Formulation of a consortium-based Zn biofertilizer, and its quality control, to improve Zn status of wheat grains and Wistar rat blood plasma
Abstract
Zn-deficiency causes immense losses to agriculture and leads to various human health issues adding to the burden on the global healthcare system. Growing zinc-dense cereals using Zn-biofertilizer is one of the most enticing solutions to the problem. In this study a Zn-biofertilizer containing a consortium of Zn-solubilizing strains of Streptomyces sp., Pseudomonas sp. and Zinc oxide nanoparticles as source of Zn was prepared. Strains showing an excellent Zn-solubilization efficiency (>200%), additional plant-growth-promoting traits, abiotic stress tolerance, and root colonization were selected. Seven experiments, mainly comparing the influence of bulk and nano-ZnO as Zn-sources in combination with the prepared Zn-biofertilizer on wheat plant growth and grain Zn-fortification were performed. When wheat plants were grown in the presence of prepared biofertilizer and nano-ZnO a significant increase in plant vegetative growth and grain yield was observed. A 35.1%, 60.5% and 67.2% increase in total- plant length, fresh and dry-weight respectively, was observed compared to the control. Similarly, wheat grains per spike, grain yield, and grain protein increased by 17.0%, 13.9%, and 47.5%, respectively. The Atomic Absorption Spectroscopy and SEM–EDX of wheat grains grown with biofertilizer and nano-ZnO reveal a high Zn-content (43.0 ± 0.5 mg kg−1) in the grains. The AAS analysis of the blood from Wistar rats fed with Zn-dense wheat flour obtained in the study shows a higher Zn-content (7.79 ± 0.18 μg ml−1) in the blood than those fed with control flour. This study conclusively proves that the prepared Zn-biofertilizer with ZnO-nanoparticles can improve the Zn-content of wheat, consequently increasing blood Zn-content in rats.
1 INTRODUCTION
Zinc (Zn) deficiency is a significant global concern adversely affecting the health and growth of both plants and animals. Since this essential micronutrient is involved in various biochemical pathways and acts as a structural constituent and regulatory co-factor in enzymes and proteins, it plays crucial roles in gene expression, enzyme activation, chlorophyll synthesis, signal transduction, and plant defense against diseases (Alloway and Brussels, 2001, Khan et al. 2022, Saleem et al. 2023b). The deficiency of Zn can reduce crop yield, restrict plant development, and lower the nutritional quality of agricultural products (Ozturk et al. 2006). The regular use of agricultural products with low micronutrient density, especially Zn, leads to malnutrition and health conditions especially affecting, children, expecting mothers, and elderly. This micronutrient deficiency is also referred to as “hidden hunger” (van Dijk et al. 2021, Younas et al. 2023). Various human health conditions like adverse pregnancy outcomes, stunted growth, premature death and failure to recover from COVID-19 are now attributed to the deficiency of Zn (Prasad 2008, Khan et al. 2021, Khan et al. 2022).
Since Zn deficiency is mainly due to its unavailability in agricultural soil or due to its availability as insoluble Zn, bacteria capable of solubilizing these insoluble Zn forms play an important role in improving Zn bioavailability in soil. Therefore, among various available solutions, the use of biofertilizers for Zn biofortification offers an ecofriendly, effective, non-toxic, low cost, and socially acceptable solution (Khan and Khan 2022, Khan et al. 2022). As cereal crops are the primary source of dietary Zn, particularly for the population in developing countries suffering the most from malnutrition, the biofortification of cereal crops is of utmost importance. The Zn status of deficient wheat grains having a Zn concentration of <15 mg kg−1 can be significantly improved by the formulation and use of an effective Zn-solubilizing bacteria based biofertilizer (Saleem et al. 2023a). Bacteria facilitate the solubilization of metals and minerals in soil through the production of siderophores and organic acids (Kamran et al. 2017, Costerousse et al. 2018). Various heterotrophic bacteria like Acinetobacter sp., Bacillus sp., Burkholderia sp., Gluconacetobacter sp., Klebsiella sp., Pseudomonas sp., Ralstonia sp., and Serratia sp. are known to perform this function (Kamran et al. 2017, Costerousse et al. 2018). Additionally, it is also found that Zn-solubilizers foster soil microbial diversity by increasing soil fertility (Hussain et al. 2018, Saleem and Khan 2022). Many Zn solubilizers are also known to have additional plant growth-promoting traits and hence can contribute to plant growth through other mechanisms too (Saleem et al. 2023a).
Although Zn solubilization by various bacteria has been reported in previous studies, a systematic study on the subject is missing. More specifically: reports on extensive isolation of Zn solubilizing bacteria, the formulation of a consortia-based Zn biofertilizer, its quality control and effectiveness in improving the plant growth, and Zn content in edible parts are missing. Careful selection of strains capable of effective Zn solubilization, preferably through more than one mechanism like siderophore production and organic acid production is key for the formulation of a good Zn-biofertilizer. It is also desirable that these strains have additional plant growth promoting (PGP) traits and survive well in soil, even under abiotic stress (Yadav and Chandra 2014, Dinesh et al. 2018, Saleem and Khan 2022). Since, microbial consortia based bioinoculants have many bacterial strains, the consortia can have a different set of desirable PGP traits making such inoculants better for the plants than monoculture based bioinoculants (Averill et al. 2022, Khan 2022). Furthermore, nanotechnology has revolutionized many industries and the use of nanomaterials in agriculture is also increasing (Khan et al. 2015, Khan et al. 2016, Khan and Malik 2019). Zinc oxide (ZnO) is used as a source of Zn, and nanoforms of ZnO are known to illicit better plant growth responses than the bulk ZnO (Saleem et al. 2023b, a). However, it has not yet been studied that how the ZnO nanoparticles (ZnO-NPs) influence the survival of Zn solubilizing bacteria in the soil and their PGP activities.
This study, for the first time, presents a comprehensive protocol for the systematic selection of efficient Zn-solubilizing strains based on their zinc solubilization efficiency, additional plant growth-promoting traits, ability to colonize plant root, co-survival in a consortium, and tolerance to abiotic stresses for the preparation of an efficient Zn biofertilizer. We also report for the first time the quality control procedure of the prepared biofertilizer. The ability of the prepared biofertilizer to improve the Zn content of wheat grains was also verified. Finally, the Zn fortified wheat grains obtained in this study were used as feed for Wistar rats to verify that their blood's Zn status has improved.
2 MATERIALS AND METHODS
2.1 Isolation of effective Zinc solubilizing bacteria
2.2 Quantitative assessment of Zn solubilization by isolated bacterial strains using atomic absorption spectroscopy
Strains exhibiting high Zn solubilization efficiency with a solubilization zone diameter of 14 mm or more on basal medium containing insoluble Zn were chosen for detailed study. For quantitative assessment flasks containing 50 mL of basal broth and 0.1% insoluble Zn (ZnO) were inoculated with 1 mL of log phase culture of the strains to be tested. The flasks were incubated at 30°C for 10 days on a shaker set at 150 rpm. The samples from the flasks were collected after 2, 5, and 10 days of incubation, and centrifuged at 12 298 × g for 10 minutes. From the supernatant 1 mL was transferred to a flask, and distilled water was added to bring the final volume to 50 mL. This sample was then used to quantify the available Zn using atomic absorption spectrophotometer (AAS, GBC 932 Plus, GBC Scientific Equipment Ltd.). Medium without culture was used as control. The change in the pH of the broth due to the growth of a test strain was determined using a pH meter (LMPH-9, Labman). The experiments were conducted in triplicates.
