Identification of cis-acting elements recognized by transcription factor LlWOX11 in Lilium lancifolium
Abstract
WOX transcription factors play important roles in plant developmental processes and mainly bind to the WOX-binding element to regulate gene expression. Previously, we characterized a WOX gene from Lilium lancifolium, LlWOX11, positively regulating bulbil formation, and showed that it bound to the motif of TTAATGAG. However, whether LlWOX11 could bind to other motifs is unclear. In this study, Transcription Factor-Centered Yeast One Hybrid (TF-Centered Y1H) was utilized to study the motifs recognized by LlWOX11, and five motifs with seven bases were obtained. In addition to three motifs containing known cis-acting elements: TCAACTC (CAREOSREP1), AGAAAGA (DOFCOREZM/POLLENILELAT52), ACAGTAT (CACTFTPPCA1), we identified that LlWOX11 could bind to two new motifs: TGCGAAA, TCCATCA. We further searched for the core sequences of these motifs by Y1H. Dual-luciferase assays (LUC), Electrophoretic mobility shift assays (EMSA) and chromatin immunoprecipitation (ChIP) were performed to further determine that these motifs were bound by LlWOX11 in the plant. In addition, we found that LlWOX11 inhibited the transcription of LlRR9 by binding to the screened motifs in the promoter and further promoted bulbil formation. These findings will help to further reveal the functions of the WOX protein and the molecular mechanism of bulbil formation regulated by LlWOX11.
1 INTRODUCTION
The WUSCHEL-related homeobox (WOX) family is a class of transcription factors containing homologous domains, which is divided into 15 families. There are three distinct clades through phylogenetic analyses in the WOX gene family: an ancient clade (WOX10, WOX13, WOX14), an intermediate clade (WOX8, WOX9, WOX11, WOX12), and a modern/WUS clade (WUS, WOX1, WOX2, WOX3, WOX4, WOX5, WOX6, WOX7; Van Der Graaff et al., 2009). The characteristic structural feature of WOX family proteins is an N-terminal homeodomain (HD) which consists of 60–66 amino acids (He et al., 2022). The HD binds DNA through a helix-turn-helix (HTH) structure. The WOX proteins also contain the specific WUS-box motifs, in the form of T-L-X-L-F-P-X-X (X can be any amino acid) at the C-terminal in the genes in the WUS clade. In addition, some WOX proteins contain an activator domain between the HD and the WUS box consisting of acidic amino acids (Sloan et al., 2020).
WOX family gene transcription factors play a wide variety of roles in plant developmental processes (van der Graaff et al., 2009; Willoughby et al., 2021). WOX10, WOX13, and WOX14 participate in root development, callus formation, and drought tolerance (Ikeuchi et al., 2022; Lv et al., 2023). WOX2, WOX3 and WOX8 promote somatic embryo development in plants (Hassani et al., 2022; Long et al., 2023; Xu et al., 2023). WOX11 and WOX12 play roles in root development and organogenesis (Baesso et al., 2018; Xu et al., 2023). As a member of the WOX family intermediate clade PgWOX11 binds to the PgCLE45 promoter to regulate adventitious root branching (Liu et al., 2020). A new study shows that OsWOX11 works together with OsSLG2 to regulate grain width by affecting the expansion of cells within the spikelet shell in rice (Xiong et al., 2023). Liao et al. (2023) found that WOX11 regulates seed dormancy through mediating phytochrome B (PHYB) signaling during the induction stage. WOXs play a role in the regulatory network by binding to the motif in the downstream target gene promoters (Liu et al., 2020). Currently, motifs of sequences with a TAAT and a G-Box-like, have been identified which are the binding sites of WOX genes (Leibfried et al., 2005; Yadav et al., 2011; O'Malley et al., 2016). QHB, WOX3, and WOX11 proteins also bind to the TTAATGG sequence in rice (Kamiya et al., 2003; Zhao et al., 2009). WOX11 and WOX12 directly bind to the known WOX-binding cis-elements (TTAATGG) in the promoters of WOX5 and WOX7 to promote root primordia initiation and organogenesis (Hu and Xu, 2016). In L.lancifolium, LlWOX11 can bind to “TTAATGAG” in the promoter of LlRR9 to inhibit its transcription and enhance cytokinin signaling to promote bulbil development (He et al., 2022). The WUS protein could also specifically recognize the sequence CACGTG (Busch et al., 2010), and the appraisal of two binding core sequences for WOX13 (CAAT and TTAA) has been accomplished (Franco-Zorrilla et al., 2014). At present, the cis-elements of reported WOXs are mainly WOX-binding elements, and more binding elements need to be discovered to complete the regulatory pathway of WOX genes.
