Transcriptomic analysis reveals the participation of NTRC in iron homeostasis in Arabidopsis
Abstract
NADPH-dependent thioredoxin reductase C (NTRC) plays a central role in redox regulation of chloroplast photosynthetic metabolism. Accordingly, Arabidopsis (Arabidopsis thaliana) NTRC-null mutants show defective photosynthetic performance and growth inhibition. Remarkably, these mutants show almost a wild-type phenotype at the seedling stage, which raises the question of whether NTRC plays different functions throughout plant development. In this work, we have addressed this issue by performing transcriptome comparisons of Arabidopsis wild-type and ntrc mutant lines at seedling and adult stages of development. In contrast with the high impact of NTRC on leaves from adult plants, the low transcriptomic differences in seedlings suggested a less relevant function of NTRC at this stage of plant development. Notably, the ntrc mutant showed transcriptomic changes resembling the response to Fe excess throughout plant development, though this response was almost unique at the seedling stage. The lack of NTRC caused altered levels of Mn, Zn, Cu, S, P, K and Na, but no significant differences in the content of Fe, as compared with the wild type. Moreover, at the seedling stage, the lack of NTRC caused hypersensitivity to Fe deficit but a protective effect in response to Fe excess, most likely due to lower ROS accumulation in the mutant seedlings. Our results reveal the different impacts of NTRC throughout plant development and identify Fe homeostasis as a process highly affected by NTRC, most notably at the seedling stage.
1 INTRODUCTION
Plant chloroplasts, the organelles that perform photosynthesis, are the source of metabolic intermediates that support plant growth. Furthermore, these organelles have an important sensor activity able to monitor changes in environmental conditions, which influence photosynthesis performance (Schwenkert et al. 2022; Tano and Woodson 2022). A central regulatory mechanism that allows the rapid response of chloroplast photosynthetic performance to environmental cues, such as light intensity and darkness, is based on the protein dithiol-disulfide exchange, which is the basis of thiol-dependent redox regulation (Mittler and Jones 2024; Sies et al. 2024). This regulatory mechanism, which largely relies on the protein disulfide reductase activity of thioredoxins (TRXs), is universally found in any type of organism, from bacteria and fungi to animals and plants.
In contrast with heterotrophic organisms, which harbour two, at most three, TRXs that use reducing power from NADPH via the participation of an NADPH-dependent TRX reductase (NTR), the TRX gene family in plants is remarkably complex (Meyer et al. 2012). Among plant cell compartments, chloroplasts are the organelles with the highest content of TRXs (Zaffagnini et al. 2019; Geigenberger et al. 2017), which are grouped into typical and atypical isoforms (Chibani et al. 2021). Though it remains to be established the source of reducing power for all chloroplast TRXs, it is known that typical TRXs, such as those of the types m and f, which are the most abundant in plant chloroplasts (Okegawa and Motohashi 2015), are reduced by ferredoxin (FDX), the final acceptor of the photosynthetic electron transport chain via the action of a FDX-dependent TRX reductase (FTR) (Schürmann and Buchanan 2008). Thus, the FDX-FTR-TRXs redox system links the redox regulation of chloroplast enzymes to light. Chloroplasts harbour an additional redox system, NTRC, an NTR with a TRX domain at the C-terminus (Serrato et al. 2004), which has a high affinity for NADPH (Bernal-Bayard et al. 2012). The finding that the hydrogen peroxide scavenging enzyme 2-Cys peroxiredoxin (PRX) is efficiently reduced by NTRC (Alkhalfioui et al. 2007; Moon et al. 2006; Pérez-Ruiz et al. 2006), led to the proposal of an antioxidant function for this enzyme. However, the Arabidopsis ntrc mutant, which is devoid of NTRC, shows a rather severe growth inhibition phenotype (Lepistö et al. 2009; Pérez-Ruiz et al. 2006; Serrato et al. 2004), and impairment of different redox-regulated processes supporting the proposal that NTRC acts as a central hub in chloroplast redox regulation, as recently reviewed (Cejudo et al. 2021).
The finding that Arabidopsis mutants combining the deficiencies of NTRC and 2-Cys PRXs recover a WT-like phenotype uncovered the essential function of 2-Cys PRXs in controlling the reducing capacity of chloroplast TRXs, which allows optimization of the photosynthetic performance of the organelle in response to changes in light intensity (Pérez-Ruiz et al. 2017; Yokochi et al. 2021). Moreover, NTRC is essential for the activity of TRXs of the types x and f during the early stages of plant development (Ojeda et al. 2017). However, ntrc seedlings develop normal green cotyledons (Lepistö et al. 2009; Ojeda et al. 2021), and neither the lack nor the overexpression of NTRC has any effect on embryogenesis in developing seeds (Gallardo-Martínez et al. 2023) in contrast with its relevant role at latter stages of plant development. Hence, NTRC has differential functions throughout plant development: a poor contribution at early stages of plant development when chloroplast biogenesis occurs, whereas in fully differentiated chloroplasts from mature leaves, this enzyme plays a key role in the redox regulation of photosynthesis in response to changes in light intensity.
