Warm temperature and mild drought remodel transcriptome and alter Arabidopsis responses to mite herbivory
Abstract
In the context of climate change, increased temperature and decreased water availability are expected to have profound effects on plant-herbivore interactions. To gain further insight into this issue, this work focuses on the dissection of the response of the Arabidopsis thaliana plant to the mite pest Tetranychus urticae under environmental conditions that resemble climate change. Phenotypical and molecular changes were analyzed in plants grown under mild drought and/or warm temperatures. When the transcriptome results were compared in standard and altered climate conditions, a large number of genes were found to be differentially expressed. Mite infestations in these plants showed that basal alterations conditioned the subsequent response of the plant to a specific biotic stressor. Warm temperatures favored mite performance and decreased jasmonic acid accumulation. Reduced plant damage in mild drought conditions was correlated with a higher jasmonic acid accumulation and the up-regulation of many genes involved in the production of defensive compounds. In conclusion, the use of ambient conditions that resemble climate change highlighted the drastic alterations in gene expression that may occur in nature. Understanding how these changes affect specific plant-herbivore interactions is crucial to determining how global warming will affect crop production in the future.
1 INTRODUCTION
Climate change is a global threat associated with rising atmospheric CO2 concentrations and global temperatures, changing precipitation regimes, and increased nitrogen deposition (Bellard et al., 2012). The latest measurements confirm that the mean temperature has increased globally in the past decade. The temperature is predicted to increase by at least 1.5°C in the next decades, which is expected to be associated with a decrease in soil moisture in temperate and subtropical regions (IPCC, 2023; https://www.ipcc.ch/report/sixth-assessment-report-cycle). Predictions estimate that the prevalence of pests will increase as a result of global warming. New pest problems will arise from the introduction of invasive species into agroecosystems and pests that had previously minor significance, will emerge as major threats (Hamann et al., 2021). Additionally, as the number and complexity of simultaneous stresses on a plant increase, its growth and survival will decline, even if the effect of each stress is minimal or insignificant on its own (Zandalinas et al., 2024). In particular, temperature and water availability are expected to have profound effects on plant-herbivore interactions. Elevated temperatures accelerate pest development and increase their reproductive potential, inducing more generations per season and resulting in exponential pest growth (Altermatt, 2010; Jönsson et al., 2013). This enhanced performance is likely to reach a maximum since further temperature increases lead to a rapid decline in performance (Kingsolver, 2009). Drought stress has a more variable effect on pest performance, which can be positive or negative depending on the feeding guild and the severity of the stress (Gely et al., 2020). Combined rising temperatures and drought stresses are expected to induce greater food consumption by herbivores (Hamann et al., 2021).
Altered climate conditions also have effects on plants, which have evolved complex regulatory strategies to overcome abiotic stresses (Claeys & Inzé, 2013). Under water-deficient conditions, plants limit their growth to reduce water losses and tend to enhance plant resistance against herbivory (Lin et al., 2023). However, different regulatory strategies are applied to cope with mild drought or severe drought stress (Skirycz et al., 2011). Plants also show different responses to increasing temperature. Warm temperatures induce moderate developmental changes through the thermomorphogenesis process (Casal & Balasubramanian, 2019), while excessive heat stress has a strong negative impact on plant growth and development. Combined high temperature and drought stress further decrease plant growth and yield compared to their individual effects (Xu et al., 2023). Phytohormones play an important role in responses to these abiotic stresses. Abscisic acid (ABA) accumulates rapidly during drought conditions (Christmann et al., 2005). Jasmonates (JA), which play an essential role in plant defense against pests, also influence tolerance to abiotic stresses (Kazan, 2015). The accumulation of another defense-related hormone, salicylic acid (SA), is compromised under drought conditions and temperatures greater than 23–24°C (Choudhary & Senthil-Kumar, 2022; Kim et al., 2022). Some metabolites, such as anthocyanins, also accumulate and act in defense against drought (Naing & Kim, 2021; Nakabayashi et al., 2014). Anthocyanins are synthesized through the phenylpropanoid pathway in plants and have been described to play an important role in tolerance to both biotic and abiotic stresses by absorbing ultraviolet, high light irradiation, and ROS (Xu et al., 2017). Anthocyanins also accumulate under cold conditions, while high ambient temperatures repress their biosynthesis (Kim et al., 2017; Zhang et al., 2011). Transcriptomic analyses have been performed to better understand plant responses to abiotic stresses. ABA-related genes are primarily differentially expressed in response to drought while genes encoding heat shock proteins in response to heat. Combined drought and heat stresses cause a different response from that provoked by individual stresses, but this characterization has only been performed in severe heat and drought conditions (Xu et al., 2023). Therefore, there is a lack of understanding of how a moderate temperature increase and mild drought conditions, which are related to the early onset of climate change, will affect plant-herbivore interactions.
The two-spotted spider mite Tetranychus urticae and the model plant Arabidopsis thaliana have been largely used to explore the response of plants to herbivory (Arnaiz et al., 2018; Santamaria et al., 2020). Although A. thaliana is a nonpreferred host for the London mite reference population, which is adapted to feed on beans, the outstanding ability of the polyphagous T. urticae to adapt to new hosts makes possible the use of the reference mite population in this model system. Transcriptomic analyses of the A. thaliana response to T. urticae found jasmonates as the main modulators of induced defenses and pointed to the role of indole glucosinolates and anthocyanins as key defensive compounds (Santamaria et al., 2021; Zhurov et al., 2014). Indole glucosinolates and additional JA-regulated compounds were demonstrated to be necessary to assure defense against T. urticae herbivory (Widemann et al., 2021). Regarding climate change conditions, T. urticae has been predicted to cause more damage to plants in increased temperature and decreased water availability conditions. When T. urticae infested red raspberry, temperatures between 25 and 30°C cause shorter developmental times and higher fecundity, leading to high population rates (Bounfour & Tanigoshi, 2001). Similarly, T. urticae reproduction and developmental rates were higher at lower relative humidity in different plant species, such as cucumber, tomato, and barley (Santamaria et al., 2018; Shibuya et al., 2016; Ximénez-Embún et al., 2017), and mite egg hatching was inhibited at high humidity (Ubara & Osakabe, 2015). The combination of mild drought and warm temperatures has not been tested on the performance of T. urticae or the growth and development of A. thaliana plants.