Transcription factors (TFs) promote or inhibit downstream gene expression via binding to specific cis-acting elements (Ambrosini et al., 2020). Hence, the research on the specific cis-acting element recognized by a TF is of great significance for revealing its function and regulatory mechanisms. At present, the main methods of cis-acting element identification include the Systematic evolution of ligands by exponential enrichment technology (SELEX), chromatin immunoprecipitation (ChIP), Chromatin targeted cleavage and labeling technology (CUT & Tag; Kaya-Okur et al., 2019), Yeast one-Hybrid (Y1H) and Transcription Factor-centered Yeast one Hybrid (TF-centered Y1H). Compared to other technologies, TF-centered Y1H is a new method to analyze the function of specific transcription factors which was developed in 2014 (Ji, Wang, et al., 2014). It was conducted on the basis of Y1H and determined the possible protein and DNA interactions in a short time, which is increasingly being used in plant research due to its advantage of simplicity and efficiency.
Previous studies showed that LlWOX11 could promote bulbil formation of L. lancifolium (He et al., 2022). Further studies showed that LlWOX11 could regulate directly the transcription of LlRR9 via binding to its promoter containing the sequence motif of “TTAATGAG” (He et al., 2022). However, whether LlWOX11 can bind to other motifs remains unknown. In this work, we identified the motifs recognized by LlWOX11 using a TF-Centered Y1H assay (Ji, Wang, et al., 2014) and verified it through LUC reporter assays, EMSA and ChIP-PCR. These results suggested that LlWOX11 recognizes more motifs than we previously knew. The identified motifs recognized by LlWOX11 will facilitate to understand the functions of LlWOX11 in-depth and lay a solid foundation for revealing the molecular mechanism of bulbil formation regulated by LlWOX11 in lily.
2 MATERIALS AND METHODS
2.1 Plant materials
The autotriploid species Lilium lancifolium was used as plant material in this study. L. lancifolium bulbs of uniform size with 1–2 cm buds were grown in peat soil containing perlite in a greenhouse at the Institute of Vegetables and Flowers, Chinese Academy of Agricultural Sciences, Beijing, China, in September of 2020. Samples of the leaf axil were collected by hand dissection with a double edge blade and quickly frozen in liquid nitrogen for RNA extraction and vector construction.
2.2 Construction of the bait vector
Total RNA was isolated from leaf axils of L. lancifolium according to the instructions of the RNAprep Pure Plant Kit (TIANGEN). The cDNA of leaf axils of L. lancifolium was then obtained by using the TransScript® Uni All-in-One First-Strand cDNA Synthesis SuperMix Kit (Trans). The coding sequence (CDS) of LlWOX11 was amplified and cloned into the vector pGADT7-Rec2 (Clontech) to obtain the bait vector pGADT7-LIWOX11 using the yeast recombination method (Matchmaker™ One-Hybrid Library Construction & Screening Kit).
2.3 TF-centered Y1H screening
We constructed a random DNA library with 7 base lengths according to Ji, Wang, et al. (2014). The pGADT7-LIWOX11 vector and DNA sequence library were transformed into the Y187 yeast strain together and then were cultivated in the medium of TDO+ 3-AT (3-Amino-1, 2, 4-triazole; 0, 10, 20, 30, 40, 50, 75, 100 mM). A Y1H filter was used to discover the motifs recognized by LlWOX11 (He et al., 2022). The inserts in the positive pHIS2 plasmids were sequenced. The insertion sequences together with the left and the right insertion flanking sequences (“GGG” and “CCC”) were analyzed using the programs of PlantCARE (http://bioinformatics.psb.ugent.be/webtools/plantcare/html/) and New PLACE (https://www.dna.affrc.go.jp/PLACE/?action = newplace) to identify whether there are known cis-acting elements.
