Suppression of MdPRP6 enhances adaptation of apple plants to long-term drought
Abstract
Apples are one of the world's four most economically significant fruits, and drought stress is an important factor limiting the development of the global apple industry. Here, we demonstrate that a proline-rich protein (PRP), MdPRP6, is an important factor regulating the long-term drought adaptation of apple plants. Suppression of MdPRP6 in apple plants (MdPRP6-Ri) enhances their adaptation to long-term moderate drought conditions, as indicated by their significantly higher biomass and relative water content (RWC) compared with wild-type (WT) plants. Under drought stress, the net photosynthetic rate (Pn), intercellular CO2 concentration (Ci), stomatal conductance (Gs), and transpiration rate (Tr) were higher, and photosystem II (PSII) damage was lower in MdPRP6-Ri plants than in WT plants. Suppression of MdPRP6 increased the activity of antioxidant enzymes, including superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), which reduced oxidative damage to apple leaves under drought stress. The stomatal openings of MdPRP6-Ri plants were larger than those of WT plants; the WUEI and WUEL were thus higher in MdPRP6-Ri plants than in WT plants under long-term moderate drought stress. We also found that suppression of MdPRP6 increased the wax content of the leaf epidermis, which limits water evaporation caused by non-stomatal factors under drought stress. In sum, our findings suggest that MdPRP6 negatively affects the long-term drought adaptation of apple plants, possibly by modulating both stomatal and non-stomatal water loss.
1 INTRODUCTION
Drought is a critical environmental stressor with global significance, substantially impacting agricultural productivity and contributing to nearly 50% of total crop losses attributed to meteorological disasters worldwide (Zhao et al., 2023). Apple (Malus domestica), one of the most widely produced and consumed fruits globally, is particularly vulnerable to drought stress. China, the world's largest apple producer, contributes to over 50% of the global apple yield; thus, the sustainability of China's apple industry is essential for the global apple supply (China Apple Industry Association, Agricultural Planning and Design Institute of China Agricultural University, 2023). Nevertheless, the majority of China's apple orchards are situated in arid and semi-arid regions, where irregular annual precipitation, particularly low rainfall in spring and summer, coupled with insufficient irrigation infrastructure, presents considerable challenges. Additionally, factors such as the natural environment, sparse vegetation, and suboptimal human management practices further hinder the growth and development of apple trees, which adversely affect fruit quality and yield (Wang et al., 2013). In light of these challenges, much research has focused on characterizing drought tolerance mechanisms; for example, the physiological, molecular, and genetic mechanisms in apples have been used to address similar issues worldwide (Mihaljević et al., 2021). The advancement of drought-resistant apple cultivars through genetic and molecular techniques is recognized as a crucial strategy for alleviating the negative impacts of drought on apple production (Liu et al., 2024) and ensuring the maintenance of apple quality and yield amidst increasingly unpredictable climate conditions.
Plants require sufficient water during their growth, development, and reproductive cycles. Insufficient continuous precipitation, excessive cultivation, overexploitation of water resources, and pollution of water resources can lead to a decrease in the available water content in the soil and exacerbate water scarcity, resulting in a decrease in the amount of water absorbed by plants from the soil. By contrast, higher transpiration rates in the atmosphere can induce water loss in plants, leading to drought stress (Mishra and Singh 2010). When there is a lack of water, the permeability of the cell membrane weakens, the compartmentalization of the membrane system is disrupted, and excess reactive oxygen species (ROS) are produced. This results in an increase in relative electrolyte leakage (REL) and the malondialdehyde (MDA) content and the destruction of the double layer structure of the cell membrane, which induces the loss of its selective permeability, the inactivation of proteases on the membrane, and metabolic disorders of cells (Jogawat et al., 2021). When plants experience severe drought stress, the structure of their chloroplasts becomes compromised, resulting in damage to the thylakoids and inhibition of chlorophyll synthesis (Chaves et al., 2009). Plant photosynthesis is also severely affected by drought stress, as decreases in stomatal opening and intercellular carbon dioxide absorption lead to a reduction in the photosynthetic rate (Sala et al., 2021). Drought can also damage the photosynthetic electron transport chain, thereby reducing the rate of photosynthesis (Jing et al., 2024).