The interactions in agroecosystems are complex and we currently do not have a good understanding of how plants under changing climate conditions respond to herbivory. To obtain further insight into this issue, this work focuses on the dissection of the response of A. thaliana to T. urticae when plants are grown under warm temperatures and/or mild drought conditions. Transcriptomic analyses showed that a large number of genes were found to be differentially expressed when the environmental conditions were altered. Basal alterations conditioned the subsequent response of the plant to T. urticae. Whereas warm temperatures favored mite performance, mild drought conditions reduced plant damage. Our results highlight the relevance of mild alterations in climate conditions for the complex molecular interactions between plants and herbivores.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
A. thaliana Columbia (Col-0) seeds were surface sterilized in 70% ethanol for 2 min and in a solution of 5% (V/V) sodium hypochlorite +5% (W/V) SDS for 10 min, and then washed with sterile distilled water. Sterilized seeds were sowed on 150 mL pots (6.5 x 6.5 x 5.5 mm) containing peat moss and vermiculite (3:2 v/v) and stratified in darkness at 4°C for 5 days. Then, plants were kept in growth chambers (Sanyo MLR-351-H) with a 16 h/8 h day/night photoperiod and an environmental relative humidity of around 75% for 2 weeks at different temperatures and soil water content conditions. For temperature experiments, plants were grown at either 23, 25, or 27°C. For soil water content experiments, the relative water content of the pots was kept at 2.2 g water g−1 dry soil (normal condition), 1.2 g water g−1 dry soil (minimal drought stress), or 0.7 g water g−1 dry soil (mild drought stress).
2.2 Measurement of physiological parameters
Forty-eight plants for each water treatment (normal watering, minimal drought stress, or mild drought stress) were grown at 23, 25, or 27°C. Soil water content was determined using an HH2 Moisture Meter (Delta-T Devices). Twenty-four three-week-old rosettes from each combined treatment condition were scissed and the fresh weight of each rosette was determined in an analytical balance (Denver Instrument SI-234). Then, they were kept in a 50°C oven for 48 h and their dry weight was measured similarly. Rosette water content was considered as the weight lost during rosette drying and was then determined as fresh weight − dry weight. The other twenty-four three-week-old rosettes were used for rosette size and petiole length calculation. Images were captured using an HP Scanjet 5590 Digital Flatbed Scanner series with 1200 dpi of resolution. Rosette size was analyzed using Adobe Photoshop CS software and petiole length was calculated using Fiji software (https://fiji.sc/).
2.3 Pigment quantification
Chlorophyll and carotenoid pigments were extracted in 80% ice-cold ethanol from each of three pools of six three-week-old rosettes from each treatment condition. Extracts were centrifuged at 12,000 g for 10 min at 4°C, and the absorbance at 645, 663, and 470 nm was measured for chlorophyll a, chlorophyll b, and carotenoid determination. The concentration of pigments was calculated as previously described (Lichtenthaler, 1987) and expressed as mg g−1 fresh weight. Anthocyanin content was measured as previously described (Vandenbussche et al., 2007). Extraction of anthocyanins was performed in 300 μL of methanol containing 1% HCl for 24 h at 4°C. Subsequently, the samples were centrifuged for 10 min at 10,000 g at 4°C. The absorbance of supernatant at 530 nm and 657 nm was measured. The anthocyanin content was calculated as OD530–0.25 × OD657 and expressed as μg g−1 fresh weight.
2.4 Spider mite infestation
T. urticae London strain (Acari: Tetranychidae) was reared in Phaseolus vulgaris plants and maintained in growth chambers (Sanyo MLR-350-H) at 25 ± 1°C, >70% relative humidity and a 16 /8 h day/night photoperiod. Twelve three-week-old Col-0 plants from each treatment condition were infested with 20 T. urticae age-synchronized female adults per plant for periods of 30 min for RNA isolation, 3 h for hormone measurements, or 4 d for damage quantification. Each plant was covered by a cylinder to prevent the movement of mites from infested plants. Infested plants and control plants without mites were transferred to growth chambers at optimal mite conditions. For plant damage determination, mites were removed from the plants and the entire rosettes were excised and scanned using a size reference on an HP Scanjet (HP Scanjet 5590 Digital Flatbed Scanner series). All the images were taken in Adobe RGB colour mode, with 1200 dpi of resolution. Plant damage was identified as the total area of chlorotic spots detected after spider mite feeding. Image processing and quantification of the feeding damage were performed using Ilastik as described (Ojeda-Martinez et al., 2020). Independent plants non-infested and mite-infested were used to quantify rosette areas. Fecundity assays were performed using detached leaves from three-week-old Col-0 plants subjected to different temperature and soil water content conditions. The newest emerging leaf (about 1 cm long) from each plant was placed in a Petri dish filled with water and covered in parafilm, with the leaf petiole inserted into a hole made in parafilm contacting water and infested with 10 age-synchronized adult females. After 36 h of infestation, the number of eggs laid in the leaf or the dish was counted. Eight biological replicates were used for each treatment condition.
2.5 RNA isolation
Six A. thaliana three-week-old rosettes grown at different temperatures (23 and 27°C) or soil water content conditions (normal and mild drought) were pooled for each of the three biological replicates used for each treatment condition. Control plants and plants infested with mites for 30 min were frozen in liquid nitrogen, and stored at -80°C until use. Total RNA was extracted using RNeasy Plant Mini kit (Qiagen) according to the manufacturer's instructions and DNA contamination was digested using RNase-Free DNase set (Qiagen). Quantification and quality of DNA-free RNA were assessed using a Nanodrop ND-1000 Spectrophotometer.
