Sex and salt stress response – physiological and biochemical aspects of hydroponic culture of dioecious Rumex thyrsiflorus Fingerh
Abstract
This study investigates the sex-specific physiological and biochemical responses to salt stress in male and female Rumex thyrsiflorus plants under hydroponic culture conditions. In vitro regenerated plants were exposed to different sodium chloride (NaCl) concentrations (0, 43, and 86 mM), and the resulting changes in morphology, photosynthetic performance, and biochemical profiles were analyzed. Salt stress resulted in significant morphological adaptations, including reduced leaf area and closed stomata, particularly in the male plants, indicating adaptive strategies to minimize water loss and ion toxicity. Photosynthetic efficiency, especially the photochemical performance of photosystem II, decreased under elevated NaCl levels, with a marked reduction observed at 86 mM. Biochemical analyses revealed remarkable responses, including increased enzymatic antioxidant activities and the accumulation of free proline, a known compatible osmolyte, as well as branched-chain amino acids, soluble proteins, and carbohydrates. These shifts in metabolite profiles varied by sex, with male plants showing a greater increase in compounds such as proline, γ-aminobutyric acid, methionine, and the osmoprotectant sucrose, highlighting sex-specific patterns of metabolic adaptation. Females showed higher chlorophyll retention and greater resistance to oxidative damage, suggesting a range of different adaptive strategies. The study highlights the importance of identifying sex-specific stress responses in R. thyrsiflorus, which has implications for breeding programmes aiming to improve crop resilience. These results expand our understanding of plant stress biology and provide valuable insights for further research into how dioecious plants respond to environmental challenges.
1 INTRODUCTION
The world population is expected to grow to 9.6 billion people by 2050, which will require a 70% increase in food production. However, rising environmental stressors like salinity, which currently affects more than 20% of farmland, make this challenge more difficult. By 2050, more than half of the world's arable land could contain enough salt to cause plants' salt stress, which could reduce agricultural production by 50% (Islam et al., 2019; EL Sabagh et al., 2021). Initial exposure to salt impairs the ability of plants to take up water from the soil and alters the water balance of cells, limiting cell expansion and growth rate. In addition, stomatal opening and transpiration are reduced, limiting the plant's ability to take up CO2 and thus reducing RUBISCO activity and the efficiency of the Calvin cycle (Abogadallah et al., 2010). Prolonged exposure to salinity leads to ionic stress, primarily due to sodium chloride (NaCl), causing nutritional imbalances and oxidative stress, with increased levels of reactive oxygen species (ROS), changes in enzyme activity and dysfunction of photosynthetic and mitochondrial electron transport chains, ultimately leading to shifts in cell metabolism and premature ageing of mature leaves (Hasanuzzaman et al., 2021; Dell'Aversana et al., 2021 and references therein). Plants exhibit sophisticated and advanced mechanisms to adapt to the osmotic and ionic stress caused by elevated salinity. This adaptation primarily involves the strategic adjustment of osmoregulation by specific osmotic regulators, including proline (Pro) (Munns, 2005; Najar et al., 2020). Moreover, plants respond to ionic/oxidative stress by two other enzymatic and non-enzymatic intracellular mechanisms. The first mechanisms involve specific enzymes such as catalase (CAT), peroxidase (POX) and superoxide dismutase (SOD), while the non-enzymatic mechanisms are controlled by molecules such as plant secondary metabolites (SMs) (Golkar et al., 2019; Datta et al., 2021). Additionally, compatible osmolytes such as Pro, alanine (Ala), and aminobutyric acid (GABA) may play an important role as antioxidants and osmolytes (Carillo, 2018; Carillo et al., 2019b).
SMs are responsible for the survival of plants under harmful environmental conditions (González Mera et al., 2019). Under laboratory conditions, the production of specific SMs can be significantly increased by triggering plant immune responses through various biochemical techniques, such as elicitation (Golkar et al., 2019). Elicitors used under in vitro conditions include a range of physical factors such as UV radiation, osmotic pressure, and temperature fluctuations, as well as chemical factors such as heavy metals, antibiotics, and salinity (Datta et al., 2021). For instance, in the callus tissue of dioecious Rumex thyrsiflorus, the most appropriate concentration of NaCl (salt stress) to increase SMs production in the callus suspension obtained from the hypocotyls of female seedlings was 129 mM NaCl, while in male seedlings it was 43 mM NaCl in the culture medium (Gozdur et al., 2024).
Stress factors can disrupt the photosynthetic process, leading to a reduction in energy dissipation through the photochemical pathway. As a result, there is an increase in dissipation through the non-photochemical pathway, specifically through chlorophyll fluorescence (Cetner et al., 2016). Measuring the fluorescence of chlorophyll a from photosystem II (PSII) is a practical, non-invasive method commonly used by plant physiologists. This technique provides valuable insights into the functioning of PSII, particularly in plants exposed to environmental stress, making it a crucial tool for assessing the effects of various stressors on photosynthetic efficiency (Sulkiewicz and Ciereszko, 2016; Ashraf and Harris, 2013).
The family Polygonaceae Juss. includes the thyrse sorrel (Rumex thyrsiflorus Fingerh.), a model taxon for the study of the karyology and evolution of plant sex chromosomes (Grabowska-Joachimiak et al., 2012). Its dioecious nature makes it possible to study the effects of a particular stress factor on sex. A study conducted on this species (Gozdur et al., 2023) confirms that the response to heat stress depends on sex and that the activity of enzymatic antioxidants such as SOD and CAT plays an important role in the response to high-temperature stress. The healing properties of the Polygonaceae family have been widely used in the treatment of various diseases. The therapeutic qualities of the genus Rumex are attributed to its SMs, which exhibit a range of beneficial effects (Tonny et al., 2017). R. thyrsiflorus contains various biologically active phenolic compounds (PCs), including flavonoids such as catechin and myricetin as well as phenolic acids (e.g. chlorogenic acid and caffeic acid) (Dziedzic et al., 2020; Gozdur et al., 2024). In addition, Rumex contains anthraquinone derivatives, in particular emodin and chrysophanol, which have an inhibitory effect on bacteria (Abu Thaher, 2022; Gozdur et al., 2024).
The present study investigated the concentration- and sex-dependent effects of NaCl treatment on in vitro regenerated female and male thyrse sorrel plants grown in hydroponic culture. It focuses in particular on changes in plant morphology, photosynthetic activity, photosynthetic pigment content, stomatal apparatus parameters, enzymatic antioxidant activity, i.e. SOD and CAT, as well as soluble proteins, free amino acids (AAs) including Pro, carbohydrates, and PC content. The hypothesis is that the response to salt stress varies significantly depending on the sex of the plant. If these differences are confirmed, they could provide valuable insights for future research and help to develop targeted strategies for breeding and cultivating salt-tolerant plants, which is becoming increasingly important in view of the current environmental challenges.
2 MATERIALS AND METHODS
2.1 Plant material
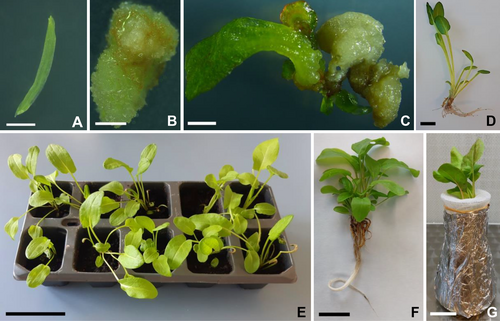
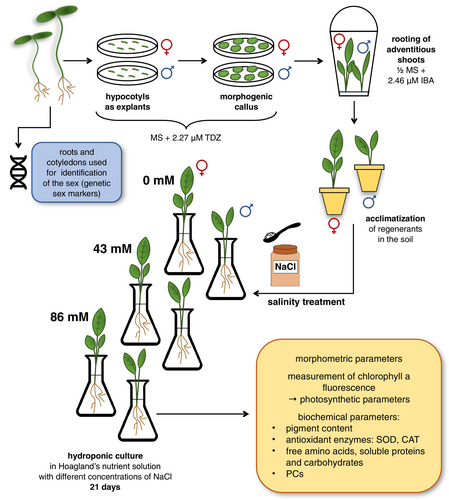
The seeds of Rumex thyrsiflorus Fingerh. (POLAN©) were first sterilized in 70% (v/v) ethanol for 1 min, followed by 50% (v/v) commercial bleach (<5% sodium hypochlorite) for 12 min. The seeds were then rinsed 3 times in sterile distilled water. Germination lasted 11 days in a culture room at 25 ± 3°C, 16 h/8 h photoperiod (day/night), and fluorescent white light (intensity: 60–90 μmol m−2 s−1). Hypocotyls (ca 5 mm) were isolated from seedlings and placed on MS medium (Murashige and Skoog, 1962) containing 3% (w/v) sucrose, 2.27 μM thidiazuron (TDZ), and 0.8% (w/v) agar, as described by Ślesak et al. (2017). The explants (Figure 1A) were lined up on a Petri dish (60 mm) (5 explants/per Petri dish). The experiment was repeated twice (total 150 explants/30 Petri dishes). The transfer of callus tissue induced on hypocotyls to fresh medium took place every 4 weeks (Figure 1B). Arisen adventitious shoots (Figure 1C) were rooted in ½ MS medium supplemented with 2% (w/v) sucrose, 0.8% (w/v) agar, and 2.46 μM indole-3-butyric acid (IBA). After 10 weeks, the regenerated plants that had developed a root system (Figure 1D) were transferred to pots with soil (Figure 1E) and acclimated to the ex vitro conditions in the culture room (conditions as described above). After 8 weeks, selected regenerants (Figure 1F) of both sexes were removed from the soil and transferred to flasks containing Hoagland's nutrient solution (Figure 1G) (Hoagland and Arnon, 1950) (Table S1) for 7 days of adaptation.
