Regulatory role of ABA-responsive element binding factors in plant abiotic stress response
Abstract
As sessile organisms, plants are inevitably threatened by various abiotic stresses. Abiotic stresses seriously affect plant growth and development and crop yield. Plants have evolved complex regulatory networks to resist stresses that occur during their life cycle. The plant hormone abscisic acid (ABA) is accumulated under osmotic stress conditions such as drought, salt, and others. The ABA signaling pathway plays a key role in plant response to abiotic stresses, in which ABA-responsive element binding factors (ABFs) play a crucial role in the whole process. ABFs are a class of basic leucine zipper proteins (bZIPs) that specifically recognize ABA response elements and belong to the a subfamily of the bZIP family. The discoveries of ABFs-mediated tolerance to multiple abiotic stresses, including salinity, drought, cold, and heat stress are highlighted and discussed in this review. The aim of this review is to further analyze the mechanisms of abiotic stress regulation mediated by ABFs and to lay the foundation for breeding superior stress-resistant crop varieties.
1 INTRODUCTION
Plants are often affected by various environmental stresses due to the changes in the global climate in recent years. During the long-term evolutionary process, plants have developed complex regulatory networks to cope with these adverse circumstances. (Junaid et al., 2024). Abscisic acid (ABA) is a plant hormone discovered and identified in the 1960s that plays an important role in plant growth and their response to stress (Vishwakarma et al., 2017). The ABA-dependent and ABA-independent pathways of gene expression play a vital role in regulating transcription under abiotic stresses (Hussain et al., 2021). Transcription factors have a key function in gene expression by selectively binding to cis-acting elements in the promoters of target genes and controlling the expression of downstream genes as trans-acting elements (Asayama 2006).
ABA-responsive elements binding factors (ABFs) are a class of basic leucine zipper that specifically recognize abscisic acid responsive elements (ABREs) and belong to the subfamily A of the bZIP family (Lindemose et al., 2013). ABFs are alternatively referred to as ABA-responsive element-binding proteins (AREBs). The main members of the ABFs in the model plant Arabidopsis thaliana are ABF1, AREB1/ABF2, AREB2/ABF4, and ABF3 (Pan et al., 2023). These family members are mainly expressed in vegetative tissues. In Arabidopsis, ABF1 was significantly induced by low-temperature stress, and AREB1/ABF2, AREB2/ABF4, and ABF3 were induced by osmotic stresses such as dehydration, and high salt, as well as by the phytohormone ABA. (Fujita et al., 2013). Along with the extensive study of ABFs in Arabidopsis, they have also been identified and studied in other plants such as rapeseed (Brassica napus), carrot (Daucus carota), cotton (Gossypium hirsutum), and alfalfa (Medicago sativa). The ABFs/AREBs play an important role in adaptive responses to various abiotic stresses (Kim 2006). In this review, we will highlight recent advances in ABF-mediated tolerance to a variety of abiotic stresses including salt, drought, cold, and heat stress.
2 STRUCTURAL AND CIS-ACTING ELEMENTS OF ABF TRANSCRIPTION FACTORS
Typical members of the ABFs/AREBs family have five highly conserved structural motifs, C1, C2, C3, and a bZIP region at the N-terminal end of the amino acid sequence that can bind DNA sequences, and a C4 structural domain at the C-terminal end, respectively (Noguero et al., 2013). These conserved structural domains are enriched in the R-X-X-S/T sequence (Figure 1), which can be phosphorylated by calcium-dependent protein kinase (CDPK) and sucrose non-fermenting related protein kinase 2 (SnRK2) to activate the transcriptional activity of ABFs (Roychoudhury and Banerjee. 2017). ABFs are activated by phosphorylation, which in turn binds to ABRE elements in the promoter regions of downstream genes to regulate downstream stress-responsive metabolic pathways (Ma et al., 2024).

ABA-responsive elements (ABREs) are the major cis-acting elements involved in regulating ABA-responsive gene expression (Cai et al., 2024). These elements are essential for the plant's ability to respond to abiotic stress, including drought, salt, and other environmental factors (Wang et al., 2025). The core sequence of ABRE, PyACGTGGC, is specifically recognized by ABF (ABA response element binding) transcription factors, with the core motif ACGT being highly conserved (Meng et al., 2024). This recognition was first discovered in the wheat Em gene and the rice RAB16 gene (Azad et al., 2024).