2.3 Selection of effective Zn solubilizing strains for the development of the biofertilizer
For the preparation of the Zn-solubilizing bacteria-based biofertilizer, the strains were selected based on their calculated Zn solubilization efficiency (ZSE %). The selected strains were then screened for the presence of various PGP traits like the production of indole acetic acid, ammonia, siderophore and EPS and for the solubilization of phosphate and potassium as detailed in Saleem et al. (2023b, a). The production of hydrolyzing enzymes by the strains, including lipase, cellulase, amylase, and protease, was tested on Luria agar plates with tween-20, basal medium supplemented with 1.0% cellulose, starch agar plates and skim milk agar plates, respectively (Vedder 1915, Sierra 1957, Hendricks et al. 1995, Gopinath et al. 2005, Lal and NJASfM 2012, Dinesh et al. 2018). To check IAA production, the bacteria were grown to log phase in tryptone/peptone yeast extract broth for 3–5 days at 30°C in the dark. Subsequently, 1.5 mL of this broth was centrifuged at 11 200 × g for 10 minutes and 1.0 mL of Salkowski reagent was added to 1.0 mL of the supernatant. The mixture was then incubated at 30°C in the dark for 1.0 h and the appearance of a pink color in the medium indicated a positive test for IAA production. To check the ammonia production by bacteria, the strains were grown in 10 mL sterile peptone broth. To which 1.0 mL of Nessler's reagent was added and the appearance of a yellow-brown precipitate indicated the production of ammonia (Cappuccino and Sherman, 1992). The exopolysaccharide (EPS) by the bacteria was checked using the method described by Mody et al. (1989). The strains were cultured in 100 mL flasks containing 50 mL nutrient broth supplemented with 5% glucose. The broth was incubated at 30°C for five days on a rotary shaker incubator set at 120 rpm. After incubation, the culture was centrifuged at 7168 × g for 30 minutes and three volumes of chilled acetone was then added to one volume of the supernatant (3:1 v/v). The EPS floating on the surface was collected, washed, and transferred to a filter paper (Whatman No. 42). The ability of bacteria to solubilize phosphorus (P) was tested by spotting 10 μL of freshly grown culture on Pikovskaya agar plates and the plates were incubated at 30°C for four to five days. The formation of a halo zone was identified as a positive test for phosphorus solubilization. The ability of bacterial strains to solubilize potassium (K) was tested on Aleksandrov agar medium (Aleksandrov et al., 1967). The freshly grown culture of the strain was spot inoculated on Aleksandrov agar plates and the plates were incubated at 30°C for a duration of five days. The formation of a halo zone around the spot was measured using a scale and recorded. Briefly, lipase production was tested by spot inoculation of the strains on tween 20 agar plates and the plates were incubated for 5–6 days at 30°C (Sierra et al. 1957). The occurrence of an iridescent sheen after five days of incubation indicated a positive test. For cellulase the strains to be tested were spot inoculated on basal agar plates containing 1% cellulose and the plates were incubated for five days at 30°C. After five days, the medium was flooded with 0.01% Congo red solution for 15 minutes, and the plates were destained using 1% NaCl solution for 5 minutes. The presence of a clear zone against the red background indicated a positive test for cellulase production (Hendricks et al. 1995). The production of α-amylase by the strains was tested by spot inoculating the strains on a starch agar plate (Vedder 1915). After five days Gram's iodine solution was added to the starch agar plates and a zone of clearance indicated a positive test. While production of protease by the strains was tested by spot inoculation on skim milk agar plates. Following a five day incubation period at 30°C, the formation of a clear zone around the spot indicated a positive test.
The method of Diabankana et al., (2021) was used to check the ability of the strains to inhibit the growth of the plant pathogen Fusarium oxysporum (ITCC-188) and Alternaria alternata (NAIMCC-F-02141, Diabankana et al. 2021). The Fungal mycelium was spot-inoculated at the centre of the PDA plates. Subsequently, pure bacterial cultures were streaked on the same plate around the fungal spot and the plates were incubated for 10 days at 30°C. Following the incubation period, inhibition of fungal growth by the bacteria was checked. The potential to tolerate abiotic stresses like salinity and drought stress was assessed by checking the ability of the strains to grow on nutrient agar and broth supplemented with different concentrations of sodium chloride (50, 100, 200, 400, 600, 800, and 1000 mM) and polyethylene glycol 6000 (5, 10, 15, 20, and 25% w/v), respectively (Haque et al. 2020). To formulate a consortia-based biofertilizer it is important to ensure that the strains do not inhibit the growth of one another. The in-vitro co-survival of these strains was checked by cross-streaking their log-phase cultures on nutrient agar (Dinesh et al. 2018). The growth was observed after an incubation at 30°C for 2–3 days.
2.4 Polyphasic characterization of selected Zn-solubilizing strains
The selected strains were subjected to a polyphasic characterization for their correct phylogenetic identification including 16S rRNA gene sequencing, and biochemical characterization using standard protocols. Gram staining was performed using the standard protocol of Hans Christian Gram (Smith and Hussey 2005). The morphology of the cells grown to log phase was checked under a scanning electron microscope (JEOL JSM-6510, JEOL) at a magnification of 2,000–25 000 x g and an accelerating voltage of 15 kV. The strains were subjected to various biochemical tests for their correct phylogenetic characterization. The tests included the production of indole, citrate, catalase, oxidase, urease and acid production from sugar and gelatin hydrolysis were performed according to the standard protocols detailed in Cowan and Steel's manual (III edition), (Barrow and Feltham 1993). The phylogenetic position of the strains was also determined based on their partial 16S rRNA gene sequences as described by Khan et al. (2010). The sequences determined in this study were compared with the 16S rRNA gene sequences available in the DNA databank using the BLAST program (Altschul et al. 1997). The MEGA X program was used to calculate the Neighbor-joining phylogenetic tree based on the partial 16S RNA gene sequences and the tree topology was confirmed through a bootstrap analysis from 1000 re-samplings (Kumar et al. 2018).
2.5 Mechanism of Zn solubilization by the isolated strains
Since bacteria produce siderophores and organic acids to solubilize zinc, the strains were tested for the production of these compounds.
2.5.1 Siderophore production
Siderophore production was checked in Modi medium (10 mL) inoculated with 1 mL of inoculant containing ~108 cells ml−1. The inoculated tubes were maintained at 30°C for five days. The cultures were centrifuge at 4427 × g force, and the catechol-type siderophores (specifically salicylic acid; SA and 2,3-dihydroxybenzoic acid; DHBA) in the supernatant were quantified using the Hathway reagent (Reeves et al. 1983). Equal volumes (1:1, v v−1) of Hathway reagent and 0.1 M potassium ferricyanide were added to the supernatant and the solutions were mixed thoroughly. The absorbance at λ560 nm was determined to estimate the concentrations of salicylates. The concentrations were calculated using sodium salicylate as a standard. The concentration of 2,3-DHBA type siderophore was measured by determining the absorbance at λ700 nm and by comparing it to a standard 3-DHBA curve.
2.5.2 Production of organic acid
Organic acid production by the selected Zn-solubilizing strains was checked using Gas Chromatography Mass Spectrometry (GC–MS; Thermo Fisher Scientific) as detailed in Saleem et al. (Saleem et al. 2021, Saleem et al. 2023a). Briefly, the supernatant of the cultures grown to log phase were collected and filtered using 0.45 μM filters and the organic acids were extracted from cell free supernatant using n-hexane. The extracted organic acids were analyzed using a variant 240 GC–MS system equipped with an HPG 1800C series II gas chromatographic detector (GCD) and an HP-5MS column (30 m × 0.25 mm, 0.25-μm film thickness). Helium was used as a carrier gas (16.3 mL/min), with ion source temperature set at 220°C and interface temperature of 270°C.
2.6 Production of a consortium-based Zn-biofertilizer and its quality control
2.6.1 Biofertilizer preparation
Consortium-based Zn biofertilizers were prepared using effective Zn solubilizing bacterial strains. Among the effective Zn solubilizers, strains having multiple PGP traits were selected for the biofertilizer preparation. Three different carrier materials —charcoal, peat, and vermiculite—were evaluated for their suitability to support microbial count in the biofertilizer (Herrmann and Lesueur 2013). Thirty grams of each carrier material was sterilized for 30 minutes at 121°C in an autoclave to eliminate any potential contaminants and spores. A single colony from the pure culture grown on nutrient agar was inoculated in test tubes containing 10 mL of sterile nutrient broth. An aliquot of 2.5 mL from this culture was then aseptically transferred to a 100 mL flask containing 50 mL of sterile nutrient broth. The flasks were incubated at 30°C on a rotary shaker set at 200 rpm for 2–4 days depending on the strain's growth rate. The cultures grown to log phase were then aseptically transferred to 500 mL bottles containing sterile carrier material to achieve a viable count of 109 cells g−1 of biofertilizer slurry as detailed in Saleem and Khan (Yadav and Chandra 2014, Saleem and Khan 2022). The prepared biofertilizer was then subjected to quality control assessments.
2.6.2 Quality control
To determine the pH of the prepared biofertilizer, the biofertilizer was suspended in water (1:1 w/v). The pH of the suspended solution was determined using a pH meter.
2.6.3 Survival of Zn solubilizing strains in soil
To check the survival of the biofertilizer in the soil, the biofertilizer strains were selected based on resistance to rifampicin as a selection marker. The prepared biofertilizer was coated on wheat seeds and the seeds were sown in the soil to study the survival of inoculated biofertilizer in soil. While uninoculated soil was taken as a control. After 15 DAS (Days after sowing), 1 g of soil from the rhizosphere of wheat plants undergoing various treatments were collected and were serially diluted in NSS. From appropriate dilutions, an aliquot of 100 μL was spread on various agar media. Nutrient agar, basal agar medium containing 0.2% of ZnO and King's B medium containing 0.02 g ml−1 of rifampicin were used to determine total aerobic bacterial, total Zn solubilizing bacteria, and total population of biofertilizer strains, respectively in the soil samples.
2.6.4 Root colonization
To determine the suitability of strains for wheat crops, their ability to colonize wheat plant roots was checked. Wheat seeds were grown in-vitro with or without the prepared biofertilizer, and root samples were collected 7–10 days after germination. The root samples were fixed in 2.5% glutaraldehyde for 2–3 hours and subsequently washed with sterile phosphate buffer (Spencer 2019). The samples were dehydrated in a series of ethanol (20%, 40%, 60%, 80%, and 99%) and were observed under SEM with an accelerating voltage of 15 kV to study the bacterial colonization of the root surface.
