Seaweed extract combined with boron promotes the growth of sugar beet by improving the photosynthetic performance under boron deficiency
Abstract
Biostimulants can improve mineral nutrient effectiveness, but the physiological mechanisms by which seaweed extract, a natural biostimulant, and boron (B) fertilizer promote the growth of sugar beet under B deficiency are not clear. In this study, B and seaweed extract were applied under B-deficiency (0.32 mg B kg−1 soil) and potentially B-deficient conditions (0.69 mg B kg−1 soil), and the growth and photochemical properties of sugar beet seedlings were investigated. The results indicated that B application alone or with seaweed extract promoted sugar beet growth, with the combined application having a significantly enhanced effect. When comparing the two soils with different B content (deficient and moderately deficient), the spray of seaweed extract under the B-deficient condition was more effective. After the addition of seaweed extract, the B content of the shoots and roots increased by 28.56% and 12.64%, respectively, under B deficiency(0.32 mg B kg−1 soil). Furthermore, the content of chlorophyll a (Chla), chlorophyll b (Chlb), and the net photosynthesis rate (Pn) increased by 8.96%, 30.57%, and 13.74%, respectively. The addition of seaweed extract significantly improved the light saturation point (Pm), with a 35.00% increase compared to the control. Additionally, it increased the quantum yield (ETO/CSm) of leaf electron transfer per unit area and reduced the absorption of light energy loss of the PS reaction center (DIO/RC) and per unit area (DIO/CSm), thereby improving photosynthetic performance and significantly increasing aboveground dry matter accumulation by 19.05%. In conclusion, seaweed extract can enhance B absorption, light energy capture and utilization ability. It provides the theoretical evidence for the regulation of B nutrition in sugar beet and the rational application of biostimulants.
1 INTRODUCTION
Biostimulants are a class of substances that stimulate plant growth by improving nutrient utilization efficiency and have been widely used in agricultural production in recent years (Samuels et al., 2022). Seaweed extract is a vital category of biostimulant, rich in polysaccharides, vitamins, and amino acids (Xu et al., 2022). Seaweed extract can enhance crop tolerance to nutrient stress by promoting plant nutrient absorption and improving photosynthetic performance (Yadav et al., 2016; Ali et al., 2021; Zafar et al., 2022), thus improving crop plants yield and quality (Hussein et al., 2021; Xu et al., 2022; Vila et al., 2023). Seaweed extracts have recently gained attention due to their safety characteristics, high efficiency, and environmental friendliness (Arioli et al., 2023; Raja and Vidya, 2023).
Sugar beet (Beta vulgaris L.) is a biennial herb that belongs to the Chenopodiaceae family, and it is distributed in over 50 countries worldwide (Tayyab et al., 2023). According to Monteiro et al. (2018), sugar produced from beets represents 30% of the total global sugar production. In China, sugar beet is largely distributed in North China, Northeast China, and Northwest China. Boron deficiency has emerged as a global agricultural crisis, affecting 132 major crops across more than 80 countries, resulting in decreased yield and quality (Liu et al., 2024). Soil boron (B) deficiency is often found in sugar beet planting areas (Shukla et al., 2021). Moreover, sugar beet has a high requirement for B, which makes it more susceptible to growth limitations caused by B-deficiency stress. Previous studies have shown that B-deficiency inhibited the development of sugar beet chloroplasts and chlorophyll content, thus reducing the rate of photosynthesis, and ultimately limiting sugar beet growth (Song et al., 2019; Song et al., 2022). Boron deficiency is currently a significant factor that limits the expansion of sugar beet cultivation (Monteiro et al., 2018). Boron supplementation can promote leaf growth of sugar beet, photosynthetic pigment content, leaf photosynthetic properties, and photoassimilate transport and accumulation (Wu et al., 2021). This has become a routine method to alleviate soil B deficiency stress (Tariq et al., 2022), but its needs further improvement. Seaweed extracts are effective to enhance plant tolerance to abiotic stresses (Van Tol de Castro et al., 2024). They enhance the tolerance of lettuce and orange to potassium and calcium deficiency, and improve growth and yield (Chrysargyris et al., 2018; Alebidi and Abdel-Sattar, 2024). However, little is known about the impact of supplementing B fertilizer with seaweed extract on the growth of sugar beet plants under B deficiency, especially concerning the physiological mechanism of photosynthesis.
