2016). Contrastingly, the higher AGE inhibition activity in unprimed Suraj under stress could be owing to elevated endogenous melatonin content and its inherent tolerant nature. Primed seedlings of both varieties exhibited significantly alleviated ROS accumulation (lesser staining of DAB, NBT, and H2O2 content) and therefore decreased the EL levels and MDA under PEG-induced drought stress conditions, justifying the beneficial effect of melatonin in regulating redox homeostasis (Figures 3A–E and 4B).
4.2 Melatonin Maintained Intracellular MGO Homeostasis by Improving Glyoxalase, the Nonglyoxalase System, the Glutathione Pool, and Glycolysis
The interplay between disrupted redox potential and increased MGO levels creates a vicious cycle of oxidative stress, compromising seed germination and seedling growth under drought conditions. To mitigate the overaccumulation of this potential cytotoxic compound, plants initiate glyoxalase systems to detoxify and convert MGO into less harmful compounds (Talaat and Todorova 2022). Seedlings of unprimed L-799 exhibited significantly higher intracellular MGO with upregulated MGO biosynthesis genes (MGS1 and SSAO1), which correlated with the decreased enzymatic activities (GLY I, II, III) and transcript expressions of GLY I, II, III, MGR1, MGD1, and LDH5, showing the negative effect of drought stress in L-799 (drought-sensitive variety). Interestingly, melatonin priming notably elevated activities (GLY I, II, III) and expressions of GLY I, II, III, MGR1, MGD1, and LDH5 in L-799, suggesting a beneficial effect of melatonin on the glyoxalase system that could have reduced intracellular MGO levels under PEG-induced stress. Nevertheless, the reduced MGO levels, coupled with decreased expression of MGS1 and SSAO1, along with augmented activities and expressions of GLY I, II, and III in unprimed Suraj under stress conditions, suggest its inherent potential to withstand stress (Figures 4A–C and, 5A–E and Figures S2C, D and S3A–F). These observations are consistent with reports showing that melatonin can improve glyoxalase and nonglyoxalase antioxidant systems, potentially enhancing MGO detoxification and cellular homeostasis (Hussain et al. 2018; Kaya et al. 2023).
GSH is a fundamental player in maintaining cellular redox homeostasis by scavenging ROS and MGO under stress (de Bari et al. 2020). Yadav et al. (2005) reported that an increase in MGO levels correlated with a decrease in the GSH levels under stress. In this study, primed seedlings with significantly increased GSH and GSH/GSSG ratio were associated with enhanced GR1 expression compared to unprimed seedlings under drought stress in L-799, supporting a possible role of melatonin in maintaining the GSH pool under stress, consistent with the findings of Kaur and Bhatla (2016). Contrastingly, nonsignificant differences in GSH levels and GR1 expressions between primed and unprimed seedlings of Suraj under drought stress (Figure 5D,E and Figure S4A–D) suggest no additional beneficial effect of melatonin in the tolerant variety as reported by Supriya et al. (2022).
Abiotic stress, particularly drought stress-mediated injuries, results in sugar accumulation (Kaur et al. 2021). Sugar metabolism is directly linked to MGO production, as reported by Borysiuk et al. (2018). Elevated sugar levels enhance glycolysis, resulting in an increased production of MGO. This concomitantly reduces the activity of glycolytic enzymes such as triose TPI and impairs its downstream triphosphate formation, thereby limiting the capacity to manage the increased glucose flux. Melatonin-primed L-799 displayed a significant decrease in the glucose content (2.24 times) along with elevated pyruvate content (6.58 times) compared to unprimed seedlings under stress. Melatonin priming lowered the expression of upstream glycolysis gene HK3 but upregulated TPI1, PGK5, and PK1 compared to unprimed seedlings in L-799 under stress conditions. Conversely, in Suraj, the levels of glucose, pyruvate, and the expression of glycolysis genes exhibited opposite trends compared to L-799 in primed and unprimed seedlings under stress conditions. Thus, it can be suggested that melatonin aids in metabolizing accumulated sugars to pyruvate by enhancing glycolysis, especially downstream genes, thus reducing MGO formation. The increased TPI1 expression levels owing to melatonin priming under stress are supported by Sharma et al. (2012) findings, where increased expression of TPI regulates the swift equilibrium between DHAP and GAP, thereby reducing the toxic levels of MGO (Figure 6A,B and Figure S5A–F).