2.7 Pot experiment to test the efficiency of the prepared Zn biofertilizer for the Zn fortification of Triticum aestivum
The pH of soil used in the study was determined using a pH meter as discussed above, and the DTPA (diethylenetriaminepentaacetic acid)-extracted Zn concentration to quantify bioavailable Zn in the soil was determined using Flame Atomic Absorption Spectroscopy (Lindsay and WJSssoAj 1978). Seven sets of pots were used to grow the wheat (Triticum aestivum) variety PBW343 under various treatments as detailed in Supplementary table 2. The first set of 3 control (con) pots wheat was grown in unamended soil without any fertilizer. While the second set of 3 pots (con_NPK) was fertilized with 120, 60, and 40 Kg ha−1 of nitrogen, phosphorous and potassium (N, P, and K) fertilizers respectively, which is the recommended dose of NPK fertilizer for wheat (Firdous et al. 2018). In the third treatment (con_NPK_biof), the prepared Zn-biofertilizer was used (20 g fertilizer Kg−1 of wheat) to coat the seeds before sowing and the soil was fertilized with NPK fertilizer at the previously mentioned dosages. In the fourth treatment (NPK_Zn_bulk), in addition to standard doses of NPK fertilizers, bulk ZnO as a source of Zn was added to the soil at a dose of 5 Kg ha−1. While, in the fifth treatment (NPK_Zn_nano), instead of bulk ZnO, ZnO NPs were used as a source of Zn in addition to NPK fertilizers. In the sixth treatment (Zn_bulk_biof), standard NPK fertilizers, and bulk ZnO (5 Kg ha−1) were added to the soil and seeds were coated with the prepared Zn biofertilizer. And in the seventh treatment (Zn_nano_biof), ZnO NPs were added at the same dose along with NPK fertilizers to the soil and the seeds were coated with the prepared Zn biofertilizer.
2.8 Plant growth parameters, grain yield, Zn content, Zn uptake and localization in wheat grains
2.8.1 Plant growth parameters
Where, V = Volume (ml) of HCl used for titration.
22 = Equivalent weight of CO2.
N = Normality of HCl (36.5).
W = Weight (mg) of leaves used.
2.8.2 Grain yield
The grain yield was determined, in terms of grains per spike, grain yield (weight of 1000 seeds in g) and straw yield after harvesting the crop at 120 DAS. The protein content in the wheat grains was determined by grinding seeds using a mortar and pestle and extracting one gram of seeds with 3 mL of 50 mM phosphate buffer (pH 7.8), containing 1 mM EDTA, and 2% w/v polyvinylpyrrolidone. The extract was centrifuged for 10 min at 4°C. Double-distilled water to a final volume of 1 mL was added to 0.2 mL of supernatant, the protein content in the prepared sample was determined by the standard method of Lowry et al. (Lowry et al. 1951, Saleem et al. 2023a). For the estimation of the protein, to each sample sodium carbonate 2% (w/v) was added and samples were incubated at room temperature for 10 minutes to denature the proteins. After which Copper sulfate (0.5% w/v) was added to the solution and samples were incubated at room temperature for 10 minutes to facilitate the formation of a copper-protein complex. Then Folin–Ciocalteu reagent (1% v/v) was added to each solution, and solutions were mixed well, and incubated for 30 minutes at room temperature. The absorbance of each solution was measured at 750 nm using a microplate reader.
2.8.3 Grain Zn content
The Zn content in the grains was determined using the modified method of Rathje as detailed in our previous study (Rathje 1959, Saleem et al. 2023a). The seed samples from different treatments were homogenized in a bio-homogenizer. For digestion a finely powdered seed sample was mixed with 15 mL of the acid mixture containing nitric acid (71%), sulfuric acid (97%), and perchloric acid (70%) in a ratio of 9:2:1 vol/vol, respectively (Rathje, 1959). The mixture was kept on a hot plate set at a temperature of 100–120°C until the solution became colorless. The digested solution was diluted to 100 mL and was filtered using Whatman's filter paper number 40 (Whatman, Sigma). The Zn concentration in the samples was determined using Flame Atomic Absorption Spectroscopy.
2.8.4 Uptake and localization of Zn in wheat grains
To study the uptake and localization of Zn in wheat grains grown under various treatments, SEM–EDX analysis and dithizone staining of the wheat grains was performed. For the SEM–EDX analysis the sections of the seeds were prepared after soaking the seeds in water for 1 hour. The Paraffin wax block containing dry seeds were prepared and the blocks were hardened using standard protocol (Spencer 2019). The longitudinal sections of seeds were prepared with the help of a sharp scalpel blade. The sections were subjected to SEM–EDX analysis, and dithizone staining to determine the Zn content in the prepared sections. For SEM–EDX analysis, the sections were fixed in a solution of 2.5% glutaraldehyde in 25 mM PIPES buffer having a pH of 6.9 for 2–4 hrs. After 4 hours the sections were washed with 25 mM PIPES buffer for 3–15 min. The fixed samples were dehydrated in an ethanol series and were visualized under SEM–EDX.
Seeds were stained with dithizone to localize Zn density in the grains as described by Ozturk et al. (Ozturk et al. 2006). The sections were stained for 30 minutes in a dithizone solution. The dithizone solution was freshly prepared by dissolving 500 mg of dithizone (1–5- diphenyl thiocarbazone) per liter of analysis grade pure methanol. Samples were then observed under Light Microscope (Olympus BX60, Model BX60F5, Olympus optical Co. limited) equipped with VGA color camera (Sony, Model no. SSC-DC58AP).
2.9 In-vitro Wistar rat-based study to check the influence of Zn fortified wheat on plasma Zn level
To understand whether feeding the rats with Zn-dense wheat flour obtained in this study improves their blood Zn content, 12 female Wistar albino rats weighing 210 ± 20 g were used. The rats were kept in an in-house animal facility, maintained at 22 ± 3°C and 55% ± 5% relative humidity with a 12 h light–dark cycle. The rats were maintained under laboratory environment with ad libitum and were provided free access to tap water. Animal use was reviewed and approved by the Institutional Animal Ethics Committee (IAEC) at Aligarh Muslim University (D.No. 697/CAH, 09 December 2023). After acclimatization, the rats were divided into two groups, the Wistar albino rats in control group (T1) were fed with a normal diet of ad libitum coated with control wheat flour (unfortified wheat without any treatment) and tap water. In the second experimental group (T2) the rats received ad libitum coated with Zn dense wheat flour (Zn_nano_biof; wheat grown in the presence of prepared Zn biofertilizer and zinc oxide nanoparticles as source of Zn). The rats were maintained on this diet for a period of one month, and their Zn status was monitored through AAS analysis of the blood.
Blood samples were collected on 0 and 30 days of feeding. The blood (1 mL per rat) was collected from the tail vein of the rats using a syringe. And the collected sample was placed in a sterile glass test tube containing EDTA as an anticoagulant solution. The test tubes were held in a slanting position at 25°C for 6 h and were stored at 20°C. To check the concentration of Zn, the 1 mL of blood was placed in a clean 50 mL flask, and 5 mL of an acid mixture (HNO3, H2SO4 and HClO4 in a ratio of 4:2:1 v v−1) was added. The mixture was heated on a hot plate at 150°C for 30 minutes, later the final volume was adjusted to 25 mL with double-distilled water. The prepared blood samples were then analyzed for zinc concentration using GBC 932 Plus Flame Atomic Absorption Spectroscopy.
2.10 Statistical Analysis
The computer software SPSS-25 (SPSS Inc., Chicago) was used for statistical analysis of the data obtained. Duncan's Multiple Range Test (DMRT) was applied at p ≤ 0.05 in addition to calculation of standard deviation (SD) to ascertain the significance of differences between the values to be compared. The graphs were plotted using ggplot2 package of R program.
3 RESULTS
3.1 Isolation of Zn solubilizing bacteria and quantitative assessment of Zn solubilization
A total of 280 strains isolated from different soil samples as shown in supplementary table 3, were found to solubilize ZnO. Among these 32 strains showing a solubilization zone of more than 14 mm on basal medium containing ZnO were identified as effective Zn solubilizers and were selected for further study. When the Zn solubilizing efficiency (ZSE) was calculated, it was found that on the basal medium containing insoluble ZnO, all the strains show a ZSE value of at least 150%. While, 51.5% of the strains exhibited a ZSE values exceeding 200%. Interestingly the strain B89 shows a maximum solubilization zone diameter of 30 mm on basal medium supplemented with ZnO and a ZSE value of 300%.