2.6 RNA-Seq library preparation, sequencing, alignment, and DEG analysis
Total RNA was sent to Novogene (UK) Company Limited (Cambridge, UK). Double-stranded cDNA libraries obtained from poly A enriched mRNA were sequenced using an Illumina Novaseq platform and 150 bp paired-end reads were generated. More than 40 M paired-end reads were obtained for each sample. Reads were mapped to the Arabidopsis reference genome (ensemble release 39, TAIR10) using Hisat2 v2.0.5. Mapped reads were quantified using featureCounts v1.5.0-p3. Differential expression analysis was performed with the DESeq2 R package (1.20.0). Differentially expressed genes (DEGs) were considered those genes showing a p-value <0.05 and a fold change higher than 1.5. The set of lists of DEGs for each experiment was used to search the Gene Ontology (GO) database using the Metascape tool (http://metascape.org) (Zhou et al., 2019). Gene enrichment analyses for each comparison were performed with the Bonferroni step-down test using the ClueGO package (Bindea et al., 2009) in Cytoscape (Shannon et al., 2003). Venn diagrams, heatmaps, and violin plots were created with DeepVenn (https://www.deepvenn.com), ClustVis (https://biit.cs.ut.ee/clustvis), and SRPlot (http://www.bioinformatics.com.cn/srplot). The datasets generated during the current study are available in the ArrayExpress repository, accession number E-MTAB-13675.
2.7 Gene families and network analyses
The full set of Arabidopsis transcription factors was downloaded from the Plant Transcription Factor Database (PlantTFDB v5.0) in the plant regulatory data and analysis platform PlantRegMap (Tian et al., 2020). Arabidopsis genes belonging to intracellular and cell membrane receptors were extracted from the literature (Garcia et al., 2021). The KEGG (Kyoto Encyclopedia of Genes and Genomes) database was primarily used to identify genes related to metabolic pathways (Kanehisa & Goto, 2000). Identified genes were classified into metabolic functional categories by using the Pathway Tools Omics Dashboard (Karp et al., 2019). Schematic representations of metabolic pathways were based on the experimentally supported and computationally predicted metabolic pathways available in the AraCyc v17.2.0 database (https://plantcyc.org/content/aracyc-17.2.0). The STRING database v12.0 (https://string-db.org) was used to construct Protein–Protein Interaction (PPI) networks. The CytoNCA plugin for Cytoscape (https://apps.cytoscape.org/apps/cytonca) was used for node centrality analyses in networks. Coexpression networks were identified using the WGCNA tool in the iDEP platform (http://bioinformatics.sdstate.edu/idep11) and visualized in Cytoscape.
2.8 Hormone determination
For plant hormone quantification, six rosettes from three-week-old Col-0 plants from each of the different treatment conditions were pooled per each of the three biological replicates used. Plant hormones (JA, SA, ABA, and indoleacetic acid, IAA) were quantified at the Plant Hormone Quantification Service of the Instituto de Biología Molecular y Celular de Plantas (IBMCP, Valencia, Spain). Extraction and separation of plant hormone profiles were performed as described (Seo et al., 2011). Extracts were passed consecutively through reverse phase, cationic exchange, and ionic exchange columns (Oasis 30 mg, Waters). The final residue was dissolved in 5% acetonitrile and 1% acetic acid and separated by reverse phase UHPLC chromatography with a reverse Accucore C18 column (2.6 mm inner diameter, 100 mm length; Thermo Fisher Scientific) with a 2 to 55% acetonitrile gradient. Hormones were analyzed by electrospray ionization and targeted-SIM using a Q-Exactive spectrometer (Orbitrap detector, Thermo Fisher Scientific). Isotope-labelled hormones were used as internal standards. The concentrations of plant hormones in extracts were determined using embedded calibration curves and the Xcalibur and TraceFinder programs (ThermoFisher Scientific).
2.9 Statistical analysis
Statistical differences among treatments were analyzed by two-way analysis of variance (ANOVA). Shapiro–Wilk tests were conducted to check normality and Levene's tests to assess the homogeneity of variance in the model residual values. Soil water content (W), temperature (T), and its interaction (WxT) were analyzed in the experiments of physical parameters, pigment accumulation, hormonal measurements, rosette damage, and mite fecundity. Tukey's honest significant difference (HSD) post hoc test was applied to compare treatments when more than two groups were compared. Student's t-test was used for pairwise comparisons between two means. Statistical tests are described in the figure legends. The Statgraphics 19 software (https://www.statgraphics.com) was used for all statistical analyses. Model parameters and statistical results, including exact P-values, are reported (Dataset S1).
3 RESULTS
3.1 Mild changes in climate conditions cause slight phenotypical effects
To know how small variations in temperature and soil water content affect the growth and development of Arabidopsis plants, combinations of different environmental conditions were applied. Plants were grown under standard conditions (23°C and normal soil water content) or were subjected to small temperature increases (25 or 27°C) and soil water content reductions (minimal drought or mild drought). After treatments, several physical parameters were quantified (Figure 1). A significant interaction effect between soil water content and temperature was found for all parameters quantified except for rosette size (Dataset S1). Great effects were observed when plants were grown at 27°C and mild drought. These plants had lower rosette size, rosette fresh and dry weight, and rosette water content than plants grown under standard conditions, as well as longer petioles. These results under conditions resembling predicted climate change supported the decision to use only the most divergent tested temperatures (23°C and 27°C) and soil water content conditions (normal and mild drought) for the next experiments.
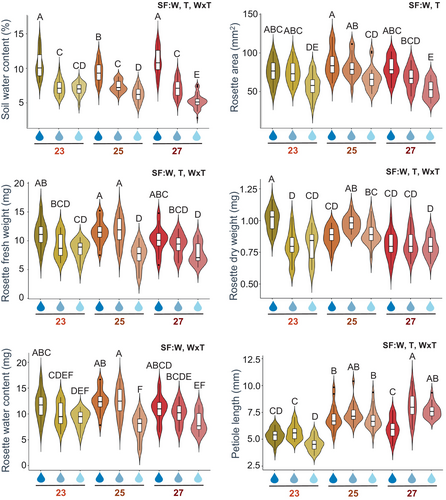
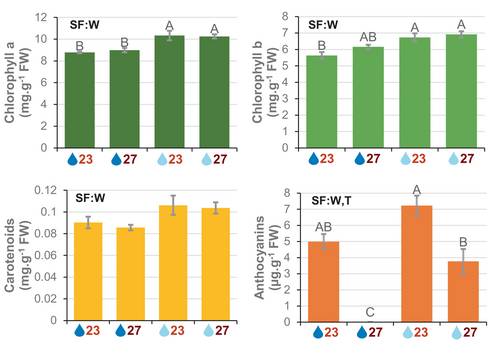
Pigment content was quantified in plants grown under the selected temperatures and soil water content conditions (Figure 2). No significant interaction effect between soil water content and temperature variables was found for any pigment (Dataset S1). Chlorophylls and carotenoids increased significantly in mild drought conditions and did not vary with temperature. Anthocyanins were accumulated to a higher degree in mild drought and their content was drastically reduced in warm temperature conditions.
