Exogenous Quercetin Treatment Provides Insights Into Transcriptional Regulatory Network for Salt Tolerance of Triticum urartu
Lei Han and Xiaohan Wu contributed equally to this study.
ABSTRACT
Quercetin, a well-known antioxidant, plays a crucial role in the response of plants to biotic and abiotic stresses. Triticum urartu is the donor of the A subgenome of common wheat. This study aimed to reveal the mechanism by which quercetin treatment alleviates salt stress in T. urartu. The quercetin treatment resulted in the reduction of Na+ content and enhancement of K+ content in the stressed leaves, while it elevated the Na+ content in the stressed roots. This indicated that under salt stress, quercetin treatment can promote transporting K+ to the leaves and retaining Na+ in the roots. Transcriptome analysis showed that in the roots, quercetin treatment increased the expression level of several genes encoding the rate-limiting enzyme for ethylene biosynthesis, core ethylene signaling proteins, and negative regulators of the core ABA signaling pathway (abscisic acid 8′-hydroxylase and protein phosphatase 2C) under salt stress, revealing that quercetin treatment may induce ethylene signal and suppress ABA signal. We observed that quercetin treatment increased the expression level of several respiration genes in roots under salt stress, including 36 glycolysis genes, 18 mitochondria-related genes, and 4 ATP synthase genes. This displayed that quercetin treatment may enhance the respiration of T. urartu. The enhancement of respiration will provide more energy and carbon sources for salt stress response. Overall, quercetin treatment alleviates the negative effect of salt stress in T. urartu plants via multiple mechanisms, offering potential for improving plant salt tolerance.
1 Introduction
Salinity is one of the most severe environmental factors limiting plant growth, development, and production (Ashraf and Munns 2022; Liu et al. 2022; Mäkelä et al. 1998). The crop plants living in saline soils suffer from osmotic stress, ion imbalance, oxidative damage, nutritional imbalance, and metabolic disruption (Munns and Tester 2008; Yang and Guo 2018). Salt stress-induced reactive oxygen species (ROS) can lead to membrane lipid peroxidation, protein denaturation, carbohydrate oxidation, DNA damage, and decreased enzyme activity (Liang et al. 2024). Quercetin, as a flavonoid compound, facilitates photosynthesis, growth, development, germination, pollen development, and antioxidant activity (Singh et al. 2021). Due to the three-hydroxyl group-based redox-active centers, quercetin exhibits powerful antioxidant activity, which contributes to the tolerance of plants to biotic and abiotic stresses (Agati et al. 2012; Ghiotto et al. 2004; Parvin et al. 2019; Vollmannová et al. 2024). Quercetin also serves as a signaling molecule that plays a key role in abiotic stress tolerance by regulating auxin distribution, which participates in the control of plant organ development under water deficit and salt stress conditions (Naramoto 2017; Peer et al. 2013). Exogenous flavonoid application has been revealed to mitigate abiotic stress in numerous plants. A study by Fu et al. (2022) showed that treatment with naringenin significantly decreased the electrolyte leakage rate and malondialdehyde (MDA) levels in Triticum urartu under salt stress. In Brassica oleracea, the application of liquiritin alleviated salt-induced oxidative stress and reduced levels of MDA and H2O2 (Akram et al. 2022). Yildiztugay et al. (2020) reported that exogenous naringenin treatment could reduce oxidative stress caused by NaCl or PEG via the glutathione–ascorbic acid redox systems in bean plants. Arikan et al. (2023) reported that quercetin and kaempferol can alleviate oxidative damage by enhancing the activity of antioxidant enzymes under arsenic stress. A study on Trigonella corniculata showed that the exogenous application of quercetin can alleviate chromium toxicity by improving photosynthesis and the antioxidant defense system (Aslam et al. 2022). Although previous studies have shown that exogenous flavonoid treatment can alleviate salt-induced injury, no omics analysis has been used to elucidate the mechanisms. Exogenous quercetin treatment can verify the role of quercetin in plant stress tolerance, and transcriptome analysis can reveal the downstream signaling networks mediated by quercetin (as a signaling molecule).