Therefore, in this study, the sugar beet KWS1176 was used as a test variety under two soil conditions with different B content: B-deficient (0.32 mg B kg−1 soil) and moderately B-deficient (0.69 mg B kg−1 soil). The aims of this study were: (1) To explore the promoting effect of adding seaweed extract on B absorption and utilization; (2) To clarify the improvement effect of adding seaweed extract on the growth of sugar beet; and (3) To elucidate the changes in photosynthetic reaction, light response curve, and electron transport after adding seaweed extract. The findings of the present study will provide the theoretical basis for nutrient management measures involving seaweed extract in crops.
2 MATERIALS AND METHODS
2.1 Experimental Material
The two kinds of soil used for the test were obtained from Farm 291 in Heilongjiang Province (Shuangyashan City, 131°45′E, 46°96’N), including B-deficient soil and potentially B-deficient soil. The soil type was white slurry soil. According to the classification of soil B deficiency, the soil with an available B content of 0.32 mg kg−1 is classified as B-deficient soil, and the soil with an available B content of 0.69 mg kg−1 is classified as potentially B-deficient soil (Ahmad, 2012). The sugar beet (Beta vulgaris L.)variety KWS1176, which was sourced from KWS SAAT SE & Co. KGaA, is characterized by its high yield and elevated sugar content; this variety is the primarily cultivated sugar beet variety in Northeast China (Song et al., 2021). The B fertilizer was boric acid (containing 17% B). Additionally, the tested seaweed extract contained no less than 100 g L−1 active components (polysaccharides, vitamins and amino acids) of the seaweed extract, no less than 90 g L−1 compound sugar alcohol, and no more than 30 g L−1 nutrient elements (S, Cl, Na).
2.2 Experimental Design
The experiment was conducted in the climate control chamber of the National Sugar Improvement Center (Harbin) from November 9, 2021, to December 30, 2021. Two B deficiency conditions were set: B-deficient soil (BD, containing 0.32 mg kg−1 B) and potentially B-deficient soil (PBD, containing 0.69 mg kg−1 B). Each basin (11 cm diameter, 9.5 cm high) was filled with 500 g dried soil, and the amount of soil fertilization was based on the field application amount (N 120 kg ha−1, P2O5 90 kg ha−1, and K2O 120 kg ha−1). The amount of fertilizer applied in the soil was 0.082 g per basin (Zhao et al., 2023). According to previous research (Wu et al., 2021), the sugar beet seedlings were sprayed once with water, boric acid (0.2%), or boric acid (0.2%) + seaweed extract (0.026%) on both BD and PBD soils, after the full unfolding of the 2nd pair of true leaves. The spraying volume is determined by the need for the leaves to be uniformly coated with the treatment agent, ensuring no droplets are present. The treatment groups were BDCT (sprayed with water on BD soils), BDB (sprayed with boric acid on BD soils), BDS + B (sprayed with boric acid+ seaweed extract on BD soils), PBDCT (sprayed with water on PBD soils), PBDB (sprayed with boric acid on PBD soils), and PBDS + B (sprayed with boric acid+ seaweed extract on PBD soils), with six replicates for each treatment. The plants were illuminated for 14 hours per day, and the positions of the pots were rotated daily to ensure uniform light exposure for all plants. The temperature was 25°C/20°C (14 h/10 h), and the relative humidity was 65%–70%.
2.3 Sugar Beet Seedling Growth and Biomass
7 days after spraying treatment, the leaf length, leaf width, and plant height of sugar beet were measured with a scale. Relative chlorophyll content was recorded by a SPAD-502 (Minolta) instrument before spraying and 2, 7, and 14 days after spraying. On the 15th day after spraying, the fresh weight of the plant was recorded, and the samples were dried until constant weight and then the dry weight was determined.