3.2 Environmental conditions affect plant damage and egg deposition upon T. urticae infestation
Plants that grew in the selected conditions were subjected to mite infestation. After 4 days of infestation, phenotypical differences were measured between non-infested and mite-infested plants. Non-infested plants were bigger in size than mite-infested plants under any environmental condition (Figure 3A). When the rosette areas of non-infested or mite-infested plants were analyzed, a dependent interaction between soil water content and temperature conditions was found (Figure 3B, Dataset S1). Plants that grew under warm temperature and normal soil water content had the largest rosette area, whereas plants that grew under warm temperature and mild drought were significantly smaller. Leaf damage quantifications showed a significantly minor rosette-damaged area in the plants grown under mild drought than in normal soil water conditions. No significant effects were found due to temperature increase (Figure 3C, Dataset S1).
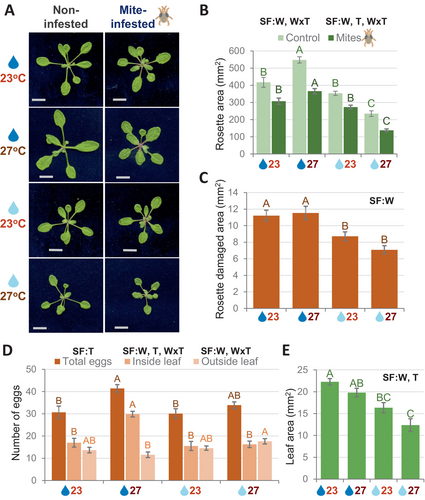
To evaluate mite performance, fecundity assays were carried out and the number of eggs laid inside and outside detached leaves was measured at 36 h after mite infestation (Figure 3D). Whereas the number of eggs deposited inside or outside leaves depended on the interaction between soil water content and temperature, significant differences in the total number of deposited eggs were only due to temperature changes (Dataset S1). Similar numbers of eggs were laid inside and outside in all conditions except for the normal soil water/warm temperature condition. The number of eggs laid inside leaves was higher than outside leaves at this condition, which correlated with a higher number of total oviposit eggs at warm temperature than at standard temperature. Leaf area quantifications after 36 h mite infestation showed lower values under mild drought or mild drought/warm temperature conditions (Figure 3E). More than 80% of the mites survived the 36 h of the experiment in all leaves.
3.3 Mild changes in soil water content and temperature differently affect gene expression
Since the slight phenotypical differences observed in plants grown at different environmental conditions affected mite performance, transcriptomic analysis was carried out in plants grown under the selected conditions (mild drought, warm temperature, and combined mild drought/warm temperature) before and after 30 min of mite infestation. The goal was to detect changes in gene expression underlying the phenotypical effects associated with the different environmental conditions and how the short-term transcriptomic response of the plant to the mite was affected. Using the first three components in the PCA analysis, a good separation of the samples coming from the three biological replicates was found for the temperature and the mite factors (Figure S1 A). A preliminary bulk analysis for gene coexpression showed the presence of abiotic-responsive genes but also biotic-responsive genes in the non-infested and mite-infested samples (Figure S2).
DEGs were obtained for each altered climate condition compared to standard conditions (Dataset S2). The highest number of DEGs was found in the warm temperature/mild drought condition and the lowest in the mild drought condition (Figure 4A). No clear differences were found between the number of down- or up-regulated genes. An analysis of the overlaps of DEGs between the different environmental conditions showed a high number of genes specifically regulated in each condition, a low number of genes regulated in all three conditions, and a substantial number of genes whose regulation is associated with temperature (Figure 4A). Interestingly, the detected DEGs associated with each altered ambient condition were largely different in infested and non-infested samples (Figure S3). Likewise, the expression of the DEGs observed after mite infestation under standard climate conditions changed considerably under altered ambient conditions (Figure 4B). A heatmap was constructed comparing the different altered environmental conditions to control conditions, in both mite-infested and non-infested samples (Figure 4B). Interestingly, many DEGs upregulated in response to the mite infestation at control conditions had a lower expression when plants grew at altered environmental conditions and were infested with mites, mainly at warm temperature.

3.4 Transcriptomic specificities are associated with diverse biological processes
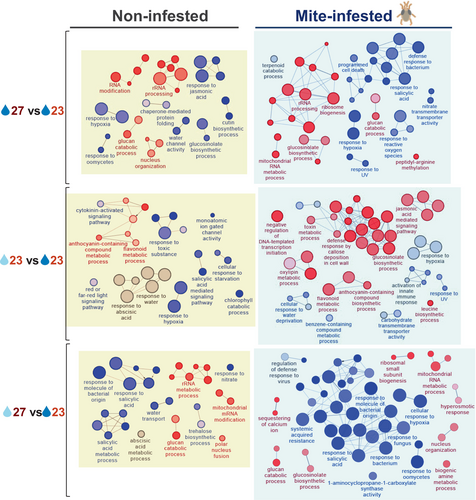
Large variations in gene expression suggested the involvement of diverse biological processes of the plant. All detected DEGs were used to identify the enriched biological processes in the plant triggered by altered environmental conditions (Figure 4C). As expected, some ontology terms related to abiotic responses were found. Besides, a large number of terms related to biotic defense were identified. Many of them were associated with the sets of down-regulated genes, but some also with sets of up-regulated genes. Interestingly, many of the GO terms associated with the sets of down-regulated genes were found in the up-regulated gene set after mite infestation under standard climate conditions.