T. urartu (diploid, AA) is the donor of the A-subgenome of common wheat (Triticum aestivum, BBAADD) (Dvořák et al. 1993; Peng et al. 2011). T. urartu exhibits a high photosynthetic rate and strong abiotic resistance, and it is an important gene source for improvements of economic traits and abiotic resistance of common wheat (Peng et al. 2011). Qiao et al. (2019) reported that the “phenylpropanoid biosynthesis” pathway was enriched in the roots of T. urartu under cadmium treatment. A study about the synthetic allotetraploid wheat (SlSlAA) and T. urartu (AA) showed that, compared with the synthetic allotetraploid wheat, T. urartu plants have a clear defect in the synthesis/accumulation of flavonoids (Fu et al. 2022). The above research revealed that phenylpropanoid compounds, especially flavonoids, may play an important role in the abiotic stress tolerance of T. urartu. However, the roles of flavonoids in the response of T. urartu to salt stress are unclear. In this study, exogenous quercetin treatment was applied to reveal the role of quercetin in the salt tolerance of T. urartu. Physiological measurements and transcriptomic analyses were performed to investigate the mechanisms underlying salt tolerance alteration induced by exogenous quercetin treatment in T. urartu plants, which will help to explore the salt tolerance regulatory network mediated by quercetin.
2 Materials and Methods
2.1 Plant Materials and Growth Conditions
T. urartu was used in this study. The seeds used in this study were provided by Professor Bao Liu from Northeast Normal University, China. The seeds were germinated in deionized water at 28°C for 3 days, and then the plants were transplanted into hydroponic boxes in a growth chamber (25°C during the day, 18°C during the night, with a photoperiod of 16-h of light/8-h darkness). The 10-day-old T. urartu plants were subjected to the following four treatments: (1) control (CT): 0 mM NaCl + 0 mg l−1 quercetin (CAS number: 117-39-5), (2) Q: 0 mM NaCl +0.5 mg l−1 quercetin, (3) S: 100 mM NaCl +0 mg l−1 quercetin, and (4) SQ: 100 mM NaCl +0.5 mg l−1 quercetin. All treatment solutions were composed of half-strength Hoagland nutrient solution and 0.005% ethanol. After 7 days of treatment, leaves and roots were collected for physiological measurements and RNA-seq analysis, and the phenotype was recorded by photographing at 14 d after the treatments. There were three biological replicates for each treatment and each tissue, and each biological replicate consisted of five plants.
2.2 Physiological Measurements
2.3 RNA Sequencing and Quantitative Real-Time PCR
For RNA sequencing analysis, the RNA library was prepared with the Hieff NGS Ultima Dual-mode RNA Library Prep Kit (Yeasen). The Agilent 2100 Bioanalyzer was used for quality control of RNA. The cDNA library was then sequenced on an Illumina NovaSeq 6000 sequencing instrument. The RNA-Seq reads were mapped to the T. urartu reference genome (Ling et al. 2018) using HISAT2 (v2.2.1; Kim et al. 2019). The KEGG enrichment was performed with the clusterProfiler package for the DEGs (Wu et al. 2021). Genes were then clustered according to their expression patterns. The clustering results were visualized by the “Mfuzz” package. The weighted correlation network analysis was performed by the “WGCNA” package in R (Langfelder and Horvath 2008). The expression levels of all genes were used for WGCNA. The network was visualized using Cytoscape 3.10.2 (Shannon et al. 2003). Quantitative real-time PCR was performed using an ABI Real-Time PCR System and the specific primers (Table S1). The expression levels of target genes were normalized using the actin gene (Giménez et al. 2011; Ravel et al. 2009).
2.4 Statistical Analysis
A completely randomized experimental design was adopted. The statistical significance of physiological measurements was determined by a Fisher's LSD post hoc test, while the statistical significance of quantitative real-time PCR data was analyzed by Student's t-test. Differential gene expression analysis was performed using the R package DESeq2 (Love et al. 2014). Differentially expressed genes (DEGs) were defined as adjusted p < 0.05 and |log2 fold change| ≥ 1.
3 Results
3.1 Effects of Exogenous Quercetin Treatment on Physiological Parameters of T. urartu Under Salt Stress
The NaCl-only treatment (S treatment) limited the growth of T. urartu plants compared to the control (CT; Figure 1). The SQ treatment showed an improvement in plant growth parameters compared to the S treatment. Compared to the S treatment, SQ treatment significantly enhanced shoot growth, with a 12% increase in shoot length and a 1.21-fold elevation in shoot fresh weight (Figure 1B,C). Compared with the S treatment, SQ treatment enhanced photosynthetic efficiency by 3.45-fold higher in net photosynthetic rate, 2.96-fold in transpiration rate, 19% in intercellular CO2 concentration, and 3.22-fold in stomatal conductance (Figure 1D).