ABREs share sequence similarities with other cis-acting elements, notably the G-box (CACGTGGC), which is found in the promoters of genes regulated by various environmental signals (Hua et al., 2024). ABREs that contain a G-box-like sequence are referred to as G-ABREs (RoyChoudhury et al., 2008). These elements are predominantly found in gene promoters induced by environmental stress factors such as drought and salt (Hussain et al., 2021). The wheat Em gene, for example, contains the G-ABRE motif (Em1a: GGACACGTGGC), which is involved in its ABA and stress-induced expression (Wang et al., 2002). G-ABREs play a crucial role in the regulation of stress-responsive genes by ABF transcription factors. Interestingly, while ABFs primarily bind to ABREs, the synergy between C-ABREs and G-ABREs has been well-documented. Studies have shown that these elements can be recognized together by ABFs, forming a composite regulatory module. Such as HVA22 and HVA1 in barley and Rab16B in rice, ABREs and C-ABREs are separated by less than 20 base pairs and can cooperatively form an ABA response complex (ABRC), highlighting the complex and coordinated regulatory mechanisms underlying ABA-responsive gene expression (Busk and Pagès, 1998).
The determination of the core sequence is of utmost importance during the process of ABFs recognizing and binding to cis - elements (Zhang et al., 2024). For instance, the substitution of the A in the ACGT core sequence with a G (CGCGTG) results in the loss of ABF binding activity, highlighting the specificity of the ABF-ABRE interaction (Tang et al., 2024). This specificity is essential for the precise regulation of ABA-dependent gene expression, activating only those genes necessary for stress tolerance.
ABF transcription factors regulate plant stress responses through interactions with multiple cis-acting elements beyond canonical ABREs (ABA-responsive elements; Bulgakov et al., 2019). While a single ABRE is often insufficient to activate ABA-dependent transcription, most ABA-responsive genes harbor clustered ABREs that function cooperatively as ABA-responsive complexes (ABRCs). For example, wheat (Triticum aestivum) HVA1 and HVA22 promoters contain paired ABREs coupled with CE1 or CE3 motifs, forming ABRCs essential for drought-inducible expression (Shen et al., 1996). Similarly, Arabidopsis RD29B requires dual ABREs for ABA-triggered activation in seeds and vegetative tissues, underscoring the necessity of multi-element synergy (Rehman et al., 2021).
CE3-like motifs enhance transcriptional activation by coordinating with ABREs (Sato et al., 2016). In barley, the proximity of the CE3 motif to ABREs in the HVA1 promoter facilitates chromatin looping, enabling ABF transcription factors to recruit cofactors such as MYB/WRKY. This coordination integrates both ABA-dependent and ABA-independent signals, including osmotic stress, through composite regulatory modules (Xiong et al., 2025). Moreover, ABFs bind both ABRE and dehydration-responsive element (DRE) / C-repeat (CRT) motifs, enabling cross-talk with dehydration-responsive element-bindings protein (DREB) / C - repeat binding factor (CBF) pathways to regulate responses under combinatorial stresses (Dutta et al., 2024).
Evolutionarily, the ABRC architecture is conserved across plant species, with functional diversification in motif organization. Both monocots (e.g., Triticum aestivum) and dicots (e.g., Arabidopsis thaliana) exhibit a conserved spacing of 10–20 bp between ABREs and coupling elements (CEs; Islam et al., 2021). This spatial constraint is critical for cooperative transcription factor (TF) recruitment and enhancer looping (Clarisse et al., 2024). CE3-ABRE modules are preferentially recruited to activate drought-responsive genes, playing a central role in ABA-dependent osmotic stress adaptation. These multi-element clusters outperform single ABRE systems, enabling rapid transcriptional reconfiguration under varying stress conditions by synchronizing the activation of osmotic regulators and the reactive oxygen species (ROS) detoxification enzymes. The evolutionary conservation of ABRC architecture thus optimizes resource allocation, allowing plants to respond efficiently to dynamic abiotic stress conditions.