An analysis of the enriched GO terms showed strong differences among individual comparisons (Figure 5). Notably, biological terms related to the regulation of RNA molecules were found in the up-regulated genes in response to the warm temperature condition, mainly in standard soil water content conditions. Similarly, biological terms related to abiotic stimuli were more enriched in samples subjected to mild drought, mainly at standard temperature conditions. Again, the most remarkable findings were related to defense categories. Salicylic acid-related terms were highly abundant in the down-regulated gene sets from samples subjected to warm temperature/mild drought condition with and without mite infestation and those mite-infested after warm temperature condition. Jasmonic acid-related terms were found among the down-regulated genes in samples at warm temperature and, interestingly, in the up-regulated set of genes of the mite-infested plants under mild drought. Anthocyanin-related terms were only found in the up-regulated genes in mild drought conditions.
3.5 Altered environmental conditions negatively affect the expression of receptors
Enrichment in certain biological processes in response to altered environmental conditions or mite infestation suggests changes in how the plant perceives external cues. Consequently, de novo or enhanced expression of receptors is expected to manage these stimuli. The number of transcript reads showed that at any environmental condition, most receptors up-regulated after mite infestation had already a basal expression in non-infested plants. For example, 67 of the 68 receptors up-regulated after mite treatment at standard environmental conditions had more than 0.1 FPKMs per sequence in non-infested plants at the same conditions (Figure 6A, Dataset S3). When the expression of receptors in non-infested or mite-infested plants at altered conditions was compared with their expression at standard conditions, a down-regulation of most receptors was found. This down-regulation was more relevant in the warm temperature/mild drought condition and lower in mild drought and comprises both intracellular and cell membrane receptors (Figure 6B, Dataset S3). A heatmap was built to show how the expression of receptors induced by mites under standard conditions changes in non-infested or mite-infested plants under altered conditions (Figure 6C). Minor changes were found under mild drought. In contrast, the expression of many receptors induced by the mite under standard conditions was down-regulated at warm temperature, mainly when it was accompanied by mild drought.
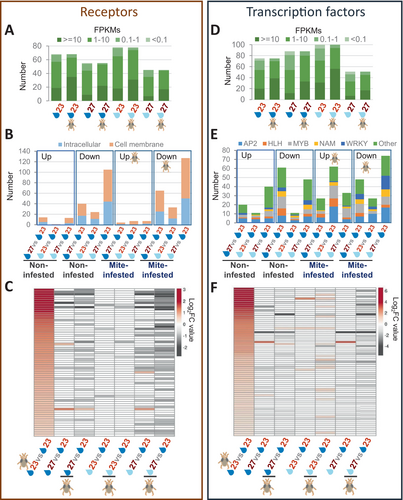
3.6 Specific environmental conditions differentially affect the expression of transcription factors
It is expected that variations in the expression of receptors are accompanied by changes in the expression of the transcription factors (TFs) involved in signaling pathways. As for receptors, the number of reads showed that, at any environmental condition, most TFs up-regulated after mite infestation were already expressed in non-infested plants (Figure 6D, Dataset S4). Different patterns were found when the expression of TFs in non-infested or mite-infested plants at altered conditions was compared with their expression at standard conditions (Figure 6E, Dataset S4). In the comparisons at warm temperature, the down-regulated TFs were always more abundant than those up-regulated. In samples under mild drought only, a few TFs were differentially expressed in basal conditions but an elevated number of them after mite infestation, higher in the set of the up-regulated genes. Among the main TF families involved in plant defense, the most relevant results are the high numbers of TFs from the AP2 and MYB families differentially regulated in all the sets, the high number of HLH TFs up-regulated after mite infestation in the mild drought condition, and the elevated number of NAM and WRKY TFs in the sets of down-regulated genes. The expression of TF differentially regulated in response to mites under standard conditions was also compared with their expression under altered environmental conditions in infested and non-infested plants. A clear pattern was not found in the heatmap (Figure 6F).
3.7 Several TFs act as hubs in the global response to mites under altered environmental conditions
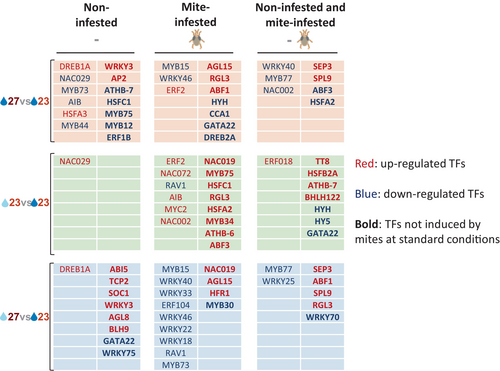
The different expression patterns observed suggest that specific TFs are important in the response to mites when temperature and soil water content change. The TFs that vary with environmental conditions showed an intricate network including a large number of TFs involved in the response to biotic and abiotic stresses, as well as in the development of the plant (Figure S4). To find the most relevant TFs in the network, we chose the closeness parameter to infer centrality. A subnetwork with the 55 TFs with the highest closeness values was built (Figure S5A). The expression of these key TFs was characterized for the different transcriptomic comparisons. Twenty-one of these TFs are induced by mites under standard conditions (Figure S5B). In altered conditions, differentially expressed TFs are likely to be associated with mite infestation, temperature, and/or soil water content variations (Figure 7, Figure S5C). The expression of some of these TFs was only altered in plants not infested with mites. Differentially expressed TFs present in both mite-infested and non-infested networks are likely involved in the response to abiotic stresses. Increased temperature was associated with a decrease in the expression of NAC002, MYB77, WRKY40, ABF3, and HSFA2 and an increase in the expression of SEP3 and SPL9. In mild drought, the expression of HY5, HYH, and GATA22 decreased, and the expression of ERF018, TT8, BHLH122, ATHB-7, and HSFB2A increased. Finally, the warm temperature/mild drought condition was associated with a decrease in the expression of WRKY70, WRKY25, and MYB77, and an increase in the expression of SPL9, RGL3, ABF1, and SEP3. Interestingly, some of these TFs (NAC002, MYB77, WRKY40, ERF018, WRKY25) were induced by mites in standard conditions. Remarkably, several TFs not induced by mites in standard conditions were differentially expressed in altered conditions only in mite-infested plants (Figure 7). On the other hand, TFs that are up-regulated under altered environmental conditions only in mite-infested plants are likely to be closely related to the specific response of the plant to the mite infestation that depends on the environmental conditions. These TFs were mostly up-regulated in mild drought conditions and down-regulated at warm temperatures and combined conditions (Figure 7). Among them, several TFs previously related to defense were specifically up-regulated in mild drought conditions (MYC2, MYB34, MYB75) and down-regulated in warm temperature/mild drought conditions (ERF104, MYB30, WRKY18, WRKY22, WRKY33, WRKY40).