Despite the extensive analysis of the function of NTRC in adult leaves, hence, with fully differentiated chloroplasts, the function of this enzyme at earlier stages of plant development remains poorly known. In this work, we have addressed this issue by performing a comparative analysis of the transcriptomes of the Arabidopsis ntrc mutant, as compared to WT, in seedlings and leaves from adult plants. Our results show a low transcriptomic impact of the lack of NTRC at the seedling stage, in contrast to the severe impact on adult leaves. Remarkably, these analyses revealed that the lack of NTRC affects the expression of genes involved in Fe homeostasis, triggering transcriptomic changes resembling the response to Fe excess, which is more relevant at the seedling stage.
2 MATERIALS AND METHODS
2.1 Biological material, growth conditions, and Fe treatments
Arabidopsis thaliana WT (ecotype Columbia) and ntrc mutant (Serrato et al. 2004) were routinely grown in soil in growth chambers under short-day (8/16 h light/ dark) photoperiod at 22 and 20°C during light and dark periods, respectively, and light intensity of 125 μmol m−2 s−1. WT and ntrc seedlings were grown on Murashige and Skoog (MS) medium containing 0.35% (w/v) Gelrite (Duchefa) under continuous light at 22°C and 125 μmol m−2 s−1.
Fe treatments were performed in synthetic media (pH 5.5, 0.7% agar) containing 5 mM KNO3, 2.5 mM KH2PO4, 2 mM MgSO4, 2 mM Ca(NO3)2, 70 μM H3BO3, 14 μM MnCl2, 10 μM NaCl, 1 μM ZnSO4, 0.5 μM CuSO4, 0.2 μM Na2MoO4, 4.7 mM MES (Rodríguez-Celma et al. 2013) and a variable concentration of Fe-EDTA as source of Fe. For Fe treatments at the seedling stage, seeds were germinated in synthetic media containing either 50 μM Fe-EDTA (Fe-sufficient, control condition), 500 μM Fe-EDTA (Fe-excess), or medium without Fe-EDTA and supplemented with 100 μM ferrozine (sodium 4-[3-(pyridin-2-yl)-6-(4-sulfophenyl)-1,2,4-triazin-5-yl]benzene-1-sulfonate) to chelate residual Fe (Fe-deficient). Fe treatments at the adult stage were performed on plants grown in Fe-sufficient medium for 21 days and transferred to Fe-sufficient (control conditions), Fe-excess or Fe-deficient media for 9 days.
2.2 RNA extraction, RNA-Seq and RT-qPCR analyses
Two different transcriptomics experiments, at the adult and seedling stages of plant development, were carried out. For adult samples, eight-week-old short-day grown WT and ntrc plants were randomly selected, and young rosette leaves were collected during the day period (after 2 h of illumination at 125 μmol m−2 s−1). The experimental design consisted of three biological replicates for each genotype, each of them containing leaves from three individual plants. WT raw RNA-Seq data from the adult stage can be found in Gene Expression Omnibus, identified with accession number GSE147793. RNA extraction was performed from pooled leaf samples using the Sure Prep kit (Fisher), following the manufacturer's instructions. RNA concentration and purity were tested by an Agilent 2100 Bioanalyzer, a microfluidics-based platform that performs quality control of DNA and RNA samples before sequencing. Library construction of cDNA molecules was carried out following the manufacturer's instructions using the TruSeq Stranded Total RNA with Ribo-Zero kit (Illumina). The generated DNA fragments were sequenced with the Illumina HiSeq 4000 platform at STAB-VIDA (Caparica, Portugal), yielding approximately 60–80 million 150 base pairs long paired-end reads for each sample. For seedling samples, six-day-old WT and ntrc mutant seedlings, grown under continuous light in MS medium and lacking true leaves, were collected. The experimental design consisted of three biological replicates for each genotype. RNA extraction and quality control were carried out following the above indicated procedure for adult samples. The cDNA libraries were constructed according to the manufacturer's instructions using the Illumina Stranded mRNA prep ligation kit (Illumina). Sequencing of the DNA fragments was performed with the Illumina NextSeq 500 platform at Centro Andaluz de Biología Molecular y Medicina Regenerativa (CABIMER, Seville, Spain), yielding approximately 18–26 million 75 base pairs long single-end reads for each sample.
For all samples, the FastQC software package was used to control the quality (http://www.bioinformatics.babraham.ac.uk/projects/fastqc/). Once the good quality of all samples had been determined, read mapping to The Arabidopsis Col-0 TAIR 10 reference genome (https://plants.ensembl.org/) and transcript assembly were performed with the software tools HISAT2 and StringTie (Pertea et al. 2016) using default parameters. Differential expression analysis was carried out with the Ballgown (Pertea et al. 2016) and LIMMA (Ritchie et al. 2015) R packages. Differentially expressed genes (DEGs) were selected using a fold change of ± log2(2) and P-value <0.05 computed according to a moderated t-test. Volcano plots and Venn diagrams were generated using the R packages ggplot2 and eulerr. Gene Ontology (GO) term enrichment analysis over the different gene sets was performed with the enrichGO function of the Bioconductor R package ClusterProfiler (Yu et al. 2012). From the same R package, the compareCluster function was used to compare the different gene ontology terms of adult and seedling samples. Principal Component analysis was carried out using the prcomp function from the R package stats of the R Core Team.