3 THE ABA-DEPENDENT SIGNALING PATHWAY MEDIATED BY ABFS
Progress in understanding ABA receptors and signal transduction has recently been made (Zhang, Mao, et al., 2022). It has been shown that the key regulatory mechanism of the abscisic acid signaling pathway lies in the interaction of protein phosphatase 2C (PP2C) which is a core component protein in the ABA signaling pathway, together with sucrose non-fermentation-associated protein kinase 2 (SnRK2; Hasan et al., 2022). The regulatory pattern of the ABA signaling pathway is that ABA binding to its receptor (PYRABACTIN RESISTANCE PROTEINS/PYR-LIKE PR-OTEINS/REGULATORY COMPONENTS; PYR/PYL/RCAR) inhibits the activity of the negative regulator PP2C, decreases PP2C inhibition of SnRK2 kinase, thereby activating SnRK2 inhibition of ABFs/AREBs (Tailor et al., 2023). The expression of abscisic acid-inducible genes was up-regulated by direct phosphorylation of ABFs/AREBs by SnRK2 (Puranik and Prasad, 2013). ABFs can bind to the promoters of some PP2C and mediate their rapid expression, forming a negative feedback regulatory system that tightly regulates the ABA signaling pathway under abiotic stress (Jung et al., 2020). After describing the role of ABFs/AREBs in regulating ABA-dependent gene expression, we present a comprehensive model summarizing the key components and interactions of this pathway (Figure 2).
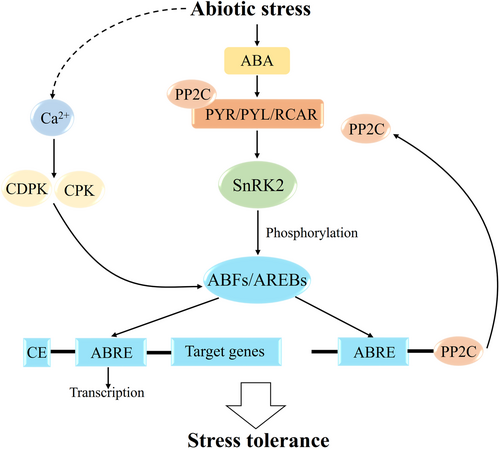
The phosphorylation recognition motifs (RXXS/T) of ABFs/AREBs are commonly found at sites that are phosphorylated by Ser/Thr protein kinases, such as SnRK2 and calcium-dependent protein kinases (CPKs; Kagaya et al., 2002; Furihata et al., 2006). AtCPK32 has been proven to physically bind to AtABF4 (Kilburn et al., 2023). AtCPK12 phosphorylates both ABF1 and ABF4 in vitro (Zhang, Mao, et al., 2022). Likewise, AtCPK6 phosphorylates and interacts with AtABF3 to increase its transcriptional activity (Valmonte-Cortes et al., 2025). StCDPK2 interacts with and is able to phosphorylate StABF1 in a calcium-dependent manner in vitro (Grandellis et al., 2016). Similarly, group A PP2Cs interact directly with phosphorylation-specific AtABFs and AtCPK11 and can be dephosphorylated, thus providing a mechanism for restoring homeostasis in vivo through inactivation and possible destabilization of ABF/AREB (Lynch et al., 2012). Recently, it has been shown that SnRK2 kinases phosphorylate and activate ABFs (Furihata et al., 2006; Fujii et al., 2007; Sirichandra et al., 2010; Kline et al., 2010). In Arabidopsis thaliana, four AREB/ABFs, AREB1/ABF2, AREB2/ABF4, ABF3 and ABF1, have been shown to act downstream of the kinases SnRK2.2, SnRK2.6 and SnRK2.3 (Liu et al., 2019). It remains to be further verified whether these four ABFs are activated by phosphorylation (Wang et al., 2019).
4 INTERACTIONS BETWEEN ABFS AND OTHER TFS
Evidence for interactions between the ABFs and CBFs/DREBs have been reported (Figure 3). Arabidopsis AREB1, AREB2 and ABF3, were shown to interact with DREB1A, DREB2A and DREB2C (Kim et al., 2011). Many abiotic stress inducible genes contain the DRE/CRT as well as ABRE in their promoter regions (Agarwal et al., 2017). The DRE/CRT sequence in the promoter of the AtRD29A gene can function as the CE of ABRE (Krishna et al., 2022). AtAREB1, AtAREB2, AtAREB3 can bind to and activate the AtDREB2A promoter in an ABA-dependent pathway. Moreover, they can affect the response of AtDREB2A to osmotic stress through ABA-dependent and ABA-independent pathway (Chen et al., 2022). New insights have recently emerged suggesting that ABA- and CBF-independent pathways do not respond independently to cold temperatures (Wang et al., 2024). Some COR genes such as COR15A, COR47, RD29A, and RD22 contain both DRE and ABRE in their promoters (Sun et al., 2021). And there are 2052 genes in Arabidopsis that have the two cis-elements (Mishra et al., 2014), indicating that they may be co-regulated by the ABFs/AREBs and CBFs/DREBs in response to stress (Figure 4).
