Grafting improves nitrogen efficiency and stabilizes yield and quality of cucumber by enhancing the NO3− uptake
Abstract
Grafting can promote the growth and nitrogen use efficiency (NUE) of cucumber seedlings under reduced nitrogen (N) application, however, its underlying mechanisms and effects on mature plants remain unknown. For this purpose, self-grafted and rootstock-grafted cucumber plants were treated with two N levels (7 and 4 mM) throughout the entire growth period. The long-term reduced-N treatment significantly limited the growth, root morphology, nitrate (NO3−) uptake, NUE traits, photosynthesis, phenylalanine ammonia-lyase (PAL) activity, yield, and fruit quality of self-grafted plants but had no influence on rootstock-grafted plants, it even improved their NUE traits, total phenolic and flavonoid contents, and PAL activity. Furthermore, the expression of the NRT1.2, NRT1.5, NRT2.2, and NRT2.5 genes were significantly down-regulated in self-grafted plant roots, while they and the transcription factors NLP6 and LBD38 were up-regulated in rootstock-grafted plant roots under reduced-N environments. Correlation analysis showed that plant growth, root surface area, N-accumulation, N-uptake efficiency (NUpE), NUE, photosynthesis, PAL activity, yield, and fruit quality were all positively correlated with each other; meanwhile, the root morphology, NRT1.2 and NRT2.1 gene expression were all positively correlated with NUpE and NUE. The results demonstrate that under reduced-N application, rootstock grafting can enhance NO3− uptake and N accumulation to improve the NUE of cucumber plants and resist reduced-N environment through secondary metabolism, maintaining growth, photosynthesis, yield, and fruit quality without adverse effects. The up-regulation of NRT genes and related transcription factors regulates the NO3− uptake in rootstock-grafted plants. Rootstock grafting will be beneficial for fertilizer conservation and efficient cucumber production. yield and fruit quality.
1 INTRODUCTION
Nitrogen (N) is an essential nutrient required for crop growth. It participates in the light energy absorbing process and the synthesis of substances that support plant growth, development, and fruit formation (Xu et al., 2021). Consequently, when fertilizers are applied at an appropriate rate, they will be an important foundation for plant growth, maximizing nitrogen use efficiency (NUE), along with optimizing the yield and quality of agricultural production (Wan et al., 2017). China is the country with the highest N-fertilizer usage in the world, and overuse of N-fertilizers has gradually become a common practice to increase crop yields (Chen et al., 2022). Nevertheless, China's N fertilizer utilization rate is only 20–35%, significantly lower than the global average (Ti and Yan, 2020). Excessive N fertilizer application reduces nitrogen use efficiency (NUE), increases nitrogen losses, contributes to environmental pollution, and negatively impacts plant growth, yield, and fruit quality (Du et al., 2020). Therefore, reducing N-fertilizer application moderately without affecting plant growth and development is an important challenge for achieving sustainable agriculture.
Grafting is widely used in vegetable crops to alleviate the damage caused by soil-borne diseases (Gilardi et al., 2013) and to improve the tolerance of sensitive plants to environmental stresses, like heat, drought, and salt stress (Balfaón et al., 2022; Chen et al., 2020; Mozafarian et al., 2023). Moreover, grafting can also enhance the plant's nutrient absorption by “Replacing roots” as the rootstock's root system serves as the primary organ for nutrient acquisition. This process can enhance the ability of grafted plants to obtain inorganic nutrients (Chen et al., 2022). However, the efficacy of grafting mainly depends on the genotype of the rootstock (Colla et al., 2010), and pumpkin is the main rootstock in the grafting production of gourd family vegetables. Choosing suitable pumpkins as rootstocks can increase yield, improve quality, and enhance the disease and stress resistance of cucurbits. Thus, researchers have screened a lot of appropriate rootstocks to enhance nutrient absorption and utilization of specific scion varieties (Ulas et al., 2021; Ulas et al., 2022) and found that appropriate rootstock grafting can quickly and effectively enhance the N absorption and utilization capacity of plants (Ulas et al., 2019).
- N-
fertilizer affects organ formation and development, enhancing photosynthetic characteristics and other ecological processes, which leads to complex plant responses to N fertilizer. Some studies indicate that a suitable amount of N application can improve the photosynthetic ability of plants, thereby increasing yield and fruit quality (Yang et al., 2023). Thus, for researchers in cucumber cultivation techniques, exploring appropriate N application rates to improve yield and fruit quality is of great significance. In our previous work, we showed that grafting with a pumpkin rootstock can significantly increase the NUE of cucumber seedlings without sacrificing plant growth under short-term reduced-N treatment (Liang et al., 2021). However, for mature plants, the impact of pumpkin rootstock grafting on cucumber fruit yield and quality exposed to long-term N reduction environments still needs further verification. Besides, nitrate (NO3−) is one of the most important forms of N that higher plants can directly utilize, and it is the most easily absorbed nitrogen oxide compound by plants (Zhen et al., 2018). NO3− uptake by plants is carried out through nitrate transport proteins (NRTs) located on the plasma membrane (Li et al., 2013). Numerous research in Arabidopsis and field crops have revealed that the genes encoding these transport proteins are involved in regulating the absorption and transportation of NO3− (Li et al., 2022; Wei et al., 2021; You et al., 2022). However, there are few studies on these in vegetable crops.
For this purpose, we will explore (1) the effects of rootstock grafting on NUE, yield, and fruit quality of mature cucumber plants exposed to long-term N reduction environment and (2) whether the enhanced NO3− uptake of grafted cucumber plants is regulated by NRT genes at the transcriptional level. The results will offer a theoretical foundation for N fertilizer saving and efficiency, enhancing the production of cucumber plants by grafting.