For Real-time quantitative PCR (RT-qPCR) analysis, total RNA was extracted using the Trizol reagent (Invitrogen) and cDNA synthesis was performed with 1 μg of total RNA using the Maxima first-strand cDNA synthesis kit (Thermo Scientific) according to manufacturer's instructions. RT-qPCR was performed using an IQ5 real-time PCR detection system (Bio-Rad) with oligonucleotides listed in Supplemental Table S1. Expression levels were normalized using ACTIN2, UBIQUITIN10 and UBIQUITIN-CONJUGATING ENZYME9 as reference genes.
2.3 Measurements of chlorophyll levels and determination of photosynthetic parameters
Chlorophyll levels were measured as previously described (Pérez-Ruiz et al. 2006). Room temperature chlorophyll a fluorescence was measured using a pulse-amplitude modulation fluorometer IMAGING-PAM M-Series (Walz). The maximum quantum yield of PSII was assayed after incubation of plants in the dark for 30 min by calculating the ratio of the variable fluorescence, Fv, to maximal fluorescence, Fm (Fv/Fm). Induction-recovery curves were performed using red (635 nm) actinic light at 81 μmol m−2 s−1 for 10 min. Saturating pulses of red light at 10,000 μmol m−2 s−1 intensity and 0.6 s duration were applied every 60 s, and recovery in darkness was recorded for up to 10 min. The parameter Y(II), corresponding to the respective quantum yield of PSII photochemistry, was calculated according to reported equations (Kramer et al. 2004).
2.4 Determination of anion superoxide content
The detection of superoxide anion content was performed with nitroblue tetrazolium (NBT) staining, as previously described (Cordoba et al. 2016), with some modifications. Briefly, at least six seedlings of WT and ntrc mutant grown in Fe-deficient, Fe-sufficient (Fe-control) and Fe-excess media were randomly selected and incubated in staining buffer (10 mM phosphate pH 7.6, 10 mM NaN3 and 0.1% NBT (Sigma)) for 2.5 h at room temperature in agitation. Then, the NBT solution was removed, and a bleaching solution (ethanol: acetic acid: glycerol; 3:1:1) was added. Once the chlorophyll had been removed, a new bleaching solution was added to the seedlings, which were stored at 4°C until images were taken with an Olympus Microscope. For the quantification of the area of cotyledons stained with NBT, colour thresholds were used in the ImageJ software.
2.5 Ionomics determinations
For WT and the ntrc mutant, whole rosette samples of 8-week-old plants grown in soil under short-day conditions and 4-day-old seedlings grown under continuous light in MS medium were lyophilized for determination of ion content by inductively coupled plasma mass spectrometry (ICP-MS) at the Servicio de Análisis, Instituto de Recursos Naturales y Agrobiología de Sevilla (IRNASE, CSIC), Seville, Spain. In brief, 4 mL of HNO3 (65%) was added to the weighed lyophilized material. The digestion was carried out under pressure in a microwave oven (MARS ONE CEM). The treated samples were diluted with ultrapure water and passed through nylon filters. Ion determination was performed using an AGILENT 7800 ICP-MS. Principal Component analysis was carried out using the prcomp function from the R package stats of the R Core Team.
2.6 Statistical analysis
Statistical analyses were performed using GraphPad Prism version 6.01 (GraphPad Software, Inc.), and the experimental results were analyzed using two-tailed Student's t-tests.
3 RESULTS
3.1 Comparative transcriptomic analyses reveal a poor impact of NTRC at the seedling stage of plant development
With the aim of determining the impact of NTRC at different stages of plant development, we have performed a comparative transcriptomic analysis of Arabidopsis WT and the ntrc mutant at seedling and adult stages. To that end, genome-wide transcriptomes were determined by RNA-Seq analysis of three independent biological samples harvested from young leaves of 8-week-old Arabidopsis WT and ntrc mutant plants grown under short-day photoperiod (adult stage) and from seedlings that had been grown for 6 days on MS medium under continuous light (seedling stage) (Figure 1A, C). Though the ntrc mutant contained lower chlorophyll levels at both developmental stages, the difference was more significant at the adult stage (Figure 1B, D). The sequencing quality of the samples was assessed by the high percentage of mapped reads and correlation between biological replicates (around 99%) (Figure S1A-D). A principal component analysis showed that the ntrc and WT seedling replicates clustered together, whereas replicates from adult ntrc clustered separately from the WT (Figure S2), thus indicating a more relevant impact of NTRC on the transcriptomes at adult than at seedling stages.