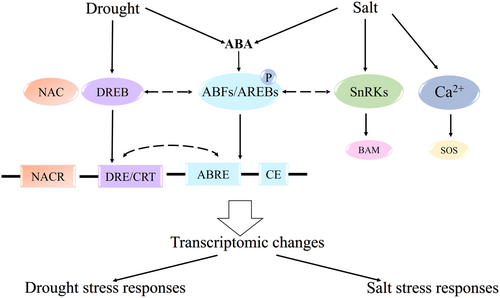
It was revealed that ABFs can interact with NACs, and ABFs expression is controlled by NACs (Figure 3). AtNAC016 repress AtABF2/AtAREB1 expression under drought (Sakuraba et al., 2015). Similarly, AtNAP functions as a negative regulator in osmotic stress by inhibiting AtABF2/AtAREB1 (Seok et al., 2017). ANAC096 of Arabidopsis contains both an ABRE and DRE binding domain, and can interact with AtABF2 to synergistically activate AtRd29A expression (Jia et al., 2022). Likewise, ANAC072, which has high homology to ANAC096, interacts with AtABF3 to enhance AtRD29A expression and reduces AtRD29B activity (Jia et al., 2022).
Recently, there has been a continual discovery of the interactions between ABFs and other transcription factors (Linden et al., 2021). In Arabidopsis, ABF2/AREB1 was found to be engaged in ABA-GA crosstalk to ensure an efficient response to drought stress (Wang et al., 2020). However, more studies are needed to understand the interactions and mechanisms of action of ABFs with other transcription factors.
5 ABF-MEDIATED RESPONSES TO ENVIRONMENTAL STRESSES
The ABF transcription factors are important regulators of plant response to stress, which activate the expression of downstream stress response genes by binding to ABA response element (ABRE; Hossain et al., 2010). ABFs regulate stress tolerance through osmoregulation, antioxidant response, gene activation, and interaction with other regulatory proteins (Table 1), which are largely dependent on signal transduction of the plant hormone abscisic acid (ABA). ABA is not only the main signaling molecule of ABF activation, but also plays an important role in the stress response of plants through multiple signaling pathways (Kumar et al., 2019).
| Name | Source | Function | References |
|---|---|---|---|
| ABF2D/GhABF2D | Gossypium hirsutum | Tolerance to drought. | Kerr et al., 2018 |
| Reduced photosynthetic rate and slowed gr-owth. | |||
| AtABF1 | Arabidopsis thaliana | Tolerance to drought. | Yoshida et al., 2015 |
| Barbosa et al., 2013 | |||
| AtABF3 | Arabidopsis thaliana | Tolerance to drought and low temperature. | Oh et al., 2005 |
| Increased stomatal closure and reduced water loss. | Vanjildorj et al., 2005 | ||
| Yoshida et al., 2010 | |||
| Choi et al., 2013 | |||
| Wang et al., 2016 | |||
| Nam et al., 2019 | |||
| Kerr et al., 2018 | |||
| AtABF4 | Arabidopsis thaliana | Tolerance to drought. | Lin et al., 2020 |
| Reduced transpiration under stress. | |||
| AtABP9 | Arabidopsis thaliana | Tolerance to heat and drought. | Zhang et al., 2008 |
| AtAREB1/AtABF2 | Arabidopsis thaliana | Tolerance to drought. | Fujita et al., 2015 |
| Roca Paixão et al., 2019 | |||
| Lin et al., 2020 | |||
| Wang et al., 2020 | |||
| de Melo et al., 2020 | |||
| AtAREB2/AtABF4 | Arabidopsis thaliana | Tolerance to drought and salt. | Yoshida et al., 2010 |
| Lin et al., 2020 | |||
| Pan et al., 2020 | |||
| Muñiz García et al., 2018 | |||
| AtDPBF3 | Arabidopsis thaliana | Tolerance to drought. | Qian et al., 2019 |
| Interacted with AtADF5 to regulate stomatal opening and closing. | |||
| BnaABF2 | Brassica napus | Tolerance to drought and salt. | Zhao et al., 2016 |
| Reduced water deficit rate and regulated stress response genes. | |||
| CbABF1 | Chorispora bungeana | Tolerance to freezing and drought. | Yue et al., 2019 |
| CmABF1 | Cucumis melo | Tolerance to low temperature. | Li et al., 2022 |
| CmABF3 | Cucumis melo | Tolerance to drought. | Xu et al., 2020 |
| Activated the expression of ABA-responsive genes | |||
| DcABF3 | Daucus carota | Activated expression of genes involved in stomata development. | Wang et al., 2021 |
| Increased the number of stomata. | |||
| GhABF2 | Gossypium hirsutum | Tolerance to drought and salt. | Liang et al., 2016 |
| Regulated genes related to ABA, drought, and salt response. | |||
| GhABF3 | Gossypium hirsutum | Tolerance to drought and salt. | Zhang et al., 2022 |
| GmbZIP1 | Glycine max | Tolerance to drought, salt and low temperature. | Gao et al., 2011 |
| IbABF4 | Ipomoea batatas | Tolerance to drought, salt and oxidative stresses. | Wang et al., 2019 |