2.4 Photosynthetic Pigment Content
The photosynthetic pigment was extracted by using 95% ethanol and determined using a spectrophotometer. The concentrations of Chl a, Chl b, and carotenoids were calculated based on the absorbance values at each wavelength as follows (Song et al., 2022).
Concentration of Chl a (mg g−1 FW) = 13.95 A665–6.8 A649.
Concentration of Chl b (mg g−1 FW) = 24.96 × A649–7.32 × A665.
Concentration of carotenoid (mg g−1 FW) = (1000A470–2.05Chl a – 114.8Chl b) / 245.
2.5 Photosynthetic Parameters and Chlorophyll Fluorescence Parameters
Photosynthetic parameters of sugar beet leaves were determined using a portable photosynthetic meter (Hansatech). Measurements were taken at a light intensity of 200 μmol m−2 s−1 between 10:00 and 11:00 am (Deligios et al., 2019). Light response curves were measured at different light intensities using artificial LED white light, set across 9 light intensity gradients. These curves were fitted using a right-angle hyperbolic modified model, with a coefficient of determination between 0.996 and 0.999. This modified model accurately reflects the response process of the leaf to photosynthetically active radiation (PAR) (Wang et al., 2022).
Chlorophyll fluorescence parameters were measured using a Pocket PEA portable Chl fluorimeter (Handy). Measurements were taken from the second pair of compound leaves under light. Chlorophyll fluorescence and gas exchange parameters were determined at the same part of the leaves. The OJIP curve consists of four individual points: O, J, I, and P. These points correspond to moments at 0, 2, 30, and 1000 ms, with relative fluorescence intensities denoted as FO, FJ, FI, and Fm, respectively. The OJIP curves were standardized for the O-J and O-P segments, followed by JIP-test analysis was carried out to get fluorescence parameters, including FV/Fm, PItotal, PIABS, and energy flow parameters per unit cross-sectional area and reaction center (Huo et al., 2022).
Wt = (Ft – F0) / (FJ – F0); Wk = (FK – F0) / (FJ – F0).
Vt = (Ft – F0) / (Fm – F0); VJ = (FJ – F0) / (Fm – F0).
ΔWt = Wt – WCK; ΔVt = Vt – VCK.
The relative fluorescence intensity at different time points is represented by Ft. In the standardized curve, the relative variable fluorescence of the two characteristic points K and J are denoted as WK and VJ, respectively. ΔVt and ΔWt represent the difference between the Vt and Wt curves and the control (CT). The relative variable fluorescence intensity (VJ) at point J reflects the electron transport efficiency from QA to QB, with a larger VJ value indicating lower transmission efficiency.
2.6 Boron Content and Boron Accumulation in Plants
Boron accumulation (μg plant−1) = B content (μg g−1) × Dry mass of corresponding parts (g plant−1).
2.7 Available Boron Content in the Soil
Ten grams of air-dried soil sample (fineness of 2 mm) were weighed into a 50 mL polyethylene bottle, and magnesium sulfate solution was added, and heated in a 100°C water bath for 65 minutes. After cooling, the supernatant was filtered. An acidic potassium permanganate solution was poured into the filtrate and shaken well for 2–3 minutes, with the addition of an ascorbic acid solution to decolorize the solution. A mixed-color developer was then added. The absorbance was measured after 80 minutes at 415 nm by a spectrophotometer (Huo et al., 2022). The B content calculation formula was the same as in section 2.3.4.
2.8 Data Analysis
Data processing (e.g., mean, standard deviation) and statistical analysis ANOVA were performed using Excel 2010 and SPSS 12.0. The LSD method was used to compare data groups (p < 0.05), and Origin 2022 was used to graph the results of the principal component analysis (PCA).