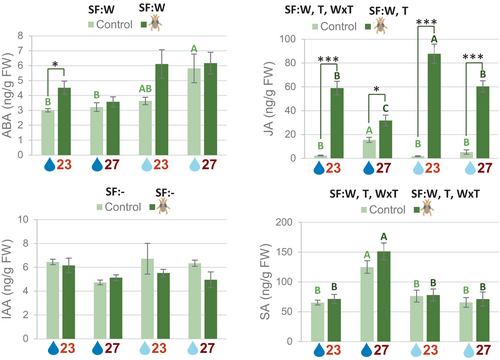
3.8 Environmental conditions affect defense-related hormonal responses
Variations in the expression of TFs are expected to eventually affect the regulation of genes involved in different metabolic pathways. Hormone quantifications in the different biotic and abiotic stress conditions showed differences in the accumulation of ABA, JA, and SA but not for IAA (Figure 8, Dataset S1). ABA accumulation depended on the soil water content and was higher in mild drought conditions. A significant increase in ABA content after mite infestation was only found at standard conditions. JA accumulation in non-infested plants depended on the interaction between soil water content and temperature and was significantly higher in plants grown at normal watering and warm temperature. After mite infestation, the content of JA increased in all environmental conditions. This content was conditioned by the additive and opposite effects of mild drought (positive) and warm temperature (negative). Variations in the accumulation of SA were only found in the warm temperature condition and no significant changes were found after mite treatment in any environmental condition.
Heatmaps with the expression of the differentially expressed enzymes and regulators involved in the jasmonic acid and salicylic acid hormonal metabolic routes showed strong differences when altered environmental conditions were compared with normal conditions (Figure 9). At warm temperature, several negative regulators of JA accumulation were down-regulated in non-infested plants. After mite stress, this down-regulation was not found and some negative regulators were up-regulated. In mild drought, a strong up-regulation of genes involved in the synthesis and degradation of active jasmonates, as well as positive and negative regulators were found after mite infestation. Mild drought/warm temperature conditions showed an intermediate response to environmental conditions in non-infested and mite-infested plants. Regarding SA, down-regulation of positive regulators of the SA route, as well as of genes involved in the synthesis and degradation of SA, was found at warm temperature and mild drought/warm temperature conditions (Figure 9). Under mild drought, minor changes were found in the genes involved in the SA pathway, with an up-regulation of BMST after mite treatment suggesting a possible over-accumulation of the MeSA derivative.
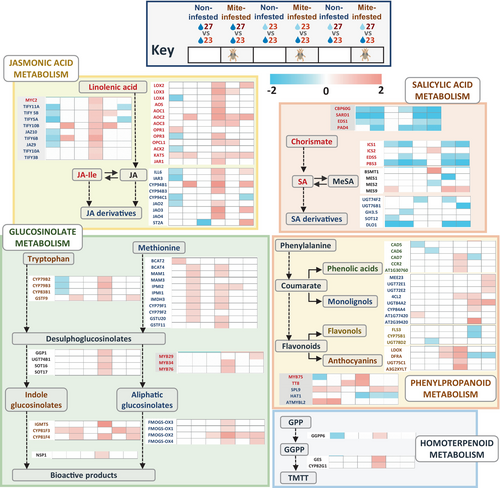
3.9 Metabolic routes for defense-related metabolites are affected by environmental conditions
A careful analysis of the metabolic pathways altered by environmental conditions leads us to select the pathways related to the metabolism of several secondary metabolites involved in defense (glucosinolates, phenylpropanoids, and homoterpenoids). As for hormones, heatmaps with the expression of enzymes and regulators showed differences between altered environmental conditions and normal conditions (Figure 9). Mild drought stress caused a strong up-regulation of most enzymes and TFs involved in the production of indole and aliphatic glucosinolates, phenolic acids, anthocyanins, and the homoterpenoid (E,E)-4,8,12-trimethyltrideca-1,3,7,11-tetraene (TMTT) after mite infestation (Figure 9). In contrast, warm temperature and warm temperature/mild drought conditions were associated with minor up-regulation of glucosinolate routes after mite stress, and with down-regulation of the pathways leading to the production of phenolic acids, flavonols, and anthocyanins. Additionally, the combined altered condition caused the up-regulation of several enzymes involved in the formation of monolignols.
4 DISCUSSION
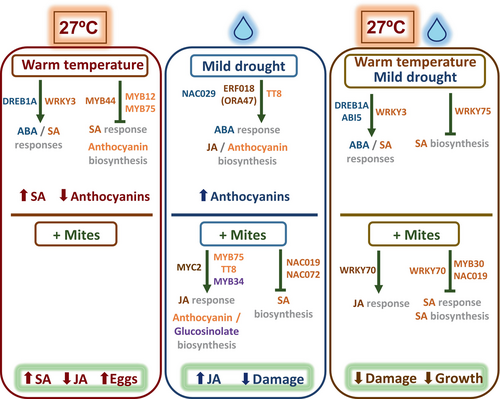
The main goal of this work was to better understand the molecular effects in plants caused by changing climate conditions. As these conditions are highly variable and cannot be accurately predicted, we have selected mild homogeneous variations in temperature and soil water content to partially resemble climate change conditions. Two major questions were targeted as key points to be addressed. The first question is related to the basal changes caused by variations in the ambient conditions. The second question aims to characterize the effect of seed basal changes of the plant during modulated environmental conditions on the subsequent response of the plant to a specific biotic stressor. A schematic representation compiling the most relevant findings has been depicted (Figure 10).