| Under stress photosynthetic efficiency was high and endogenous ABA and ROS content was low. | |||
| Induction of LEA gene expression under stress. | |||
| Seed germination was little affected by salt and osmotic stress. | |||
| MdABF1 | Malus×domestica | Tolerance to drought. | Rui et al., 2022 |
| MeABFs | Manihot esculenta | Keeps cells in osmotic balance under dehydration stress | Feng et al., 2019 |
| OsABF1 | Oryza sativa | Tolerance to drought and salt. Activated stress response genes. | Hossain et al., 2010 |
| OsABF2 | Oryza sativa | Tolerance to drought and salt. | Hossain et al., 2010 |
| OsAREB1 | Oryza sativa | Tolerance to drought and heat. | Jin et al., 2010 |
| OSBZ8 | Oryza sativa | Response to salt stress. | Mukherjee et al., 2006 |
| Regulated ABA-mediated transcription under salt. | Paul et al., 2017 | ||
| Basu and Roychoudhury 2014 | |||
| Gupta et al., 2012 | |||
| OsbZIP16 | Oryza sativa | Tolerance to drought. | Chen et al., 2012 |
| Pandey et al., 2018 | |||
| OsbZIP23 | Oryza sativa | Tolerance to drought and salt. | Xiang et al., 2008 |
| OsbZIP46 | Oryza sativa | Tolerance to drought, salt and oxidative stress. | Tang et al., 2012 |
| Involved in ABA-dependent regulation of stress response genes including WRKY, auxin response, and regulation of seed germination under ABA. | Tang et al., 2016 | ||
| OsbZIP72 | Oryza sativa | Tolerance to drought. Regulation of seed germination under ABA, activation of chlorophyll catabolism genes. | Lu et al., 2009 |
| PeABF3 | Populus tomentosa | Tolerance to drought. | Yang et al., 2020 |
| Interaction with PeADF5 to induce stomatal closure. | |||
| PtrABF | Poncirus trifoliata | Tolerance to drought. Interaction between PtrABF and PtICE1 resulted in reduced stomatal density and stomatal index. | Zhang et al., 2015 |
| PtrABF4 | Poncirus trifoliata | Tolerance to drought. Involvement in PtrBAM3-mediated amylolytic pathway. | Zhang et al., 2023 |
| PtrABR1 | Poncirus trifoliata | Tolerance to drought. Involvement in PtrBAM3-mediated amylolytic pathway. | Zhang et al., 2023 |
| PtrAREB1-2 | Poncirus trifoliata | Tolerance to drought. | Li et al., 2019 |
| RhABF2 | Rosa hybrida | Interaction with RhFer1 enhances dehydration tolerance. | Liu et al., 2017 |
| SlAREB1 | Solanum lycopersicum | Tolerance to drought and salt. | Orellana et al., 2010 |
| StABF1 | Solanum tuberosum | Induced by low temperature. | Muñiz García et al., 2012 |
| TaABP1 | Triticum aestivum | Tolerance to drought. | Cao et al., 2012 |
| Increased water use efficiency. | |||
| TaAREB3 | Triticum aestivum | Tolerance to freezing and drought. | Wang et al., 2016 |
| VvABF2 | Vitis vinifera | Tolerance to osmotic stress. Induction of LEA gene expression under duress and better ROS scavenging ability. | Liu et al., 2019 |
| ZmABP2 | Zea mays | Tolerance to drought and salt. | Zong et al., 2018 |
| ZmABP9 | Zea mays | Tolerance to drought and salt. | Zhang et al., 2011 |
| ZmbZIP4 | Zea mays | Tolerance to salt. | Ma et al., 2018 |
| ZmbZIP72 | Zea mays | Tolerance to salt. | Ying et al., 2012 |
5.1 Drought and salt stress
The ABF transcription factor family is a key regulator of plant response to abiotic stresses such as drought and salt (Yoon et al., 2020). As an important component of the ABA signaling pathway, ABFs promote drought and salt tolerance in plants by regulating the expression of genes associated with osmotic stress (Yang et al., 2024). The function of ABF is mainly realized through the ABA signaling pathway, but studies have shown that ABA is not the only signaling molecule that regulates the stress response (Yang et al., 2024). ABA functions through two pathways: the ABA-dependent pathway and the ABA-independent pathway. In the ABA-dependent pathway, ABFs, as downstream target genes for ABA signaling, directly respond to ABA accumulation activating the expression of stress-related genes (Soma et al., 2021). In the ABA-independent pathway, other signaling molecules and transcription factors (such as DREB/CBF) interact synergistically with ABF to regulate the stress adaptation mechanism of plants (Amudha and Balasubramani, 2015).