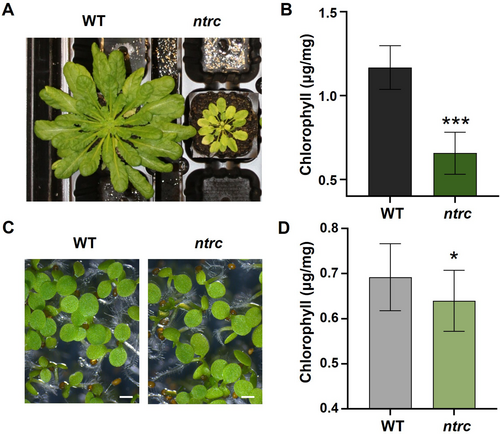
The comparison of the transcriptomes from leaves of the adult plants of the WT and the ntrc mutant, using a fold change of gene expression of ±log2(2) and p < 0.05, identified a large set of 535 DEGs in the ntrc mutant, 358 upregulated and 177 downregulated (Figure 2A). Strikingly, a relatively low number of DEGs (94), of which 35 were upregulated and 59 downregulated (Figure 2B), were identified in the comparison of the WT and the ntrc mutant transcriptomes at the seedling stage. The lists of upregulated and downregulated genes in the ntrc mutant at both developmental stages are available as supplemental information (Supplemental Appendix S1). A Venn diagram representation of DEGs at adult and seedling stages identified an overlapping set of 24 DEGs common to both stages (Figure 2C). Thus, the higher number of DEGs between the ntrc mutant and the WT at the adult stage confirms the deep impact of NTRC on plant growth, in line with the severe growth inhibition phenotype of the ntrc mutant (Figure 1A, B), whereas the modest transcriptomic changes shown by the ntrc seedlings reveals the poor impact of NTRC at this early developmental stage, which is in line with the almost WT-like phenotype of the ntrc seedlings (Figure 1C, D). Moreover, the low number of DEGs commonly detected at seedling and adult stages indicates that the participation of NTRC throughout plant development is restricted to a few biological processes.
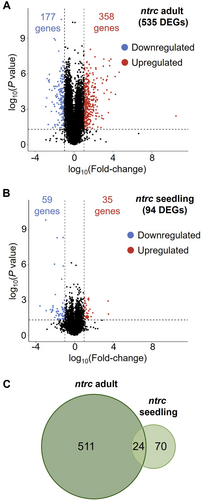
3.2 The ntrc mutant shows transcriptomic changes resembling the response to Fe excess
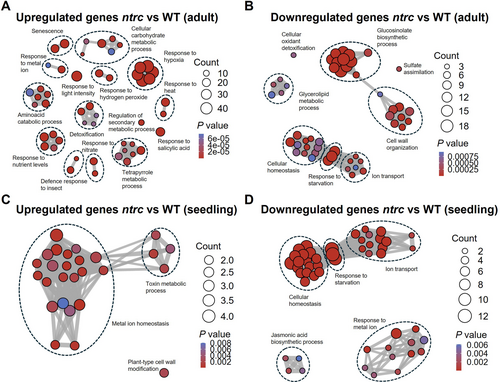
To identify the processes affected by the lack of NTRC at adult and seedling stages, a gene ontology (GO) term enrichment analysis was performed (Supplemental Appendix S2). In line with the severe growth inhibition phenotype of the ntrc mutant, the comparison of the WT and ntrc mutant transcriptomes at the adult stage showed the pleiotropic effects caused by the absence of NTRC, affecting a wide range of biological processes, as previously reported (Lepistö et al. 2009). Upregulated genes in the ntrc mutant at this developmental stage are enriched in GO terms involved in metabolism (carbohydrates, amino acids and tetrapyrroles), abiotic stress response (hypoxia, nutrient level, nitrate, light, heat, metal ion), biotic stress response (insect, salicylic acid), and detoxification (Figure 3A). In contrast, GO terms related to glucosinolate and glycerolipid metabolisms, cell wall organization, cellular homeostasis, response to starvation and ion transport were the biological processes identified among the downregulated genes in the ntrc mutant (Figure 3B). At the seedling stage, most enriched GO terms among the upregulated genes in the ntrc mutant were related to metal ion homeostasis (Figure 3C), whereas GO terms associated with cellular homeostasis, response to starvation and ion transport, as well as response to metal ion and jasmonic acid biosynthesis, were identified among the downregulated genes (Figure 3D).
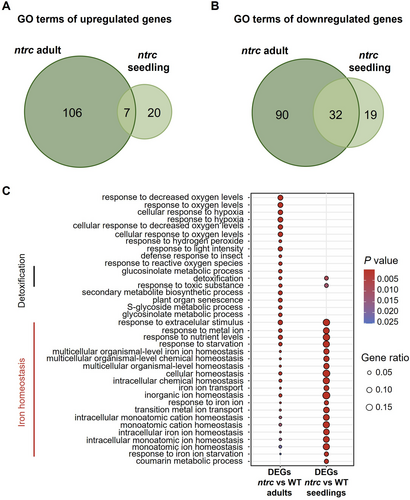
To further analyze the impact of NTRC at different stages of plant development, Venn diagrams were constructed identifying common GO terms among upregulated and downregulated genes in the ntrc mutant at adult and seedling stages (Figure 4A, B and Supplemental Appendix S3). This analysis identified GO terms related to detoxification and homeostasis of Fe enriched at both stages of development (Figure 4C). The fact that many of the DEGs between the ntrc mutant and the WT from adult and seedling stages identified terms such as “Intracellular iron homeostasis”, “Iron ion homeostasis”, “Multicellular organismal-level iron homeostasis”, “Response to iron ion” and “Response to iron starvation” (Figure 4C) revealed the effect of NTRC on the transcription of genes involved in Fe homeostasis at both developmental stages here analyzed, a previously unrecognized function of NTRC, which seems more relevant in seedlings than in leaves from adult plants.