2.2 Identification of sex
Genomic DNA was extracted from the leaves of regenerated plants using the hexadecyltrimethylammonium bromide (CTAB) method (Gawel and Jarret, 1991) with modifications (Kwolek and Joachimiak, 2011). A PCR-based technique using DNA markers on the Y chromosome was used to determine plant sex. Primers developed by Korpelainen (2002) were used, which amplify the male-specific RAYSI sequence on the Y chromosomes of Rumex acetosa and its close relatives (Navajas-Pérez et al., 2006): RAY-F (5′-ACTCGAATGTAAGCATTTGGTCCTA-3′) and RAY-R (5′-ACTACACGATTGTCCATAAAGTGGA-3′). In addition, primers URG08-F (5′-CCAATTGGTCTCAACTAGAACA-3′) and URG08-R (5′-TGTTATAGGTTTTGGACTGCCA-3′) were used, which amplify the male-specific repeat sequence RAYSII in R. acetosa (Mariotti et al., 2009). To check the quality of the template DNA, primers amplifying the autosomal sequence of RAE730 were used: R730-A (5′-CTCGGACCAATTATCTCAT-3′) and R730-B (5′-CATTATTTGGAGCCGAT-3′) (Navajas-Pérez et al., 2005). The amplification reaction was performed in a T100 thermocycler (BioRad) (for details, see Ślesak et al., 2015). PCR products were separated on a 1% (w/v) agarose gel in 1× TBE buffer with Simply Safe (EURx) for 60 min at a constant voltage of 120 V. Perfect Plus 100 bp DNA Ladder (EURx) was used as molecular standards.
2.3 Experimental design and salinity treatment
- the length of the aboveground part and the length of the root were measured, the leaves were counted, and the area of each leaf was calculated,
- 9 random leaf discs of 1 cm2 were taken for relative water content (RWC) analysis,
- imprints were taken from the abaxial leaf surface of 3 random individuals of each sex,
- the fluorescence of chlorophyll a from PSII was measured using a HandyPEA fluorimeter (Hansatech),
- 3 randomly selected leaves (pooled samples) from male and female individuals were used for analysis of photosynthetic pigment content (0 day and 21 day), antioxidant enzymes: SOD, CAT, soluble proteins, free AAs, carbohydrates, and PCs (21 day).
To clarify the scheme of the experiment, see Figure 2.
2.4 Morphometric parameters and RWC
Where:
LL – leaf length,
W – maximum width of the leaf,
K – shape factor with a value of 0.75.
Where:
FW – fresh weight,
TW – saturated weight,
DW – dry weight.
2.5 Analysis of the parameters of the stomatal apparatus
To analyze the stomatal apparatus, surface imprints were made using clear nail polish on 3 randomly selected leaves of each sex from day 0 and day 21 of each treatment. The varnish was applied to an area of approximately 1 cm2 of the mid-blade abaxial leaf surface (avoiding the leaf margin and the main vein). After drying, the nail polish was removed with tweezers and mounted on a microscope slide. The following parameters of the stomata were measured: length of stomatal apparatus (LSA), width of stomatal apparatus (WSA), length of stomatal pores (LSP), width of stomatal pores (WSP), area of stomatal pores (ASP), and width of guard cells (WGC).
To measure the parameters, images of the slides were taken using a Nikon DS-Fi2 microscope with a Nikon 60i camera and NIS-Elements D 4.00.00 4.0 software.
Where:
NS – number of stomata in the field of view,
NE – number of epidermal cells in the field of view.
2.6 Measurements of chlorophyll a fluorescence
Photosynthetic activity was measured using a HandyPEA fluorimeter based on the fluorescence of chlorophyll a from PSII. The measurements were performed on the leaves of the regenerants on day 0 of the experiment and at the end of the salt stress (day 21). The following parameters were analyzed: time to maximal fluorescence (TFm), density area over the Chlorophyll Fluorescence (ChF) transient delimited by a horizontal line at Fm (Area), variable fluorescence (Fv), maximum quantum yield of PSII photochemistry (Fv/Fm), maximum ratio of quantum yields of photochemical and concurrent non-photochemical processes in PSII (Fv/F0), overall performance index of PSII (PI) and performance index (potential) for energy conservation from photons absorbed by PSII to the reduction of intersystem electron acceptors (PIABS) (further details in Gozdur et al., 2023) (Kalaji et al., 2018). After each measurement of chlorophyll fluorescence, 3 leaves were collected from each individual plant, quickly frozen in liquid nitrogen, and stored in a deep freezer for subsequent analysis.
2.7 Biochemical studies
2.7.1 Photosynthetic pigments
The content of photosynthetic pigments was analyzed in freeze-dried (Labconco Freezone 77530–32, A. G. A. Analytical) leaves of three randomly selected male and female regenerants at each stage of the experiment (the procedure is described in detail in Gozdur et al., 2023). Lyophilised plant tissue (10 mg) was homogenised in 100% acetone cooled to −20°C and a small amount of MgCO3. The homogenate was then centrifuged 1500 × g at 4°C for 5 min. The supernatant was then diluted 1:4 into cuvettes containing 200 μL of extract and 800 μL of 100% acetone. Absorbance measurements were performed using a spectrophotometer (SmartSpec Plus, BioRad) at three different wavelengths: 662 nm, 645 nm, and 470 nm. The analysis was carried out according to Lichtenthaler and Buschmann (2001) with some modifications. The contents of chl a, chl b, and carotenoids (car) were calculated using Lichtenthaler's (1987) equations and expressed in terms of dry weight (mg ⋅ g−1 DW).
2.7.2 Determination of SOD activity spectrophotometrically
The extraction method has already been described in detail in Peharec Štefanić et al. (2018). In summary, 0.5 g of deep-frozen leaf tissue was pulverized in 1.5 mL of 100 mM potassium phosphate buffer (pH 7.0) in a cooled mortar, along with 50 mg of PVP. The resulting homogenates were centrifuged at 18600 × g for 15 min at 4°C. The supernatants were then transferred to fresh tubes and centrifuged for a further 45 minutes under the same conditions. The protein concentrations in the obtained supernatants were determined by the Bradford assay using bovine serum albumin (BSA) as a standard (Bradford, 1976).
The activity of SOD (EC 1.15.1.1) was assessed using the method of Beauchamp and Fridovich (1971), which is based on the inhibition of the reduction of nitroblue tetrazolium (NBT). To determine the maximum absorbance, a reaction mixture containing 13 mM methionine (Met), 75 μM NBT, 0.1 M EDTA, and 2 mM riboflavin was incubated for 8 min in a 15 W light box. The formazan produced by NBT photoreduction was then measured at 560 nm (Genesys 180 UV–VIS, Thermo Fisher Scientific). The volume of protein extract required for 50% inhibition of the NBT reduction rate, defined as one unit (U) of SOD activity, was added to the reaction mixture and measured as previously described. The specific SOD activity was expressed as U · mg−1 protein.
2.7.3 Determination of CAT activity spectrophotometrically
Approximately 200 mg of the leaf tissue was ground to a fine powder in liquid nitrogen using a pre-cooled mortar and pestle. The exact weight of each sample was noted before homogenization in 1.2 mL of 0.2 M phosphate buffer (pH 7.8 with 0.1 mM EDTA). The samples were centrifuged at 21000 × g for 20 min at 4°C. The supernatant was collected, the pellet resuspended in 0.8 mL of the buffer, and centrifuged again. The combined supernatants were used for the determination of CAT activity (Elavarthi and Martin, 2010).
The CAT activity (EC 1.11.1.6) was measured according to the method described by Aebi (1984). In this assay, the CAT activity is determined in a reaction mixture of 50 mM phosphate buffer (pH 7.5) and 30% (w/v) H2O2, which is adjusted until the absorbance at 240 nm is between 0.500 and 0.550. The reaction is started by adding 20 μL of crude extract to the mixture. The reaction was carried out at 25°C for 2 min, and the decrease in absorbance at 240 nm, indicative of H2O2 consumption, was monitored using a spectrophotometer, with a decrease of 0.0145 absorbance units corresponding to the degradation of 1 μmol of H2O2. The resulting CAT activity, expressed in μmol of H2O2 degraded, was normalized to the protein content of the sample. The protein concentration was determined according to the Bradford method (Bradford, 1976) using the same assay as above.
2.7.4 In-gel assay of SOD
Extraction of enzyme: Approximately 1 or 1.5 g fresh weight (FW) of material was mixed with homogenization buffer (100 mM Tricine, 3 mM MgSO4 · 7H2O, 1 mM DTT, 3 mM EGTA, pH 8.0) at a ratio of 1:3 (g FW: mL buffer), using the method of Miszalski et al. (1998) with modifications. The extract was then centrifuged at 1500 × g for 5 min at 4°C. The concentration of soluble protein was determined according to the Bradford method (Bradford, 1976) with the Thermo Fisher Scientific Protein Assay (USA), using BSA (Merck) as a protein standard.
Native PAGE and staining: Native polyacrylamide gel electrophoresis (PAGE) was performed according to the method described by Miszalski et al. (1998). After protein quantification, the samples were separated on a 12% polyacrylamide gel, for the analysis of SOD activity staining, at 4°C and 180 V.
For detection of SOD activity in the gels, the method by Beauchamp and Fridovich (1971) was applied. The gels were incubated in a standard staining phosphate buffer (50 mM, pH 7.8) containing 1 mM EDTA, 2.8 mM TEMED, 22 μM riboflavin, and 245 μM NBT on a shaker at room temperature in the dark for approximately 20 min. Subsequently, the gels were exposed to white light on a transilluminator (UVP) until the SOD activity bands became visible.
To inhibit Cu/ZnSOD activity, the gels were incubated in a buffer supplemented with 3 mM KCN. To inhibit both Cu/ZnSOD and FeSOD activity, H2O2 was added to the staining buffer to achieve the final concentration of 5 mM. Additionally, 10 mM 3-amino-1,2,4-triazole was included in the standard staining buffer to eliminate bands indicating the presence of CAT.
The polyacrylamide gels were scanned using an EPSON Perfection V600 Photo scanner.