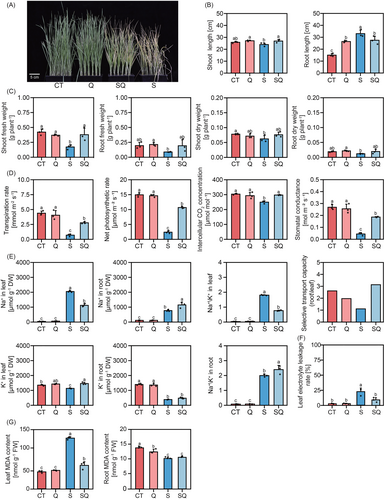
SQ treatment affected ion homeostasis, increasing leaf K+ content by 29% while reducing leaf Na+ content by 45.3% compared with S treatment (Figure 1E). Compared with the S treatment, SQ increased root Na+ content by 49% (Figure 1E). Compared to the S treatment, SQ treatment decreased the Na+/K+ ratio in leaves and increased the Na+/K+ ratio in roots (Figure 1E). Quercetin application enhanced the ST value of stressed plants, indicating an increased ability to transport K+ to the leaves while retaining Na+ in the roots (Figure 1E). Compared to S treatment, SQ treatment reduced leaf MDA content by 53.9% and leaf electrolyte leakage rate by 59.3% (Figure 1G).
3.2 Enrichment Analysis of Differentially Expressed Genes
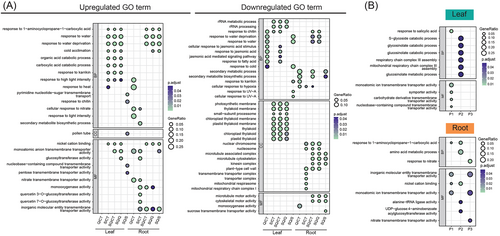
In the leaves, there were 8266 DEGs between CT and S (S/CT), 5647 DEGs between CT and SQ (SQ/CT), and 2271 DEGs between S and SQ (SQ/S; Figure 2A). In the roots, there were 3407 DEGs in S/CT, 6043 DEGs in SQ/CT, and 3142 DEGs in SQ/S (Figure 2B). The KEGG enrichment analyses for DEGs between each treatment were performed. In the leaves, the genes downregulated by SQ compared with S were enriched in “Cytochrome P450” (Figure 2C). Compared to S, the genes upregulated by SQ treatment in the roots were significantly enriched in “butanoate metabolism” and “glycolysis/gluconeogenesis” and the genes downregulated by SQ in the roots were enriched in “phenylpropanoid biosynthesis,” “cysteine and methionine metabolism,” “cytoskeleton proteins,” “DNA replication,” and “cytochrome P450” (Figure 2C). We also performed GO enrichment analysis for DEGs between S and SQ (SQ/S). The upregulated genes in the leaves were enriched in “pyrimidine nucleotide−sugar transmembrane transport” (GO:0090481), “response to chitin” (GO:0010200), “pollen tube” (GO:0090406), “nucleobase−containing compound transmembrane transporter activity” (GO:0015932), and “pentose transmembrane transporter activity” (GO:0015146; Figure 3A), and the upregulated genes in the roots were enriched in “response to 1-aminocyclopropane-1-carboxylic acid” (GO:0009961), “response to water” (GO:0009415), “response to water deprivation” (GO:0009414), “cold acclimation” (GO:0009631), “nickel cation binding” (GO:0016151), “monoatomic anion transmembrane transporter activity” (GO:0008509), and “inorganic molecular entity transmembrane transporter activity” (GO:0015318; Figure 3A).