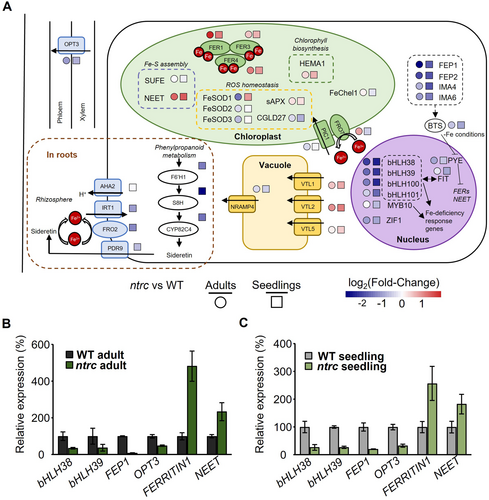
In plants, Fe homeostasis involves different organs and tissues including roots and the vascular system and, in leaf photosynthetic cells, different cell compartments (Liang, 2022). Thus, to figure out the effect of NTRC on Fe homeostasis at different developmental stages, DEGs involved in Fe homeostasis or response to Fe in the transcriptomic analyses of seedling and adult samples were selected, and their fold-changes in the level of expression were represented in different organs and cell compartments (Figure 5A). Genes encoding peptides (FEP1, FEP2, IMA4, IMA6) that positively regulate the transcription factors bHLH38, bHLH39, bHLH100 and bHLH101, all of them involved in activation of the expression of genes responsive to Fe deficiency, were found among the most downregulated genes in seedlings and, at lower extent, in adult leaves of the ntrc mutant (Figure 5A), hence indicating that the lack of NTRC triggers transcriptomic changes resembling the response to Fe excess. This notion was further supported by the identification of genes involved in the transport of Fe through the phloem and xylem, such as OLIGO PEPTIDE TRANSPORTER 3 (OPT3) among the downregulated genes at both stages. Furthermore, genes encoding the Fe transporter in root cells IRON REGULATED TRANSPORTER 1 (IRT1) and enzymes of the sideretin biosynthetic pathway were also downregulated in seedlings of the ntrc mutant (Figure 5A). These genes showed differences only at the seedling stage due to the nature of the adult samples, which excluded root tissues. In leaves cells, the comparative transcriptomic analyses revealed upregulation of genes encoding transport of Fe to the vacuoles, VACUOLAR IRON TRANSPORTER-LIKE 1, 2 and 5 (VTL1, VTL2, VTL5) and the chloroplast PERMEASE IN CHLOROPLASTS 1 (PIC1), both at adult and seedling stages (Figure 5A). Furthermore, genes involved in Fe-S assembly (NEET) and tetrapyrrole biosynthesis (HEMA1) into the chloroplast and, remarkably, genes encoding ferritins (FER1, FER3 and FER4) were upregulated in adult and seedlings samples of the ntrc mutant, further supporting the notion that the lack of NTRC triggers a Fe excess transcriptomic response. On the contrary, CONSERVED IN THE GREEN LINEAGE AND DIATOMS 27 (CGLD27), which is required for growth under Fe starvation (Urzica et al. 2012), was downregulated in the ntrc mutant at both developmental stages. Among genes encoding enzymes involved in the scavenging of reactive oxygen species (ROS), formed in the presence of Fe via the Fenton reaction, stromal APX (sAPX) was upregulated in the ntrc mutant, whereas FeSOD1-3 genes were downregulated in mutant seedlings and FeSOD1 upregulated in adult leaves. Finally, several of the DEGs between the ntrc mutant and the WT were chosen to validate the results of the RNA-Seq data by RT-qPCR analysis. The lower level of transcripts of the bHLH38, bHLH39, FEP1 and OPT3 genes and the higher levels of the FER1 and NEET genes in adult leaves (Figure 5B) and seedlings (Figure 5C) of the ntrc mutant validated the results of the transcriptomic analysis.
3.3 Effect of Fe excess and deficit treatments on photosynthetic performance
To gain a deeper insight into the function of NTRC in metal ion homeostasis, we sought to analyze the effect of Fe deficiency and excess treatments on the ntrc mutant performance at the two developmental stages analyzed. For seedling treatments, WT and ntrc seeds were germinated and grown for 4 days under continuous light in a synthetic medium supplemented with 50 mM Fe-EDTA (Fe-control, sufficiency conditions), 100 mM ferrozine and no added Fe-EDTA (Fe-deficiency conditions) and 500 mM Fe-EDTA (Fe-excess conditions) (Figure 6A). To analyze the effect at adult stage, WT and ntrc plants grown for 21 days under short-day photoperiod and Fe-sufficient conditions (Fe-control) were transferred to Fe-control, Fe-deficiency or Fe-excess synthetic media and grown for 9 days. The photosynthetic parameters Fv/Fm, which reflects PSII stability, and quantum yield of PSII photochemistry (Y(II)) were determined as readouts of the treatments (Figure 6A-C).