2.7.5 Analyses of soluble proteins, free AAs, and carbohydrates
A modified assay from Bradford et al. (1976) was used to determine the soluble protein content. 5 mg of lyophilized leaf was extracted in 0.25 mL of a 200 mM Tris–HCl, pH 7.5, 500 mM MgCl2 buffer at 4°C for 24 hours. After centrifugation (16 000 × g, for 10 min at 4°C), a 20 μL supernatant aliquot was mixed with 180 μL diluted Bio-Rad protein assay reagent. Protein concentrations were measured using BSA standards and expressed as mg × g−1 DW. Free AAs were determined according to Carillo et al. (2019a). Plant samples (10 mg) were mixed with 0.5 mL ethanol (40:60 v/v), incubated overnight at 4°C, and centrifuged at 14 000 × g (5 min). The supernatants were pooled and analyzed by HPLC using a Shimadzu Nexera X2 UHPLC system (Shimadzu) after pre-column derivatization with o-phthalaldehyde (OPA). The extract (20 μL) was derivatized with OPA reagent (40 μL) for 3 min in the autosampler needle, injected onto the column (ZORBAX Eclipse Plus C18, 250 × 4.6 mm internal diameter; Agilent Technologies Italia S.p.A) and eluted at a flow rate of 1 mL/min at 25°C with a discontinuous gradient. Solvent A: 50 mM sodium acetate (pH 6.4), tetrahydrofuran 0.3% (v/v) and methanol 20% (v/v), solvent B: methanol, and the gradient elution occurred according to the scheme: 0 min: 0% B, 1 min: 0% B, 11 min: 3% B, 13 min: 7.2% B, 17 min: 7.2% B, 32 min: 10% B, 38 min: 18% B, 48 min: 38% B, 57 min: 60% B, 60 min: 80% B, 62 min: 95% B, 72 min: 100% B, 80 min: 0% B. The amino acid-OPA derivatives were detected by their fluorescence with excitation at 330 nm and emission at 450 nm. The HPLC peaks were identified and quantified by comparing their retention times and area data with those obtained from the standards. For the standards preparation, 10 μL of the amino acid standard solutions (A9906 Fluka, Sigma-Aldrich) were combined with 10 μL of ethanol:water in the ratio 40:60 (v/v) and derivatized with OPA reagent (40 μL) for 3 min in the autosampler needle, injected onto the column and eluted in the same way as the sample. Results were expressed in μmol · g−1 DW.
Pro content was also determined from the same extract according to a procedure adapted by Bates et al. (1973) and previously outlined in Carillo et al. (2019a). A 100 μL aliquots of extract and Pro standards were placed in 1.5 mL screw-cap tubes. Each tube received 200 μL of a reaction mixture containing 1% ninhydrin in 60% acetic acid and 20% ethanol. The tubes were sealed, mixed, and heated at 95°C for 20 min. After cooling and brief centrifugation, 100 μL of the mixture was transferred to a polypropylene microplate. Absorbance at 520 nm was measured with a microplate reader (Synergy HT, Bio-Tek), and Pro concentration was determined using the standard calibration curve. The results were expressed in μmol · g−1 DW.
A 10 mg plant sample was extracted twice with 150 μL of 80% ethanol and re-extracted with 150 μL of 50% ethanol at 80°C for 30 min. The supernatants of the three extractions were combined after centrifugation at 13 500 × g for 10 min at 4°C and stored at −20°C for carbohydrate analysis. The remaining pellet, which contained starch, was treated with 350 μL of 0.1 M KOH at 95°C for 2 h. After cooling and adjusting to pH 4.5, it was mixed with a hydrolysis buffer (50 mM sodium acetate, pH 4.5, 2 U/mL α-amylase, 20 U/mL amyloglucosidase) and incubated at 37°C for 18 h. The mixture was centrifuged and the supernatant containing the hydrolyzed glucose was collected. Glucose, fructose, and sucrose were quantified using a pyridine nucleotide coupled enzymatic assay according to Carillo et al. (2019a) and expressed as μmol · g−1 DW.
2.7.6 Analyses of phenolic compounds
Lyophilized leaf tissue (8–73 mg of each sample) was extracted in falcon tubes in 3 mL of methanol (HPLC-grade pure, Merck) by sonification (Sonic 2, POLSONIC Palczyński ultrasonic bath), 2 times over 30 min at room temperature. The extracts were centrifuged for 8 min at 1500 × g (MPW-223E, MPW). The supernatant was filtered through syringe filters (pore diameter = 0.2 μm Millex® GP, Filter Unit, Millipore). Analysis of PCs was conducted using the HPLC-DAD method (Sułkowska-Ziaja et al., 2017; Szopa et al., 2017a, 2017b). A liquid chromatograph apparatus (Merck-Hitachi) was used, which is equipped with a DAD detector (L-245) in the UV light range (200–400 nm). The analytical column, Purospher® RP-18e (4 × 250 mm, 5 μm; Merck), which utilizes a gradient program with a flow rate of 1 mL/min at 25°C, a detection wavelength 254 nm, and an injection volume of 10 μL. The mobile phase consisted of: A: methanol: 0.5% acetic acid in a ratio of 1:4 (v/v), B: methanol, and the gradient elution occurred according to the scheme: 100% A 0–20 min; 100–80% A 20–35 min; 80–60% A 35–55 min; 60–0% A 55–70 min; 0% A 70–75 min; 0–100% A 75–80 min; 100% A 80–90 min at a flow rate of 1 mL/min. All samples were injected in triplicate. The identification of PCs was based on comparing their spectra with UV spectra and retention times of reference substances obtained from Sigma-Aldrich (Germany) and Chemfaces (China): phenolic acids: gallic, neochlorogenic, protocatechuic, chlorogenic, cryptochlorogenic, vanillic, caffeic, p-coumaric, ferulic acids; flavonoids: catechin, epicatechin, vitexin, rutoside, hyperoside, isoquercetin, myricetin, quercitrin, quercitin, apigenin, rhamnetin; anthraquinones: emodin, chrysophanol. A quantitative analysis of the compounds was performed using the calibration curve method.
2.8 Data analysis
Statistics for all results on plant morphometric parameters, RWC, SI, stomatal apparatus parameters, photosynthetic parameters, and photosynthetic pigment content of the Rumex thyrsiflorus plants used for the salt stress experiment are presented as means ± SEM (standard error of the mean). Differences between means were analyzed using two-way ANOVA and Tukey's post hoc test. The results of the PC measurements were expressed as mean values ± SD (standard deviation). Differences between means within the same sex were analyzed using one-way ANOVA and Tukey's post hoc test. In addition, differences between the different sexes within NaCl concentration were analyzed using the unpaired Student's t-test. Statistically significant differences were verified for *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001. The statistical analysis was performed using GraphPad Prism software (v. 10.2.3).
To determine the relationship between female and male regenerants subjected to two different salinity treatments and the aboveground parts length, root length, leaf number, leaf area, RWC, SI, soluble proteins, free AAs, and carbohydrates, the data were subjected to Principal Component Analysis (PCA) performed in Minitab 18 statistical software.
To evaluate the relationship between the female and male regenerants exposed to the two salinity treatments and PC content, the data were also subjected to PCA analysis in GraphPad Prism software (v. 10.2.3) and plotted as a heat map created in R v 4.3.2 using the geom_tile function. The results were visualized using the ggplot2 package.
3 RESULTS
3.1 Molecular analysis
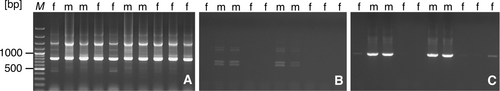
Analysis of the reaction products with primers that amplify the autosomal sequence RAE730 confirmed the high quality of the template DNA (Figure 3A). In the male specimens, PCR reactions with primers URG08-R and URG08-F, targeting the RAYSII sequence, showed two bands of approximately 700 bp and 600 bp in size (Figure 3B). Additionally, the use of RAY-R and RAY-F primers, amplifying RAYSI sequences in male individuals, resulted in a product of approximately 930 bp (Figure 3C). A total of 78 female individuals and 31 male individuals were successfully identified. The ratio of male-to-female plants regenerated in vitro was M:F = 1:2,52.
3.2 Effect of salinity on morphological properties, RWC and SI
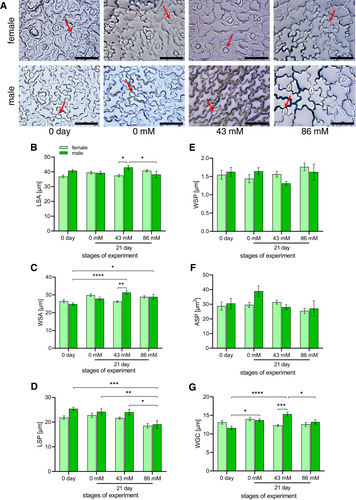
After 21 days, a deterioration in the physiological state of the regenerated leaves was observed at 43 mM NaCl and 86 mM NaCl media, mainly in male plants (red arrows; Figure 4). Significant responses (p < 0.05) were observed in the morphometric parameters tested for salt tolerance, except for RWC (Table 1). For the length of the aboveground part, a significant difference was only observed between 0 day and 0 mM NaCl for both female and male plants (Table 1). Root length showed significant sex differences at day 0. For both sexes, each subsequent stage of a particular sex was statistically different from 0 day (Table 1). Leaf number showed differences between the sexes only on day 0 and between males on day 0 and 0 mM NaCl, as well as between females on day 0 vs. 0 mM and on day 0 vs. 43 mM NaCl (leaf sampling also affected the number of leaves in this case) (Table 1). Leaf area decreased with increasing salt stress in all individuals grown in NaCl solution. Within the sexes, significant differences occurred between day 0 and the next stages with different NaCl concentrations (Table 1). Stomatal index (SI) significantly increased in males at 43 mM and 86 mM compared to 0 day and at 43 mM in males compared to 0 mM NaCl. No significant statistical changes were observed for females (Table 1).