The quantitative assessment of Zn solubilization at different time intervals was checked in basal broth supplemented with different concentration of insoluble ZnO using AAS. The strains Bh48, B89, 75, 6, S10, 3, Bh2 and B83, were found to solubilize i.e., 138.2, 135.3, 128.6, 120.2, 115.4, 112.9, 107.7 and 104.4 mg of Zn l−1 of broth respectively on the 10th day of incubation. A total of 56.2% of strains solubilized more than 85 mg l−1 of Zn in the tested broth compared to control. The growth of Zn solubilizing strain was coupled with a slight decrease in pH of the spent broth. The strain Bh48 shows maximum Zn solubilization (138.2 mg l−1) at 10th day of incubation which was coupled with a maximum drop in pH to 4.4. While, with the growth of other strains a decrease in pH of only 4.7–6.4 was observed (Table 1).
| S.No. | Strain | Zone of Zn solubilization diameter (mm) | Zn-solubilized in liquid medium (mg l−1) | |||||
|---|---|---|---|---|---|---|---|---|
| 2 days | pH | 5 days | pH | 10 days | pH | |||
| 1 | uninoculated control | 0.0 ± 0.0 | 6.0 ± 0.7 | 7.0 ± 0.4 | 9.1 ± 0.7 | 7.0 ± 0.0 | 14.08 ± 0.6 | 7.0 ± 0.1 |
| 2 | 6 | 27.0 ± 0.0 | 32.9 ± 1.6 | 6.7 ± 1.1 | 74.0 ± 2.1 | 6.6 ± 0.0 | 120.2 ± 1.4 | 4.7 ± 0.0 |
| 3 | 3 | 20.0 ± 0.0 | 8.6 ± 1.5 | 7.0 ± 0.0 | 70.6 ± 1.4 | 6.7 ± 0.0 | 112.9 ± 1.9 | 5.4 ± 0.0 |
| 4 | pp6 | 21.0 ± 0.0 | 24.1 ± 0.9 | 6.6 ± 1.1 | 55.7 ± 1.3 | 6.1 ± 0.0 | 85.3 ± 2.4 | 6.1 ± 0.0 |
| 5 | pp7 | 22.0 ± 0.0 | 31.5 ± 1.1 | 6.3 ± 0.0 | 65.1 ± 1.5 | 5.8 ± 0.0 | 93.5 ± 1.1 | 5.4 ± 0.0 |
| 6 | pp8 | 24.0 ± 0.0 | 23.7 ± 2.1 | 7.0 ± 0.0 | 67.9 ± 1.0 | 5.8 ± 0.0 | 89.3 ± 0.3 | 5.2 ± 0.0 |
| 7 | Bh2 | 24.0 ± 0.0 | 10.5 ± 1.3 | 5.7 ± 0.0 | 67.4 ± 0.6 | 5.4 ± 0.0 | 107.7 ± 5.6 | 5.2 ± 0.0 |
| 8 | Bh48 | 27.0 ± 0.0 | 10.3 ± 2.0 | 6.3 ± 0.0 | 94.2 ± 2.5 | 5.7 ± 0.1 | 138.2 ± 1.3 | 4.4 ± 0.1 |
| 9 | 22 | 27.0 ± 0.0 | 25.7 ± 3.2 | 7.0 ± 0.0 | 66.0 ± 2.6 | 6.5 ± 0.0 | 94.3 ± 2.1 | 5.1 ± 0.0 |
| 10 | 75 | 28.0 ± 0.0 | 23.8 ± 2.4 | 7.0 ± 0.0 | 67.6 ± 2.0 | 6.3 ± 0.0 | 128.6 ± 1.5 | 4.7 ± 0.1 |
| 11 | S10 | 25.0 ± 0.0 | 25.1 ± 3.6 | 6.9 ± 0.0 | 71.4 ± 0.9 | 5.8 ± 0.0 | 115.4 ± 2.7 | 5.2 ± 0.0 |
| 12 | T5 | 19.0 ± 0.0 | 12.5 ± 2.3 | 7.0 ± 0.0 | 34.4 ± 2.7 | 6.5 ± 0.0 | 46.9 ± 2.3 | 5.2 ± 0.1 |
| 13 | T2 | 18.0 ± 0.0 | 10.9 ± 0.8 | 7.0 ± 0.0 | 26.4 ± 0.9 | 6.8 ± 0.0 | 33.2 ± 1.3 | 6.1 ± 0.0 |
| 14 | OE1 | 15.0 ± 0.0 | 10.3 ± 0.7 | 7.0 ± 0.0 | 20.7 ± 1.1 | 6.8 ± 0.0 | 28.6 ± 0.8 | 6.4 ± 0.0 |
| 15 | I7 | 15.0 ± 0.0 | 9.0 ± 0.9 | 7.0 ± 0.0 | 20.0 ± 1.6 | 6.8 ± 0.0 | 28.5 ± 1.2 | 6.1 ± 0.0 |
| 16 | B82 | 18.0 ± 0.0 | 13.7 ± 1.5 | 6.8 ± 0.0 | 23.1 ± 0.9 | 5.7 ± 0.0 | 30.4 ± 1.8 | 4.7 ± 0.1 |
| 17 | S5 | 16.0 ± 0.0 | 11.0 ± 0.9 | 7.0 ± 0.0 | 21.5 ± 1.2 | 6.8 ± 0.0 | 27.0 ± 0.4 | 6.4 ± 0.0 |
| 18 | FB4 | 16.0 ± 0.0 | 11.7 ± 0.2 | 7.0 ± 0.0 | 22.2 ± 2.2 | 6.8 ± 0.0 | 27.7 ± 0.7 | 6.4 ± 0.0 |
| 19 | 2 | 15.0 ± 0.0 | 9.6 ± 0.6 | 7.0 ± 0.0 | 19.5 ± 1.2 | 6.8 ± 0.0 | 26.7 ± 0.7 | 5.4 ± 0.0 |
| 20 | ZnS28-3 | 17.0 ± 0.0 | 12.1 ± 0.6 | 7.0 ± 0.0 | 22.2 ± 2.2 | 6.8 ± 0.0 | 27.7 ± 0.7 | 6.4 ± 0.0 |
| 21 | F7 | 15.0 ± 0.0 | 9.3 ± 0.4 | 7.0 ± 0.0 | 18.5 ± 0.5 | 6.8 ± 0.0 | 23.7 ± 0.7 | 5.4 ± 0.0 |
| 22 | 31 | 15.0 ± 0.0 | 9.6 ± 0.6 | 7.0 ± 0.0 | 19.5 ± 1.2 | 6.8 ± 0.0 | 29.5 ± 0.9 | 5.4 ± 0.0 |
| 23 | AF5 | 15.0 ± 0.0 | 9.6 ± 0.6 | 7.0 ± 0.0 | 19.2 ± 1.0 | 6.8 ± 0.0 | 29.0 ± 0.4 | 6.4 ± 0.0 |
| 24 | Fl4 | 15.0 ± 0.0 | 10.0 ± 1.1 | 7.0 ± 0.0 | 18.9 ± 1.0 | 6.8 ± 0.0 | 31.0 ± 1.7 | 6.4 ± 0.0 |
| 25 | B1 | 22.0 ± 0.0 | 32.5 ± 0.6 | 6.3 ± 0.0 | 65.4 ± 1.7 | 5.8 ± 0.0 | 94.5 ± 0.5 | 5.4 ± 0.0 |
| 26 | B9 | 20.0 ± 0.0 | 27.8 ± 1.0 | 6.3 ± 0.0 | 64.1 ± 0.6 | 5.8 ± 0.0 | 92.5 ± 0.5 | 5.4 ± 0.0 |
| 27 | B57 | 18.0 ± 0.0 | 12.1 ± 0.6 | 7.0 ± 0.0 | 22.2 ± 2.2 | 6.8 ± 0.0 | 27.7 ± 0.7 | 5.4 ± 0.0 |
| 28 | B96 | 20.0 ± 0.0 | 23.4 ± 1.9 | 7.0 ± 0.0 | 62.6 ± 1.4 | 6.7 ± 0.0 | 92.7 ± 0.6 | 5.4 ± 0.0 |
| 29 | B83 | 21.0 ± 0.0 | 24.8 ± 1.3 | 7.0 ± 0.0 | 66.3 ± 1.0 | 6.7 ± 0.0 | 104.4 ± 1.9 | 5.4 ± 0.0 |
| 30 | B5 | 25.0 ± 0.0 | 25.4 ± 0.7 | 7.0 ± 0.0 | 68.3 ± 0.5 | 5.8 ± 0.0 | 90.0 ± 1.5 | 5.2 ± 0.0 |
| 31 | B81 | 22.0 ± 0.0 | 33.1 ± 0.9 | 6.3 ± 0.0 | 64.7 ± 1.5 | 5.8 ± 0.0 | 93.9 ± 1.4 | 5.4 ± 0.0 |
| 32 | B7 | 26.0 ± 0.0 | 25.4 ± 0.7 | 7.0 ± 0.0 | 68.3 ± 0.5 | 5.8 ± 0.0 | 90.0 ± 1.5 | 5.2 ± 0.0 |
| 33 | B89 | 30.0 ± 0.0 | 29.4 ± 1.4 | 7.0 ± 0.0 | 80.6 ± 2.8 | 5.8 ± 0.0 | 135.3 ± 1.5 | 5.2 ± 0.0 |
| 34 | B-00333* | 18.0 ± 0.0 | 13.7 ± 1.5 | 6.8 ± 0.0 | 23.1 ± 0.9 | 5.7 ± 0.0 | 31.4 ± 1.7 | 5.5 ± 0.1 |
- Note: B-00333* is the standard strain.