2.4 Core sequences of the screened motif analysis
In order to identify the core sequences of the screened motifs, base deletion was performed one by one from the left and right ends of each motif sequence. Serial sequences were generated (Figure 2) and interacted with LlWOX11 to determine the core sequence of the screened motif. The sequence inserted into pHIS2 vector was repeated three times. The pHIS2 element library plasmid was co-transformed into Y187 with the pGADT7-LlWOX11 vector. Then SD/−Leu/−Trp (DDO) and SD/-His/−Leu/−Trp (TDO) medium supplied with 3-AT were used to select the transformed yeast cells according to Ji, Zheng, et al. (2014).
2.5 Electrophoretic mobility shift assay (EMSA)
The CDS of LlWOX11 was incorporated into the MAL-C5X vector (Trans). The recombinant plasmid was transformed into the E. coli strain BL21. The expression of the sequence encoding LlWOX11 fused with the Maltose-binding protein (MBP) was induced by 1 mM isopropyl β-D-1-thiogalactopyranoside (IPTG) at 37°C for 3 h. The LlWOX11 protein was isolated and purified according to the instruction manual of the pMAL Protein Fusion & Purification System (NEB). SDS-PAGE was used to examine he purity of the recombinant LlWOX11 protein. The probes labeled with biotin were synthesized by Sangon. The same probe without biotin labeling was used as the competitor. The probes were then incubated with LlWOX11 protein and assayed using a LightShift Chemiluminescent EMSA Kit (Thermo).
2.6 Chromatin immunoprecipitation (ChIP) assay
To confirm whether the LlWOX11 protein binds to the screened motifs, ChIP experiment was conducted on Nicotiana benthamiana and L. lancifolium expressing 35S::LlWOX11-GFP. The ChIP assay was performed applying the EpiQuik™ Plant ChIP Kit (EpigenTek). Firstly, plants were cross-linked with 1% formaldehyde, and chromatin was sheared into fragments with 0.2–0.8 kb length through sonication. The chromatin fragments were immunoprecipitated with an anti-GFP antibody as a positive control (ChIP+) or a mouse IgG antibody (Beyotime) as a negative control (ChIP-). Immunoprecipitated DNA was purified after reversing the cross-linking. The primers used for the ChIP assay are shown in Table S3. The promoter regions of the cell cycle-related gene (NW_017670315.1) in N. benthamiana were searched for ChIP quantitative PCR, and the promoter fragments containing only the screening motifs were used to detect the binding of LlWOX11 and motifs. The promoter regions of LlRR9 and the promoter fragments containing selected motifs were used for the ChIP assay. The ChIP-qPCR data were analyzed according to the improved approach of Shi et al. (2014).
2.7 Dual-luciferase (LUC) reporter assay in Nicotiana benthamiana
Transient expression analysis in vivo was carried out to further verify the results of Y1H. Four-week-old tobacco plants (Col-0) were used to test the bindings of LlWOX11 and the screened motifs according to the method of He et al. (2022). Three tandem copies of the core sequence of “AACT”, “AAAG”, “AGTA”, “CGAA” and “CATC” were introduced into the pluc-35Rluc vector to drive the LUC reporter gene. The CDs of LlWOX11was inserted into pCAMBIA3301 with the 35S promoter (named as pCAM-LlWOX11) as the effector plasmid using the pEASY-Basic Seamless Cloning and Assembly Kit (Trans). These reporters and effectors were transformed into the Agrobacterium tumefaciens strain EHA105. The empty pCAMBIA3301 was also transformed as a control. Distinct reporters were then co-infiltrated with the effector into N. benthamiana leaves using a syringe. The incubated leaves were sprayed with D-luciferin potassium salt (Beyotime), and the fluorescence was detected by a chemiluminescent imaging system (Tanon).
2.8 Searching for DNA motifs in the promoters of genes
Previous studies showed that LlWOX11 binds to the promoter of LlRR9 and inhibits its expression (He et al., 2022). In addition, we found a positive correlation between the expression levels of some genes concerned with plant development and LlWOX11 in L. lancifolium. In order to comprehend the distribution of screened motifs and due to the lack of the genome of L. lancifolium, a 1000 bp promoter sequence before the “ATG” of these genes from Arabidopsis thaliana were retrieved from the NCBI database (https://www.ncbi.nlm.nih.gov/) for analysis.