| NaCl concentration | ||||||||
|---|---|---|---|---|---|---|---|---|
| 0 day (before experiment) | 0 mM (control) | 43 mM | 86 mM | |||||
| Morphometric parameters | F | M | F | M | F | M | F | M |
| aboveground part length [cm] | 17.00 ± 1.07a | 16.74 ± 1.13a | 27.64 ± 2.58b | 23.40 ± 2.34b | 19.74 ± 2.13ab | 20.94 ± 2.22ab | 19.43 ± 2.01ab | 18.76 ± 2.60ab |
| root length [cm] | 12.54 ± 0.70a | 13.08 ± 0.66b | 24.79 ± 2.42cdefgh | 23.93 ± 2.24defgh | 31.72 ± 1.88efgh | 26.37 ± 5.49fgh | 24.37 ± 1.95gh | 24.90 ± 2.56h |
| leaf number | 6.35 ± 0.64a | 11.36 ± 1.19bde | 15.63 ± 2.22bcde | 26.25 ± 5.56c | 11.60 ± 1.28be | 18.86 ± 2.56bce | 9.00 ± 1.29ae | 13.71 ± 1.69bce |
| leaf area [cm2] | 5.93 ± 0.59ad | 3.40 ± 0.33b | 16.90 ± 2.09ce | 8.23 ± 1.00ad | 16.10 ± 1.66c | 9.10 ± 1.72de | 9.80 ± 3.34abcd | 7.63 ± 2.03abcd |
| RWC | 85.17 ± 0.55a | 80.99 ± 80.99a | 86.54 ± 86.54a | 84.25 ± 2.03a | 89.95 ± 4.37a | 98.63 ± 5.50a | 86.35 ± 4.17a | 90.39 ± 3.68a |
| SI | 14.36 ± 0.45abc | 12.03 ± 0.31a | 12.55 ± 0.43ac | 12.73 ± 0.50ac | 15.26 ± 0.33abc | 16.84 ± 1.14bc | 15.09 ± 0.39abc | 15.40 ± 1.41c |
- n = 7–92; different letters indicate significant differences among means as determined using two-way ANOVA and Tukey's post-hoc test (p < 0.05).
Observation of stomata showed that most stomata were closed during NaCl treatment, especially at 86 mM NaCl (red arrows; Figure 5A). Significant responses (p < 0.05) were also observed for the stomatal parameters tested for salt tolerance, with the exception of WSP and ASP (Figures 5E,F). The largest stomata were observed at 43 mM NaCl on the leaves of male plants (Figures 5B,C), which were also characterized by large guard cells (Figures 5G). Significant differences in these parameters occurred between female and male specimens at 43 mM NaCl (*p < 0.05, **p < 0.01, ***p < 0.001, respectively). A significant decrease in the stomatal pore length was observed in males at 86 mM, which tended to decrease with increasing NaCl concentration (Figure 5D). A significant increase in the WSA parameter occurred in the male individuals after 21 days on 43 mM and 86 mM NaCl compared to day 0 of the experiment (Figure 5C). There were significant differences in the LSA and WGC parameters between 43 mM and 86 mM NaCl in the males (Figures 5B,G). Additionally, in the WGC parameter, a *p < 0.05 difference appeared in males between day 0 and 21 day on 0 mM NaCl and ****p < 0.0001 between day 0 and 21 day on 43 mM NaCl (Figure 5G). There were no significant differences between individuals at different NaCl concentrations in females (Figure 5).
3.3 Effect of salinity on photosynthetic activity
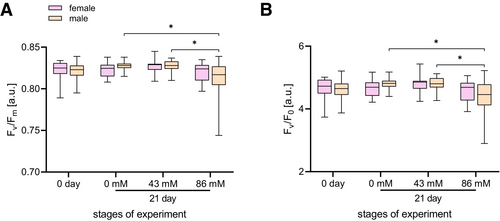
Statistically significant differences (p < 0.05) were found only for two photosynthetic parameters (Fv/Fm, Fv/F0) among the seven photosynthetic parameters tested for salt tolerance (Figure 6). The analysis of the maximum photochemical yield of photosystem II (Fv/Fm) (Figure 6A) showed a statistical decrease between male individuals cultured on 43 mM and 86 mM NaCl and between 0 mM and 86 mM NaCl. Analysis of the mean Fv/F0 value also showed the same significant differences (Figure 6B).
3.4 Effect of salinity on biochemical properties
3.4.1 Content of photosynthetic pigments
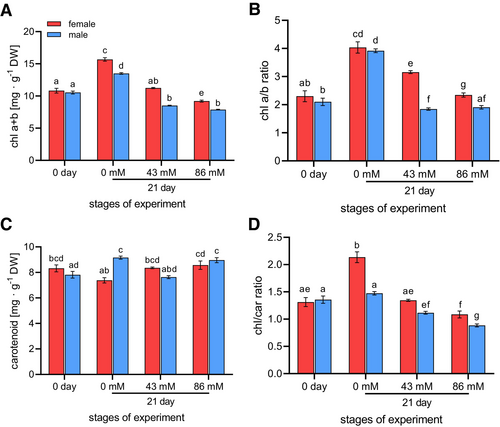
Before the start of the experiment (day 0), there were no statistically significant differences in pigment content between females and males (Figure 7). Our results showed a decreasing trend in total chlorophyll content (Figure 7A) and the chl a/b ratio (Figure 7B) after 21 days of salt stress, with female plants clearly dominating. The trend was very similar for the male plants, except that there was no significant difference between 43 mM and 86 mM NaCl. A significant increase in chl a + b and the chl a/b ratio was observed between day 0 and day 21 on 0 mM in both sexes. Furthermore, on day 21, significant differences between the sexes occurred only at 0 mM and 86 mM for chl a + b and at 43 mM and 86 mM NaCl for the chl a/b ratio. The differences also occurred in female individuals between 0 mM and 43 mM and between 0 mM and 86 mM (Figures 7A,B). The carotenoid content showed a significant difference between females and males only at 0 mM NaCl. However, a significant increase from 0 mM to 86 mM NaCl was observed in females and from day 0 to 0 mM NaCl in males (Figure 7C). There were significant differences between the sexes in the chl/car ratio at 0 mM and 86 mM NaCl. Additionally, a significantly decreasing trend was observed after 21 days of salt stress in both sexes (Figure 7D).
3.4.2 SOD and CAT activity
Manganese (MnSOD), iron (FeSOD) and copper-zinc (Cu/ZnSOD) forms of SOD were identified (Figure 8A). The presence of Cu/ZnSOD and MnSOD was detected in both female and male plants under all culture conditions. However, FeSOD was only detected in female plants in a medium with the highest NaCl concentration (86 mM NaCl) (Figure 8A).
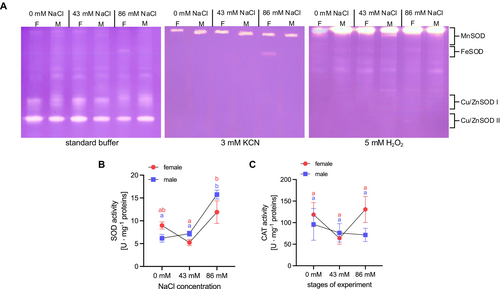
A significant increase in total SOD activity was observed in male plants grown on medium containing 86 mM NaCl when compared with both male control plants (0 mM NaCl) and plants treated with 43 mM NaCl (Figure 8B). In the female plants, a significant increase in total SOD activity was observed in the variant with 86 mM NaCl compared to the plants from a medium with 43 mM NaCl (twofold higher) (Figure 8B). The presence of NaCl in the culture medium had no effect on significant changes in CAT activity (Figure 8C).
3.4.3 Primary metabolites
The analysis revealed significant changes in the content of free AAs, soluble proteins, and carbohydrates in thyrse sorrel plants under the different salinity treatments (0, 43, and 86 mM NaCl) (Figures S1 and S2). Ala content increased significantly in male plants treated with 86 mM NaCl compared to the control, suggesting an adaptive response to high salinity (Figure S1A). The Arg (arginine) content in female plants treated with 86 mM NaCl was significantly lower than that in the control group (Figure S1B). The Asn (asparagine) content decreased significantly in the female plants treated with 86 mM NaCl compared to the plants treated with 43 mM NaCl (Figure S1C). GABA content remained relatively low in all treatments, but increased slightly at higher salinity levels, especially in the male plants (Figure S1E). This suggests that GABA may play a role in the stress response of male plants. A significant decrease was also observed in the female plants treated with 43 mM and 86 mM NaCl compared to the control (Figure S1E). Gln (glutamine) content differed significantly between female and male individuals at 86 mM NaCl (Figure S1F). The Glu (glutamic acid) content decreased significantly in female plants treated with 86 mM NaCl compared to plants in the control group (Figure S1G). Gly (glycine) content varied significantly between NaCl concentrations in male plants and between the sexes at each stage (Figure S1H). Its level decreased significantly at 86 mM NaCl compared to the control in females. The highest His (histidine) concentration was observed in the males at 43 mM NaCl (Figure S1I). The Leu (leucine) content increased significantly at 43 mM and decreased at 86 mM in the female plants (Figure S1K). For Lys (lysine), a statistically significant difference was observed between female and male plants at 43 mM NaCl (Figure S1L). A significant decrease in MEA (monoethanolamine) was observed in females on 43 mM and 86 mM NaCl and an increase in males on 86 mM NaCl (Figure S1M). A significant increase in Met in males on 86 mM NaCl compared to controls was noted (Figure S1N). Orn (ornithine) content was higher in male plants. However, a significant decrease was observed at 86 mM NaCl (Figure S1O). Phe (phenylalanine) content was higher in male plants grown with 86 mM NaCl (Figure S1P). A significant increase in Pro content at 43 mM and 86 mM NaCl was observed in both sexes. The Pro content at 86 mM NaCl was more than 6-fold higher in male plants than at 0 mM NaCl (Figure S1Q). Ser (serine), a photorespiratory amino acid, also increased strongly in males, especially at 86 mM NaCl (Figure S1R). Trp (tryptophan) levels were significantly higher in males than in females at 86 mM NaCl (Figure S1T). Val (valine) levels increased significantly in males at 43 mM compared to 0 mM NaCl. It was also 1.5 times higher than in the females at the same concentration, suggesting its role in nitrogen storage and protein synthesis under stress (Figure S1V). The content of total AAs shows that the proportion of AAs in male plants at a concentration of 86 mM NaCl is significantly higher than in female plants (Figure S1W).