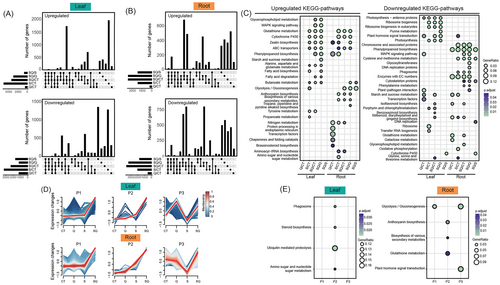
3.3 Gene Expression Response Induced by Quercetin Application
To understand the expression response of T. urartu plants to quercetin treatment, DEGs were clustered into three patterns: Pattern 1 (P1), expression level in SQ > CT or SQ > S, but no significant difference between S and CT treatments; Pattern 2 (P2), the expression levels were higher in S treatment than in CT treatment, while SQ treatment showed higher expression level than S treatment; Pattern 3 (P3), expression level in SQ > S and S < CT. The KEGG enrichment was conducted for the DEGs with the three patterns. The P2 genes in the leaves were enriched in “phagosome,” “steroid biosynthesis,” “ubiquitin mediate proteolysis,” and “amino sugar and nucleotide sugar metabolism” (Figure 2E). In the roots, P1 genes were enriched in “glycolysis/gluconeogenesis” (Figure 2E), P2 genes were enriched in “anthocyanin biosynthesis,” “glutathione metabolism,” and “biosynthesis of various secondary metabolites,” and P3 genes were enriched in “glycolysis/gluconeogenesis pathway” and “plant hormone signal transduction pathway” (Figure 2E). GO enrichment analyses for P1, P2, and P3 were conducted. The top enriched GO terms in the leaves for P1 genes were “response to salicylic acid” (GO:0009751), “monoatomic ion transmembrane transporter activity” (GO:0015075), “symporter activity” (GO:0015293), “carbohydrate derivative transmembrane transporter activity” (GO:1901505), and “nucleobase−containing compound transmembrane transporter activity” (GO:0015932) (Figure 3B). In the roots, P1-P3 genes were mainly enriched in “response to 1−aminocyclopropane−1−carboxylic acid” (GO:0009961), “nickel cation binding” (GO:0016151), “inorganic molecular entity transmembrane transporter activity” (GO:0015318), “monoatomic ion transmembrane transporter activity” (GO:0015075), “amino acid metabolic process” (GO:0006520), and “response to nitrate” (GO:0010167) (Figure 3B).
Many DEGs with P1–P3 patterns in the leaves encode crucial tolerance proteins and signal proteins, including eight peroxidases (PODs), six glutathione S-transferases (GSTs), one high-affinity potassium transporter (HAK), one sodium/hydrogen exchanger (NHX), one salt overly sensitive 1 (SOS1), 26 transcription factors, six Ca2+-sensing proteins (including calcium-binding proteins, caleosin, calmodulin-binding protein, calcium-dependent phosphotriesterase protein, and calcium-dependent lipid−binding protein), 60 kinases and three phosphatases (Figures 4A and 5A). A lot of DEGs with P1-P3 patterns in the roots encode dehydrins (DHNs), late embryogenesis abundant proteins (LEAs), low temperature and salt-responsive proteins, stress-responsive proteins, universal stress proteins, PODs, GSTs, NHXs, salt overly sensitive 3 (SOS3), STELAR K+ outward rectifier (SKOR), plasma membrane H+-ATPase (AHA), HAKs, and aquaporins (Figures 4B and 5B). These genes were associated with osmotic adjustment, Na+ tolerance, and antioxidant system. The genes with P1–P3 patterns in the roots involve transcriptional regulation, signal transduction, nitrate transport and energy metabolism, including 39 transcription factor genes, nine Ca2+-sensing protein genes (calcium-binding protein gene, calcium-dependent lipid-binding protein genes, calcium-binding EF hand family protein genes, and calcium-dependent protein kinase genes), one calcium uptake protein 1 gene, two calcium-transporting ATPase genes, seven kinase genes, and seven nitrate transporter genes (Figure 5B). In the leaves, expression levels of the 4-coumarate: CoA ligase gene (TuG1812G0200001525.01) were higher in SQ treatment than in S treatment (Figure S1). In the roots, compared with S treatment, SQ treatment upregulated the expression of the flavanone 3-hydroxylase gene (TuG1812G0200005402.01) and the flavonol synthase gene (TuG1812G0600003370.01; Figure S1). This suggested that the synthesis of some flavonoids may be influenced by quercetin treatment in salt-treated plants.