2.9 Transient gene overexpression in L. lancifolium
The full-length cDNA of LlRR9 was inserted into the pCAMBIA3301 vector using the pEASY®-Basic Seamless Cloning and Assembly Kit (Transgen Biotech). The transformation of L. lancifolium was carried out through Agrobacterium-mediated vacuum infiltration using the A. tumefaciens strain EHA105. Agrobacterium cells were collected and suspended in the buffer solution comprising 10 mM MgCl2, 200 mM acetosyringone, and 10 mM MES (pH = 5.6). After being submerged in an infiltration solution, the stem segments of L. lancifolium were then subjected to a vacuum of −0.06 MPa for a duration of 3 min three times. The infiltrated segments were washed with distilled water and then grown on the medium of MS + 60 g l−1 sucrose +5 g l−1 agar (pH = 5.8) at 20 ± 1 °C under dark conditions for 1 d and then 22 ± 1 °C with a 16 h photoperiod for about two weeks, and further verified by qRT-PCR analysis. The rate of bulbil induction was determined following a two-week period of cultivation. Each treatment included three independent experimental replicates, and each replication had a total of 30 leaf axils.
2.10 Statistical analysis
The data for the analyses were presented as the means with standard deviation from three biological replicates. The SPSS 23.0 software (SPSS Inc.) was used to perform statistical analyses, and statistical significance was defined at p < 0.05. Non-overlapping letters indicated significant differences between the comparisons, based on the ANOVA analysis and Duncan's multiple range test.
3 RESULTS
3.1 Construction and screening of the random DNA insertion prey library
Through plate screening with different concentrations of 3-AT, yeast cells were not able to grow on the medium plate containing 75 mM 3-AT (Figure 1A), indicating the reporter gene could not be activated. According to the results, 75 mM 3-AT was selected for the subsequent screening concentrations. The Y187 yeast strain containing the pGADT7-LIWOX11 bait plasmid was used as the receptor strain. The motif library plasmid was transferred into it and coated on an SD-TLH screening plate containing 75 mM 3-AT. Screening through the TDO medium with 75 mM 3-AT, 155 positive clones were selected (Figure 1B).
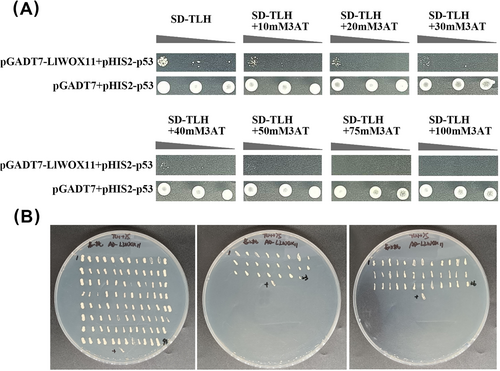
3.2 Identification of the DNA motifs bound by LlWOX11
The TF-centered Y1H system is a convenient and efficient approach to identify the DNA sequence bound by transcription factors of interest. TF-centered Y1H was performed, and five positive clones that have high binding affinity to LlWOX11 were identified. Through high stringency selection, the sequences of the five insert fragments were obtained (Table 1). Motif prediction showed that three of the sequences contained known cis-acting elements: CAREOSREP1, DOFCOREZM/POLLENILELAT52 and CACTFTPPCA1. No studied element was predicted in the other insertion sequences: “TGCGAAA” and “TCCATCA” (Table 1), and thus the two sequences might contain novel motifs recognized by LlWOX11.
| Clone number | The identified sequence (underlined) with the two sides of flanking sequences (5′-3′) | Motif prediction |
|---|---|---|
| 1 | CCCTCAACTCGGG | CAREOSREP1 |
| 2 | CCCAGAAAGAGGG | DOFCOREZM; POLLENILELAT52 |
| 3 | CCCACAGTATGGG | CACTFTPPCA1 |
| 4 | CCCTGCGAAAGGG | No result |
| 5 | CCCTCCATCAGGG | No result |
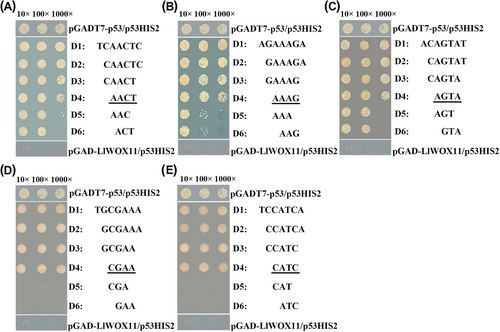
3.3 Core sequence characterization of the motifs recognized by LlWOX11
We used Y1H assays to identify the core sequence of the filtered motifs. Yeast cells containing the reporter vector with the three repeated tandem motifs having the third DNA base (A) missing from the left border of the studied motif CAREOSREP1 (TCAACTC) could not grow on TDO medium with 75 mmol l−1 3-AT, illustrating that LlWOX11 bound to the sequence “AACTC”, but not to “ACTC”. Moreover, cells with the reporter lacking the second DNA base (T) from the right border did not grow, implying that LlWOX11 is able to bind to the sequence “AACT”, but not to “AAC” (Figure 2). These observations indicated that the third DNA base (A) from the left border and the second DNA base (T) from the right border of motif CAREOSREP1 are critical for binding by LlWOX11. In the same way, the unannotated motifs were determined (Figure 2). The core sequences of the remaining two annotated motifs are “AAAG”, “AGTA”. The core sequences of two unknown motifs are “CGAA”, “CATC” respectively.