2 MATERIALS AND METHODS
2.1 Plant material and experimental treatments
The experiments were carried out in a plastic film greenhouse under natural light of 400–800 μmol.m-2.s−1, temperatures of 20–30/15–18°C (day/night), and humidity of 60–75%. Seeds of rootstock ‘Jinchun No. 4’ (Cucumis sativus L.) and ‘Ribenjingtiantaimu’ (Cucurbita moschata Duch.) were sown into 50-hole trays with a 2:1 (v/v) ratio of peat and perlite composite matrix and a relative humidity of 60–75%. The mixed substrate contains available nitrogen, phosphorus, and potassium contents of 59.7, 31.3, and 285.9 mg kg−1, respectively, conductivity (EC) value of 0.35 mS.cm−1, pH of 6.69, substrate bulk density of 0.22 g cm−3, and total porosity of 64.98%. 5 days later, the cucumber seeds used as scions were sown. Cucumber and pumpkin seeds were purchased from Tianjin Kerun Cucumber Research Institute and Shandong Shouguang Seed Industry, respectively. When the scion's cotyledon fully expanded and at the rootstock's first true leaf development stage, grafting was carried out to obtain self-grafted (J/J) plants and rootstock-grafted (J/R) plants. The “insertion grafting” method was used for grafting (Hassell et al., 2008). When the second true leaf was fully expanded, the grafted plants were transplanted into a 20 L cultivation substrate bag (70 × 20 × 8 cm) containing the same matrix formula as the seedling, each bag containing two plants. The plants were irrigated by Japan Yamazaki cucumber special nutrient solution (pH 6.4 ± 0.1, electrical conductivity 1.2 ± 0.1 mS cm−1, formula was done according to Liang et al., 2021) with two levels of NO3−-N concentration [7 mM (control) and 4 mM (reduced-N)]. The N levels were chosen based on our preliminary experiments (Figure S1). The plants were irrigated with nutrient solution every three days, 1 L each time, to maintain the water content at an appropriate value (70%). Treatments consisted of two grafting combinations and two N concentrations. These treatments were repeated three times with 20 plants in each replicate, and the experiment was conducted with the split-plot design.
2.2 Measurement of plant growth parameters
After 14 days of reduced-N treatment, 6 plants per treatment were selected (2 plants/replicate × 3 replicates), photographed and related parameters measured. Root morphology was scanned and determined using the WinRHIZO root system analyzer (Epson perfection V700 Photo). The root activity was determined by measuring the oxidation of alpha-naphthylamine (α-NA) according to the method of Ramasamy et al. (1997) and expressed as μg α-NA per gram DW per hour (μg α-NA g−1 DWh−1). At harvest, 6 plants per treatment were collected and divided into leaf, stem, and root parts, then oven-dried at 105°C for 15 min and then at 70°C for 48 h; finally, the biomass of each organ was recorded.
2.3 Measurement of NO3− uptake
At harvest, fresh root and shoot samples were taken to determine the NO3− content following the method of Zhang et al. (2017). 0.5 g fresh samples were ground in 10 mL deionized water and placed in boiling water for 30 min. Then, 0.1 mL of extraction solution was mixed with 0.4 mL of 5% salicylic acid-H2SO4 for 20 min, and 9.5 mL of 8% sodium hydroxide was added. The sample was cooled to room temperature and the absorbance was measured at 410 nm. NO3− uptake was calculated as the product of NO3− content and fresh weight.
2.4 Analysis of N content, N accumulation (NA), N uptake efficiency (NUpE), N utilization efficiency (NUtE) and NUE
To determine the N concentration of organs, dry samples were ground into a fine powder and digested by H2SO4-H2O2 at 180–250°C(Lang et al., 2018). The total N concentration was analyzed using a continuous flow analyzer (AA3, Bran + Luebbe). The calculation formula for N efficiency related parameters refers to the method of Siddiqi and Glass (1981) and Elliot and Laüchli (1985) as follows:
NA = N content × Dry weight (mg N).
NUpE = N accumulation / Root dry weight (mg N mg DW−1).
NUtE = Total plant dry weight / total N contents (mg DW mg N−1).
NUE = NUpE × NUtE (mg N mg N−1).
2.5 Determination of parameters related to leaf photosynthesis and secondary metabolism
At harvest, on sunny days from 9:30 to 11:30 am, the net photosynthetic rate (Pn) and stomatal conductance (GS) of the functional leaves (the second fully unfolded true leaf from the top) were measured using Li-6800 photosynthetic apparatus (Li-Cor). Fresh samples of leaves and roots were rapidly frozen at ultra-low temperatures for subsequent analysis. Chlorophyll (Chl) content was measured according to Hussain et al. (2019). 0.1 g of fresh leaves were placed in 10 mL of 80% acetone and soaked overnight in the dark. The extract was filtered, and its absorbance at 663, 645, and 470 nm was recorded. Then, the total chlorophyll content was calculated.
The determination of total phenolic content was done according to Ghimire et al. (2011). Ethanoic extract (100 mg) was added to 0.3% HCl methanol solution, then 2 mL of 2% aqueous sodium carbonate was added and incubated for 2 min. Finally, 50% of the Folin-Ciocalateu reagent was added, and the absorbance was measured at 725 nm.
Flavonoid content was determined using the method described by Pirie and Mullins (1976). 10 mL of 50% ethanol solution was added to 0.2 g ground sample used for extraction in an 80°C water bath for 2 h, then centrifuged at 7000 × g for 10 min. 0.3 mL of 5% (g L−1) Al(NO3)3 test solution was added, shaken and left standing for 6 min, then 4 mL of 4% NaOH and 0.4 mL of 50% ethanol aqueous solution was added, shaken l and left standing for 12 min. The absorbance value was measured at 504 nm.