3.2 Selecting effective Zn solubilizing strains for the development of consortium based biofertilizer
The strains were selected based on Zn solubilization efficiency, additional PGP traits, antifungal activity against plant pathogens and tolerance to abiotic stress.
3.2.1 Plant growth promoting traits
Thirty-two effective Zn-solubilizing strains were screened for the presence of various PGP traits. These traits include production of IAA, ammonia, HCN, siderophore, and EPS, and solubilization of phosphate and potassium, as detailed earlier (Saleem et al. 2023b, a). The strains were also screened for their ability to produce various hydrolytic enzymes including lipase, cellulase, amylase, and protease as reported in our previous paper (Saleem et al. 2023a). A total of thirteen strains (PP8, 2, S10, 22, 75, B1, B83, B7, B89, B82, B5, B81, and B-00333) tested positive for lipase production. The cellulase was produced by 11 strains (strains 3, PP6, PP7, FB4, AF5, FL4, I7, Bh2, Bh48, 22, and B57). The enzyme amylase was produced by strains PP6, PP8, 2, 28–3, S10, F7, T2, I7, Bh2, and Bh48. When tested for protease production fifteen strains (strains 3, PP6, PP8, FB4, 2, 28–3, S10, Bh2, Bh48, B83, B7, B89, B82, B5, and B1) isolated in this study, and Pseudomonas aeruginosa B-00333 tested protease positive for protease production. Among various protease producing strains, strain B89 shows a maximum zone with a diameter of 20 mm on skim milk agar plates.
3.2.2 Inhibition of plant Pathogenic fungi
The dual culture method was used to check the antagonistic activity of 32 effective Zn solubilizers against plant pathogens Fusarium oxysporum (ITCC-188) and Alternaria alternata (NAIMCC-F-02141). It was found that Fusarium oxysporum inhibited the growth of twelve strains (strain Bh2, 75, S5, FB4, 2, ZnS28-3, F7, T5, T2, 31, OE1 and AF5), while remaining twenty strains inhibited the growth of Fusarium oxysporum. It was interesting to note that all the Zn solubilizers suppressed the growth of Alternaria alternata (Saleem et al. 2023b).
3.2.3 Tolerance to abiotic stress
The Zn-solubilizing strains were evaluated for their tolerance to salt and drought stress. The majority of the strains (90.6%) were able to grow even in the presence of a salt concentration as high as 1000 mM, exhibiting significant tolerance to salt stress. Interestingly the majority of the effective Zn solubilizers (78.1%) chosen for the study grew even in the presence of 15% PEG, demonstrating significant tolerance to drought stress. While 18.7% of the strains showed growth in the presence of 20% of PEG, and 12.5% of the strains namely strain 6, PP7, PP8, and S10, showed exceptional drought stress tolerance as these strains weerre able to grow even with 25% of PEG (Saleem et al. 2023b, a).
3.2.4 Growth in co-cultures
For a consortium-based biofertilizer it is important to ensure that the strains included in the consortium do not inhibit the growth of the other strains in a co-culture. Therefore, the ability of the strains to grow in co-culture was checked. Growth in 1,024 co-culture combinations of 32 strains was checked. Notably, in 62.5% of the combinations, the presence of one strain supported the growth of the other strain indicating a positive interaction. In 37.5% of the combinations that included strains PP8, B83, B7, B89, B82, B5, and B81, the inhibition of the growth was observed.
Hence, on the basis of Zn solubilization efficiency, additional PGP traits, abiotic stress tolerance and co-survival compatibility, four strains viz. strain 3, Bh2, Bh48, and PP7 were selected for the development of a consortium based biofertilizer.
3.3 Polyphasic characterization of selected Zn solubilizing strains
3.3.1 Colony morphology and cell shape
The strain 3 formed tan colored, circular, embedded colonies on nutrient agar medium. After three days of incubation spores were produced, imparting a cottony white appearance to the colony (Figure 1 A(i)). The cell shape and arrangement were observed under scanning electron microscope (SEM). The aerial mycelia are characterized by a limited branching, and were predominantly straight or slightly curved and irregular. These filaments lack septation (Figure 1 C(i)). Furthermore, the distal segments of the aerial hyphae are frequently subdivided into unbranched chains of oidial spores. These spores can be stained using crystal violet as shown in Figure 1 B(i). Strain Bh48 forms a light brown to a dusty pink colored colony on nutrient agar medium after three days of incubation (Figure 1 A(ii)). The aerial mycelia of this strain are predominantly straight, and occasionally spiral (Figure 1 B(ii)). When grown on shaker produced a dusty pink pigment. The mycelium fragments into arthrospores and rods of varying lengths (Figure 1 C(ii)).
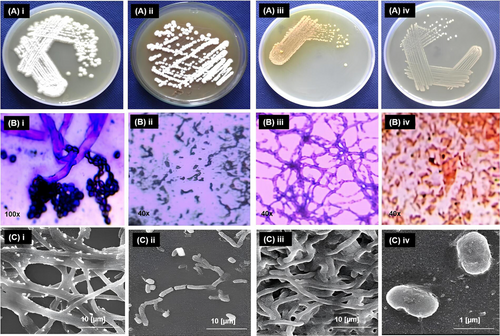
Strain Bh2 forms yellow-colored colonies on nutrient agar medium (Figure 1 A(iii)). The aerial mycelium has biverticillate spiral structure, presenting yellow pigmentation. Notably, these filaments lack septation (Figure 1 B(iii) and C(iii)). Strain PP7 formed cream-colored colonies on nutrient agar medium (Figure 1 A(iv)). When examined using scanning electron microscopy, Strain PP7 appears to have a rod-shaped morphology. Cells of strain PP7 were short rods having a length of 0.7–1.0 μm and a width of 0.4–0.6 μm (Figure 1 B(iv) and C(iv)).
3.3.2 Biochemical properties
The biochemical tests were performed to compare the selected strains with their phylogenetic neighbor. Strain 3, Bh48 and Bh2 tested positive and strain PP7 tested negative in Gram staining. Strain 3 tested positive for spore formation, and gelatin liquefaction and negative for indole production. The spores of strain 3 were gram positive. Glucose, fructose, mannose, lactose, maltose, and glycerol were used as carbon sources by the strain. Strain Bh2 tested positive for spore formation and negative for indole production. This strain utilized glucose, fructose, maltose, sucrose, and mannitol as carbon sources. Strain Bh48 tested positive for spore formation and gelatin liquefaction and negative for indole production. Glucose, maltose, sucrose, and mannitol were used as carbon sources by this strain. Strain PP7 produced acid from glucose, fructose, maltose, and mannitol and tested negative for gelatin liquefaction and indole production (Supplementary table 4).
3.3.3 16S rRNA gene sequencing
The gene for 16S rRNA was amplified and sequenced to determine the correct phylogenetic position of the strains. The sequences determined in the study were submitted to GenBank and the accession numbers of the sequences are given in Figure 2. The results of the BLAST searches showing the closest phylogenetic neighbour of the strains are also shown in Figure 2. The strain 3, Bh2, Bh48 and PP7 show maximum similarity of 99, 99.5, 100, and 99.8%, with the Streptomyces venezuelae strain T9 (KX389563.1), the Streptomyces fimbriatus strain QMA44 (MT525252.1), the Streptomyces laurentii strain NPS17 (OQ096629.1) and the Pseudomonas sp. strain AAUGM-9 (MT549901.1), respectively. The neighbor-joining phylogenetic tree calculated using the partial 16S rRNA gene sequences of the strains and their phylogenetic neighbor are shown in Figure 2A, B, C and D. The trees clearly show that the strain 3, Bh2, Bh48 and PP7 make a monophyletic clade with Streptomyces venezuelae, Streptomyces fimbriatus, Streptomyces laurentii and Pseudomonas sp., respectively.
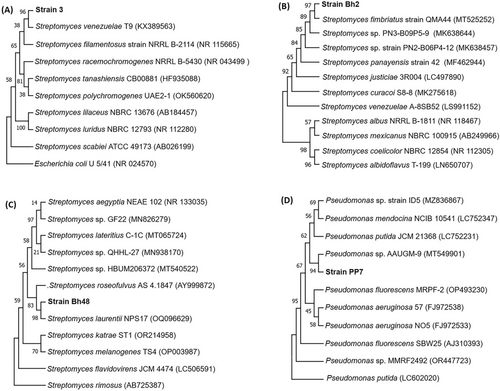
Based on the results of the 16S rRNA gene sequence analysis, morphology and biochemical tests, it is concluded that strain 3, Bh2, Bh48 and PP7 belong to Streptomyces venezuelae, Streptomyces fimbriatus Streptomyces laurentii, and Pseudomonas sp. respectively.