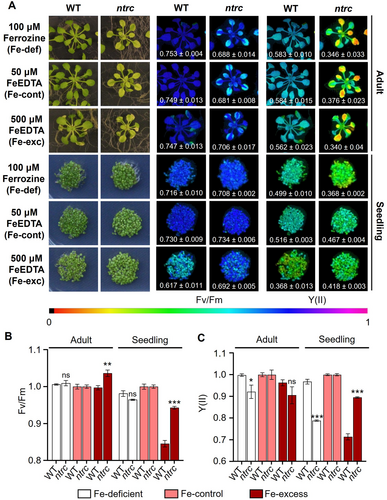
The Fe deficiency treatment caused no significant differences in the levels of Fv/Fm either at adult or seedling stages of the WT and ntrc mutant (Figure 6A, B). However, the quantum yield of PSII (Y(II)) revealed a slight effect of this treatment at the adult stage in the ntrc mutant, which was more severe at the seedling stage (Figure 6A, C), hence indicating the sensitivity of the ntrc mutant to Fe deficiency, which is higher at early stages of development. The Fe excess treatment, which did not significantly affect the WT at the adult stage, caused slightly higher levels of Fv/Fm (Figure 6A, B), but no significant variation of Y(II) (Figure 6A, C) in adult ntrc plants. In contrast, this treatment had a severe effect on WT seedlings, as revealed by the lower levels of Fv/Fm (Figure 6A, B) and Y(II) (Figure 6A, C). Remarkably, the lack of NTRC triggered a significant tolerance to Fe excess at the seedling stage, as shown by the higher levels of Fv/Fm (Figure 6A, B) and Y(II) (Figure 6A, C) in seedlings of the ntrc mutant.
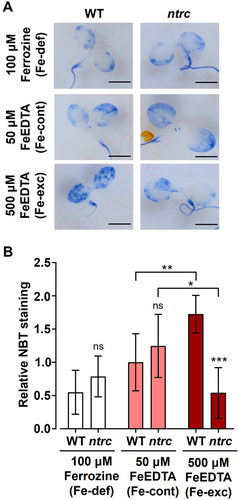
It is known that Fe excess may be potentially toxic for plants by triggering ROS formation by the Fenton reaction (Halliwell and Gutteridge 1992). Thus, the better performance of the ntrc mutant seedlings under Fe excess might be due to altered ROS homeostasis caused by the lack of NTRC. To test this possibility, seedlings of the WT and the ntrc mutant subjected to Fe excess and deficiency treatments were stained with NBT to detect the contents of superoxide anion (Figure 7A). No significant differences in NBT staining between the WT and ntrc mutant seedlings were observed under control conditions or in response to Fe deficiency; in contrast, the treatment with Fe excess caused higher levels of NBT staining, hence of superoxide anion, in WT, but not in the ntrc mutant seedlings (Figure 7B). Thus, Fe excess provoked oxidative stress in seedlings of the WT, but not of the ntrc mutant, suggesting that the alteration of ROS homeostasis caused by the absence of NTRC protects from the negative effects of Fe excess.
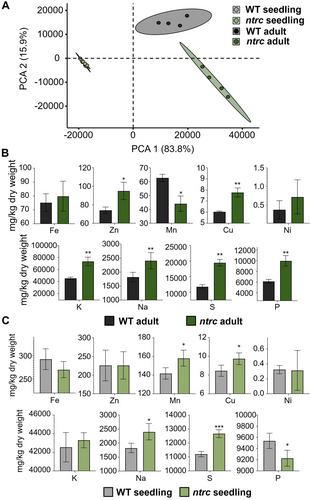
Finally, to further explore the impact of NTRC on metal ion homeostasis, a global ionomic analysis was performed in the WT and the ntrc mutant at the seedling and adult stages. As in the transcriptomic study (Figure S2), a principal component analysis clustered the WT and ntrc seedling samples, while the adult samples were found separately (Figure 8A), further confirming the different impact of the lack of NTRC in these developmental stages. Remarkably, despite the transcriptomic changes affecting genes involved in Fe homeostasis caused by the lack of NTRC, no significant differences in the contents of Fe in the WT and the ntrc mutant were detected either at adult (Figure 8B) or at seedling stages (Figure 8C). However, the lack of NTRC caused higher contents of Na, Cu and S, both at adult (Figure 8B) and seedling stages (Figure 8C), whereas the levels of K, which were higher in adult leaves of the ntrc mutant (Figure 8B), showed no significant differences in WT and ntrc mutant seedlings (Figure 8C). In contrast, opposite changes in the levels of Mn and P were detected in adult (Figure 8B) and seedlings (Figure 8C) of the ntrc mutant, as compared with the WT.