4.3 Melatonin Priming-Induced MGO Homeosis Was Associated With Enhanced Autophagosome Formation
Autophagy plays a crucial role in plant survival and stress responses (Su et al. 2020). Cui et al. (2018) found that melatonin can ameliorate seed germination by improving energy production and activating a metabolic cascade related to autophagy, protein degradation, and other processes under PEG stress. Higher MDC-stained (autophagosome) bodies correlated with upregulated expression of ATGs protein intensities (ATG8-PE:ATG8) in primed L-799, pointing towards a positive correlation between melatonin priming and autophagy induction under stress conditions, similar to the results of Supriya et al. (2022). However, we recognize that MDC staining is not specific to autophagosomes and that increased ATG8 expression or lipidation alone does not conclusively demonstrate autophagic flux. Therefore, these outcomes should be interpreted as indicative of enhanced autophagy activation but not definitive evidence of enhanced autophagy flux. Cellular energy status and autophagy are interdependent attributes, as energy deprivation incites autophagy activation (Feng et al. 2024) and simultaneously, autophagy also improves cellular energy status by reusing the nonfunctional macromolecules (He 2022). The decreased ATP content in L-799 primed seedlings under stress, compared to controls, is consistent with an energy-deprived state that may trigger autophagy activation. But, the decreased ATP content in unprimed seedlings compared to primed seedlings can be the reason for weak autophagy signal as depicted by lesser autophagosome formation and ATGs expression in L-799, as already reported by Supriya et al. (2024). Lower ATP content correlated with higher autophagosome and ATGs expressions in unprimed Suraj, justifying the incitation of autophagy under energy deprivation conditions. Surprisingly, no change in energy (ATP) content along with lesser expression levels of ATGs in primed stressed seedlings compared to unprimed control plants substantiates the intracellular balanced state of Suraj. Autophagy is known to prevent MGO-induced apoptosis through activation of AMPK (Park et al. 2020). Furthermore, in primed stressed plants of L-799, the increased expression of genes and proteins (SnRK2.6, SnRK1.1, and KIN10) involved in autophagy promotion (Yang et al. 2023), along with the reduced expression of the autophagy-suppressing gene (COST1; Bao et al. 2020), indicates stronger induction of autophagy. Interestingly, higher expression of ATGs and ATG-PE:ATG8, MDC-stained bodies, upregulated SnRK2.6, SnRK1.1, and KIN10 expressions, along with alleviated expressions of COST1 in unprimed Suraj, suggest improved autophagy, which might be owing to higher endogenous melatonin contents. Moreover, the reduced autophagosome formation and lower expression of ATG genes in primed Suraj plants, compared to unprimed ones, under stress indicate that exogenous priming does not further improve stress responses in this drought-tolerant variety (Figures 6C and, 7A–C and Figures S6A–F, S7B).
4.4 MGO Signaling Might Activate Autophagy via MAPK, Independent of ABA
Abscisic acid (ABA) plays dual roles as a negative regulator of seed germination and a positive regulator of autophagy (Sirko et al. 2021). Under drought conditions, increased levels of ABA compared to controls show the negative effect of stress on germination in unprimed L-799. Conversely, melatonin priming mitigated this effect by reducing ABA levels, thereby promoting seedling growth in L-799 compared to unprimed seedlings under stress conditions. Despite melatonin's antagonistic relationship with ABA, it paradoxically induced autophagy, presenting a scientific conundrum. In a recent study, we reported that melatonin-induced autophagy is independent of ABA through the activation of MAPK (Supriya et al. 2024). Notably, previous studies in animals demonstrated that the interaction of AGEs with plasma membrane receptors known as receptors for advanced glycation end-products (RAGEs) activated MAPK cascades in response to defense (Lee et al. 2014; Jeong and Lee 2021). Here, primed seedlings displayed a significant increase in the expression of RAF18 and MAPK6, which correlated with higher transcript levels of SnRK2.6, SnRK1.1, and KIN10 in L-799 compared to unprimed seedlings under stress, suggesting a positive impact of melatonin on autophagy under stress similar to the findings of Supriya et al. (2024). Honig et al. (2012) reported that overexpression of Atg8-interacting proteins (ATI1 and ATI2) stimulated seed germination in the presence of ABA, which explains the elevated levels of ABA along with autophagy in unprimed Suraj under stress. These findings suggest that melatonin may reduce MGO levels under drought and influence stress signaling, potentially contributing to MAPK activation and autophagy, which are beneficial for seed germination (Figure 7B–D, Figures S6E,F and S7A).