The regulatory pattern of ABFs to salt stress is very similar to that of drought stress. ABA plays a central role in regulating plant responses to environmental stresses, mainly through two pathways: ABA-dependent and ABA-independent. ABFs/AREBs, as key transcription factors, regulate the expression of drought- and salt-responsive genes by binding to ABRE and coupling elements (CE), and interacting with NAM, ATAF1/2, and CUC2 (NAC) and DREB to co-regulate gene expression. Under drought and salt stress, SnRKs enhance the activity of ABFs/AREBs by phosphorylating them, while Ca2+ signals activate the salt overly sensitive (SOS) pathway to participate in drought and salt responses. The ABA-dependent plant salt tolerance pathway mediated by ABFs, like the drought tolerance pathway, mainly enhances salt tolerance by regulating stomatal conductance, enhancing the efficiency of reactive oxygen metabolism, activating ion transport pathways, or regulating the expression of stress genes related to the ABA-dependent pathway, and the tolerance of plants to drought stress and salt stress is often enhanced simultaneously (Figure 3).
In Arabidopsis, the ABF subfamily genes ABF2, ABF3, and ABF4 are upregulated under abiotic stresses such as high salinity, drought, freezing, and cold, enhancing plant tolerance to these adverse conditions (Yang et al., 2024). Drought and salt stress induce cellular dehydration, and ABF transcription factors mitigate this by regulating the expression of genes involved in osmotic adjustment, thereby maintaining cellular osmotic homeostasis and improving stress resilience (Li et al., 2024). The ectopic expression of FtbZIP12 in Arabidopsis enhances seed germination, mitigates taproot damage, and improves seedling tolerance to osmotic stress (Weng et al., 2022). ABF transcription factors play a pivotal role in P5CS1-mediated proline biosynthesis under extreme osmotic conditions (Weng et al., 2022). Overexpression of PvP5CS2 in Arabidopsis augments salt resistance by enhancing proline synthesis capacity (Shah et al., 2024). Similarly, transgenic Arabidopsis overexpressing LrP5CS exhibit improved osmotic, drought, and salt tolerance without adverse effects under non-stress conditions (Wei et al., 2016). In barley, proline accumulation driven by the P5CS1 allele enhances drought stress tolerance (Maghsoudi et al., 2018). Additionally, GhP5CS1 in cotton likely modulates salt stress responses by regulating proline biosynthesis (Fang et al., 2025).
ABF transcription factors enhance plant tolerance to drought and salt stress by modulating stomatal aperture to minimize water loss. For instance, AtABF3 overexpression promotes stomatal closure, improving drought resistance in creeping bentgrass, alfalfa, peanut, and cotton (Wang et al., 2016; Zhang et al., 2022). Similarly, IbABF4 overexpression in Arabidopsis thaliana and sweet potato enhances ABA sensitivity and stress tolerance (Wang et al., 2019), while BnABF2 and AtABF4 confer improved drought and salt resistance in Arabidopsis thaliana and potato (He et al., 2013; Pang et al., 2024). In rice, OsABF1 and OsABF2 interact with ABRE-mediated target genes to positively regulate stress tolerance (Hossain et al., 2010; Zhang et al., 2017). Overexpression of stress-responsive ABFs (e.g., ABF1, ABF2, ABF3, ABF4, AREB1, AREB2) induces ABA hypersensitivity, reduces transpiration rates, and significantly enhances drought tolerance. These findings highlight the pivotal role of ABFs in coordinating stomatal regulation and stress-responsive gene expression to improve plant resilience under abiotic stress.