The protein content decreased significantly in male plants at 43 mM NaCl compared to the control, while a significant increase was observed in male plants at 86 mM NaCl compared to female plants at the same salinity level, suggesting different rates of protein synthesis or degradation under different salinity conditions and between the two sexes (Figure S2A).
Regarding carbohydrates, starch levels increased significantly on medium with 43 mM and 86 mM NaCl in females, while they decreased in males on 43 mM but increased on 86 mM NaCl (Figure S1B). The glucose content increased significantly in both female and male plants at 86 mM NaCl, indicating a metabolism more focussed on carbohydrate storage and growth (Figure S2C). Fructose content also increased significantly at 43 mM and 86 mM salinity levels, indicating changes in carbohydrate metabolism under stress conditions (Figure S2D). Sucrose content, which is much lower compared to hexoses, increased significantly in female plants at higher salinity levels, especially at 86 mM NaCl, emphasizing its role in osmotic regulation and energy supply under stress (Figure S2E).
3.4.4 PCA of morphometry, RWC, and primary metabolites
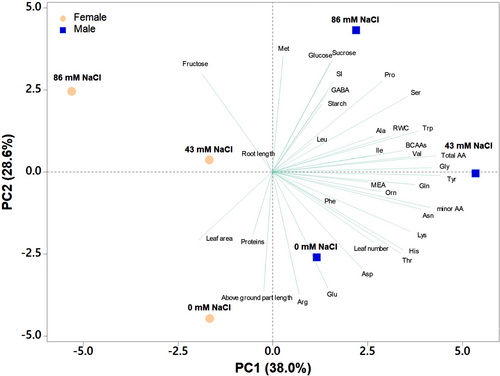
PCA was performed on female and male plants to investigate the different responses of female and male regenerants to different salinity treatments (0, 43, and 86 mM NaCl) (Figure 9). The first four principal components explained 94.4% of the total variance, with PC1, PC2, PC3, and PC4 accounting for 38.0%, 28.6%, 17.5%, and 10.4%, respectively (Table S2). Sex contributed to the highest variability, leading to PC1 of male samples located on the positive side of PC1 and female ones on the negative side of PC1. The second major source of variability was the saline treatment, with samples treated with 0 mM NaCl positioned on the negative side of PC2 and those treated with 86 mM NaCl situated on the positive side of PC2 (Figure 9). PC1 correlated positively with Gln, Asn, Gly, total and minor AAs, Thr (threonine), BCAAs (branched-chain amino acids), His, Lys, Val, and Trp, while it correlated negatively with leaf area and fructose. PC2 correlated positively with Met, sucrose, glucose, fructose, Pro, SI, and GABA, while it correlated negatively with Arg, aboveground part, Glu, and Asp (aspartic acid). Male plants treated with 86 mM NaCl showed the highest amounts of protective metabolites (sucrose, GABA, and Pro), while male and female samples at 0 mM NaCl showed the highest morphometric parameters (shoot length, leaf number, leaf area), Glu, and Arg. Interestingly, the male samples treated with 43 mM NaCl showed the highest contents of total and minor AAs (including BCAAs), MEA, and RWC values (Figure 9).
3.5 Effects of salinity on phenolic compound production
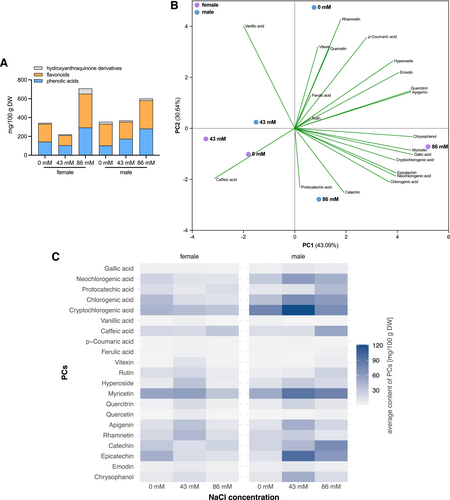
Phenolic acids, flavonoids, and hydroxyanthraquinone derivatives (Figure 10A) were identified in the analyzed methanolic leaf extract of regenerated female and male plants cultured in Hoagland's solution. A total of nine different types of free phenolic acids were identified in the methanolic leaf extracts of the regenerated plants, including gallic acid, neochlorogenic acid, protocatechuic acid, chlorogenic acid, cryptochlorogenic acid, vanillic acid, caffeic acid, p-coumaric acid, and ferulic acid. In addition, the analysis revealed ten flavonoids: vitexin, rutin, hyperoside, myricetin, quercitrin, quercetin, apigenin, rhamnetin, catechin, and epicatechin. Moreover, two hydroxyanthraquinone derivatives, emodin and chrysophanol, were identified (Figure S3). Differences in PC content were found according to sex and different NaCl concentrations. Table 2 shows the average amounts of phenolic acids, flavonoids, and hydroxyanthraquinone derivatives in R. thyrsiflorus treated with different NaCl concentrations (0 mM, 43 mM, and 86 mM). The average content ranges were wide, ranging from 0.10 mg/100 g DW (vanillic acid, in females at 43 mM NaCl) to 120.80 mg/100 g DW (cryptochlorogenic acid in females at 86 mM NaCl) for phenolic acids, 0.07 mg/100 g DW (quercetin at males at 43 mM NaCl) to 96.60 mg/100 g DW (epicatechin in females at 86 mM NaCl) for flavonoids, and 2.61 mg/100 g DW (emodin in females at 43 mM NaCl) to 48.28 mg/100 g DW (chrysophanol in females at 86 mM NaCl) for hydroxyanthraquinone derivatives. The female regenerants showed the highest PC concentration when cultured at 86 mM NaCl, but for caffeic acid, the peak occurred in leaf extracts after 43 mM NaCl treatment and for protocatechuic acid in nutrient solution without NaCl. The males also showed the highest PC concentration when cultured on nutrient solution supplemented with 86 mM NaCl, but also on nutrient solution without NaCl (rhamnetin and emodin) (Table 2).
| Phenolic compounds | NaCl concentration | |||||
|---|---|---|---|---|---|---|
| female | male | |||||
| 0 mM (control) | 43 mM | 86 mM | 0 mM (control) | 43 mM | 86 mM | |
| Gallic acid | 3.33 ± 0.04a | 1.81 ± 0.02b | 9.36 ± 0.56c | 3.49 ± 0.08a | 3.43 ± 0.13a | 5.88 ± 0.52b |
| Neochlorogenic acid | 21.82 ± 0.28a | 10.91 ± 1.08b | 61.02 ± 2.47c | 12.15 ± 0.66a | 28.50 ± 1.34b | 46.46 ± 1.24c |
| Protocatechuic acid | 16.58 ± 0.13a | 15.35 ± 0.20b | 12.00 ± 0.77c | 8.73 ± 0.15a | 9.14 ± 0.46a | 39.15 ± 0.53b |
| Chlorogenic acid | 37.45 ± 0.56a | 12.06 ± 0.16b | 71.71 ± 2.32c | 15.12 ± 4.40a | 34.47 ± 1.61b | 60.42 ± 2.08c |
| Cryptochlorogenic acid | 43.22 ± 0.98a | 31.56 ± 7.65a | 120.80 ± 3.01b | 37.11 ± 15.85a | 77.25 ± 3.61b | 71.76 ± 2.70b |
| Vanillic acid | 0.40 ± 0.07a | 0.10 ± 0.01b | 0.23 ± 0.05b | 3.68 ± 0.40a | 2.74 ± 2.12a | 0.15 ± 0.01a |
| Caffeic acid | 18.43 ± 0.49a | 29.84 ± 0.37b | 15.46 ± 0.49c | 16.55 ± 0.43a | 14.82 ± 0.80a | 54.13 ± 1.45b |
| p-Coumaric acid | 1.02 ± 0.11a | 1.24 ± 0.57a | 2.22 ± 1.07a | 2.73 ± 1.93a | 0.99 ± 0.17a | 0.92 ± 0.91a |
| Ferulic acid | 0.71 ± 0.54a | 1.14 ± 0.63a | 1.02 ± 0.30a | 2.90 ± 1.18a | 2.03 ± 0.20a | 3.12 ± 1.84a |
| Vitexin | 0.81 ± 0.89a | 1.25 ± 0.51a | 1.04 ± 0.17a | 11.04 ± 3.96a | 0.78 ± 0.35b | 5.27 ± 3.26ab |
| Rutin | 14.55 ± 1.86a | 6.98 ± 0.33b | 7.06 ± 2.94b | 19.36 ± 5.54a | 4.56 ± 1.21b | 22.35 ± 5.29a |
| Hyperoside | 6.61 ± 1.19a | 5.58 ± 0.24a | 27.10 ± 1.97b | 32.13 ± 5.06a | 11.92 ± 1.14b | 17.06 ± 3.53b |
| Myricetin | 54.38 ± 2.67a | 30.97 ± 0.44b | 90.25 ± 2.83c | 58.14 ± 16.33a | 50.06 ± 3.98a | 78.23 ± 6.98a |
| Quercitrin | 3.39 ± 0.78a | 3.74 ± 0.17a | 22.13 ± 0.56b | 16.49 ± 10.33a | 5.87 ± 0.77a | 9.75 ± 3.89a |
| Quercetin | 0.19 ± 0.02a | 0.08 ± 0.06a | 0.80 ± 0.14b | 7.26 ± 3.54a | 0.07 ± 0.02a | 3.33 ± 4.46a |
| Apigenin | 13.65 ± 1.73a | 9.16 ± 0.64b | 46.51 ± 0.49c | 33.87 ± 4.74a | 12.04 ± 0.90b | 19.06 ± 0.19b |
| Rhamnetin | 18.55 ± 2.05a | 14.90 ± 0.55a | 26.45 ± 0.19b | 39.80 ± 7.73a | 21.02 ± 0.83b | 10.29 ± 0.01c |
| Catechin | 29.73 ± 0.96a | 22.01 ± 0.44b | 40.59 ± 1.91c | 15.06 ± 2.15a | 20.94 ± 0.61a | 71.94 ± 2.94b |
| Epicatechin | 46.63 ± 1.25a | 12.84 ± 4.80b | 96.60 ± 3.88c | 16.33 ± 1.62a | 53.19 ± 4.66b | 62.65 ± 2.90b |
| Emodin | 3.78 ± 0.21a | 2.61 ± 0.08b | 8.58 ± 0.61c | 7.04 ± 0.37a | 4.62 ± 0.17b | 3.29 ± 0.47c |
| Chrysophanol | 7.88 ± 0.16a | 6.16 ± 0.05b | 48.28 ± 3.07c | 15.02 ± 0.25a | 10.21 ± 0.33b | 16.08 ± 0.96a |
- n = 3; DW – dry weight; different letters indicate significant differences among means as determined using one-way ANOVA and Tukey's post-hoc test (p < 0.05).