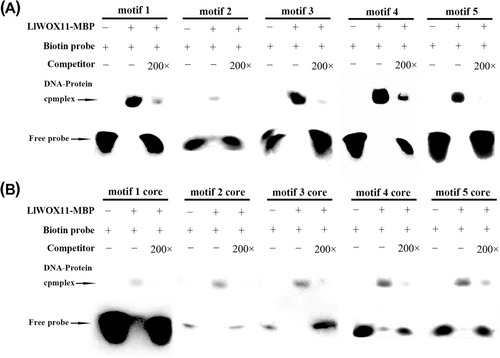
3.4 EMSA analysis of the bindings of the selected motifs with LlWOX11
To further determine whether these motifs were bound by LlWOX11, EMSA assays were subsequently performed. The EMSA results of five motif probes revealed a retarded band, which demonstrates that DNA–protein complexes could be observed when LlWOX11 interacted with these five motifs, and the signal decreased when the same unlabeled competitor probe was added (Figure 3A), showing that LlWOX11 could bind to these selected motifs, which was consistent with the results of Y1H. The core sequence of the screened motif was also subjected to EMSA analysis, and the results showed that the core sequence binds to the LlWOX11 protein (Figure 3B), consistent with the Y1H results.
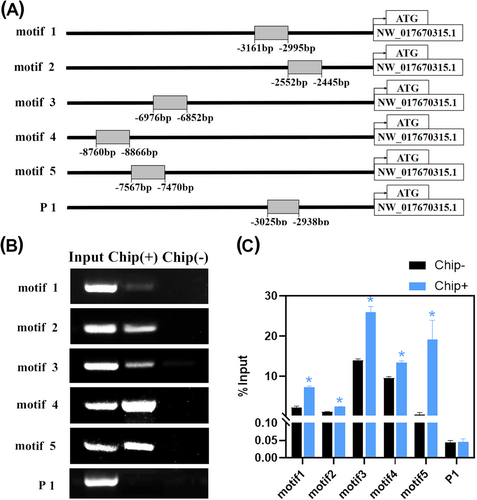
3.5 Interaction of LlWOX11 with selected motifs occurs in plants
We performed ChIP analysis to determine whether bindings between LlWOX11 and the screened motifs occur in plants or not. The LlWOX11-GFP fusion gene was transformed into tobacco (N. benthamiana) plants. The N. benthamiana genes (NW_017670315.1), which contained the screened motifs in promoter regions, were used in this experiment (Figure 4A). The promoter fragments containing specific motifs were captured by ChIP using a GFP antibody. Then, the enriched truncated promoter identified by ChIP-PCR was utilized for ChIP-qPCR, and both the promoter fragments containing the selected motifs were significantly enriched (Figure 4B, C). Meanwhile, the promoter fragment (P1) lacking any sequences of the selected motifs was used as negative controls, and it failed to be enriched. The results suggested that the bindings between LlWOX11 and the screened motifs to regulate gene expression occurred in plants.