4.5 Melatonin Regulated Drought-Induced Cell Death During MGO Homeostasis
MGO can induce both apoptosis and autophagy, but the determining conditions between the two pathways remain unclear (Park et al. 2020). MGO, as a precursor for AGEs formation, induces intracellular damage by increasing ROS levels and mitochondrial damage, leading to apoptosis (Park et al. 2020). In parallel, existing research on animals and humans reports that autophagy plays a crucial role in mitigating MGO-induced apoptosis (Park et al. 2020), which has not yet been addressed in plant systems. Under drought, increased MGO levels with higher cell death and elevated expressions of BAG2, BAG6, MC9, and SOG1 in unprimed seedlings compared to controls in L-799 show that the drought-induced cell death could be a result of elevated intracellular MGO contents. Similarly, significantly downregulated MGO content, with reduced expression of necrosis-related genes and decreased cell death in primed L-799 seedlings under stress, substantiates the beneficial role of melatonin against cell death under drought, further supported by higher autophagosome formation. Interestingly, unprimed Suraj seedlings under stress exhibited elevated expression of BAG6 with higher cell death, although it was lower than L-799, which correlated with increased ROS levels and ATGs expression. This suggests a mechanism in which both cell death and autophagy help in balancing cellular status during stress, highlighting Suraj's inherent drought tolerance. These findings align with studies showing ROS can trigger autophagy (Yoshimoto et al. 2009; Wang et al. 2015) and has a dual role, supporting survival through autophagy at optimal levels but leading to necrotic cell death when produced at excess levels during prolonged exposure (Sadhu et al. 2019). In Suraj, melatonin priming did not significantly alter MGO content or the expression of BAG2 and SOG1; however, it reduced BAG6, MC9 expression, and ROS levels and associated cell death without compromising its inherent tolerant nature (Figure 8 and Figure S7C–F).
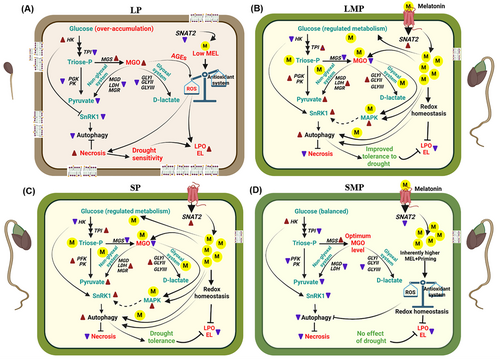
This study contributes toward a better understanding of the role of melatonin in the regulation of drought responses beyond its known antioxidant role. Although previous research has examined MGO detoxification and autophagy independently, our findings suggest a possible connection between melatonin-mediated MGO homeostasis and autophagy activation under drought stress during seed germination. This association may involve MAPK-SnRK1 signaling and could operate independently of ABA, although further experimental validation (e.g., using inhibitors, mutants, or overexpression lines) is required to confirm this regulatory pathway.
5 Conclusion
This study has shed light on melatonin-mediated regulation of intracellular methylglyoxal (MGO) during seed germination under PEG-induced drought stress in drought-sensitive (L-799) and tolerant (Suraj) varieties of upland cotton. Melatonin priming enhanced endogenous melatonin levels and improved glyoxalase and nonglyoxalase systems, thereby reducing MGO accumulation in L-799. Despite decreased ABA content in primed seedlings of L-799 under stress, the melatonin-mediated MGO homeostasis might have activated autophagy by elevating the expression of MAPK6 and other autophagy signaling components (SnRK2 and KIN10). In contrast, Suraj did not showcase significant differences in these attributes between primed and unprimed seedlings under stress, likely owing to higher endogenous melatonin content and its stress-tolerant nature (Figure 9). To our knowledge, this is the first report to suggest that MGO might act as an inducer of autophagy under drought stress in upland cotton. Although these findings provide new insights into the melatonin–MGO–autophagy nexus, further research using transgenic or gene-editing approaches (e.g., overexpression or knockout lines of genes involved in melatonin, methylglyoxal, and autophagy) will be essential to functionally validate these regulatory relationships toward better understanding of the mechanisms underlying plant stress tolerance.
Author Contributions
P.G., D.D., and L.S. contributed to the conceptualization and experimental design. D.D. conducted the experiments, collected the data, prepared the graphs, analyzed and interpreted the data, and wrote the manuscript. L.S. performed a few experiments, interpreted the data, corrected the manuscript, and helped with the pictorial representation of the summary. A.K. performed a few experiments. P.G. analyzed and interpreted the results and corrected the manuscript. All authors contributed to the work and edited the manuscript.
Acknowledgments
We express our grateful thanks to Dr. B. Sree Lakshmi, Principal Scientist and Head, Cotton Section, Regional Agricultural Research Station, Acharya N. G. Ranga Agricultural University (ANGRAU), Guntur, Andhra Pradesh, India, for providing seeds of the L-799 variety, and the Director, ICAR-Central Institute for Cotton Research (CICR), Nagpur, India, for seeds of the Suraj variety used in the research work. We would also like to thank Ayon Chatterjee and Arpan Chatterjee, Department of Biochemistry, University of Hyderabad, for their help in carrying out the experiments involving HPLC and Western Blot.
Conflicts of Interest
The authors declare no conflicts of interest.
Open Research
Data Availability Statement
Data sharing is not applicable to this article as all newly created data is already contained within this article. The data that support the findings of this study are available in the supplementary material of this article.