4 DISCUSSION
The Arabidopsis ntrc mutant shows a severe growth inhibition phenotype at adult stages of development (Serrato et al., 2004; Lepistö et al. 2009), which is in clear contrast with the minor alteration of growth at earlier stages, as indicated by the slightly lower content of chlorophyll in seedlings of the ntrc mutant than in those of the WT (Figure 1B, D). Thus, the growth phenotype of the ntrc mutant indicates different contributions of NTRC throughout plant development. Whilst it is well-established the relevant contribution of NTRC to redox regulation of chloroplast photosynthetic metabolism in leaves from adult plants (Ojeda et al., 2021; Pérez-Ruiz et al. 2017; Yokochi et al., 2021), its function at earlier stages of plant development remains to be analyzed. In this work, we have addressed this issue by a comparative transcriptomic analysis of the WT and the ntrc mutant at seedling and adult stages of development. The high transcriptomic changes between the leaves of adult plants of the WT and the ntrc mutant, in contrast with the low transcriptomic changes detected in seedlings (Figure 2A, B), agree with the growth phenotype of the ntrc mutant and confirm the different contribution of NTRC throughout plant development. The high variety of processes identified by GO analysis of the DEGs from adult leaves of WT and ntrc mutant (Figure 3A, B) is in line with previous transcriptomic analysis (Lepistö et al. 2009), and supports the central function of NTRC in chloroplast redox regulation, which impacts on primary and secondary metabolisms, nutrient assimilation, and response to abiotic and biotic stresses. Seedling development is associated with chloroplast differentiation (Cackett et al. 2022; Waters and Langdale 2009), which precedes active photosynthesis at this early stage of plant growth. Thus, the almost WT phenotype of the ntrc mutant seedlings (Figure 1C, D; Ojeda et al. 2017), in line with the low transcriptomic differences between WT and ntrc mutant at this stage of development (Figure 2B), suggests that NTRC has a minor contribution to chloroplast differentiation. In agreement with these results, it was found that seed development (Gallardo-Martínez et al. 2023) and seedling growth (Ojeda et al. 2021) are unaltered in the ntrc mutant, further confirming the limited contribution of NTRC at early plant development.
The finding of GO terms related to “Metal ion homeostasis” as the most represented among the upregulated genes in the ntrc seedlings (Figure 3C), in addition to the identification of GO terms “Response to metal ion” and “Ion transport” among the downregulated genes (Figure 3D) revealed a previously unknown participation of NTRC in the regulation of the expression of genes involved in the response to metal ions and the control of metal ion homeostasis. The transcriptomic comparisons reveal that this effect of NTRC is exerted both at seedling and adult stages of plant development (Figure 4C). However, due to the low transcriptomic changes observed in the ntrc mutant seedlings, this effect is more relevant at this stage of development. The global analysis of the transcriptomic changes in the ntrc mutant, as summarized in the scheme of Figure 5A, indicates that the lack of NTRC triggers transcriptomic changes resembling the response to Fe excess. This proposal is supported by the following findings. (1) Of the complex network of transcription factors involved in the response of Arabidopsis to Fe deficiency (Gao et al. 2019), genes encoding transcription factors bHLH38, bHLH39, bHLH100 and bHLH101, which are known to be induced under Fe deficiency (Wang et al. 2007), are among the most downregulated genes in the ntrc seedlings, as compared with the WT, and, to a lesser extent, also in adult leaves. Since it is known that in the presence of Fe, the proteasomal degradation of transcription factors turns off Fe signalling (Vélez-Bermúdez and Schmidt, 2022), the downregulation of transcription factors bHLH38, bHLH39, bHLH100 and bHLH101 is compatible with a Fe excess transcriptomic response in the ntrc mutant. (2) Transcription factors bHLH and FIT form heterodimers to activate the expression of the FRO2 and IRT1 genes, involved in the reduction of Fe3+ to Fe2+ and the transport of Fe2+ in roots, respectively (Wang et al. 2013; Yuan et al. 2008). Thus, the downregulation of the FRO2 and IRT1 genes in seedlings of the ntrc mutant agrees with the lower expression of genes encoding bHLH and FIT transcription factors, as expected of a transcriptomic response to Fe excess. And (3), genes encoding chloroplast-localized proteins involved in chlorophyll biosynthesis, such as HEMA1 (Rodríguez-Celma et al. 2013), Fe-S cluster assembly, such as NEET, and ferritins, which are induced in response to Fe excess (Tissot et al. 2019), were upregulated both at seedling and adult stages in the ntrc mutant.
The differences detected in the ionomic analysis of adult leaves and seedlings of the WT and the ntrc mutant (Figure 8B, C) confirm the relevance of NTRC in ion homeostasis, as suggested by the transcriptomic data. However, it is worth noting that no significant differences were detected in the contents of Fe in adult leaves (Figure 8B) or seedlings (Figure 8C) of the ntrc mutant, suggesting that the effect of the lack of NTRC on the expression of genes involved in Fe homeostasis is triggered by impaired signalling from altered chloroplast redox state in the ntrc mutant. In support of this possibility, it is well-known that altered chloroplast function affects Fe homeostasis. In this regard, it was reported that PSI photoinhibition treatments, which provoke damage to Fe-S clusters (Tiwari et al. 2016), trigger the upregulation of genes involved in Fe excess response (Kilic et al. 2023), thus resembling the transcriptomic response of the ntrc mutant. Moreover, the Arabidopsis mutant affected in the plastid protease ClpC1 shows altered Fe homeostasis (Wu et al. 2010), and mutants of the PAP/SAL1 retrograde signalling pathway show upregulation of genes encoding ferritins (Balparda et al. 2020). The fact that the SAL1 sensor activity of the chloroplast redox poise was found to be altered in the ntrc mutant (Chan et al. 2016) indicates that chloroplast retrograde signalling is affected by the redox state of the organelle. Altogether, these results lend support to the notion that the lack of NTRC affects the expression of genes involved in Fe homeostasis due to altered chloroplast-to-nucleus retrograde signalling.