3.4 Mechanism of Zn solubilization by selected Zn solubilizing strains
3.4.1 Siderophore production
The quantitative assessment of siderophore production by four selected Zn solubilizing strains used for consortium based Zn biofertilizer preparation was checked. All the four strains were found to produce a catechol type siderophore. Strain 3, PP7, Bh48 and Bh2 produced 64.5, 89.8, 101.4 and 59.3 μg of salicylic acid per ml of broth, respectively (Table 2). In addition to catechol type of siderophores these strains also produced small quantities of DHBA (2,3 Dihydroxybenzoic acid) type of siderophores. The strain 3, PP7, Bh48 and Bh2 produced 6.3, 2.7, 7.7 and 1.6 μg ml−1, of DHBA respectively in each ml of broth.
| S. No. | Strains | Colony Diameter (mm) | CAS agar Diameter (mm) | Solubilization efficiency (SE) | Solubilization Index (SI) | Siderophores (μg mL−1) | |
|---|---|---|---|---|---|---|---|
| SA | 2,3- DHBA | ||||||
| 1 | 3 | 10.3 ± 0.0 | 12.0 ± 0.0 | 116.5 | 2.1 | 64.5 ± 2.0 | 6.3 ± 0.4 |
| 2 | pp7 | 12.0 ± 0.0 | 20.0 ± 0.0 | 166.6 | 2.6 | 89.8 ± 0.7 | 2.7 ± 0.0 |
| 3 | Bh48 | 10.6 ± 0.5 | 13.3 ± 0.5 | 125.4 | 2.2 | 101.4 ± 4.0 | 7.7 ± 0.3 |
| 4 | Bh2 | 10.6 ± 0.5 | 13.0 ± 0.0 | 122.6 | 2.2 | 59.3 ± 2.2 | 1.6 ± 1.1 |
3.4.2 Production of Organic acid
Organic acids produced by microorganisms help in improving the Zn uptake mainly through lowering the soil pH. The production of various organic acids by the Zn solubilizing bacteria used for biofertilizer production was checked using Gas Chromatography Mass Spectrometry. The GC–MS analysis confirmed the production of various organic acids by the strains selected for biofertilizer production. Most of the strains produced varying quantities of more than 10 organic acids. Strain Bh48, produced various quantities of thirteen organic acids. These include 2-thiobarbituric acid (5.14%), benzoic acid (4.96%), orotic acid (3.01%) acetic acid (0.57%), oxalic acid (0.03%), and salicylic acid (0.03%; Table 3). Strain PP7 also produced thirteen organic acids, however carbonic acid was the major organic acid produced by the strain accounting for 31.1% of the total acids produced (Table 3). Thirteen organic acids were also produced by strain 3, benzoic acid being the most abundant accounting for 16.61% of the total acids produced (Table 3). Interestingly strain Bh2, produced isophthalic as the main organic acid alone accounting for 85.72% of the total acids (Table 3).
| Organic acids | Peak area (%) | |||
|---|---|---|---|---|
| 3 | PP7 | Bh48 | Bh2 | |
| Propanedioic acid | 0.02 | - | - | - |
| Oxalic acid | 5.84 | 0.43 | 0.03 | 0.07 |
| Benzeneacetic acid | 0.79 | 0.62 | 8.77 | 0.26 |
| Formic acid | 0.01 | - | - | - |
| Sulfurous acid | 3.73 | 1.46 | 0.87 | - |
| Acetic acid | 1.9 | 0.07 | 0.57 | 0.02 |
| Propanoic acid | 0.23 | - | - | - |
| Benzoic acid | 16.61 | 3.7 | 7.47 | 0.55 |
| Thiocyanic acid | 0.14 | - | - | - |
| Phthalic acid | 0.21 | 0.31 | - | 0.08 |
| Diglycolic acid | 0.05 | - | - | - |
| Carbonic acid | 0.25 | 31.1 | 3.01 | 0.38 |
| Orotic acid | 14.62 | - | - | 0.35 |
| Thiobarbituric acid | - | 1.5 | 5.14 | 0.93 |
| Salicylic acid | - | 2.59 | 0.03 | 0.32 |
| Isophthalic acid | - | - | - | 85.72 |
| Stearic acid | - | 0.94 | - | - |
| 2-amino benzoic acid | - | 0.93 | - | - |
| Pentanoic acid | - | 0.01 | - | - |
| Phosphonoacetic acid | - | - | 2.4 | - |
| 3–4 Dihydroxymandelic acid | - | - | 4.02 | - |
| 2-Butynoic acid | - | - | 0.06 | - |
- ‘-’ sign indicates that the organic acid was not detected.
3.5 Consortium based Zn biofertilizer preparation and its quality check
Four strains, namely Streptomyces venezuelae strain 3, Streptomyces laurentii strain Bh48, Streptomyces fimbriatus strain Bh2, and Pseudomonas sp. strain PP7 having Zn solubilization efficiencies of 200, 270, 240, and 220%, respectively. Those strains were selected for the formulation of a consortia based Zn biofertilizer. These strains also have at least three additional PGP traits as discussed earlier. The additional PGP traits of the strains in the consortia will collectively augment plant growth and will contribute to soil fertility. The strains show resistance to rifampicin which was used as a selection marker to study the survival of these strains in soil. Furthermore, it was confirmed that the strains within the consortium do not exhibit antagonistic growth.
Three carrier materials namely charcoal, peat, and vermiculite were tested for their ability to support the viability of the strains in the biofertilizer. The pH of the biofertilizers prepared with charcoal, peat, and vermiculite was 7.2, 6.8, and 7.1, respectively. While, the moisture content of the biofertilizer was found to be 38.8, 43.8 and 45.9% when charcoal, peat, and vermiculite, respectively were used as carrier material. After seven days of manufacturing, the total viable count for charcoal, peat, and vermiculite based biofertilizers was 1.35 ± 0.02 × 1010, 2.2 ± 0.41 × 109, and 1.21 ± 0.02 × 1010 CFUs g−1 of biofertilizer, respectively. The viable count at the end of shelf life (180 days) was 6.6 ± 0.20 × 107, 1.4 ± 0.23 × 106, and 5.4 ± 0.23 × 107 CFUs g−1 of biofertilizer in charcoal, peat, and vermiculite based biofertilizer, respectively. No contamination was observed in any of the biofertilizer formulations during the 180 days of shelf life (Table 4).
| Biofertilizer Properties | Charcoal | Vermiculite | Peat | Recommended |
|---|---|---|---|---|
| pH | 7.2 | 7.1 | 6.8 | 6.5–7.5 |
| Moisture (%) | 38.8 | 43.8 | 45.9 | 35–40 for Charcoal 40–50 for peat and Vermiculite |
Viable count (CFU g−1 of slurry) |
5 × 107 g−1 of Biofertilizer | |||
Time of manufacture 0–7 days |
1.35 ± 0.02 × 1010 | 2.2 ± 0.41 × 109 | 1.21 ± 0.02 × 1010 | |
| 30 days | 1.89 ± 0.06 × 109 | 6.4 ± 0.30 × 108 | 9.1 ± 0.41 × 109 | |
| 60 days | 8.2 ± 0.41 × 109 | 9.8 ± 0.30 × 107 | 8.7 ± 0.20 × 108 | |
| 120 days | 9.4 ± 0.20 × 108 | 6.2 ± 0.30 × 107 | 8.5 ± 0.30 × 107 | |
| At expiry (180 days) | 6.6 ± 0.20 × 107 | 1.4 ± 0.23 × 106 | 5.4 ± 0.23 × 107 | 107 g−1 of Biofertilizer |
Contamination during 180 days |
Nil at 105 dilution | Nil at 105 dilution | Nil at 105 dilution | Nil at 105 dilution |
Zone of Zn solubilization with ZnO |
Strain 3: 20 mm Strain Bh2: 24 mm Strain PP7: 22 mm Strain Bh48: 27 mm | Strain 3: 20 mm Strain Bh2: 24 mm Strain PP7: 22 mm Strain Bh48: 27 mm |
Strain 3: 20 mm Strain Bh2: 24 mm Strain PP7: 22 mm Strain Bh48: 27 mm | >10mm |
Particle size of carrier material |
0.045–0.180 mm | 0.5-4 mm | 0.15–0.25 mm | Shall pass through 0.15–0.212 mm IS sieve for charcoal, 4–8 mm for vermiculite, 0.15–0.25 mm for peat |
The tests for different carrier materials and quality control parameters clearly demonstrated that biofertilizers prepared with all the three carrier materials meet the required standard and are suitable for useage in agriculture. However, charcoal was the best carrier material among the three followed by peat and vermiculite, as it supports maximum CFU g−1 of the biofertilizer (Table 4). When the survival of the strains in soil inoculated with three different biofertilizers was checked, it was found that charcoal-based biofertilizer increased the total aerobic bacterial count in soil by 23.18% compared to control 15 DAS. The population of Zn solubilizing strains increased by 266.6% and 112.5% in soil as checked on basal medium containing ZnO and King's B medium (containing rifampicin) respectively, compared to control (Supplementary table 5). This clearly indicates that the inoculated Zn solubilizers survive well in the soil.
The ability of biofertilizer strains to colonize the wheat plant root surface was checked by using a root surface colonization assay. The results of the assay revealed that all the selected strains exhibit a noteworthy ability to colonize root surfaces as shown in Figure 3C and D in comparison to the control (Figure 3A and B). The scanning electron micrographs clearly show the presence of inoculated strains on root epidermis, indicating a successful attachment and colonization.