ABRE-like motifs are not essential for ABA-mediated regulation of certain stress-induced genes, such as rd22 (Roychoudhury et al., 2013). The expression of RD26 is induced by drought, ABA, and high salinity, with its promoter region containing a dehydration-responsive element (DRE), two MYB recognition sites, and one MYC recognition site (Fujita et al., 2004). Overexpression of OsMYB2 reduces ROS accumulation under salt stress, enhancing oxidative stress tolerance (Yang et al., 2012). Similarly, TaMYB33 and MdMYB46 improve salt and drought tolerance by restoring osmotic balance and enhancing ROS scavenging (Wei et al., 2021). Transgenic Arabidopsis overexpressing CBF3/DREB1A exhibits elevated free proline levels under stress, while slmyc1 tomato plants show reduced stress resistance due to shorter roots and higher ROS levels (Feng et al., 2023). In sweet potato, IbMYC2 enhances salt and drought tolerance by regulating anthocyanin accumulation and ROS scavenging mechanisms (Hu et al., 2024).
Although the ABA-dependent pathway is central to plant stress responses, plants have also evolved ABA-independent mechanisms to cope with osmotic stress (Muhammad Aslam et al., 2022). In the ABA-independent pathway, APETALA2 (AP2)-type transcription factors, such as DREBs, activate stress-responsive genes by binding to dehydration-responsive elements (DREs; Xie et al., 2019). For instance, AtDREB1A/CBF3 and its ortholog PeDREB2 are induced by drought and salt stress in an ABA-independent manner, enhancing stress tolerance and antioxidant enzyme activities (Zhou et al., 2012). Similarly, GmDREB1B;1 activates GmPYL21 expression and promotes ABRE-mediated gene expression independently of ABA (Kidokoro et al., 2015). However, DREB2A requires post-translational modifications, such as phosphorylation, for activation, as its overexpression alone does not confer stress tolerance (Agarwal et al., 2017). While DREB2A downstream genes contribute to drought tolerance, they are insufficient for freezing stress resistance (Zhang et al., 2023). Additionally, certain dehydration-induced genes are unresponsive to cold or ABA, indicating the presence of alternative ABA-independent pathways. NAC domain proteins, such as ATAF1, act as negative regulators in drought signaling, while SNAC1 in rice induces stomatal closure and enhances drought and salt tolerance under field conditions (Ahmed et al., 2024).
5.2 Temperature stress
As discussed earlier, ABF transcription factors are known to be involved in various stress responses, including drought, salt, and low temperature stress. In particular, several ABF family members have been shown to play crucial roles in mediating plant adaptation to cold stress. The interplay between ABA-dependent and CBF-dependent pathways is integral to the plant's ability to cope with low temperature stress (Feng et al., 2025). It has been emphasized in the previous section that ABF1 is mainly induced by low temperature stress (Kim et al., 2006). In melon (Cucumis melo), CmADC acts as a downstream intersection of the ABA-dependent pathway and CBF-dependent pathway in response to low temperature, and CmCBF4 and CmABF1 are regulating the molecular mechanism of CmADC expression to promote putrescine synthesis in response to low temperature (Li et al., 2022). CbABF1, which is from the alpine subnival plant (Chorispora bungeana) can be induced by cold, drought, and abscisic acid (Yue et al., 2019). Moreover, the ectopic expression of CbABF1 in tobacco improved plant tolerance to freezing and drought stress (Yue et al., 2019). StABF1 from potato (Solanum tuberosum) was strongly induced by low temperature, and its expression increased with increasing time of low-temperature stress (Grandellis et al., 2016). Some other ABFs can also respond to low-temperature stress. Heterologous overexpression of AtABF3 in lettuce (Lactuca sativa) enhanced the tolerance to low temperature and drought in transgenic lettuce (Li et al., 2025). TaAREB3 was induced by ABA and low temperature, and its heterologous overexpression in Arabidopsis thaliana resulted in enhanced freezing and drought resistance in transgenic plants and an up-regulated sensitivity of transgenic plants to ABA (Wang et al., 2016). Similar to TaAREB3, overexpression of GmbZIP1 in Arabidopsis thaliana improved transgenic plants' tolerance to low temperature, drought, and salt (Askari-Khorasgani and Pessarakli, 2021).
The entry of plants into a dormant state under low-temperature stress is an important way to resist low temperature. ABF1 of Arabidopsis thaliana is involved in regulating seed dormancy and germination at low temperatures and has an important role in seedling establishment at low temperatures (Sharma et al., 2011). PpyABF3 specifically binds to the second ABRE cis-element within the PpyDAM3 promoter through its bZIP domain, thereby activating PpyDAM3 transcription and co-regulating plant dormancy in response to low temperatures (Yang et al., 2020). In another study, PpyABF3 was demonstrated to interact with PpyWDR5a, and activate the expression of PpyDAM4 and PpyGA2OX1, thereby regulating bud dormancy in pears (Yang et al., 2023). These studies demonstrate that ABFs play an essential role in affecting plant dormancy at low temperatures.