3 RESULTS
3.1 Effect of Seaweed Extract and Boron Application on the Growth of Sugar Beet Seedlings
Both B application alone and in combination with seaweed extract effectively promoted the growth of sugar beet seedlings on both types of soil (deficient and potentially deficient), with the effect of combining seaweed extract with B being more efficient. The improvement effect was more pronounced after adding seaweed extract under B-deficient soil conditions than under potentially B-deficient conditions (Figure 1a). Under both soil conditions, the values of each index are shown as follows: B added with seaweed extract > single boric acid > control (BDS + B > BDB > BDCT / PBDS + B > PBDB > PBDCT).
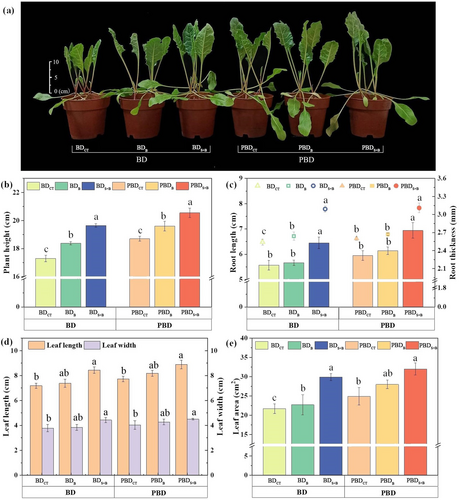
Under the seaweed extract and B treatment, plant height, root length, and root thickness increased by 7.34%, 9.34%, and 18.11% compared with the single application of boric acid in B-deficient soil (Figure 1b, c). In addition, leaf area, leaf length, and leaf width increased by 33.26%, 14.77%, and 15.50% more than boric acid-only treatment, respectively (Figure 1d, e). The values of each index were significantly (p < 0.05) different when compared with BDCT.
3.2 Effects of Seaweed Extract and B on the Accumulation of Photosynthetic Pigments and Dry Substances
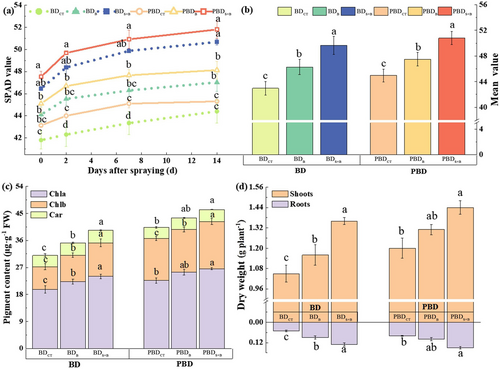
The application of both B and seaweed extract together had a significant impact on sugar beet seedlings. This led to a notable increase in the SPAD value, photosynthetic pigment content, and dry matter. The improvement effect was better in contrast to the (Figure 2a, b). Especially under B deficiency conditions, the contents of Chl a, Chl b, and carotenoids after adding seaweed extract increased by 8.96%, 30.57%, and 2.90%, respectively, in contrast to the boric acid-only treatment (Figure 2c). In addition, adding the seaweed extract to the B application resulted in a higher accumulation of dry matter; specifically, the aboveground dry weight increased by 19.05% compared to treatment with boric acid alone (Figure 2d).
3.3 Effect of Seaweed Extract and Boron on Boron Content and Boron Accumulation
The combined application of B with and seaweed extract increased B content (boron concentration in plant) and B accumulation (product of boron content and plant dry mass) in sugar beet compared to non-treated control and B-treated only (Table 1). For instance, in B-deficient soil, the B contents in the shoot and root increased by 28.56% and 12.64%, respectively, when treated with B and seaweed compared to when treated only with B, whereas the B accumulation of the aboveground and belowground parts increased by 44.43% and 49.78%, respectively.