4.1 How relevant are the altered environmental conditions on gene expression and phenotypical features?
The consequences of drought and heat stresses depend on the severity of the stress. We have applied mild stresses to avoid drastic phenotypical changes and simulate global climate change conditions. Mild drought caused reductions in the rosette area similar to that previously observed using similar soil water content conditions (Clauw et al., 2015). Mild temperature increases up to 27-28°C are non-deleterious in Arabidopsis and cause morphological and architectural changes, such as hypocotyl and petiole elongation (Casal & Balasubramanian, 2019). The higher petiole lengths found in our plants grown at 27°C agree with the expected phenotype. No obvious synergistic effects on plant phenotype traits were found when both stresses were combined.
Transcriptomic analyses using our selected conditions have not previously been performed. For warm temperature, increases from 16-17°C to 25-27°C for a short period have been assayed (Capovilla et al., 2018; Cortijo et al., 2017; Pajoro et al., 2017). However, climate warming experiments that increase the air temperature by 2–6°C for a prolonged period, as in our study, are considered necessary to better understand how plants will cope with increasing temperatures (Jagadish et al., 2021). For mild drought, progressive, short-term, or slighter conditions have been used (Baerenfaller et al., 2012; Clauw et al., 2015; Harb et al., 2010; Kumar et al., 2023). More noticeably, transcriptomic analyses combining drought and heat stresses have only been performed in severe conditions (Xu et al., 2023). Thus, this novel approach using warm temperature and mild drought stress is an innovative way to understand how gene expression is modulated by simulated climate change conditions.
Comparing our transcriptomic results with previously well-established temperature responses in Arabidopsis, molecular signatures better resemble prolonged warmth than heat shock mechanisms. HSF1A is a master TF at heat shock that is inhibited by the HSP70 and HSP90 proteins under standard temperature conditions. While heat shock and prolonged warmth repress the expression of HSP70s and HSP90s, induction of HSF1A was only found under heat shock (Wang et al., 2020). Our results also showed repression of some HSP70 and HSP90 genes and no induction of HSF1A, confirming the results expected for prolonged warmth conditions. Likewise, mild drought triggered expression changes similar to that previously observed, as TT8 TF induction (Clauw et al., 2015).
When gene expression was compared under different environmental conditions, a large number of genes were differentially expressed, mostly when ambient temperature varied. RNA processing, and in particular alternative RNA splicing (AS), is a key mechanism that enables plants to rapidly respond and acclimate to new environmental conditions (Calixto et al., 2018). Numerous lines of evidence implicate splicing in plant temperature acclimation leading to major transcriptomic rearrangements (Capovilla et al., 2018; Dikaya et al., 2021). The over-representation of GO terms related to RNA processing in the datasets comparing warm temperatures with standard temperatures supports the broad changes observed. These variations may affect the defense of the plant. The perception of biotic attackers could be diminished since temperature increases are associated with the down-regulation of many receptors. Additionally, warm temperature causes significant changes in the expression of TFs and enzymes involved in defense-related processes, such as jasmonic acid and salicylic acid metabolic pathways, or the production of glucosinolates, anthocyanins, and homoterpenoids. In contrast, minor changes were caused by mild drought, the most remarkable of which affected anthocyanin biosynthesis and the down-regulation of a set of receptors. When warm temperature and mild drought were combined, the transcriptomic analysis was more similar to that observed with only an increase in temperature, but notable differences were found. Specifically, the number of receptors down-regulated was increased and the metabolic pathways that affect the production of defense-related hormones and secondary metabolites were differently regulated. Basal alterations to an organism's transcriptome may increase or decrease its ability to withstand a second stress more successfully (Nawaz et al., 2023). Therefore, the specific alterations observed in the defensive pathways caused by warm temperatures and/or mild drought can affect the response of the plant to a second stressor in different ways.
4.2 How do differences in basal expression affect the response of the plant to a specific biotic stress?
To answer this question, we have focused on two issues: the transcriptomic response of the plant to the biotic stressor T. urticae and the resistance of the plant to the infestation. Here, we show that differential basal expression under changing ambient conditions affects mite-induced variations in gene expression. These changes could be translated into differences in the susceptibility of the plant and the performance of the mite.
We previously found that early responses after 30 min of spider mite infestation identified the highest number of DEGs (Santamaria et al., 2021). As expected, 30 min of mite infestation under different ambient conditions caused changes in the expression of an elevated number of genes. Interestingly, many of these DEGs had a differential expression when compared with the effect of the mite on the plant response under standard environmental conditions. Furthermore, at least half of these genes were not differentially regulated without the biotic stressor, which means that the expression of a substantial number of genes varied in response to the mite depending on the temperature and humidity conditions. As responses under different temperature and watering conditions caused specific responses, we can discuss the effects of each condition separately.
When mite infestation was performed at 27°C with standard soil water content conditions, no differences in plant damage were found in comparison with the infestation at standard conditions, but the performance of the mites increased as they laid a greater number of eggs on the leaf. Better mite performance was previously found in increasing temperatures. Higher rates of daily fecundity were found in T. urticae at 30°C than at 20-25°C on red raspberries (Bounfour & Tanigoshi, 2001). Development, reproduction, and life table parameters of T. urticae determined on peaches at different temperatures suggested that temperatures from 27-30°C are the most suitable conditions for mite reproduction (Rihai et al., 2013). In our experimental approach, the temperature stress affected only the plant before infestation, since the plant once infested was always reared under standard conditions. Therefore, differences in mite performance should be caused by the physiological and molecular changes in the plant. After mite stress, these plants accumulated more SA and less JA than plants that grew under standard conditions. Although both hormones play a role in the plant's response to mites, JA is the key hormone associated with an effective defensive response (Santamaria et al., 2020). Thus, lower JA levels could be responsible for increased mite performance. However, when the transcriptional response of the plant is analyzed, some unexpected results arise. The most intriguing result is the down-regulation of many genes involved in the response to salicylic acid, including some that encode known positive regulators, such as CPB60G, SARD1, EDS1, and PAD4, and the biosynthetic enzymes ICS1 and PBS3. Down-regulation of these genes was also found when plants grew at 23°C and were subjected to a heat wave at 28°C for two days (Kim et al., 2022), but in this case, SA accumulation was also reduced. Strong down-regulation of the DLO1 (S3H) gene could explain higher levels of SA in warm temperature. This enzyme converts SA to 2,3-dihydroxybenzoic acid (2,3-DHBA), which represents the main inactive form of SA (Bartsch et al., 2010). The s3h knockout mutants accumulate very high levels of SA, suggesting that plants regulate SA levels by converting them to 2,3-DHBA to prevent SA over-accumulation (Zhang et al., 2013).