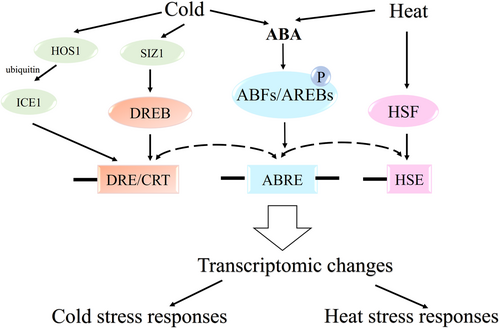
ABFs/AREBs, along with other transcription factors, regulate plant responses to cold and heat stress through ABA signaling and other pathways (Figure 4). Cold stress is primarily controlled by DREBs, which are regulated by high expression of osmotically responsive genes 1 (HOS1) and SAP and Miz1 domain containing protein 1 (SIZ1) through ABA signaling, while ABFs/AREBs enhance cold stress gene expression via ABRE (Wang et al., 2024). For heat stress, ABFs/AREBs and heat shock factor (HSF) collaboratively modulate the expression of heat-responsive genes through ABA signaling (Yadav et al., 2013). This is further substantiated by studies demonstrating that AtABP9 and OsAREB1 are integral in enhancing heat stress tolerance. Transfer of AtABP9 to Arabidopsis thaliana ensured better tolerance to heat and the combined stress condition of heat and drought due to induced expression of drought/heat stress-responsive transcription factors (DREB2H and HSF), functional genes (HIS1-3 and HSP17.4) and light-harvesting complex Lhca6 and Lhcb2.4 (Zhang et al., 2008). Likewise, OsAREB1, which can bind to ABA-responsive elements, is induced by ABA, the polyethylene glycol (PEG), and heat, indicating that OsAREB1 also has a positive impact on response to heat stress (Jin et al., 2010). In addition, OsAREB1 enhanced the resistance of transgenic Arabidopsis thaliana to heat by regulating the transcription of RD29A and RD29B (Jin et al., 2010).
6 CONCLUDING REMARKS
The response of plants to abiotic stress requires complex and precise regulation. The complex network of ABFs connected to each other is usually divided into positive or negative ABA signaling feedback loops. The ABFs/AREBs directly regulate ABRE-mediated gene expression in response to abiotic stress, the most prominent pathway being the SnRK pathway. SnRK2s are a class of protein kinases of the SnRK III subclass that act as global regulators of ABA signaling by phosphorylating as an upstream controller of ABFs/AREBs and act as a global regulator in ABA signaling. In addition to the SnRK pathway, the CDPK pathway and the CPK pathway can also activate the activity of ABFs/AREBs by phosphorylation in response to abiotic stresses. The ABFs are indispensable in the plants response to abiotic stresses. Increasing evidence indicates that ABFs can coordinate the regulation of multiple abiotic stress networks and different ABFs have different roles in response to abiotic stress. Individual and incomplete functional investigations of ABFs are insufficient to fully understand how plant cells detect external adversity signals, how these signals are transmitted to ABFs, and how this group of transcription factors controls downstream genes. Subsequent investigations should extensively employ advanced technologies like RNA sequencing and CHIP-sequencing to explore and study the superior signals received by candidate ABFs as well as new target genes downstream of the regulatory cascade. This will help establish a more comprehensive network of DERBs involved in regulating the plant's response to abiotic stresses.
Plant ABFs are widely distributed and play an important role in plant development, seed maturation, and stress signal transduction. Current research on the biological functions of ABFs in plants primarily utilize the model plants Arabidopsis thaliana and tobacco. In view of the diversity and importance of the biological functions of ABFs, the research area of ABFs can be further expanded in the future to strengthen the in-depth study of ABFs in a wider range of species and to explore their more theoretical and applied values for improving stress tolerance and elevating crop yield.
AUTHOR CONTRIBUTIONS
Y.H. and J.S. conceived the work. D.Z. and J.S. wrote the article. Y.H. prepared the illustrations. All authors read and revised the manuscript. All authors contributed to the article and approved the submitted version.
FUNDING INFORMATION
This study was supported by the National Natural Science Foundation of China (No. 32272728, 31872108).
CONFLICT OF INTEREST STATEMENT
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