3.6 The distribution of screened motifs in the promoters of genes regulated by LlWOX11
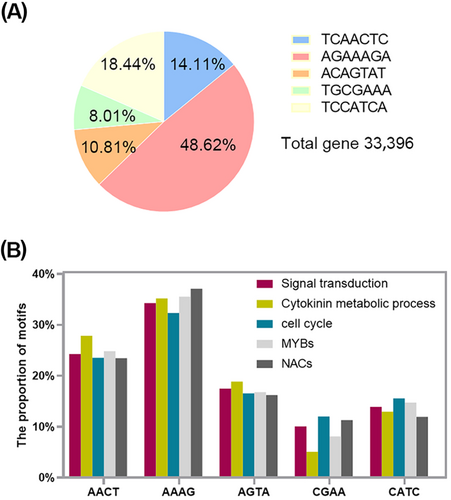
We screened for motifs in the Lilium davidii var. unicolor genome and identified 33 396 genes whose promoters contain the screened motifs. The numbers of genes containing only the “TCAACTC”, “AGAAAGA”, “ACAGTAT”, “TGCGAAA” and “TCCATCA” account for 14.11%, 48.62%, 10.81%, 8.01% and 18.44% respectively (Figure 5A). We selected 61 genes related to bulbil formation involved in “signal transduction mechanisms”, “cytokinin metabolic process” and “cell cycle” based on Cluster of Orthologous Groups of proteins (COG) analysis, and included two transcription factor families, NAC and MYB. We speculate that LlWOX11 further regulates the expression of these genes by binding to the screened motifs in the promoters. The frequency of the five motifs occurs in the promoters of different cluster genes is basically consistent with “AAAG” having the highest frequency of occurrence, followed by “AACT”, and the frequency of occurrence of “CGAA” being the smallest (Figure 5B). DOFCOREZM/POLLENILELAT52 motifs were abundant and the large number of “AAAG” may be related to the wide range of functions of this element.
3.7 LlWOX11 inhibits the transcription of LlRR9 by binding to the screened motifs in the promoter
Previous studies have shown that LlWOX11 can bind to the promoter of LlRR9 (He et al., 2022). The function of LlRR9 was analyzed during bulbil formation of L. lancifolium using methods of transient overexpression. The results of transient overexpression showed that overexpression of LlRR9 inhibited bulbil formation (Figure 6A). The induction rates of bulbil in the overexpression group were found to be considerably reduced. Overexpressing of LlRR9 significantly decreased the bulbil induction rate by approximately 53.3% compared with a control group and 46.7% compared with the empty vector group (Figure 6B). qRT-PCR analysis showed that the expression of LlRR9 was notably elevated in overexpression plants, in comparison to the control and empty treatment plants (Figure 6C).
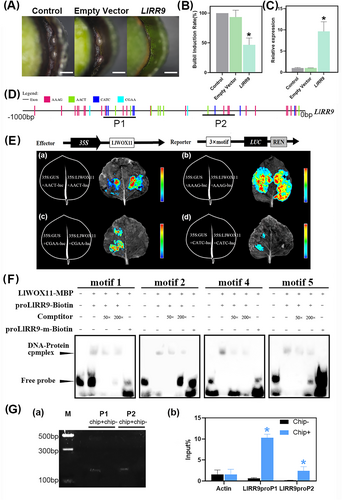
After promoter sequence analysis, 4-class selected motifs were distributed on the promoter of LlRR9 (Figure 6D). These core sequences of the 4-class motif were fused with the LUC gene to construct reporter vectors, and each reporter vector was co-transformed with the effector into tobacco plants. The results showed that LlWOX11 could bind to all the studied core sequences (Figure 6E). To determine whether LlWOX11 could bind to selected motifs to regulate gene expression, truncated promoters only containing one core sequence of the selected motifs were selected for the study. These truncated promoter fragments were used as probes for EMSA and the DNA-protein complexes generated the LlWOX11 protein bindings to the labeled probe. When more of the unlabeled probe was added, the lower signal of the bound complex was visible. When the LlWOX11 protein interacted with the mutant probes, no signal was detectable (Figure 6F). These results indicated that LlWOX11 could bind to the core sequence “AACT”, “AAAG”, “CGAA”, “CATC”. The ChIP assay in L. lancifolium showed that the promoter fragments containing selected motifs in LlRR9 were highly enriched in Chip+ products (Figure 6G). This result was consistent with that of the LUC assay, which further confirmed that LlWOX11 could regulate gene expression by binding to the core sequences “AACT”, “AAAG”, “CGAA” and “CATC”.