| Soil condition | Treatment | B content (μg g−1) | B accumulation (μg plant−1) | ||
|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | ||
| Boron deficiency (BD) | BDCT | 10.19 ± 0.15c | 9.07 ± 0.31c | 8.89 ± 0.30c | 0.73 ± 0.27c |
| BDB | 12.26 ± 0.05b | 10.22 ± 0.17b | 14.22 ± 0.75b | 0.79 ± 0.09b | |
| BDS + B | 13.55 ± 0.28a | 12.81 ± 0.06a | 18.17 ± 0.10a | 1.28 ± 0.29a | |
| Potential boron deficiency (PBD) | PBDCT | 11.18 ± 0.28b | 10.53 ± 0.12c | 12.69 ± 0.29c | 0.95 ± 0.15c |
| PBDB | 12.45 ± 0.22ab | 10.89 ± 0.16b | 16.69 ± 0.46ab | 1.09 ± 0.21b | |
| PBDS + B | 13.39 ± 0.18a | 13.71 ± 0.06a | 19.71 ± 0.18a | 1.51 ± 0.41a | |
- The data presented here are shown as mean ± SD (n = 6), BDCT/PBDCT (control), under B deficiency/potential B-deficient soil; BDB/PBDB, 0.2% boric acid under B deficiency/potential B-deficient soil; BDS + B/PBDS + B, 0.026% seaweed extract with 0.2% boric acid under B deficiency/potential B-deficient soil. Different letters indicate the significant differences between treatments at (p < 0.05) levels, respectively, according to One-way ANOVA test followed by Duncan ‘s test.
3.4 Effect of Seaweed Extract and Boron Application on Photosynthetic Parameters
The application of B fertilizer alone or with seaweed extract effectively adjusted leaf photosynthetic parameters. The photosynthetic parameters changed more significantly after adding B + seaweed extract than boric acid alone (Figure 3a-d). In the B-deficient soil condition, Pn, Gs, and Tr increased by 22.24%, 34.21%, and 43.04%, respectively, in plants treated with B and seaweed compared to plants only treated with B, and Ci decreased by 6.56%.
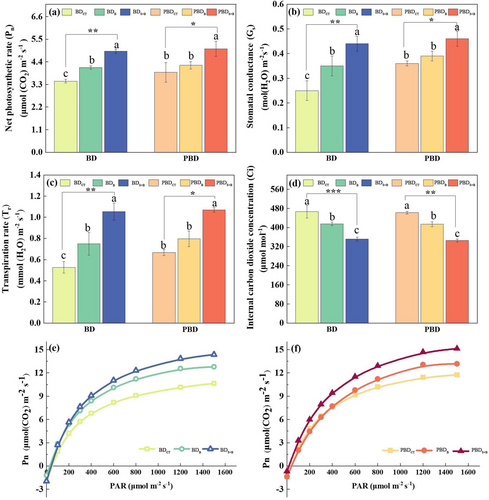
When the PAR concentration was below 200 μmol m−2 s−1, the Pn of beet leaves indicated a linear increase with increasing light intensity. After the PAR concentration was higher than 200 μmol m−2 s−1, the Pn growth rate slowed down until the PAR reached 1500 μmol m−2 s−1, reaching the light saturation point (Pm). In both B-deficient and potentially B-deficient conditions, the growth rate of Pn at the same PAR concentration was in the following order (lower to higher) depending on treatment: control treatment < single boric acid < B added with seaweed extract. Compared with the potentially B-deficient conditions, the light response curves in B deficiency showed faster growth rates of Pn during the linear growth phase and distinct Pn differences between treatments at lower PAR concentrations. The B application with seaweed extract increased the value of Pm under both B conditions but it was significant only under B deficiency conditions: Pm increased by 20.16% and 35.00% in plants treated with B or B + seaweed, respectively, compared with non-treated control, indicating a positive effect of seaweed (Figure 3e, f).