Phenylalanine ammonia lyase (PAL) activity was measured according to the method of Sánchez Rodríguez et al. (2011). 0.5 g leaf sample was ground in 5 mL of 100 mmol L−1 potassium phosphate buffer (pH 8.0) containing 1.4 mmol L−1 2-mercaptoethanol. Centrifuge the crude extract at 10000 × g for 20 min at 4°C, take 0.2 mL of the supernatant, add 0.4 mL of 100 mmol L−1 Tris–HCl buffer (pH 8.8) and 0.2 mL of 40 mmol L−1 phenylalanine. Incubate the reaction mixture at 37°C for 30 min, and add 25% trichloroacetic acid to end the reaction. Measure the absorbance at 280 nm.
2.6 Quantitative Real-time PCR (RT-qPCR) analysis
Root samples of grafted plants treated for 6 h, 1 d, 5 d, 14 d were collected and frozen immediately by liquid N2 and stored at −80°C. The Trizol reagent kit was used to isolate total RNA (Norcross). DNase 1 (TaKaRa) was used to remove DNA contamination. Recovery of RNA particles in diethyl pyrocarbonate (DEPC) water and refrigeration at −80°C ultra-low temperature for future use. The Evo M-MLV reverse transcription kit was used to synthesize the first strand of cDNA (Bioteke). The RT-qPCR was done using the PerfectStart Green qPCR SuperMix kit (TransGen Biotech), and the following cycling conditions: pre-denaturing at 94°C for 30 s, followed by 42 cycles at 94°C for 5 s, and annealing at 60°C for 30 s. The expression data was determined as previously described by using the 2-ΔΔCt method (Livak and Schmittgen, 2001). Following the principles of primer design and referring to the method of Kong et al. (2014), the primers (Table 1) were designed based on the Cucurbit Genomics Database (http://cucurbitgenomics.org) and completed by Primer 5 software.
| Grafted combinations | Gene | Accession Number | Forward primer | Reverse primer |
|---|---|---|---|---|
| J/J | CmNRT1.1 | CsaV35G039430 | GACAGGAACTATGCATTTGGGGAAT | GCGCAATGTGATGACGACTCTA |
| CmNRT1.2 | CsaV32G002800 | CTCAACGGCTGAACCTC | GTGACGATGTTGGCAGAC | |
| CmNRT1.5 | CsaV36G006280 | TGGGATGCTGCTGCTAGTGA | TTTGCTGCTTCAGCGTTTGT | |
| CmNRT2.1 | CsaV35G032350 | CTGTAGCTTCCCCGATCGTC | TCGTCAGGTTGAGGTTGTCG | |
| CmNRT2.2 | CsaV35G032220 | GAGCTGCCGAGTGACGCATC | CGGAAATGCTAGCCCGTAGTG | |
| CmNRT2.5 | CsaV31G007960 | GTGAGCGCGTTTGAGTGCCGC | GTGAGATTGCGTGGAGTTG | |
| CmNLP6 | CsaV34G006840 | CGATCATCTTGCTTCCCCTCT | GTTCTCCGTCGGCTTTTGC | |
| CmLBD38 | CsaV36G043970 | CCGAGACGGAAGTAGGATGTAC | AGTGGGAGTCAAGCGGAGAT | |
| EF1a*a | Csa 2G139820 | ACTGTGCTGTCCTCATTATTG | AGGGTGAAAGCAAGAAGAGC | |
| J/R | CmNRT1.1 | CmoCh04G017070 | TCGTAGATGGGAAGGGTATG | GAGGAAGATGAGATAAGAGGTG |
| CmNRT1.2 | CmoCh05G006220 | GTTCTCGCATTGGTGATAGTG | CTTTTGGTTGAATTGGTCCG | |
| CmNRT1.5 | CmoCh16G011260 | TGGTCTCAGCAGGCATCGT | AAACTTCAGAGGCTCCAATCAG | |
| CmNRT2.1 | CmoCh18G012010 | GCTGTCTTTGCCCGTATC | ATCCAGAATTGGGTCGAG | |
| CmNRT2.2 | CmoCh13G009540 | GAACTAAGTCATCCATGTAC | GAGGCTATGCCCGATTCGGAG | |
| CmNRT2.5 | CmoCh13G001660 | GAGCCTCGGGATGCAGTGAG | CTGTTAGGCCATTGTGCCGCAG | |
| CmNLP6 | CmoCh16G005770 | TCGGAAAGAAAGCGTGGAA | CTAACGGTAACAGGAAGAGGAC | |
| CmLBD38 | CmoCh09G002750 | AATCTTCAACTCTACCCATCCA | TATGATTCTTCGCTCTTACGG | |
| EF1a*a | Cmoch08G009890 | GCCTCAAACTCCAAGGATGA | GGCTCCTTCTCGAGTTCCTT |
- Note: All primers were designed based on a published mRNA of Cucurbita moschata on the Cucurbit Genomics Database (http://cucurbitgenomics.org) using Primer 5 software. EF1a is the reference gene. J/J: self-grafted cucumber plants; J/R: rootstock-grafted cucumber plants.
2.7 Measurements of fruit yield and quality
Fully mature fruits were harvested twice per week between May 20 and July 10. 6 plants per treatment were selected to record the fruit length, fruit number per plant, and single fruit weight separately. Then, mean fruit weight and yield per plant were calculated. 9 fruits (3 fruits/replicate × 3 replicates) per treatment were used for quality determination. Determination of total soluble solids (TSS) content using WYT-A handheld sugar meter. The soluble protein content (SP) was estimated using the Coomassie blue staining method with BSA as standard (Bradford, 1976). The free amino acid content (FAA) was estimated using the ninhydrin method described by Yemm et al. (1955). Following the method of Zhai et al. (2015), the organic acid content (OA) was measured using a neutralization titration method, vitamin C content (Vc) was measured with a UV spectrophotometer (N5000, YOKE Instrument).