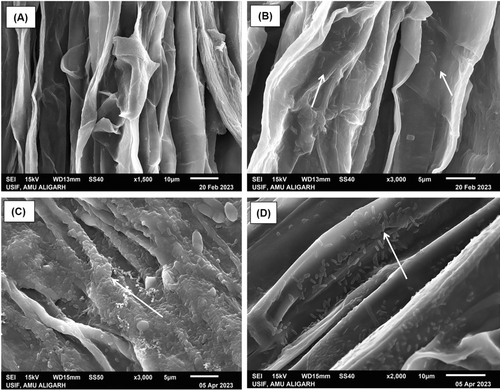
3.6 Effect of consortium based biofertilizer on wheat plant growth, grain yield, protein content, Zn content, and Zn uptake
It was confirmed that the soil used for the study had a slightly alkaline pH of 7.7–7.8 and was Zn deficient with a DTPA extracted Zn concentration of only 0.52 mg kg−1 of soil. The Wheat plant's growth response to a combination of consortia-based Zn biofertilizer and ZnO NPs (5 kg ha−1 used as source of Zn) was checked. Sterile wheat seeds, both without coating and those coated with the consortium-based Zn biofertilizer were sown. The details of different types of treatment used in the study is given in the materials and methods.
3.6.1 Impact of the Zn biofertilizer on seed germination and vegetative growth parameters
The impact of different amendments on the vegetative growth of Triticum aestivum was evaluated. In all treatments a 100% seed germination was observed. An increase in the total plant length, fresh weight and dry weight of 10.7%, 12.7%, and 15%, respectively, compared to the control was observed in the con_NPK_biof treatment where only biofertilizer in addition to standard doses of NPK was used. In the NPK_Zn_bulk treatment having NPK and bulk ZnO as source of Zn, the total plant length, fresh weight, and dry weight, increased by 20.6%, 21.8%, and 24% respectively, compared to the control. While in th NPK_Zn_nano treatment, receiving nano ZnO in addition to NPK, an increase of 21%, 32.7%, and 33.6% in total length, fresh weight, and dry weight was shown respectively, compared to the control. The plants with the Zn_bulk_biof treatment receiving bulk ZnO as source of Zn and the biofertilizer, an increase in total length, fresh weight, and dry weight of 27.1%, 45.4%, and 40.9% respectively, compared to the control was observed. And the treatment Zn_nano_biof where Nano ZnO was used as source of Zn in addition to the biofertilizer, elicits the most notable growth response, showing an increase in total length, fresh weight, and dry weight, of 35.1%, 60.5% and 67.2% respectively, compared to the control (Supplementary table 6). The change in carbonic anhydrase activity was checked in different treatments. The carbonic anhydrase activity in treatment con_NPK_biof, NPK_Zn_bulk, NPK_Zn_nano, Zn_bulk_biof, and Zn_nano_biof increased by 24.3%, 47.5%, 54.9%, 85.5% and 97.2%, respectively compared to the control (Figure 4A, Supplementary table 6). When the influence of various treatments on the photosynthetic pigments of Triticum aestivum was checked, a maximum increase was found in the Zn_nano_biof treatment. There a 7.6% increase in total chlorophyll, and a remarkable 100% increase in carotenoid content compared to the control was observed (Figure 4B, Supplementary table 6). It is also to be noted that, the use of ZnO NPs resulted in a more pronounced increase in photosynthetic pigments than the bulk ZnO.
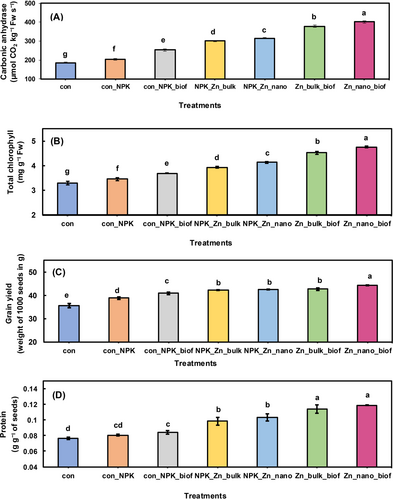
3.6.2 Grain yield parameters
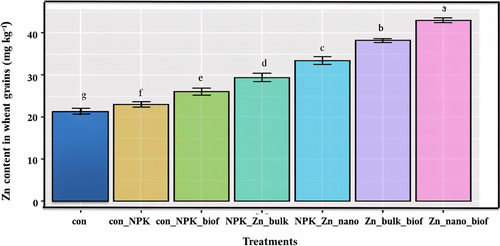
When improvement in grain yield parameters with various treatments was checked, the most notable improvement was observed in the Zn_nano_biof treatment (Table 5, Figure 4C and D). In the con_NPK_biof treatment an increase in grains per spike, grain yield, straw yield and grain protein content of only 9.8%, 5.1%, 10.5% and 5.0%, respectively was observed. In tth NPK_Zn_nano treatment an increase in grains/spike, grain yield, straw yield, and grain protein content of 12.5%, 8.7%, 17.5% and 22.5% respectively, compared to control was recorded. In the NPK_Zn_nano treatment the grains per spike, grain yield, straw yield and grain protein content increased by 12.5%, 9.2%, 33.3% and 28.7% respectively. In the Zn_bulk_biof treatment the grains per spike, grain yield, straw yield and grain protein increased by 14.3%, 10.0%, 42.1%, and 42.5% respectively. The treatment Zn_nano_biof however shows the highest increase of 17.0%, 13.9%, 50.8%, and 47.5% in grains per spike, grain yield, straw yield, and grain protein respectively (Table 5, Figure 4C and D). The most important parameter to check is the change in grain Zn content of Triticum aestivum with various treatments. It was found that the highest increase of 87.1% in wheat grain Zn content was observed with the Zn_nano_biof treatment (Figure 5, Table 5). These results conclusively prove the effectiveness of a consortium-based biofertilizer in combination with ZnO NPs to improve grain yield parameters including grain Zn and protein content in Triticum aestivum.
| Treatment | No. of grains/spike | Grain yield (1000 seeds wt. in (g) | Straw yield gram plant−1 | Grain protein (mg g−1 FW) | Grains Zn content (mg kg−1) |
|---|---|---|---|---|---|
| Con | 34.666 ± 0.577d | 35.523 ± 0.965e | 5.213 ± 0.073g | 0.076 ± 0.001d | 21.27 ± 0.81g |
| con_NPK | 37.666 ± 2.51c | 38.813 ± 0.522d | 5.763 ± 0.064f | 0.080 ± 0.001cd | 22.99 ± 0.66f |
| con_NPK_biof | 41.333 ± 1.52b | 40.883 ± 0.582c | 6.370 ± 0.050e | 0.084 ± 0.002c | 26.03 ± 0.84e |
| NPK_Zn_bulk | 42.000 ± 0.000ab | 42.246 ± 0.063b | 6.793 ± 0.047d | 0.098 ± 0.004b | 29.42 ± 1.01d |
| NPK_Zn_nano | 42.333 ± 0.577ab | 42.443 ± 0.125b | 7.660 ± 0.147c | 0.103 ± 0.004b | 33.44 ± 0.93c |
| Zn_bulk_biof | 43.000 ± 1.000ab | 42.733 ± 0.508b | 8.190 ± 0.070b | 0.114 ± 0.005a | 38.21 ± 0.47b |
| Zn_nano_biof | 44.000 ± 0.000a | 44.253 ± 0.115a | 8.620 ± 0.051a | 0.118 ± 0.000a | 43.03 ± 0.55a |
3.6.3 Zn uptake in wheat grains as checked by using SEM- EDX
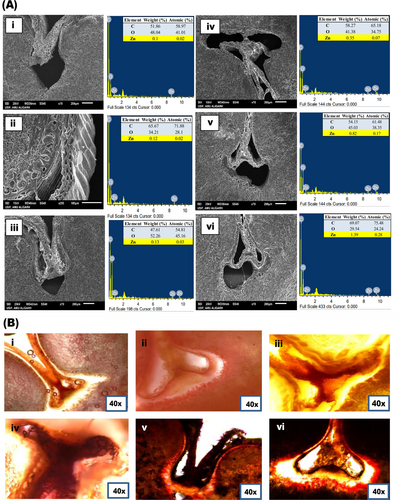
The weight percentage of Zn detected in the EDX spectra of grains obtained in various treatments is given in inset tables of each EDX spectrum of grains obtained with various treatments (Figure 6(i)). The surface composition of wheat grains exhibited peaks for carbon (C), oxygen (O), and zinc (Zn), as shown in Figure 6(i). The maximum Zn weight percentage of about 1.39% was obtained in the grains of plants grown with a combination of ZnO NPs with biofertilizer (Zn_nano_biof; Figure 6(i)F).