3.5.1 Effect of salinity on phenolic compound production within the same sex
One-way ANOVA and Tukey's post hoc test analysis revealed statistically significant differences in 18 PCs in females and 15 in males (Table 2) among the 21 compounds identified. No statistically significant differences were found for the indicated PCs in females (Table 2): p-coumaric acid, ferulic acid, and vitexin; and in males (Table 2): vanillic acid, p-coumaric acid, ferulic acid, myricetin, quercitrin, and quercetin. Significantly higher concentrations of PCs in female plants (Table 2) compared to the control (0 mM NaCl) were observed for each PC at 86 mM NaCl (only caffeic acid was significant at 43 mM NaCl), except for protocatechuic acid, vanillic acid, and rutin. In male plants (Table 2), a significant increase was observed for gallic acid, neochlorogenic acid, protocatechuic acid, chlorogenic acid, and catechin at 86 mM NaCl, and for cryptochlorogenic acid and epicatechin on both NaCl concentrations. Furthermore, a significant decrease in vitexin, rutin, and chrysophanol at 43 mM NaCl and in hyperoside, apigenin, rhamnetin, and emodin at 43 and 86 mM NaCl was observed in males.
3.5.2 Effect of salinity on phenolic compound production between the diverse sexes
The unpaired Student's t-test was used to determine statistically significant differences in PC content between the male and female regenerated plants. There were no significant differences between the sexes for p-coumaric acid and ferulic acid. Highly significant differences (p < 0.0001) were found between female and male plants at each stage of the experiment in protocatechuic acid levels (at 86 mM NaCl over threefold higher in male plants), as well as in the following compounds: neochlorogenic acid and epicatechin at 0 mM NaCl (almost threefold higher content in the female plants); gallic acid and chlorogenic acid at 43 mM NaCl (almost threefold higher content in the male plants); cryptochlorogenic acid, apigenin, and rhamnetin at 86 mM NaCl; caffeic acid at 43 mM and 86 mM NaCl (over threefold higher content in the male plants); and chrysophanol at 0 mM and 43 mM NaCl, with an additional significant difference (p < 0.001) between the sexes at 86 mM NaCl (threefold higher content in the female plants) (Table 3). Statistically significant differences (p < 0.001) between female and male plants were also found at every NaCl concentration in emodin content; at 43 mM NaCl in neochlorogenic acid (almost threefold higher content in the male plants) and rhamnetin; at 0 mM NaCl in vanillic acid (more than ninefold higher content in the male plants) and catechin; and at 86 mM NaCl in catechin and epicatechin, with an additional significant difference (p < 0.01) between sexes at 43 mM NaCl (more than fourfold higher content in the male plants) (Table 3). Further statistically significant differences (p < 0.01) were noted at 86 mM NaCl in gallic acid, neochlorogenic acid, and chlorogenic acid, as well as at 0 mM NaCl in apigenin and hyperoside (almost fivefold higher content in the male plants), where significant differences were also observed at 43 mM NaCl. At 43 mM NaCl, significant differences occurred for cryptochlorogenic acid and myricetin (Table 3). Statistically significant differences (p < 0.05) between female and male plants were found at 0 mM NaCl in vitexin (almost 14-fold higher content in the male plants) and quercetin (over 38-fold higher content in the male plants), and at 86 mM NaCl for rutin (over threefold higher content in the male plants), hyperoside, and quercitrin, with additional significant differences between the sexes at 43 mM NaCl, where a further significant difference was found for apigenin (Table 3).
| Phenolic compounds | NaCl concentration | ||
|---|---|---|---|
| 0 mM (control) | 43 mM | 86 mM | |
| Gallic acid | ns | **** | ** |
| Neochlorogenic acid | **** | *** | ** |
| Protocatechuic acid | **** | **** | **** |
| Chlorogenic acid | ** | **** | ** |
| Cryptochlorogenic acid | ns | ** | **** |
| Vanillic acid | *** | ns | ns |
| Caffeic acid | * | **** | **** |
| p-Coumaric acid | ns | ns | ns |
| Ferulic acid | ns | ns | ns |
| Vitexin | * | ns | ns |
| Rutin | ns | ns | * |
| Hyperoside | ** | ** | * |
| Myricetin | ns | ** | ns |
| Quercitrin | ns | * | * |
| Quercetin | * | ns | ns |
| Apigenin | ** | * | **** |
| Rhamnetin | * | *** | **** |
| Catechin | *** | ns | *** |
| Epicatechin | **** | ** | *** |
| Emodin | *** | *** | *** |
| Chrysophanol | **** | **** | *** |
- n = 3; ns – not significant, *p < 0.05, **p < 0.01, ***p < 0.001, ****p < 0.0001.
3.5.3 PCA and heat map analysis of PCs
To increase the interpretability of the PCs associated with the stress response to salinity, we performed PCA (Figure 10B). The first four principal components explained 98.23% of the total variance, with PC1, PC2, PC3, and PC4 accounting for 43.09%, 30.64%, 20.48%, and 4.02%, respectively (Table S3). Sex contributed the most to variability, leading to a separation on PC1 between male samples located on the positive side of PC1 (except for 43 mM NaCl) and female samples on the negative side of PC1 (except for 86 mM). The other main source of variation was saline treatment, with samples treated with 86 mM NaCl being on the negative side of PC2 and the positive side of PC1, and those treated with 43 mM NaCl being close to 0 on the PC2 side (male on the positive side, female on the negative side). Samples that were not treated with NaCl were on the opposite side of PC2. PC1 correlated positively with all PCs (except vanillic acid and caffeic acid). PC2 correlated positively with vanillic acid, p-coumaric acid, ferulic acid, vitexin, rutin, hyperoside, quercitrin, quercetin, apigenin, rhamnetin, and emodin. Female plants treated with 86 mM NaCl showed the highest amounts of most PCs; however, the lowest were female plants at 43 mM.
Increasing the NaCl concentration generally resulted in different shifts in the levels of the compounds, with some compounds showing an increased accumulation and others a marked decrease. Gender-specific responses were also observed, as certain compounds were more abundant in male or female samples at certain NaCl concentrations (Figure 10C).
4 DISCUSSION
Modern biotechnology offers a variety of methods and techniques for plant breeding with different objectives. In addition to the production of high-quality plant material, plant cell and tissue culture methods are also used today for the production of SMs. Hydroponic cultivation is also widely used to grow plants in a controlled environment, addressing the challenges of plant production under stressful conditions (Baiyin et al., 2021; Ozyigit et al., 2023). In contrast to traditional agriculture, hydroponics is soilless plant production. In this technique, plants are grown on various substrates or directly in a nutrient solution (Velazquez-Gonzalez et al., 2022). Hydroponic systems use less water than soil-based crops, thanks to the closed system and continuous circulation, making them much more environmentally friendly. Moreover, such crops are pesticide-free, so the quality of products is much higher. It is estimated that, by 2050, more than 80% of the world's population will live in urban areas, and therefore, hydroponics is an ideal solution for growing plants in such areas (Manos and Xydis, 2019).
4.1 Morphometry
The regenerated plants of Rumex thyrsiflorus obtained in vitro exhibited nearly a twofold increase in size after 21 days of hydroponic cultivation in Hoagland's nutrient solution without additional salt. This growth affected various parts of the plant, including the aboveground parts, leaf area, and roots, all of which displayed increased length after the 21-day period. The increase in morphometric parameter values was noted even without nutrient solution flow in the experiment. Baiyin et al. (2021) emphasize the importance of both nutrient solution flow and its intensity for the effective practice of hydroponic culture.
Regenerants of R. thyrsiflorus cultured on Hoagland's solution with 86 mM NaCl showed alarming symptoms, such as leaf wilting and chlorosis (yellowing of the leaves). In contrast to the control experiment, the leaf area of the plants exposed to NaCl gradually decreased over 21 days of culture. This reduction in leaf area could be an adaptive response and a protective mechanism against the negative effects of salt stress. By reducing the leaf area, the plant reduces water loss through transpiration, which promotes stomatal closure. In addition, this protective mechanism prevents the mesophyll cells from being contaminated with toxic ions under saline conditions (Islam et al., 2019).
Studies have shown that a reduction in stomata is beneficial for herbaceous plants as it increases water utilization efficiency in response to osmotic stress. Furthermore, an increase in stomatal production was observed in oil palms under salt stress conditions, which is consistent with similar observations in white poplars (Song et al., 2022). In the present experiment, a significant increase in stomatal index (SI) was observed in male plants during 21 days of culture under salt stress conditions at 43 mM NaCl.