2.8 Statistical analysis
The data difference was analyzed using Tukey's test at a 5% level of significance via SPSS 24.0 software (IBM Corp.). The Pearson's correlation analysis and Principal component analysis were performed using Origin 2022 (Origin Lab, Inc.). Using Excel 2019 software for data organization and mapping.
3 RESULTS
3.1 Plant growth differences
14 days after planting, the growth of self-grafted (J/J) plants root and shoot were significantly inhibited under the reduced-N condition, while rootstock-grafted (J/R) plants were not significantly negatively affected (Figure 1A, B). The data on root length and root surface area displayed the same results, showing a significant decrease only in J/J plants (Figure 1C, D). Although reducing N-fertilizer application improved the root activity of grafted cucumber plants, the root activity of J/R plants was significantly higher than that of J/J plants (Figure 1F). During the harvest period, the biomass of root, stem, and leaves in J/J plants under reduced-N conditions decreased by 33.0, 57.9, and 51.1%, respectively, compared to the control. Consequently, these values in J/R plants were 39.4, 132, and 19.2% higher than those of J/J plants, respectively, under the reduced-N condition (Figure 1E).
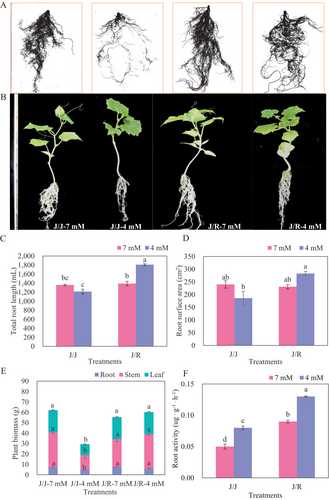
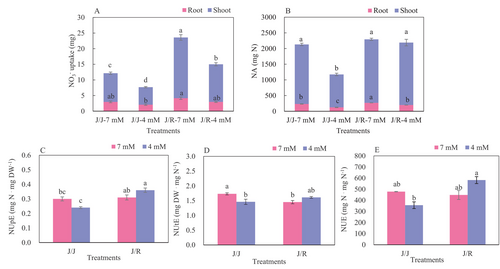
3.2 Tissue NO3− uptake, NA and NUE traits analysis
In general, the reduced-N treatment decreased the NO3− uptake in the root and shoot, however, the values were higher in J/R plants than in J/J plants irrespective of N levels (Figure 2A). For instance, the NO3 uptake in the root and shoot of J/R plants was about 2.1 and 1.4 times that of J/J plants both under control and in a reduced-N environment, separately. Similarly, the reduced-N treatment dramatically reduced the N accumulation (NA) in the root and shoot of J/J plants by 46.1 and 44.8%, respectively, relative to the control treatment, while the inhibitory effect in J/R plants was smaller (Figure 2B). Consequently, the total NA was markedly higher in J/R plants than in J/J plants, irrespective of N levels. Moreover, compared with the control, the N efficiency parameters of J/J plants all decreased due to reduced-N treatment, while those of J/R plants increased (Figure 2C-E). For instance, compared to the control, the NUpE, NUtE, and NUE of J/J plants decreased by 18.0, 15.3, and 25.2% under reduced-N treatment, respectively, whereas they increased by 17.9, 10.5 and 29.9% in J/R plants, respectively. Therefore, under reducing N application, the NUpE, NUtE, and NUE of J/R plants increased by 48.9, 10.0, and 63.4%, respectively, compared to J/J plants.
3.3 Changes in photosynthesis and secondary metabolism
Compared with control, the reduced-N treatment decreased the Chl content, Pn, and GS of J/J plants by 7.5, 4.6, and 13.3%, respectively, but had no obvious harmful influence on Pn and GS of J/R plants (Figure 3A-C). As a result, the above parameter values in J/R plants were increased by 10.7, 36.3 and 120% in contrast with J/J plants under reducing N application, respectively. Similarly, the reduced-N treatment decreased the PAL activity of J/J plants by 12.3%, but increased the total phenolic content, flavonoid content, and PAL activity of J/R plants by 15.8, 26.3 and 19.2% (Figure 3D-F). Therefore, under reduced-N treatment, the above parameter values were 18.9, 50.0, and 43.9% higher in J/R plants than in J/J plants, respectively.

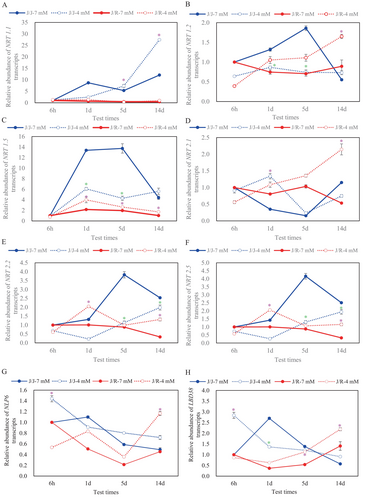
3.4 Dynamic expression of NRT genes and its related transcription factors
In order to elucidate the regulatory mechanism of rootstock grafting promoting effective absorption of NO3− by cucumber plants, the dynamic expression changes of NRT genes and related transcription factors in plant roots under N-fertilizer reduction treatment were measured. As a whole, cucumber and pumpkin roots from J/J and J/R seedlings showed different responses to the reduction in N at the transcriptional level (Figure 4). The results showed that compared to the control, in J/J plant roots, the mRNA levels of NRT1.2 and NRT1.5 genes at 1 and 5 days, NRT2.2 and NRT2.5 genes at 1, 5, and 14 days, and LBD38 at 1 day of reduced N application were all significantly down-regulated (Figure 4B-C, E-F, H), while the expression of NRT1.1 gene at 5 and 14 days were up-regulated (Figure 4A). On the contrary, in J/R plant roots, the mRNA levels of NRT1.2 and NRT2.1 genes at 14 days, NRT1.5 gene at 1, 5, and 14 days, NRT2.2 and NRT2.5 genes at 1 and 14 days, and NLP6 at 6 h and 14 days of reduced N application, were markedly up-regulated compared to the control (Figure 4B-H). In addition, compared with the control treatment, the mRNA levels of the gene in J/R plant roots and the LBD38 at 14 days of reduced N application were all significantly up-regulated (Figure 4G, H), while the expression of LBD38 in J/J plants root at 1 days of N reduction treatment was markedly down-regulated.