4 DISCUSSION
TFs are central regulators of changes in gene expression and have fundamental importance in critical aspects of plant growth and development. TFs regulate target gene expression by binding to specific cis-acting elements (Ambrosini et al., 2020), and studying the element bound by a TF is important to display its regulatory mechanisms. TF-centered Y1H is a new method to analyze the function of specific TFs which is increasingly being used in plant research. This is a useful method to research protein-DNA interactions in Tamarix hispida (Xu et al., 2017), Betula hygrometrica (Guo et al., 2018), Populus simonii×Populus nigra; Wang, Zhang, et al., 2021 and rice (Chen et al., 2022), but there is no report on lily. Previous studies showed that LlWOX11 is positively correlated with bulbil formation of L. lancifolium (He et al., 2022), but its downstream regulatory mechanism is unclear. In the present study, we applied this method in Lily for the first time to investigate the downstream motifs bound by LlWOX11. Five motifs (three known, two unknown) were obtained and laid a solid foundation for displaying the regulation mechanism on a molecular level of LlWOX11 in bulbil formation (Table 1).
WOX TFs are plant-specific transcription factors, several studies showed specifically sequences with a TAAT and a G-Box like have been identified as bound by WOXs and more components remain to be mined (Leibfried et al., 2005; Yadav et al., 2011; O′ Malley et al., 2016). In our result, five motifs containing three known cis-acting elements which were annotated separately as CAREOSREP1 (TCAACTC), DOFCOREZM/POLLENILELAT52 (AGAAAGA), CACTFTPPCA1 (ACAGTAT). Several CAREs involved in plant growth and development were identified in carrot, poplar and other plant species (Liu et al., 2019; Campos et al., 2021). BpCUC2 directly binds to CAREOSREP1 which was found extensively on the promoter in a series of IAA-related and cyclin-related genes in Betula pendula. BpCUC2 was speculated to participate in normal internode and leaf development by regulating the expression of IAA-related and cyclin-related genes (Liu et al., 2019), which provides a reference for us to search for downstream genes of LlWOX11. MdWOX11 promotes the formation of adventitious root (AR) primordia via binding to the WOX binding site (WOX-box) element CCATTAA, TTAATGG and ACC (A/T) (A/C/T) (A/C/T)” in apple (Mao et al., 2023). In our study, LlWOX11 protein could bind the sequence “TCAACTC”, in which the core sequence of “AACT” is similar with WOX-box (Figure 2A). It indicated that the conserved sequence “AA(C/T)” may be significant for the binding of WOXs. “TGCGAAA” and “TCCATCA” are novel motifs identified to be bound by WOX11, which have not been reported. Our results showed that they are significantly inhibited by LlWOX11, which suggested that the “TGCGAAA” and “TCCATCA” elements are also significant motifs recognized by WOXs, and should receive further study.
TFs bind to definite motifs and act in regulating gene expression, so their binding motifs could be used to predict their function. The DOFCOREZM/POLLENILELAT52 motif was found previously to be bound by CDF transcription factors, which have a dof domain identifying the DOFCOREZM/ POLLENILELAT52 motif (Imaizumi et al., 2005; Jin et al., 2024). CDFs regulate the expression of MYB60, SP5G by binding to the DOFCOREZM/POLLENILELAT52 motif in its promoters, and are involved in regulating potato tuberization and enhancement of activity in guard cells (Cominelli et al., 2011; Lehretz et al., 2019; Zhang et al., 2023). Therefore, the binding of LlWOX11 to the DOFCOREZM/POLLENILELAT52 motif (Figure 2B, 3, 4) suggested that it might have similar functions to the CDF TFs, such as being mediated by sucrose signals (Qi et al., 2020), and it is consistent with previous research (Hao et al., 2024), which further demonstrated that LlWOX11 is involved in the regulation of bulbil formation by responding to sucrose signaling. Gibberellin treatment promotes bulbil formation in yam (Kim et al., 2003) and the gibberellin (GA)-responsive element CAREOSREP1 was found in our screening results (Table 1), from which we speculate that LlWOX11 might play a role in the gibberellin pathway by binding to “TCAACTC”.