As a component of hemo cofactors and Fe-S clusters, Fe is essential for the photosynthetic electron transport chain; hence, altered Fe homeostasis and/or availability has a deep impact on photosynthesis and plant growth (Therby-Vale et al. 2022). Thus, once the effect of the lack of NTRC on the transcriptomic changes affecting Fe homeostasis is established, the question arising is whether these changes have any effect on the response of seedlings and adult plants to Fe availability. The fact that the Fv/Fm ratio (Figure 6A, B) and Y(II) (Figure 6A, C) parameters, which were determined as readouts of photosynthetic performance, showed more pronounced differences at seedling than adult stages both in WT and the ntrc mutant confirms the transcriptomic data indicating a more relevant effect of the lack of NTRC on Fe homeostasis at the seedling stage. The finding that seedlings of the ntrc mutant showed sensitivity to the Fe deficit treatment, as shown by the lower levels of Y(II) (Figure 6A, C), agrees with the Fe excess transcriptomic response of the ntrc mutant since the downregulation of genes involved in Fe uptake might provoke lower contents of the metal in response to this treatment. However, the similar levels of Fe in WT and ntrc mutant seedlings (Figure 8C) indicate that additional stages of Fe homeostasis contribute to the higher sensitivity to Fe deficit in seedlings devoid of NTRC.
Surprisingly, the lack of NTRC has a protective effect on the seedling's photosynthetic performance in response to Fe excess treatments, which cause more severe damage in the WT, as revealed by the levels of Fv/Fm (Figure 6A, B) and Y(II) (Figure 6A, C). The higher accumulation of superoxide anion in seedlings of the WT, as revealed by NBT staining (Figure 7A, B), suggests that the Fe excess treatment is detrimental for the WT due to ROS accumulation, which does not occur in the ntrc seedlings (Figure 7A, B). Since WT and ntrc seedlings show similar levels of Fe (Figure 8B), a likely possibility is that the overexpression of ferritins in the mutant (Figure 5A-C) captures free Fe, hence avoiding the deleterious formation of ROS by the Fenton reaction. Additionally, the gene encoding FeSOD1, which exerts a protective effect against oxidative stress (Melicher et al. 2022), showed upregulation in ntrc seedlings (Figure 5A), which suggests increased dismutation of anion superoxide to hydrogen peroxide. It is well established the role of ROS and reactive nitrogen species (RNS) in response to nutrient availability (Nieves-Cordones et al. 2019) and ion and metal homeostasis (Ramírez et al. 2011; Sandalio et al. 2023). Thus, it is likely that altered ROS and RNS caused by the lack of NTRC might affect the chloroplast-to-nucleus retrograde signalling that controls the expression of genes involved in Fe homeostasis. In support of this notion, it was shown that glutathione regulates iron homeostasis by the S-nitrosylation of bHLH transcription factors that control the expression of iron transporters in Arabidopsis (Shee et al. 2022), but more work is needed to establish the role of ROS and RNS in the NTRC-dependent signalling pathway that controls Fe homeostasis.
In summary, the comparative transcriptomic analyses between the WT and the ntrc mutant performed in this study confirm the poor effect of the lack of NTRC at early developmental stages, suggesting a minor contribution of the enzyme to chloroplast differentiation. Moreover, our results reveal the contribution of NTRC to the regulation of genes involved in metal ion homeostasis, most probably exerted by altered chloroplast-to-nucleus retrograde signalling. Remarkably, the lack of NTRC increases the tolerance to Fe excess. It is likely that the transcriptomic changes resembling the response to Fe excess in the ntrc mutant have a protective effect by avoiding the accumulation of ROS triggered by this treatment.
AUTHOR CONTRIBUTIONS
JMP-R and FJC designed the research. FRM performed the experimental work. FRM, JMP-R and FJC participated in the analysis of experimental results. FJC wrote the manuscript. All authors contributed to the manuscript and approved the submitted version.
ACKNOWLEDGEMENTS
This work was supported by Grants PID2020-115156GB-I00 and PID2023-146573NB-I00 funded by Ministerio de Ciencia, Innovación y Universidades (MICIU)/Agencia Estatal de Investigación (AEI)/10.13039/501100011033. FRM was supported by a FPI pre-doctoral contract (PRE2021-097268) from MICIU. Ministerio de Ciencia e Innovación.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The RNA-seq data sets generated in this study have been deposited in the Gene Expression Omnibus (GEO) under accession GSE285082.