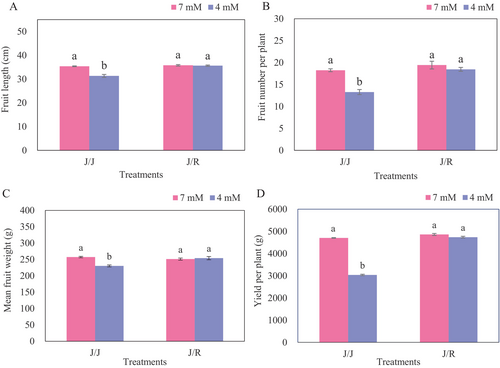
3.5 Fruit yield and quality
In relation to control, reduced-N application resulted in a decrease of 11.4, 27.2, 10.5, and 35.4% in fruit length, fruit number per plant, mean fruit weight, and yield per plant of J/J plants, respectively, while there was no significant adverse effect on J/R plants (Figure 5). Consequently, these values were remarkably higher in J/R plants than in J/J plants under reduced-N treatment. For fruit quality, reduced application of N-fertilizer resulted in a significant decrease in cucumber quality, particularly in J/J plants, while the quality performance of J/R plants was generally better than that of J/J plants (Table 2). Compared to the control, reduced-N application resulted in a decrease of 25.4, 18.4, 36.0, 10.0, and 33.6% in the content of free amino acid (FAA), soluble protein (SP), organic acid (OA), vitamin C (Vc), and total soluble solids (TSS) in J/J plants, respectively, while the FAA, SP, and OA of J/R plants were slightly reduced by 7.3, 9.3, and 1.5%, respectively, and these values reached the level of J/J plants under normal N treatment. As a result, compared to J/J plants, J/R plants exhibited higher levels of quality, irrespective of N levels.
| Treatments | Free amino acids content (mg·g−1) | Soluble protein content (mg·g−1) | Organic acid content (%) | Total soluble solids content (%) | Vitamin C content (μg·g−1) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| N treatment (N) | 7 mM | 1.85 ± 0.01a | 100% | 0.40 ± 0.00a | 100% | 1.96 ± 0.01a | 100% | 6.45 ± 0.01a | 100% | 20.1 ± 0.05a | 100% |
| 4 mM | 1.56 ± 0.01b | 84.3% | 0.35 ± 0.00b | 87.5% | 1.56 ± 0.01b | 79.6% | 5.33 ± 0.01b | 82.6% | 19.1 ± 0.35b | 95.0% | |
| Graft combination (G) J/J | 1.55 ± 0.01b | 100% | 0.35 ± 0.00b | 100% | 1.56 ± 0.01b | 100% | 5.33 ± 0.01b | 100% | 19.1 ± 0.45a | 100% | |
| J/R | 1.86 ± 0.01a | 120% | 0.40 ± 0.01a | 114% | 1.96 ± 0.01a | 126% | 6.45 ± 0.01a | 121% | 20.1 ± 0.04a | 105% | |
| Interaction (N × G) J/J-7 mM | 1.77 ± 0.00b | 100% | 0.38 ± 0.00b | 100% | 1.89 ± 0.01b | 100% | 6.40 ± 0.01a | 100% | 20.1 ± 0.07a | 100% | |
| J/J-4 mM | 1.32 ± 0.01c | 74.6% | 0.31 ± 0.00c | 81.6% | 1.21 ± 0.01c | 64.0% | 4.25 ± 0.01b | 66.4% | 18.1 ± 0.02b | 90.0% | |
| J/R-7 mM | 1.93 ± 0.01a | 100% | 0.42 ± 0.00a | 100% | 2.02 ± 0.00a | 100% | 6.50 ± 0.00a | 100% | 20.2 ± 0.03a | 100% | |
| J/R-4 mM | 1.79 ± 0.00b | 92.7% | 0.38 ± 0.00b | 90.5% | 1.90 ± 0.00b | 98.5% | 6.40 ± 0.01a | 97.4% | 20.1 ± 0.05a | 100% | |
- Note: Data indicate mean ± SE, n = 6. Different letters indicate significant differences determined by Tukey's test at P ≤ 0.05. J/J: self-grafted plants; J/R: rootstock-grafted plants.
3.6 Correlation and principal component analysis (PCA)
The correlation analysis revealed that the representative parameters for plant growth (TDW), photosynthesis (including Chl, Pn, and Gs), NUE traits (including total NA, NUpE, and NUE), yield (including mean fruit weight and yield per plant), and fruit quality (including FAA, SP, OA, TSS, and Vc) were all positively associated with each other (except for NUpE, which has no correlation with Chl content and fruit weight) (Figure 6A). The NO3− uptake was significantly positively correlated with the total NA, photosynthesis, yield, and fruit quality, meantime, the NUpE was closely related to plant root characteristics (including root length, root surface area, and root activity) and the expression of NO3− uptake related genes and their transcription factors (NRT1.2, NRT2.1, and LBD38). Moreover, other positive correlations included NUtE with TDW and NUE, total NA with root surface area, root surface area and root vitality with root length. Furthermore, the secondary metabolites in the root system (including total phenolics content, flavonoid content, and PAL activity) were closely related to the root characteristics, photosynthesis (including Pn, and Gs), NUE traits (including total NA, NUpE, and NUE), expression of NO3− uptake related genes and their transcription factors (NRT1.2, NRT1.5, NRT2.1, NLP6 and LBD38).