3.5 Effect of Seaweed Extract and Boron Application on Leaf Fluorescence Parameters
According to the OJIP curve, the leaf Fm of sugar beet seedlings showed an upward trend under different B treatments. In both B-deficient and potentially B-deficient conditions, the best application effect was seen with the combined application of seaweed extract and B, and the rapid Chl fluorescence induction curve of the PSB treatment increased faster overall (Figure 4a). Under both B-deficient soil conditions, leaf FV/Fm, Fm, PIABS, and PItotal increased in sugar beet seedlings after both B fertilizer treatments. Moreover, under B-deficient conditions, these indicators significantly increased by 2.09%, 1.34%, 18.93%, and 12.53%, respectively, in plants treated with b + seaweed compared to non-treated control (Figure 4b, c).

After standardizing the O-J segment of the OJIP curve and comparing it with the standardized Control curve, it can be seen that the relative variable fluorescence (WK) of the K point at 0.3 ms decreased after B treatment. Under B-deficient soil conditions, WK decreased by 3.86% and 4.05% in plants treated with B and B + seaweed, respectively, compared to non-treated control, indicating a slight improvement when seaweed extract was added (Figure 5a, b).
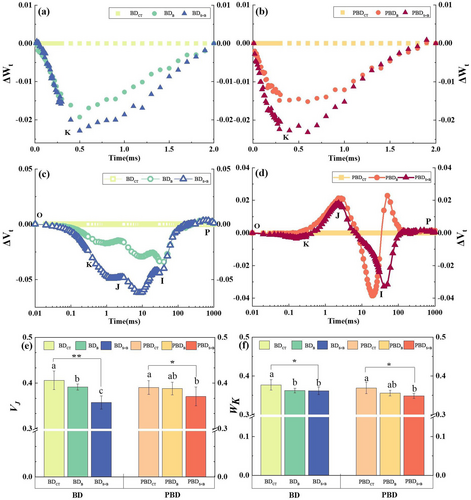
The decrease of VJ was greater than that of WK. Compared with applying boric acid alone, the treatment with added seaweed extract had a greater reduction (Figure 5c, d). Under the B deficiency condition, WK and VJ decreased by 4.05% and 11.96%, respectively, after the addition of the seaweed extract (Figure 5e, f).
The variation degree of light energy absorption and distribution parameters was more obvious after the addition of seaweed extract than the single application of boric acid and it. changed more widely under the B-deficient soil condition than potentially B-deficient condition. Under B-deficient soil condition, the unit cross-sectional area light energy allocation parameters, TRO/CSm, ABS/CSm, and ETO/CSm, increased by 5.03%, 5.48%, and 5.57%, and DIO/CSm decreased by 5.60% after dispensing of the seaweed extract with B compared to non-treated control. TRO/CSm, ABS/CSm and ETO/CSm increased by 2.14%, 2.20% and 2.61% and DIO/CSm decreased by 3.30% after seaweed extract with B compared to boric acid alone (Figure 6a, b).
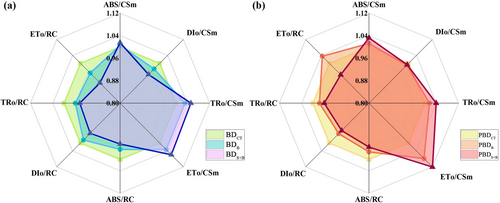
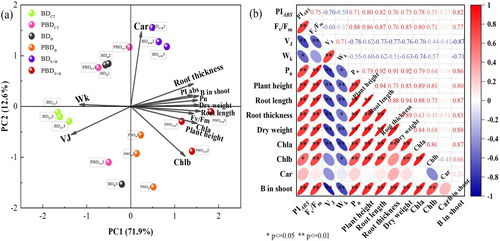
3.6 Principal Component Analysis (PCA) and Correlation Analysis
The PCA analysis of the main factors affecting beet growth was 71.90% for PC1 and 12.60% for PC2, indicating that there is an obvious separation between spraying treatment and influencing factors under different soil conditions. In PC1, the photosynthetic pigment content, Pn, VJ, and B concentration in shoots showed greater contributions, suggesting that these factors have a significant effect on plant height, root length, and dry mass (Figure 7a). The correlation matrix indicated that root length, root thickness, plant height, and dry matter were positively correlated with B concentration in shoots, photosynthetic pigment content, Pn, PIABS, FV/Fm, and negatively correlated with VJ and WK (Figure 7b).