The sequence of a cis-acting element exhibits differences in individual bases across different species (Lohmann et al., 2001; Mao et al., 2023). By screening the core sequences of the elements, the information of the sequences that play a role can be found, which contributes to the identification and analysis of elements. We further screened the core sequences “AACT”, “AAAG”, “AGTA”, “CGAA” and “CATC” of both annotated and new motifs by deleting bases separately (Figure 2). The binding of LlWOX11 to the screened motifs was verified through EMSA (Figure 3). The resulting sequence information has important implications for the study of the LlWOX11-binding elements. Within the organism, the number and positional distribution of motifs within the promoter region are associated with the transcription machinery due to the folding of the DNA in the three-dimensional structure (Casimiro et al., 2008). Our analysis found that the screening motifs were distributed on the promoters of genes related to plant organ development in Arabidopsis (Figure 5A). After the publication of the L. lancifolium genome, these motifs can be used for the screening of genes downstream of LlWOX11 and serve as important reference materials for finding the downstream genes of WOX11. The bulbil in L. lancifolium originated from the leaf axillary meristem, and the formation process was associated with cell division and regulated by cytokinins (He et al., 2022). Therefore, these genes may be the key genes involved in the bulbil formation regulated by LlWOX11. The distribution patterns are diverse and may be different in relation to their functions in bulbil formation. The high number of annotated motifs may be related to the wide function of these motifs in plants, while the number of new motifs is relatively small the we speculate that the unannotated motifs have a specific function in plant transcriptional regulation (Figure 5B).
Transcription factors can function in combination with multiple elements (Guo et al., 2018; Wang, Wen, et al., 2021). LlRR9 is a type-A RR gene whose product is a negative regulator of cytokinin signaling. We further confirmed that LlRR9 participated in the negative regulation of bulbil formation (Figure 6A, B, C). Our previous studies have found that LlRR9 was directly regulated by LlWOX11 through the WOX-binding element (TTAATGAG) in its promoter (He et al., 2022). In this study, we found sequences containing the core sequence of four classes of screened motifs (“AACT”, “AAAG”, “CGAA” and “CATC”) in the promoter of LlRR9 (Figure 6D). We carried out dual-luciferase reporter assays and EMSAs using promoter fragments containing only the screening motifs, and the results showed that LlWOX11 affect transcription from the LlRR9 promoter by binding to the screened motifs (Figure 6E, F). LlWOX11 remarkably inhibited transcription from the LlRR9 promoter containing “CGAA” and “CATC” which is consistent with previous research (Figure 6E). The fluorescence signal of “AACT” and “AAAG” has not decreased, which may be related to the extensive functions of “AAAG” and the complex regulatory network of LlWOX11 in regulating bulbil formation.
5 CONCLUSION
We constructed a prey library and combined it with high-throughput sequencing to completely determine the motifs bound by LlWOX11 in lily. We identified the motifs “TCAACTC”, “AGAAAGA”, “ACAGTAT”, “TGCGAAA” and “TCCATCA”, which are recognized by the gene LlWOX11, and they probably play significant part in LlWOX11-mediated gene expression. The identification of these five motifs will lay the foundation for the in-depth characterization of the functions of the WOX11 gene. LlRR9 impaired bulbil formation, but LlWOX11 inhibited transcription from the LlRR9 promoter containing “CGAA” and “CATC”. These results provide a reference for further research on the molecular regulatory pathway of the formation of bulbils in L. lancifolium.
AUTHOR CONTRIBUTIONS
P.P.Y. and J.M. designed the experiments. J.Y.B. performed experimental works and data analysis. J.Y.B. and P.P.Y. wrote the article. L.F.X. and M.M.B. provided suggestions for experimental operations and data analysis. J.M. and P.P.Y. provided the financial support. All authors read and approved the manuscript.
ACKNOWLEDGMENTS
We very much appreciate suggestions on the experimental operation by Dr. Guoren He (Shanghai Key Laboratory of Plant Molecular Sciences, College of Life Sciences, Shanghai Normal University). We appreciate researcher Tiegang Lu and his team (Biotechnology Research Institute of Chinese Academy of Agricultural Sciences) for their theoretical suggestions. We appreciate Ruiyuan Biotechnology (Nanjing) for its technical support during the process of TF-centered Y1H.
FUNDING INFORMATION
This work was supported by National Natural Science Foundation of China (32172612, 31902043), National key R& D program of China (2019YFD1001002).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest in relation to this work.
DECLARATIONS
The authors declare that they have no relevant financial or non-financial interests to disclose.
Open Research
DATA AVAILABILITY STATEMENT
The supporting information for this manuscript is available in the supplementary data.