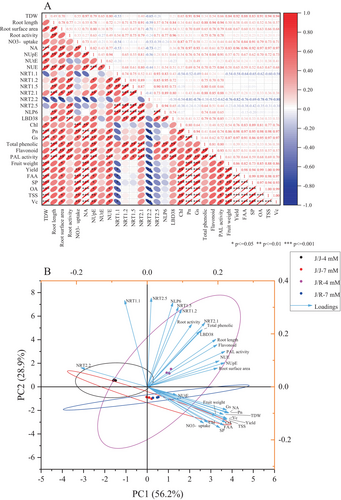
The 30 indicators of grafted cucumber plants under reduced-N treatment were used for PCA; the indicators included plant growth, root characteristics, NO3− uptake, NUE traits, gene expression of NRTs and their transcription factors, photosynthesis, secondary metabolites, yield, and fruit quality. PCA1 and PCA2 principal components were defined based on characteristic values pass 1 (Figure 6B and Table 3), and they explained 56.2 and 28.9% of the variance, respectively. The cumulative contribution was 85.1% (>80%), so the two principal components were able to cover the information of the 30 indicators relatively accurately. The results showed that the scattered points corresponding to various N treatments of self-grafted cucumber plants were clearly parted, implying that reducing N-fertilizer supply had an obvious impact on each index of self-grafted cucumber, however, the scattered points corresponding to various N treatments of rootstock-grafted cucumber plants intersect with each other, indicating that the impact of reducing N-fertilizer application on various indicators of rootstock-grafted cucumber was very mild (Figure 5B). PCA1 had strong positive loading for the growth, root morphology, NO3− uptake, NA, NUpE and NUE, NRT2.1 and LBD38, photosynthesis, secondary metabolites, yield, and fruit quality. PCA2 had high positive loading for the root activity, gene expression of NRT1.1, NRT1.2, NRT 1.5, NRT2.1, NRT 2.5, NLP6, and LBD38, and total phenolic content.
| Trait | Component | |
|---|---|---|
| PCA1 | PCA2 | |
| TDW | 0.886** | −0.307 |
| Root length | 0.799** | 0.528 |
| Root surface area | 0.816** | 0.209 |
| Root activity | 0.518 | 0.697** |
| NO3− uptake | 0.656** | −0.404 |
| NA | 0.942** | −0.292 |
| NUpE | 0.899** | 0.259 |
| NUtE | 0.345 | −0.095 |
| NUE | 0.837** | 0.289 |
| NRT1.1 | −0.265 | 0.960** |
| NRT1.2 | 0.400 | 0.861** |
| NRT1.5 | 0.363 | 0.887** |
| NRT2.1 | 0.672** | 0.712** |
| NRT2.2 | −0.777** | 0.211 |
| NRT2.5 | 0.048 | 0.989** |
| NLP6 | 0.304 | 0.934** |
| LBD38 | 0.610** | 0.619** |
| Chl | 0.702** | −0.400 |
| Pn | 0.948** | −0.288 |
| Gs | 0.922** | −0.256 |
| Total phenolic | 0.635** | 0.651** |
| Flavonoid | 0.807** | 0.479 |
| PAL activity | 0.905** | 0.395 |
| Fruit-weight | 0.798** | −0.325 |
| Yield | 0.926** | −0.355 |
| FAA | 0.890** | −0.428 |
| SP | 0.845** | −0.455 |
| OA | 0.914** | −0.396 |
| TSS | 0.925** | −0.368 |
| Vc | 0.928** | −0.355 |
| Total | 15.85 | 4.02 |
| % of variance | 56.2 | 28.9 |
| Cumulative% | 56.2 | 85.1 |
- Note: ** represents an eigenvalue that is significant (≥0.60).
4 DISCUSSION
N fertilizer is one of the indispensable and important fertilizers in agricultural production and is the “source of life” for crop growth and development (Xu et al., 2021). The current result showed that under long-term reduced-N conditions, the total root length, root surface area, and plant biomass of self-grafted cucumber seedlings were significantly restrained compared with control (Figure 1), indicating that insufficient N supply will induce severe damage to plant growth (Yang et al., 2023). However, under a long-term reduced N environment, rootstock-grafted plants were not adversely affected, and their growth status and root characteristics were all obviously better than those of self-grafted plants. This result confirms that 4 mM NO3−-N concentration is a suitable N supply level for rootstock-grafted plants, which is expected to save N-fertilizer for cucumber cultivation through grafting. The total root system and root surface area of rootstock-grafted plants increased under 4 mM NO3−-N level, possibly because NO3− not only acts as a nitrogen source but also acts as a signalling molecule to regulate their root architecture (O'Brien et al., 2016; Lin and Tsay, 2017).
NO3− is the most bioavailable form of inorganic N in higher plants, which is gradually incorporated into N-containing organic compounds after absorption and metabolism, thus the absorption capacity of NO3-, will, to some extent, determine plant growth and development (Zhen et al., 2018). This study also showed a positive correlation between NO3− uptake with NA and total plant biomass (Figure 6A). Meantime, the current result displayed that under reduced-N treatment, the NO3− uptake and NA of self-grafted plants were dramatically decreased compared to control (Figure 2A,B), indicating that long-term reduced-N treatment inhibited the NO3− uptake and N accumulation in cucumber plants. However, these values were obviously higher in rootstock-grafted plants than in self-grafted plants, irrespective of N levels, which supports the view reported by Savvas et al. (2017) that grafting using a suitable rootstock will often improve nutrient absorption and transfer to the shoot.