4.2 Photosynthesis
Abiotic factors, such as salinity, can inhibit photosynthesis, the crucial process on Earth. The inhibition results from reduced chlorophyll synthesis, restricted electron flow, and damage to photosynthetic proteins, particularly in the thylakoid membranes such as the photosystems. This impairment has a significant impact on plant growth and development. The assessment of plant response to environmental stress increasingly relies on the widely used method of chlorophyll fluorescence (Zahra et al., 2022; Nascimento et al., 2019). R. thyrsiflorus demonstrated adaptability to different salinity levels and maintained a consistent potential output of the photosynthetic apparatus' photochemical activity (Fv/Fm). This indicates that PSII was not damaged, although a slight decrease in this parameter was observed in plants cultivated in a nutrient solution containing 86 mM NaCl. Most stress factors that affect the photosynthetic apparatus of the plant lead to a decrease in the Fv/Fm parameter (Kalaji, 2011; Li and Rao, 2024; Zareei et al., 2021). In a study on Portulaca oleracea L., a significant decrease in this parameter was observed after 12 and 22 days of cultivation on a solution containing 300 mM NaCl (Hnilickova et al., 2021). In the current experiment, during culture on a nutrient solution with 86 mM NaCl, a reduction in parameter values was noted after 21 days. Some of the analyzed parameters showed a significant decrease, indicating a weakening of the photosynthetic apparatus and electron transport. In particular, the Fv/Fm parameter decreased between males at 0 mM and 43 mM and 86 mM; the Fv/F0 parameter showed a reduction at the same level. The effects of abiotic stress on R. thyrsiflorus were investigated by Gozdur et al. (2023) during the comprehensive study of the physiological and biochemical aspects of the response to heat stress. It was found that the response to this stress is sex-dependent in this dioecious species. Regenerants experienced a decrease in PSII activity when confronted with high temperatures but showed impressive adaptability to the prevailing conditions just one week after the stressor was removed (Gozdur et al., 2023).
In the present study, the chlorophyll content and the chl a/b ratio decreased after 21 days of hydroponic culture, especially in the female plants, while the carotenoid content increased, except in the male specimens where a decrease in carotenoid content was observed in the Hoagland solution with 43 mM NaCl. Misra et al. (1997) similarly reported a decrease in chlorophyll content in certain plant species under salinity influence. In the current study, a more pronounced decrease in chlorophyll a compared to chlorophyll b was observed in the leaves on day 21. This differential decrease could be due to the effects of salt stress on the synthesis and degradation of pigments. Previous studies have shown that chlorophyll a is more sensitive to salt stress than chlorophyll b. The main reason for the decrease in chlorophyll content after salt stress is chlorophyll degradation (Rahdari and Hoseini, 2011; Athar et al., 2022).
4.3 Biochemistry
4.3.1 Salinity affects SOD activity
Salinity stress has a significant impact on the activities of antioxidant enzymes such as SOD and CAT in plants. These enzymes play a crucial role in mitigating oxidative stress by scavenging ROS, such as superoxide anion radicals and H2O2, respectively, generated under various stress conditions (Miller et al., 2010; Choudhury et al., 2017; Mishra et al., 2023). The type of SOD forms in R. thyrsiflorus does not change under NaCl, although salt-induced FeSOD activity is only observed in female plants. This result may indicate that FeSOD activity under stress conditions is somehow related to the sex of the plant. However, the general increase in SOD activity in plants growing on NaCl-containing medium is not sex-specific and corresponds to a common response of SOD of different plant species to salt stress (Miszalski et al., 1998; Mishra et al., 2013; Rasool et al., 2013; Li et al., 2023). To summarize, salt stress increases SOD activity in both sexes of R. thyrsiflorus, although this effect occurs at the highest NaCl concentrations used. This could indirectly indicate a certain level of oxidative stress induced by NaCl in R. thyrsiflorus.
The fact that CAT activity did not change under salinity suggests that other antioxidant enzymes responsible for the removal of H2O2 are also active in R. thyrsiflorus, but this question requires further investigation.
4.3.2 Salinity influences amino acid metabolism
Overall, the significant changes in free AAs, carbohydrates, and soluble proteins under salinity stress highlight a multifaceted adaptive response in thyrse sorrel plants. The increase in Pro and branched-chain amino acids, such as Leu and Val, particularly in male plants, emphasizes their crucial role in stress tolerance mechanisms. Pro is known for its role in various stress responses in plants, including salt stress. Its accumulation helps in redox signaling, osmotic adjustment, protection of cell structures, and removal of ROS (Kishor et al., 2005; Szabados and Savouré, 2010; Ghosh et al., 2022).
The metabolic pathway responsible for the biosynthesis of Pro primarily involves the conversion of Glu to Pro (Glu pathway) via the intermediates glutamate-semialdehyde (GSA) and pyrroline-5-carboxylate (P5C), catalyzed by the enzymes Δ1-pyrroline-5-carboxylate synthetase (P5CS) and pyrroline-5-carboxylate reductase (P5CR). An alternative pathway involves Orn, which is first transaminated by ornithine-delta-aminotransferase (OAT), producing GSA and P5C, which is then converted to Pro (Szabados and Savouré, 2010; Mansour and Ali, 2017). In R. thyrsiflorus, as in other plant species, NaCl probably alters the expression of genes involved in Pro biosynthesis. The accumulation of Pro under salt stress conditions is often associated with the upregulation of P5CS genes (Glu pathway) and the downregulation of Pro dehydrogenase (PDH) genes (Mansour and Ali, 2017; El Moukhtari et al., 2020). The increase in Pro content under salt stress, together with the increased SOD activity, may indicate the role of Pro in scavenging excess ROS in R. thyrsiflorus. The ability to scavenge ROS in the form of H2O2 has been described, for example, in Oryza sativa in the presence of Hg2+ (Wang et al., 2009).
In addition to Pro, Asn, Ala, and GABA also accumulate in R. thyrsiflorus in response to salt stress. The accumulation of Asn can attenuate the excess ammonium released by strong photorespiration under high salinity stress, proven by the high content of free Ser (Woodrow et al., 2017) in both females and males under mild salinity. Asn can serve as a key nitrogen carrier and is involved in the storage and transport of nitrogen in the plant (Gaufichon et al., 2016). However, Asn content in female plants decreased significantly at 86 mM NaCl compared to 43 mM, suggesting that female plants may not need to reassimilate nitrogen released during photorespiration under high salt conditions because they are less sensitive to ammonia toxicity. In contrast, males appear to respond to the high ammonia release from photorespiration by accumulating other nitrogenous compounds such as Ala, GABA, and Pro, which may help prevent toxic ammonia accumulation and support protein synthesis and growth under stress. The significant increase in Ala content, particularly in male plants under high salinity (86 mM NaCl), indicates a crucial role of this amino acid in the plant's stress response. Ala synthesis involves proton-consuming reactions, particularly through the decarboxylation of malate to pyruvate by the malic enzyme and the subsequent transamination of pyruvate to Ala. This process contributes to the regulation of cytosolic pH by neutralizing excess protons and provides an energy-efficient pathway for nitrogen storage under stress. The accumulation of Ala in male plants may therefore have a dual function: buffering against cytoplasmic acidosis caused by salt stress and serving as a temporary reservoir of ammonia that can be subsequently mobilized upon relief of stress conditions (Fusco et al., 2023). Although present in relatively low concentrations, GABA shows a slight increase under higher salinity levels, especially in male plants. GABA is synthesised by the decarboxylation of glutamate, a reaction that also consumes protons, thus playing a similar role to that of Ala in maintaining cytosolic pH balance under stress. The increased GABA levels in males suggest that this amino acid may contribute to mitigating stress effects by stabilizing cellular pH and protecting against oxidative damage. Additionally, GABA can be metabolized into Krebs cycle intermediates, providing a link between stress responses and energy production, which may help maintain cellular metabolism during prolonged salinity exposure. Moreover, GABA accumulates in plant cells as an osmolyte and contributes to osmotic adjustment, stress signaling, and ROS detoxification (Shelp et al., 1999; Li et al., 2021). Furthermore, like Pro and Ala, it can be broken down after stress by the GABA shunt to provide energy and nutrients for recovery (Carillo et al., 2018). BCAAs, including Val, Leu, and Ile, are also biosynthesized, and their levels are often increased in response to salt stress (Mansour, 2000). BCAAs function as osmolytes, helping to counteract salt stress. They also serve as alternative electron donors for the mitochondrial electron transport chain. Additionally, these AAs can scavenge reactive oxygen species (ROS), though the exact mechanism remains unclear (Woodrow et al., 2017).
These results highlight the physiological and biochemical strategies that plants use to cope with salinity stress. They also underscore the role of specific AAs in adaptive responses.
Female plants appear to invest in growth and structural components even under salinity stress. This is evidenced by the lower levels of soluble proteins, free AAs, and starch at high salinity. Despite the stress, females maintain relatively high levels of fructose, suggesting a continued metabolic activity to support growth and physiological functions. The ability to maintain these functions under stress suggests that female plants have a higher salt tolerance, enabling them to continue their physiological processes almost normally. In contrast, male plants show a different response strategy to salt stress. The growth of male plants seems to be more affected by high salinity, as shown by the decrease in leaf area, which leads to an accumulation of free AAs and soluble sugars, such as sucrose and glucose. This accumulation may serve as a protective mechanism, stabilizing proteins and cell structures while maintaining osmotic balance under stress. Interestingly, the lower levels of Pro and Ser in female plants suggest that they experience less stress than male plants. Consequently, they might not be subjected to the same osmotic and ionic pressure from the salts, allowing them to exhibit greater tolerance to salinity. This tolerance could be due to the utilization of low-cost osmolytes, such as potassium ions, which compartmentalised in the cytosol (Shabala and Pottosin, 2014). By effectively managing these ions, female plants could reduce osmotic and toxic pressure on cytosolic components. Alternatively, female plants might have a better ability to exclude sodium and chloride ions from their cells. This hypothesis, regarding the different utilization of osmolytes and ion management between the sexes, will be further investigated in future studies. Understanding these mechanisms could provide deeper insights into the sex-specific adaptive strategies that enable thyrse sorrel plants to cope with salt stress.