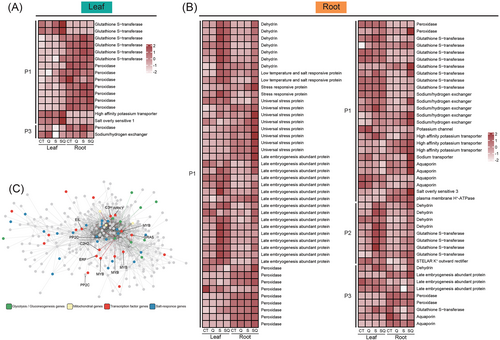
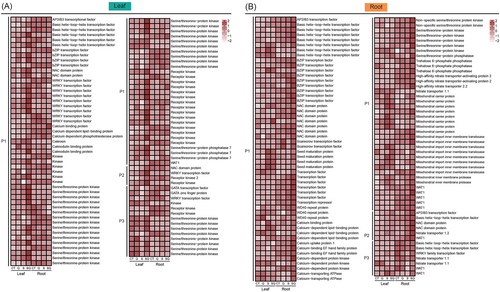
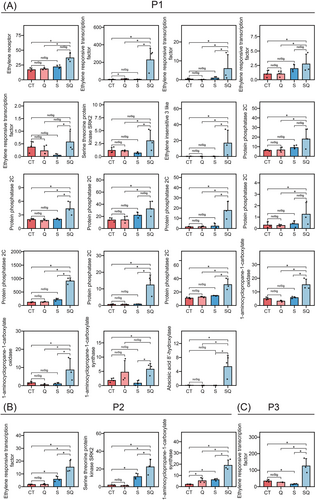
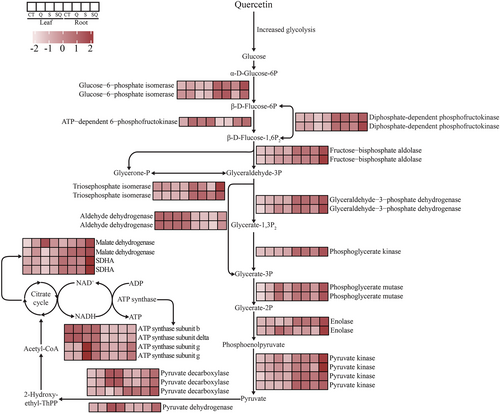
In the leaves, the expression patterns of one copper-transporting ATPase (RAN1) gene, one ethylene receptor (ETR) gene, two ethylene-responsive transcription factor (ERF) genes, and one 5′–3′ exoribonuclease 3 (XRN4) gene were identified as P1 and P3 genes (Figure S2). In addition, in the roots, one ETR, six ERFs, one ethylene insensitive 3 like (EIL3) gene, two 1-aminocyclopropane-1-carboxylate oxidase (ACO) genes, two 1-aminocyclopropane-1-carboxylate synthase (ACS) genes, eight protein phosphatase 2C (PP2C, suppressor of ABA pathway) genes, two serine/threonine protein kinase SRK2 genes, and one abscisic acid 8′-hydroxylase (ABA 8′-hydroxylase, key enzymes in ABA catabolism) gene were clustered in P1–P3 patterns (Figure 6). In the roots, many genes related to respiration were identified as P1 or P3 genes, including 26 genes encoding glycolytic enzymes, four genes involved in tricarboxylic acid cycle (TCA), 18 mitochondrial-related genes, and four ATP synthase genes (Figures 5B and 7). We next performed weighted gene co-expression network analysis (WGCNA). We noticed that the three MYB genes (TuG1812G0300001316.01, TuG1812G0300001315.01, and TuG1812G0500003602.01) targeted many genes associated with respiration and mitochondria (Figure 4C). The earlier results suggested that the expression of respiration genes and mitochondrial genes may be regulated by the MYB genes.
3.4 qRT-PCR Validation of RNA-Seq Data
We randomly selected 13 genes to validate the reliability of the RNA-Seq data. For ten out of 13 genes, fold-change values from RNA-Seq data exhibited similar trends to qRT-PCR data (Table S1), indicating that the results of RNA-Seq were reliable.