Moderate N-fertilizer supply is also beneficial for improving plant NUE (Xu et al. 2021), while NUE is jointly determined by NUpE and NUtE according to the description of Hawkesford and Griffiths (2019). These results indicated that compared to the control, the N efficiency (including NUpE, NUtE and NUE) of self-grafted plants decreased while increased in rootstock-grafted plants under long-term reduced N condition (Figure 2C-E). The close relationship between NUpE and root vitality, as well as NUE and plant growth, root morphology, NA, NUpE, and NUtE (Figure 6A), further confirms that rootstock roots have strong N absorption and utilization abilities due to their excellent root characteristics, so cucumber plants grafted onto N-efficient rootstocks effectively can improve their N efficiency. Wang et al. (2020) also reported that enhancing the ability of plant roots to obtain N is one of the effective ways to improve crop N efficiency.
The photosynthetic capacity of leaves has a close association with the dosage of N fertilizer application (Nasar et al., 2021; Wang et al., 2022), as over 60% of total N in leaves is allocated to proteins related to photosynthesis, and the N content in chloroplasts of mesophyll cells is as high as 57% (Xu et al., 2012). This research indicated that reduced-N application caused a significant decrease in Chl content, Pn, and Gs of self-grafted plants (Figure 3A-C), which is consistent with the report of Yang et al. (2023), who suggested that N-deficiency would weaken Pn and Gs of blackberry leaves. The significant positive correlation between photosynthetic parameters (Pn and GS) and NO3− uptake, NA, and NUE (Figure 6A) further supports the research results of Xiong et al. (2021) on tobacco, which suggest that the inhibition of photosynthesis caused by low-N environment is mainly due to the decrease in leaf N content, which limits the biochemical capacity of leaf flesh. However, the N-reducing environment did not have a significant adverse effect on rootstock-grafted plants, and their photosynthetic parameters were significantly higher than those of self-grafted plants under reduced-N treatment. This is consistent with the viewpoint of Fullana-Pericàs et al. (2020), who reported that rootstock grafting can improve the plant's photosynthetic performance. From the above results, we speculate that under long-term reduced-N application, grafting with excellent rootstocks would maintain the growth and photosynthetic capacity of cucumber plants by absorbing and accumulating more N. Zhang et al. (2022) reported that tomato plants grafted onto N-efficient rootstock enhanced N absorption and utilization, and maintained growth by improving light energy utilization efficiency under low-N stress. In view of this, enhancing plant photosynthetic capacity can also promote N absorption and accumulation, so plant photosynthesis is closely related to N status.
Plants produce multiple plant secondary metabolites (PSMs) to enhance their ability to interact with the surrounding adverse environment. These secondary metabolites and their related enzymes play a crucial role in the defence, adaptive regulation, signal transduction, growth and development of plant organisms (Carrington et al., 2018). Among them, phenols and flavonoids are the prominent secondary metabolites of plants. Phenylalanine ammonia-lyase (PAL) is a key rate-limiting enzyme that regulates phenolic levels under various stress constraints (Jha and Mohamed, 2022). In our study, reduced-N application treatment decreased the PAL activity of self-grafted plants but increased the total phenolic content, flavonoid content, and PAL activity of rootstock-grafted plants (Figure 3D-F). This is consistent with previous reports that low nitrogen treatment can increase the content of total phenolic and flavonoid compounds, as well as PAL activity (Rubio-wilhelmi et al., 2012; Larbat et al., 2012). Moreover, the above parameter values of J/R plants were all significantly higher than those of J/J plants under reducing N application. Correlation analysis showed that the contents of total phenolic and flavonoid and PAL activity were all positively correlated with N-efficiency (NUpE and NUE) and photosynthesis (Pn and Gs). This result indicated that pumpkin rootstock grafting could improve the antioxidant capacity of cucumber through secondary metabolism, which may be beneficial to the improvement of plant photosynthetic capacity and nitrogen efficiency to some extent. In other words, the improvement of nitrogen efficiency in grafted cucumber is a comprehensive result of the excellent characteristics of pumpkin rootstock in various aspects.
Plants take up NO3− mainly through root-specific transporters, which are divided into high-affinity (HATS) and low-affinity (LATS) transport systems. These two systems are encoded by the NRT2 and NRT1 gene families, respectively (Li et al., 2013). Some NRT1 and NRT2 genes and their related transcription factors play important roles in the absorption and transport of NO3− (Wang et al., 2012, 2018). In this study, N reduction treatment down-regulated the expression of NRT1.2, NRT1.5, NRT2.2, and NRT2.5 genes of self-grafted plant roots (Figure 4), so resulting in reduced NO3− absorption and transport (Lezhneva et al., 2014). On the contrary, these genes, along with NRT2.1, were all clearly up-regulated in rootstock-grafted plant roots under N reduction treatment, meantime, NUE was closely correlated relevant to the gene expression of NRT1.2, NRT1.5, and NRT2.1 (Figure 6A). This result reveals that rootstock grafting can improve the NO3− uptake and NUE of cucumber plants by up-regulating nitrate transporters at the transcriptional level. Research on rice and wheat has also reported that over-expressing NRT genes can increase NO3− uptake, yield, and NUE (Hu et al., 2015; Li et al., 2020b). Previous studies have shown that NRT1.5, NRT2.1, NRT2.2, and NRT2.4 were related to long-distance transport of NO3− (Wang et al., 2012; Kiba et al., 2012), AtNRT1.2 was involved in the absorption of NO3−-N under high N concentrations and ABA-mediated signal transduction (Li et al., 2020a), and NRT1.1 and NRT2.1 could affect root growth regulated by NO3−, thereby affecting NUE by affecting the total NO3− uptake by plant roots (Garnett et al., 2009). Orsel et al. (2002) suggested that NRT2.1 could be the predominant NO3− transporter in roots but that NRT2.5 also have a significant role in NO3− uptake. Li et al. (2017) reported that the AtNRT1.5 was expressed in the peri sheath of the Arabidopsis root and was responsible for loading NO3− into the xylem in the root system. Therefore, based on the results of this study, several NRT genes may be involved in the absorption and transport of NO3− in the root of rootstock-grafted cucumber plants. This process may be regulated by ABA signalling, as it has been reported that abscisic acid signalling plays a crucial role in root foraging by ABI2/PP2C under mild N deficiency, and ABA can regulate NRT2/NAR expression under nitrate-limited conditions (Wang et al., 2020). Furthermore, the expression of NRT genes is regulated by upstream transcription factors, among which NLP6 and LBD38 are key genes involved in nitrate metabolism (Castaings et al., 2009; Rubin et al., 2009). In this study, N reduction treatment significantly up-regulated the expression of NLP6 and LBD38 in rootstock-grafted plant roots, which may further regulate the downstream NRT genes through signal transduction.