4 DISCUSSION
This study found that, under B-deficient conditions, supplementing B with seaweed extract could better improve sugar beet growth parameters, such as plant height, root length, root surface area, leaf area, and dry matter accumulation, compared to adding only B, thereby promoting sugar beet growth. This may be due to the abundance of growth-active materials in the seaweed extract, such as auxin and cytokinin, which can promote cell division and elongation, thus regulating plant growth (Ilyas et al., 2021). Additionally, the mineral nutrient elements enriched in seaweed extract can increase water and nutrient absorption through roots by promoting plant root growth, thereby increasing the plant's ability to adapt to stress (Mireya Hernandez-Herrera et al., 2014; Mukherjee and Patel, 2020).
The effects of seaweed extracts vary along with crop species and application methods. For example, individual application can increase the leaf area, leaf number, and fresh weight of tomatoes and beans (Kocira et al., 2020; Vila et al., 2023). The combined application of seaweed extract and fertilizer can enhance the fertilization effect; for instance, nitrogen application can improve the utilization efficiency of barley, resulting in higher yields and lower nitrogen fertilizer input (Cozzolino et al., 2021). When seaweed extract was applied with potassium fertilizer, the height and branching number increased (Petropoulos et al., 2022). In our study, we found that seaweed extract could also promote the absorption and utilization of B, thereby improving the growth of sugar beet. The promotion effect was more significant when the soil's B deficiency was more severe. Therefore, this experiment confirmed that the addition of seaweed extract can effectively improve the application effect of B fertilizer under B deficiency conditions, providing a reference for measures to improve sugar beet growth parameters.
The rich mineral elements (K, Mg, etc.) and plant growth regulators (auxin, cytokinin) in seaweed extracts can promote the synthesis of the photosynthetic pigments and delay the degradation of Chl in plants (Chrysargyris et al., 2018; Gupta et al., 2021). Earlier studies have found that the separate application of seaweed extract could improve the photosynthetic pigment content of cowpeas (Voko et al., 2022), and the combination of seaweed extract with nitrogen fertilizer could more effectively increase the carotenoid and Chl content in lettuce leaves (Di Mola et al., 2020). In our study, combined seaweed extract and B application significantly increased the SPAD value and photosynthetic pigment content, especially the Chl b content of seedlings, by increasing the leaf B content. Compared with the single boric acid treatment, the efficiency increase with the addition of seaweed extract was more significant, further confirming the enhancement of the photosynthetic performance of sugar beet. In addition, the photosynthetic performance is also related to changes in photosynthetic parameters (Nasar et al., 2022). Previous experiments have concluded that seaweed extract significantly improved the photosynthetic parameters of melon, cucumber, and tomato, thus inducing efficient photosynthetic reactions (Lefi et al., 2023). Previous research by our group found that B deficiency considerably changed the photosynthesis parameters of sugar beet. However, this study proved that under different B-deficient conditions, the application of seaweed extract combined with B fertilizer can increase Pn, Gs, and Tr, while reducing Ci. Moreover, the effect of seaweed extract and B is more pronounced under B-deficient conditions. The reason is that sugar beet has a stronger response to the fertilizer effect of B treatment in B-deficient conditions, and seaweed extract can further improve the net photosynthetic rate by increasing the photosynthetic pigment. Eventually, improved photosynthetic performance of the sugar beet is achieved.