4 Discussion
4.1 Effects of Quercetin Application on Ion Transport and ROS Scavenging
Salt stress adversely affects plant growth and development through ion toxicity and ROS stress (Munns 2002). Compartmentation of Na+ and Cl− into the vacuole is effective and economical to resist osmotic stress and ion toxicity (Munns et al. 2016). Under salt stress, Na+ competes with K+ for the binding sites, allowing Na+ to enter the cells of the roots through K+ channels or K+ transporters (Maathuis and Amtmann 1999; Zhang et al. 2018), leading to K+ deficiencies and ion imbalance (Zhu 2003). Therefore, K+ channels or K+ transporters can mediate compartmentation of Na+ among organs or tissues. HKT1;5, the SOS1-SOS2-SOS3 pathway, NHX, and AHA play critical roles in Na+ compartmentation among tissues or within cells (Kotula et al. 2020). The SOS1-SOS2-SOS3 pathway plays a role in Na+ efflux from root cells into the rhizosphere or retraction from shoots to roots (Qiu et al. 2002; Quintero et al. 2002, 2011; Wang et al. 2023). NHX is responsible for compartmentation of Na+ into the vacuole. The NHX and SOS1 are driven by an inwardly directed proton gradient, which is generated by vacuolar H+-ATPase and AHA (Brini and Masmoudi 2012; Hualpa-Ramirez et al. 2024). HKT1;5 mediates the retraction of Na+ from shoots to roots (Ali et al. 2021; Assaha et al. 2017; Kobayashi et al. 2017; Matsushita and Matoh 1991).
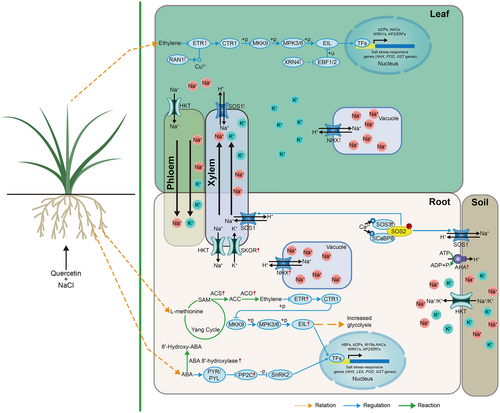
In the present study, we observed exogenous quercetin treatment enhanced the concentration of Na+ in the roots of salt-stressed T. urartu plants, but it reduced Na+ concentration in the leaves (Figure 1). The transcriptome results showed that exogenous quercetin increased the expression level of NHX and SOS1 in the leaves of salt-stressed T. urartu, and it also upregulated the expression of NHX genes, SOS3 genes, SKOR gene, and AHA gene in the roots of salt-stressed T. urartu (Figures 4 and 8). Results of Na+ and K+ suggested that exogenous quercetin treatment may promote the compartmentalization of Na+ among organs and facilitate the recirculation of Na+ from the shoots to the roots in T. urartu plants, enabling the transport of K+ to the leaves and the retention of Na+ in the roots and maintaining normal leaf functions. Our results are consistent with findings in rice (Mekawy et al. 2018) and tomato (Ekinci et al. 2024). Under salt stress, pre-treatment of apigenin to rice seeds enhanced the expression of the rice root OsSOS1 gene and reduced the Na+/K+ ratio in the sheath and leaves (Mekawy et al. 2018). In tomato plants, exogenous chrysin application alleviated salt stress damage through upregulating HKT1-1, HKT1-2, and PIP1 and enhancing the activity of antioxidant enzymes (Ekinci et al. 2024). In T. urartu plants, we also found that exogenous quercetin treatment significantly upregulated the expression of many antioxidant enzyme genes (e.g., PODs and GSTs) under salt stress (Figures 4 and 8), suggesting that quercetin as a nonenzymatic ROS scavenger can induce the upregulation of antioxidant enzyme genes. Quercetin may serve as a signaling molecule to mediate the function of salt stress response genes, at transcription, post-translational modification, and protein–protein interaction levels, which may be an important link of the gene expression regulation network of plant salt tolerance.
4.2 Gene Expression Induced by Quercetin
Flavonoids can act as signaling molecules or regulate the activity of other signaling components. For example, Schulz et al. (2015) and An et al. (2024) reported that flavonoids interact with transcription factors like MYB, bHLH, WRKY, and bZIP to activate cold-response genes. Shen et al. (2019) found that the production of anthocyanins or proanthocyanidins enhances tolerance to wounding and oxidative stress by the ABA pathway in roses and tobacco. In addition, flavonols can mediate the ABA signaling pathway via scavenging H2O2 (Brunetti et al. 2018). Compared to the CT or S treatment, the SQ treatment increased the expression levels of many transcriptional regulatory genes, such as AP2, bHLH, bZIP, NAC, WRKY, MYB, ERF, and WD40 (Figures 5, 6, 8 and S2). These transcription factors may be regulated by quercetin to respond to salt stress in T. urartu plants. ERF has been reported to regulate ROS production and ion balance in salt-stressed plants (Chen et al. 2024; Rong et al. 2014). Additionally, in T. urartu plants, we also found that exogenous quercetin treatment significantly affected the expression of key genes involved in flavonoid synthesis (Figure S1), indicating the presence of positive feedback or negative feedback regulation for flavonoid synthesis.