Plant yield will decrease under N-deficiency conditions, while under reasonable fertilization conditions, agricultural product yield can be increased by more than 40% (Xu et al., 2021). In this study, the reduced-N application significantly decreased the values of fruit size and yield of self-grafted plants but did not cause any harm to rootstock-grafted plants compared to the control treatment (Figure 5), indicating that N application with 4 mM concentration is a N deficient concentration for self-grafted plants, but a reasonable concentration for rootstock-grafted plants. Studies on kiwifruit, wheat, and blueberry have also expressed that appropriate N application has a promoting impact on yield (Guo et al., 2023; Stefaniak et al., 2020; Wang et al., 2022). The positive correlation between yield and photosynthetic performance indicated that the higher yield of cucumber plants grafted onto rootstock than onto themselves is related to their higher photosynthesis, as photosynthesis provides the main material basis for the formation of crop yield.
The impact of N on fruit quality is multifaceted. N can significantly affect the nutritional value and flavour of fruits by regulating the content and proportion of proteins and amino acids, affecting the content of vitamins and soluble solids (Yang et al., 2023). Soluble solids and organic acids are also the main water-soluble substances in fruits, which have a significant impact on their taste. The various nutrients, organic acids, sugars, and their ratios in fruits are important indicators for evaluating the intrinsic quality of fruits quality (Mikulic-Petkovsek et al., 2021). Therefore, in agricultural production, the rational application of N fertilizer is one of the key measures to improve fruit quality and yield. In this study, reducing N application significantly reduced the content of nutritional quality-related indicators in cucumber, especially in the levels of FAA, SP, OA, TSS, and Vc in self-grafted plants (Table 2). The PCA also indicated that reduced-N application had a significant impact on various indicators of self-grafted cucumber plants (Figure 6B). This is consistent with research on blackberries, which has shown that a lack of N could reduce cucumber fruit quality (Yang et al., 2023). However, the quality performance of rootstock-grafted plants was generally better than that of self-grafted plants, indicating that the grafted combination might play an important role in determining fruit quality (Zhou et al., 2022), and the formation of cucumber quality was closely related to its plant growth, NO3− uptake, NUE traits, and photosynthetic capacity (Figure 6A).
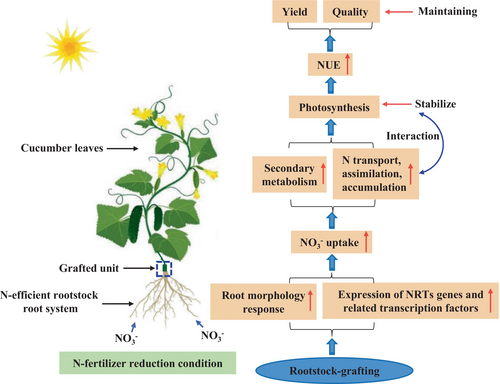
Above all, we conclude that grafting with N-efficient rootstock is a fast and effective measure to prevent the waste of inorganic N fertilizer, lower production costs, and achieve efficient cultivation and production of cucumber. Future research will identify the metabolic and signalling pathways through which NRT genes regulate NO3− uptake and utilization in plants in order to further elucidate the mechanism of rootstock grafting improving NUE in cucumber plants under reduced-N conditions at the molecular level through transgenic technology.
5 CONCLUSION
In summary, a long-term N reduction environment limited the growth, root NO3− absorption, NUE traits, photosynthesis, secondary metabolism, yield, and fruit quality of self-grafted cucumber plants but generally had no adverse effects on rootstock-grafted plants and instead increased their N efficiency. Based on our results, we suggest that rootstock grafting, with its root system advantage, promotes the absorption, transportation, and utilization of NO3−-N in cucumber plants and defends against N-reducing environments by generating secondary metabolites. Ultimately, it enables cucumber plants to maintain stable photosynthetic capacity and efficient N utilization, thereby avoiding the adverse effects of long-term N reduction conditions on plant growth, yield, and fruit quality, as presented in the simple sketch map (Figure 7). The significant up-regulation expression of NRT genes (NRT1.2, NRT1.5, NRT2.1, NRT2.2, and NRT2.5) and their related transcription factors (NLP6 and LBD38) in the pumpkin rootstock roots may exert a major regulatory role in NO3− uptake of cucumber plants at the transcriptional level.
AUTHOR CONTRIBUTIONS
Xun Wang: Writing-Original draft, Conceptualization, Methodology. Guohu Li: Visualization, Data Curation. Yanfei Yang: Investigation, Validation. Hongyan Yuan: Formal analysis, Software. Qi Huang: Data Curation, Supervision. Jiayi Liang: Conceptualization, Methodology. Ai Zhen: Writing-Reviewing and Editing, Funding acquisition, Resources, Project administration.
ACKNOWLEDGEMENTS
The authors would like to thank the Northwest A&F University for providing technical and financial supports.
FUNDING INFORMATION
This work was supported by the National Natural Science Foundation of China (NSFC) (32202580).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