When mite infestation was performed under mild drought at standard temperature conditions, the damage caused by the mite was less, but the number of eggs laid in the leaf did not vary. Previous reports associated mild drought with higher mite performance (Shibuya et al., 2016). In tomato and barley, the performance of T. urticae improved when reared in mild and moderate drought-stressed plants, with increased population growth and leaf damage (Santamaria et al., 2018; Ximénez-Embún et al., 2017). On the contrary, our Arabidopsis plants that grew under mild drought conditions were less damaged by T. urticae. In tomatoes, improved mite performance was related to a higher nutritional value of drought-stressed tomato plants (Ximénez-Embún et al., 2017). In barley, although a higher up-regulation of JA-induced defenses was shown by combined dehydration and mite treatment, the spider mite demonstrated a better performance under dehydration conditions than in well-watered plants (Santamaria et al., 2018). Furthermore, Spodoptera exigua performed less well in drought-stressed Solanum dulcamara plants than on well-watered plants, which was correlated to stronger induction of plant herbivore-induced processes under drought conditions (Nguyen et al., 2016). These findings suggest a mixed scenario where herbivore performance depends on an optimal balance of nutrients in the plant and its adaptation to plant defense compounds, which cannot be accurately predicted (Lin et al., 2023). However, insights into the transcriptomic response of Arabidopsis to T. urticae give us key points that should affect mite performance. Remarkably, the JA-related defenses are strongly induced when the mite feeds on a plant subjected to mild drought stress. Gene ontologies related to jasmonic acid signaling and defensive secondary compounds are overrepresented. These findings were correlated with a higher accumulation of JA and the up-regulation of many genes involved in JA metabolism, the production of glucosinolates and anthocyanins, and the synthesis of the volatile compound TMTT. In addition, many TFs that control these metabolic pathways are also up-regulated. From the network connecting the TFs differentially expressed, a set of TFs were considered hubs because they were the central proteins with the highest connectivity in this protein–protein interaction network. Among these hubs, key activators of mite defense-related pathways were found upon mite infestation under mild drought conditions. The relevance of MYC2 in the response to herbivory has been widely documented, with a crucial role in the JA-activated signaling pathways (Kazan & Manners, 2013). Other TFs are up-regulated under mild drought and have been associated with plant defense. ERF018 (ORA47) is an important activator of JA biosynthesis (Hickman et al., 2017). MYB34 interacts with MYC2 and contributes to the improvement of the number of active MYC-MYB complexes in herbivory to enhance glucosinolate synthesis (Schweizer et al., 2013). MYB75 and TT8 are involved in the WD-repeat/bHLH/MYB modules that activate anthocyanin synthesis in response to JA (Gonzalez et al., 2008). NAC019 and NAC072 suppresses SA accumulation by repressing the expression of ICS1 (Zheng et al., 2015).
When mite infestation was performed at warm temperature with mild drought conditions, the rosette area was significantly smaller, as well as the damaged area caused by mite feeding, but the number of eggs was similar to that observed under standard conditions. Although basal gene expression was more similar to that observed under the warm temperature treatment, the phenotypical response to the mite had more commonalities with the response of the plant to the mite under mild drought. However, the transcriptomic response differs greatly. A strong overrepresentation of ontology terms related to the salicylic acid response was found in the down-regulating set. In addition, many mite-induced receptors and TFs were down-regulated in standard conditions. Commonalities refer to the up-regulation of some enzymes that convert glucosinolates into bioactive products. Interestingly, several WRKY TFs acting as hubs, some of them induced by mites in standard conditions, were down-regulated. The role of WRKYs in defense has been well documented (Wani et al., 2021) and has been more related to a positive induction of the SA pathway than the JA pathway. WRKY75 promotes SA production by directly binding the promoter of ICS1 (Guo et al., 2017). WRKY70 has been involved in the positive regulation of SA-responsive genes and the negative regulation of JA-responsive genes (Li et al., 2004). Whether the lower damage found was mainly due to the down-regulation of the SA-related response or to changes in the nutritional value of leaves remains unknown. Furthermore, the role of ABA in mite defense has recently been demonstrated (Rosa-Díaz et al., 2024). An ABA-deficient mutant exhibited increased mite infestation which was associated with the ABA-triggered stomatal closure. The higher ABA levels observed in the warm temperature/mild drought condition could be hindering mite feeding and restricting leaf damage.
In conclusion, the use of environmental conditions that resemble climate change enables us to highlight the profound alterations in gene expression that may occur in nature. How these basal changes affect the susceptibility/resistance to biotic stressors is far to be predicted. As a consequence, general assertions on the effect of climate parameters should be avoided. An example is the preconceived assumption that drought conditions are optimal for T. urticae performance. Depending on the severity of the drought treatment and the plant species, mites can be favored or disfavored. Understanding specific climate-plant interactions is crucial to determining how global warming will affect crop production in the future.
AUTHOR CONTRIBUTIONS
MM conceived the research. EC and MM performed the experimental research. Both authors contributed to the final version of the manuscript.
FUNDING INFORMATION
This work was supported by the Spanish “Ministerio de Ciencia e Innovación” MCIN/AEI/10.13039/501100011033 (grant numbers PID2020-115219RB-I00, RED2022-134072-T).
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during the current study are available in the ArrayExpress repository, accession number E-MTAB-13675.