Flavonols exert diverse roles in auxin and ABA signaling (Taylor and Grotewold 2005). Quercetin can redirect PINFORMED (PIN) auxin-efflux facilitator proteins to mediate plant growth (Daryanavard et al. 2023). In the present study, exogenous quercetin treatment enhanced the expression level of several WAT1 genes (regulator of auxin transport) in salt-stressed T. urartu roots, indicating that quercetin treatment might mediate auxin transport of T. urartu plants to respond to salt stress (Figure 5B). Previous studies showed that PP2Cs and ABA 8′-hydroxylase (responsible for ABA degradation) act as negative regulators for the core ABA signaling pathway (De Zélicourt et al. 2016). In the present study, eight PP2Cs genes and one ABA 8′-hydroxylase gene were upregulated by exogenous quercetin treatment in the salt-stressed T. urartu roots, indicating that quercetin may negatively mediate the ABA signaling pathway via upregulation of the PP2C and ABA 8′-hydroxylase genes in T. urartu plants (Figure 6). Ekinci et al. (2024) showed that exogenous application of chrysin reduced the content of ABA under salt stress. This suggests that flavonoids may inhibit the accumulation of abscisic acid. Ethylene positively regulated the levels of flavonols in guard cells to moderate stomatal aperture (Watkins et al. 2014). It has been reported that ethylene strongly influences ABA signaling pathways through enhancing biosynthesis of flavonols to suppress ROS accumulation in tobacco (Song et al. 2023). In addition, ethylene mediates Na+ tolerance and ROS scavenging of plants by upregulating gene expression of POD, GST, and HKT (Jung et al. 2009; Mehlhorn 1990; Shu et al. 2022; Zhang et al. 2022). Our results exhibited that exogenous quercetin treatment upregulated the expression level of one RAN1 gene, one ETR gene, two ERF genes, and one XRN4 gene in salt-stressed T. urartu leaves and enhanced the expression levels of four key ethylene biosynthesis genes (two ACO genes and two ACS genes), one ETR gene, and six ERF genes in salt-stressed T. urartu roots (Figure 6). Our data revealed that quercetin may influence ethylene synthesis and signal transduction in salt-stressed T. urartu plants, and that it may interact with ABA, ethylene, and auxin to construct a gene expression regulation network to respond to salt stress (Figure 8).
In this study, we found that quercetin treatment enhanced the expression level of many respiration genes in the roots of T. urartu plants under salt stress, including 26 glycolysis-related genes, 18 mitochondria-related genes, four genes involved in the TCA cycle, and four ATP synthase genes (Figure 7). This suggests that quercetin treatment may regulate the respiration of T. urartu plants under salt stress, thereby affecting the allocation of energy between stress response and general metabolism (Figure 8). The enhanced glycolysis process plays positive roles in plant salt tolerance, and it can provide more energy and carbon sources for the synthesis of salt stress response solutes and proteins (Li et al. 2022; Lu et al. 2021). ABA inhibits glycolysis/gluconeogenesis, and ethylene promotes glycolysis and the TCA cycle (Kai-Jie et al. 2022; Mohorović et al. 2023; Song et al. 2024). We propose that quercetin treatment enhances respiration activity through crosstalk with plant hormones to provide more energy and carbon sources to resist salt stress in T. urartu plants.
Author Contributions
C.Y. and L.H. conceived and designed the study, supervised all experiments, and contributed to data interpretation and manuscript drafting. X.W., J.G., T.Z., X.O., H.W., Y.M., and S.J. performed experimental procedures, conducted data curation, and participated in initial manuscript preparation. Z.C., Y.H., J.J., L.W., and B.X. assisted in experimental execution and data collection. All authors reviewed and approved the final manuscript.
Acknowledgments
We kindly appreciate Professor Bao Liu from Northeast Normal University for providing the T. urartu seeds.
Open Research
Data Availability Statement
All raw RNA sequencing data has been uploaded to NCBI (Accession number PRJNA972256). The dataset can be obtained from the corresponding author upon reasonable request.