Chlorophyll fluorescence parameters are frequently used to assess photosynthesis potential and effectiveness. The application of seaweed extract alone has been shown to enhance stress resistance in Arabidopsis (Santaniello et al., 2017) and coconut dates (Anli et al., 2020) by regulating specific Chl fluorescence parameters (Fm, FV/Fm, PIABS, and PItotal). It can also enhance the photochemical quantum efficiency and carbon dioxide absorption capacity of lettuce (Asadi et al., 2022). When combined with other mineral nutrients, seaweed extract applied with silicon to Arabidopsis could avoid photodamage by maintaining a relatively high photochemical energy dissipation capacity (Sujata et al., 2023). It can also effectively enhance light utilization when seaweed extract is sprayed with silicon and selenium (Jalali et al., 2022). In this experiment, the seaweed extract application with B significantly improved the Chl fluorescence intensity (Ft) and the light energy conversion efficiency of photosystem II (FV/Fm) in sugar beet seedlings under B-deficient stress. Seaweed extract can increase the photosynthetic pigment content, enhance the light energy capture and utilization ability, accelerate electron transfer on the photosynthetic membrane, and thus be more conducive to the conversion of light energy into chemical energy (Al-Huqail et al., 2020; Turhan, 2021). The combined seaweed extract application and B fertilizer can improve stomatal conductance, thereby improving the photochemical quantum efficiency and carbon dioxide uptake, ultimately increasing the fluorescence intensity of Chl (Yakhin et al., 2017; Sha et al., 2024).
Previous studies have shown that salt stress leads to elevated WK and VJ levels in rice (Gao et al., 2024). This indicates that stress conditions disrupt the transport of electrons and energy in the photosystem. By applying iron fertilizer in a suitable manner, it is possible to restore the photosynthetic electron transport chain, enhance electron transmission efficiency, and ensure a balanced distribution of energy. Thus, it enhances the characteristics of electron and energy transmission in the optical system (Gao et al., 2022). In our study, we also found that seaweed extract combined with B could reduce WK and VJ, indicating that the damage to both the donor and recipient sides of PSII was reduced, and the proportion of inactivated reaction centers was decreased. Meanwhile, we discovered that the capture of light energy increased, while the energy consumption efficiency of the remaining active reaction centers did not improve. This indicates that the addition of seaweed extract during B application can alleviate the inactivation or deactivation of the reaction center per unit area under B deficiency stress. As a result, the reaction efficiency is enhanced, leading to better dissipation of energy in the electron transport chain. In summary, seaweed extract application in combination with B fertilizer can promote B absorption while simultaneously improving sugar beet fluorescence-induced parameters and energy allocation.
5 CONCLUSIONS
Under B-deficient conditions, leaf spraying with seaweed extract and B has positive effects on the growth of sugar beet leaves. The seaweed extract promoted the absorption of B by sugar beet, thereby increasing the photosynthetic pigment content. Additionally, it improved photosynthetic parameters and photochemical characteristics, enhanced the light energy capture and utilization ability of beet leaves, and subsequently boosted the accumulation of assimilation products, thus promoting the growth of beet seedlings. In summary, the seaweed extract can significantly enhance the fertilizer effect of B application and is more effective in alleviating B deficiency stress in sugar beet. This provides a rationale for the use of seaweed extracts in the production of sugar beet and highlights the potential of bio-stimulants in mitigating plant abiotic stresses.
AUTHOR CONTRIBUTIONS
Songlin Yang: performed experiments, methodology, writing original draft, investigation, software, writing-review and editing, investigation. Wenyu Wu: performed experiments, investigation, software, writing-review and editing, investigation. Baiquan Song: writing original draft, conceived and designed the experiments, supervision and funding acquisition, management of the experiments, laboratory analysis. Shangxuan Liu, Yan Wang: writing-original draft, writing-review & editing, visualization and data treating and statistics. Xiaoyu Zhao: writing-original draft, visualization and data treating and statistics. Muhammad Riaz and Muhammad Ishfaq: writing-review & editing. Abudukadier Kuerban: validation. Wang Xing: project administration.
ACKNOWLEDGMENTS
We are grateful for the experimental support provided by the Public Platform of the National Sugar Crops Improvement Center, College of Advanced Agriculture and Ecological Environment, Heilongjiang University.
FUNDING INFORMATION
This work is supported by the China Agriculture Research System (CARS-17).
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all new created data is already contained within this article.