3.6.4 Dithizone staining of wheat grains
Dithizone dye was used to localize Zn in various tissues within the wheat grains. The development of red color due to the dithizone-Zn complex formation shows the presence of Zn in both embryonic axis and endosperm transfer cells as shown in Figure 6(ii). The addition of Zn in the form of ZnO NPs and bulk ZnO led to a noticeable improvement in the red color intensity, indicating an increase of Zn content in grains. However, among various treatments, the highest intensity of red color was observed in the grains obtained in the Zn_nano_biof treatment indicating the highest concentration of Zn as shown in Figure 6(ii)F.
3.7 Effectiveness of fortified wheat to improve Zn status of Wistar rat blood
Our results demonstrated that the combination of the prepared Zn-biofertilizers with ZnO-NPs as the source of Zn significantly increased the Zn content of the wheat grains (87.1%) compared to the control. When the wistar rats were fed with this fortified wheat and the concentration of Zn in their blood was checked, a notable increase in the blood Zn content was observed. The Zn concentration in the blood of the rats fed with zinc-enriched wheat was 7.79 ± 0.18 μg ml−1, while the Zn concentration in blood of rats fed with the control wheat flour was only 4.54 ± 0.57 μg ml−1.
4 DISCUSSION
This study for the first time focuses on the development of a consortium-based Zn-biofertilizer to fortify wheat flour with an aim of increasing the Zn status of the human diet. A holistic approach was taken wherein an effective Zn-biofertilizer containing a consortium of four Zn-solubilizing bacteria and ZnO-NPs was developed, the prepared biofertilizer was subjected to quality control and was applied to soil. The survival of inoculated strains in soil was validated, and an increase in the Zn-density of wheat grains grown with the prepared biofertilizer was confirmed. When this Zn-fortified wheat flour was used as feed for Wistar rats, an increase in blood Zn concentration of Wistar rats was also observed. For the development of a consortia-based Zn-biofertilizer, strains were chosen carefully using an extensive screening of the strains for Zn-solubilization efficiency, additional PGP traits resilience to environmental stress, and an antagonistic activity against plant pathogens. It is advantageous to use a consortium over a monoculture based inoculum as multiple strains may have diverse PGP benefits to offer to plants (Khan 2022).
Earlier studies have not taken an extensive attempt to isolate such strains in high numbers or to choose strains that offer additional benefits to plants (Gontia-Mishra et al., 2017; Bhatt and Maheshwari 2020; Upadhayay et al., 2024). Interestingly, a total of 280 Zn solubilizing strains were isolated out of which 32 strains (56.2% of the total) show remarkable Zn-solubilization potential both on solid (ZSE of >140%) and liquid medium (85 mg l−1). Some strains like strain B89 and strain Bh48 even show a ZSE of 300% on basal medium and a Zn solubilization of 138.2 mg l−1 of Zn in liquid medium, respectively. These bacteria solubilized Zn either through the production of siderophores or through the production of organic acids. The production of organic acids in the broth was also evident from the drop of pH from 7.0 to 4.4. Additionally, the effective Zn solubilizers were screened for the production of various hydrolytic enzymes (lipase, amylase, cellulase, and protease), which benefits the soil ecosystem by contributing to organic matter decomposition, and nutrient cycling (Fasiku et al., 2020; Daunoras et al., 2024). The strains used for biofertilizer formulation also exhibited an antagonistic activity against soil-borne plant pathogens Fusarium oxysporum and Alternaria alternata, indicating their additional potential as biocontrol agent. Rhizospheric microorganisms can suppress fungal pathogens through mechanisms like the production of antifungal compounds, the competition for nutrients, and the induction of systemic resistance in plants (Mendes et al., 2013; Ciancio and Pieterse, 2019; Yin et al., 2021). Another important property of bacteria used for biofertilizer preparation was stress tolerance. The adaptability of the strains to saline and drought conditions, can help in mitigating abiotic stress in crops (Yang et al., 2023). It was also confirmed in-vitro that strains used for biofertilizer preparation do not inhibit the growth of one another in the prepared consortia.
It is also very important to correctly identify the strains using the guidelines of The International Committee on Systematics of Prokaryotes (ICSP). Therefore, using the recommended polyphasic approaches, strain 3, Bh48, Bh2, and PP7 were identified as Streptomyces venezuelae, Streptomyces laurentii, Streptomyces fimbriatus, and Pseudomonas sp., respectively. Members of Streptomyces and Pseudomonas are generally found in the soil and hence it was expected that these strains will easily survive in soil. Therefore, we included members of Streptomyces for the biofertilizer production although very few reports are available on the use of Streptomyces for biofertilizer production (Doolotkelvieva et al. 2015). Solubilization of Zn by the members of the genus Streptomyces and Pseudomonas have been reported earlier as well (Costerousse et al., 2018).
Bacteria solubilize Zn mainly through the production of siderophores and organic acids. All the strains selected for the biofertilizer preparation, were found to produced siderophores. The major siderophores produced by the selected strains were catechol-type siderophore, namely salicylate and 2, 3-Dihydroxybenzoic acid. These strains also produced various organic acids in addition to siderophores. The organic acids produced by ZSB like citric, malic, and oxalic acids lowers soil pH, consequently improving Zn bioavailability (Debela et al., 2013; Costerousse et al., 2018; Haroon et al., 2022). Other studies have also reported the production of various organic acids including oxalic, maleic, tartaric, fumaric, gluconic, malonic, and citric acids by the Zn solubilizing strains of Burkholderia cepacia (H1), Acinetobacter baumannii (H3), Aspergillus niger, Curtobacterium, Plantibacter, Pseudomonas, Stenotrophomonas, and Streptomyces (Costerousse et al., 2018; Upadhyay et al., 2021).
For an effective biofertilizer, other properties like survival of inoculated strains in carrier material during the shelf life are also important. Very few studies have reported the quality control parameters of biofertilizers. This study subjects the prepared biofertilizer to standard quality control. It was concluded that charcoal is the best carrier material for the biofertilizer formulation because it better supports the viability of the inoculated strains in comparison to vermiculite and peat. The colonization assays show that selected strains effectively colonize the wheat root surface. Interestingly, Zn solubilizing strains that colonize the root surface are known to improve seed germination, root length, and Zn content in wheat plants as reported in earlier studies (Kamran et al., 2017).
When tested in pots, a combination of Zn-biofertilizer with ZnO-NPs successfully improved the Zn content of the wheat grains in addition to improving vegetative growth of the plants. An increase in photosynthetic pigments (Chlorophyll and carotenoids) was also observed in this study, a similar observation was made in our previous studies also (Faizan et al., 2018, Saleem et al., 2023a). The results also proved that ZnO nanoparticles elicit stronger plant growth compared to bulk ZnO, which was observed in earlier studies also (Adil et al., 2022). Most importantly ZnO-NPs resulted in a higher Zn content (87.1% w/w) and protein content (47.5% w/w) in grains than the bulk ZnO in the presence of a Zn biofertilizer. Increase in grain protein content helps in Zn absorption in the human gut (Sandström et al., 1980). The localization of Zn in wheat grains was determined by staining the grains with dithizone dye. The highest intensity of red color indicative of highest Zn concentration was observed in the aleurone layer of grains grown with a combination of Zn biofertilizer and ZnO-NPs. When confirmed with SEM–EDX similar observations were made. It is concluded in this study that the application of ZnO-NPs in combination with a consortium based biofertilizer significantly improves the Zn content in wheat grains and has immense potential to overcome the menace of Zn deficiency. Furthermore, the feeding of Wistar rats with Zn dense wheat obtained in this study significantly improved the Zn content in Wistar rat blood. Therefore, this strategy has the potential to improve Zn nutrition and mitigate the health risks of Zn deficiency in populations relying on a cereal-based diet. The biofortification of wheat grains with Zn through the use of biofertilizers offers a sustainable approach to address micronutrient deficiencies in both plants and animals, including humans.
5 CONCLUSION
Zn-fortification of cereal crops using a combination of effective Zn solubilizing bacteria based biofertilizers and ZnO-NPs is one of the most feasible, low-cost and socially acceptable approach to deal with the global problem of Zn-deficiency. A holistic approach was taken in this study, wherein a consortia-based Zn-biofertilizer with additional PGP traits was carefully formulated and was subjected to quality control. It was proven that it is important not to only choose good Zn solubilizers, but it is equally important to investigate their survival in soil, compatibility with the carrier material in a biofertilizer. We found that inoculated strains best survived in charcoal as carrier material and the survival of biofertilizer strains in soil was also confirmed. Therefore, the Zn-biofertilizer prepared in this study can be used for crops to improve their vegetative growth, grain Zn content, and crop productivity. The fortified wheat grains obtained can improve the blood Zn-content as proven in our experiment on Wistar rat. Hence, this study for the first time proves conclusively that the microbe-based Zn-biofortification used here can help in overcoming the menace of Zn deficiency.
AUTHOR CONTRIBUTIONS
S.S. carried out all the experiments, planning and wiriting of the manuscript, S.T.K. did the planning, funding and writing of the manuscript.
ACKNOWLEDGEMENTS
We acknowledge the financial support provided by the SPARC (P594), MHRD, Govt. of India.
CONFLICT OF INTEREST STATEMENT
Authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all new created data is already contained within this article and in supplementary material.