4.3.3 Salinity affects the accumulation of sucrose
Along with Pro, sucrose accumulation in plants is frequently observed under salt stress conditions (Hasegawa et al., 2000). This response is part of the plant's general strategy to deal with osmotic stress by balancing the osmotic potential and protecting cell structures. Sucrose, together with other compatible solutes such as Pro and trehalose, helps to stabilize proteins and membranes, mitigating the damaging effects of high salinity (Parida and Das, 2005; Keunen et al., 2013).
The high increase in glucose and fructose content in the plants under saline conditions suggests that these sugars, in addition to sucrose, may be involved in some way in the osmoprotection of R. thyrsiflorus. Although these hexoses are not normally considered effective osmoprotectants compared to other sugars such as trehalose or sucrose. Nevertheless, they play an important role in the broader context of cellular stress response, particularly in energy production, antioxidant function, intracellular signaling, and osmotic adjustment (Van den Ende and Valluru, 2009; Bolouri Moghaddam and Van den Ende, 2012; Krasensky and Jonak, 2012).
4.3.4 Salinity affects the accumulation of phenolic compounds
Plant PCs are mainly derived from the phenylpropanoid pathway, a synthesis pathway in plants that can be upregulated in response to various environmental stresses (Zhou et al., 2019). Their potent antioxidant properties play a key role in biological processes through mechanisms such as neutralization of reactive oxygen species, chelation of metal ions, and regeneration of membrane-bound antioxidants (Boligon et al., 2013). Consequently, these compounds have a significant impact on human health. Studies indicate an inverse relationship between the consumption of antioxidant-rich foods and the incidence of disease in humans. The use of synthetic antioxidants is associated with negative health consequences, which is why there is an increasing focus on the study of natural antioxidants and their identification in different plant species (Aiyegoro and Okoh, 2009; Saoudi et al., 2021). The Polygonaceae family, particularly the genus Rumex, comprises about 200 plant species known for their medicinal properties (Mohammadi-Sichani et al., 2013). Extracts from aboveground parts and roots have anti-inflammatory, antioxidant, analgesic, and antimicrobial properties (Saoudi et al., 2021). Previous studies have confirmed the presence of PCs in R. thyrsiflorus root and leaf extracts, including phenolic acids, flavonoids, and other phenols (Litvinenko and Muzychkina, 2008; Orbán-Gyapai et al., 2017; Dziedzic et al., 2020).
In female plants of R. thyrsiflorus from the natural population, significantly higher levels of some PCs were found compared to male plants (Dziedzic et al., 2020). Similar observations were noted based on comparative studies of in vitro male and female cultures of the Schisandra rubriflora species subjected to elicitation stress conditions, where higher contents of PCs, including certain lignans, were also found in extracts from female lines (Szopa et al., 2022; Szopa et al., 2024).
When comparing the PC content between the natural populations of R. thyrsiflorus and the in vitro regenerated plants grown in Hoagland's nutrient solution with 0 mM NaCl, we observed that the PC content was higher in some samples in the hydroponically grown plants. In the female plants: protocatechuic acid (10.50 mg/100 g DW in the natural population and 16.58 mg/100 g DW in the hydroponic culture), chlorogenic acid (24.96 mg/100 g DW and 37.45 mg/100 g DW, respectively), cryptochlorogenic acid (34.52 mg/100 g DW and 43.22 mg/100 g DW) and caffeic acid, the concentration of which was three times higher in regenerated plants in both sexes (6.72 mg/100 g DW and 18.43 mg/100 g DW in females; 5.06 mg/100 g DW and 16.55 mg/100 g DW in males, respectively). In addition, in males: protocatechuic acid (5.21 mg/100 g DW and 8.73 mg/100 g DW, respectively), chlorogenic acid (12.73 mg/100 g DW and 15.12 mg/100 g DW, respectively), cryptochlorogenic acid (24.35 mg/100 g DW and 37.11 mg/100 g DW, respectively), vitexin (7.07 mg/100 g DW and 11.04 mg/100 g DW, respectively), apigenin (26.91 mg/100 g DW and 33.87 mg/100 g DW, respectively). In contrast, in the current study with hydroponic culture (0 mM NaCl), the following compounds showed about five times higher contents in male plants: hyperoside (male: 32.13 mg/100 g DW versus female: 6.61 mg/100 g DW) and quercitrin (male: 16.49 mg/100 g DW versus female: 3.39 mg/100 g DW). Concentrations of chlorogenic acid and catechin were found to be twice as high in the female plants. The most significant quantitative difference was observed for vitexin, the content of which was 14 times higher in male plants (11.04 mg/100 g DW) than in female plants (0.81 mg/100 g DW). In a study by Gozdur et al. (2024), the effect of different NaCl concentrations was also studied on the concentrations of PCs, but in callus suspension cultures. Thus, the myricetin concentration in leaf extracts of female regenerants from hydroponic cultures was almost 90 times higher than in female callus, both cultivated on 86 mM NaCl. The quercitrin content was 0.88 mg/100 g DW in the female callus, while it was 22.13 mg/100 g DW in the female regenerants. Thus, respectively: apigenin: 15.23 mg/100 DW in the female callus, 46.51 mg/100 DW in the female regenerants; epicatechin: 17.09 mg/100 DW in the female callus, 96.60 mg/100 DW in the female regenerants and chrysophanol: 5.05 mg/100 DW in the female callus, 48.28 mg/100 DW in the female regenerants. The same applies to the male callus and regenerants: chlorogenic acid: 8.79 mg/100 DW in the male callus, 60.42 mg/100 DW in the male regenerants and caffeic acid: 5.98 mg/100 DW in the male callus, 54.13 mg/100 DW in the male regenerants.
The predominant PCs in all samples of both male and female plants were cryptochlorogenic acid, myricetin, and, among the hydroxyanthraquinones, chrysophanol. Similar in structure to chlorogenic acid, cryptochlorogenic acid also exhibits anti-inflammatory properties, preventing the production of inflammatory cytokines induced by LPS from bacteria (Ma et al., 2022). The concentration of this acid significantly increased almost threefold in females and almost twofold in males in 86 mM NaCl compared to controls. One of the beneficial biological effects that myricetin possesses is its ability to protect the nervous system. It has shown promising effects in preclinical studies related to Alzheimer's, Parkinson's, Huntington's diseases, and amyotrophic lateral sclerosis (Taheri et al., 2020). The concentration of this compound significantly increased under 86 mM NaCl stress conditions in tissues of both sexes of regenerants, with predominance in female individuals. In extracts from female leaves of R. thyrsiflorus, a sixfold increase in chrysophanol content was observed under 86 mM NaCl. Like emodin, chrysophanol is a hydroxyanthraquinone derivative that has numerous pharmacological applications, e.g. as an anti-cancer, anti-diabetic, anti-ulcer, anti-bacterial, anti-viral, and anti-fungal agent. It also has a positive effect on digestion and reduces lipid absorption, thereby alleviating obesity (Prateeksha et al., 2019).
The results show how plant sex influences responses to salt stress and offer potential strategies to improve plant resilience under challenging conditions. These findings may help improve salt tolerance in other plant species and support further research on sex-specific adaptations to environmental stressors.
5 CONCLUSION
In summary, this study elucidates the sex-specific physiological and biochemical adjustments of Rumex thyrsiflorus to salt stress and reveals important adaptive strategies that minimize osmotic damage and ion toxicity. The observed reduction in leaf area under salt stress indicates an adaptive mechanism aimed at reducing water loss and preventing the accumulation of toxic ions, which is crucial in saline environments. Variations in the stomatal apparatus and chlorophyll fluorescence measurements showed the plant's adaptive capacity at different salinity levels, with a slight decrease in photosynthetic efficiency at the highest NaCl concentration (86 mM). Biochemically, the accumulation of free Pro, the decrease in chlorophyll content and the changes in phenolic compounds (PCs) show the metabolic adaptations of the plant to osmotic and ionic stress. Notably, male R. thyrsiflorus showed marked accumulations of specific AAs, including Pro, BCAAs, GABA, and Met, along with the osmoprotective sugar sucrose under high salinity, suggesting possible sex-specific differences in stress metabolism. These results emphasise the possible influence of the plant's sex on the metabolism of these compounds under stress conditions and warrant further research into the mechanisms underlying this sex-specific response.
Understanding these differences enriches our knowledge of how dioecious plants cope with environmental stressors, with implications for breeding salt-tolerant crops. The knowledge gained here forms the basis for targeted research that could help improve plant resilience in the face of increasing salinity challenges in global agriculture.
AUTHOR CONTRIBUTIONS
in vitro and hydroponic culture experiments, molecular, and phytochemical analysis, statistical analysis, data interpretation, original draft preparation: KG; statistical analysis, data interpretation, proofreading the manuscript: PC; phytochemical analysis, data interpretation, proofreading the manuscript: ASz; data interpretation, proofreading the manuscript: IŚ; biochemical analysis: RN; biochemical analysis: RB; in vitro culture experiments, molecular analysis: JT; study conception and design, in vitro and hydroponic culture experiments, data interpretation, writing a manuscript: HŚ. All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
We thank Dr. Agnieszka Marek for her technical help in measuring SOD.
FUNDING INFORMATION
The study was supported by the program BioS Priority Research Area – structural and translational biology “Excellence Initiative – Research University”, edition 2021 at the Jagiellonian University in Kraków and by the statutory research funds N18/DBS/000002 (2022) of the Institute of Botany, Faculty of Biology, Jagiellonian University in Kraków.
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated during and/or analyzed during the current study are available from the corresponding author upon reasonable request.