Following exposure to drought stress, plants undergo a series of morphological, physiological, and molecular changes, which help to balance water loss through transpiration with water absorption by the roots, thereby mitigating drought-induced damage (Thapa et al., 2011; Qi et al., 2021). Approximately 90% of the water in plants is lost through open stomata; therefore, water loss can be reduced by regulating stomatal opening and density under drought stress (Wang et al., 2009, 2019). Greater drought tolerance is exhibited by transgenic apple plants with smaller stomatal openings compared with WT plants (Zhao et al., 2021). In addition to stomatal closure, an increase in plant epidermal wax can reduce water loss and enhance the drought tolerance of plants (Zhu et al., 2013). Osmotic regulation is also a key approach for coping with drought stress. The accumulation of osmoregulatory substances, such as proline, soluble sugars, betaine, organic acids, and sugar alcohols, in the plant body increases their concentration in cell fluid, reduces the cell osmotic potential, improves the cell water absorption and retention capacity, and thus reduces the damage caused by drought stress to plants (Farooq et al., 2010, Farooq et al., 2017; He et al., 2018). Moreover, the regulation of the drought stress response in plants is significantly influenced by plant hormones. Abscisic acid (ABA), auxin, cytokinin, and jasmonic acid have all been shown to be closely associated with various sophisticated regulatory pathways in plants related to the drought response (Emenecker and Strader 2020; Li et al., 2021; Wang et al., 2020).
The cell wall serves as the first line of defence in higher plant cells against biotic and abiotic stresses. It is primarily composed of cellulose, hemicellulose, pectin, and various structural proteins, all of which play a crucial role in protecting plants under stress conditions (Varner and Lin 1989). Proline-rich proteins (PRPs) have been identified as integral components of the cell wall and play important roles in the formation and maintenance of cell wall structure (Showalter et al., 1993). They are known to contribute to plant responses to various stresses by stabilizing cellular structures and maintaining osmotic balance (Ingram and Bartels 1996). OsPRP3 in rice leads to increased cell wall integrity, which improves cold tolerance in transgenic plants (Gothandam et al., 2010). FOCL1, which encodes a PRP protein in Arabidopsis, is specifically expressed in guard cells; mutations in this gene cause the stomata to be covered with epidermal cells, which reduces transpiration and enhances drought tolerance (Hunt et al., 2017). Under stress conditions, plants often degrade PRP proteins while accumulating free proline, a response that enhances stress tolerance. For example, under drought stress, the expression of PRPs rapidly decreases, and the free proline content increases, which enhances drought resistance (Gujjar et al., 2018; Gujjar et al., 2019; Zhang et al., 2023c). Additionally, PRPs not only function as reservoirs for proline but are also thought to play a role in cell wall signalling cascades (Kishor et al., 2015). The hybrid proline-rich proteins (HyPRPs), in particular, facilitate the transport of signalling molecules within specific cellular locations (Banday et al., 2022). AZTI (Azelaic acid-induced 1), a type of HyPRP, may act as a membrane-associated signalling protein in the response of plants to stress after phosphorylation (Pitzschke et al., 2014a, 2014b). Thus, PRPs play multifaceted roles in reinforcing plant cell walls and modulating stress responses, which makes them vital to the development of stress-resilient crops.
In recent years, numerous drought-related genes have been identified in apple plants, including regulator genes and functional genes (Joshi et al., 2016). For example, the FERONIA receptor kinase MdMRLK2 enhances water use efficiency in apple plants under long-term water deficit conditions (Jing et al., 2024). The transcriptional factors MdbHLH160 and MdDREB2A-like play essential roles in activating ABA-mediated drought responses, further improving water use efficiency and photosynthetic performance under drought stress (Mao et al., 2024). The functional genes also attract much attention. The autophagy-related genes, MdATG8i, MdATG18a, and MdATG10 all have a positive role in apple plants responding to drought stress (Sun et al., 2018; Jia et al., 2021; Xiang et al., 2024). Other functional genes like MdMIPS1 (involved in the synthesis of myo-inositol) and MdTyDC (involved in the synthesis of dopamine) improve water use efficiency or drought tolerance in apple plants (Hu et al., 2022; Liu et al., 2024). Although the cell wall proteins are important in many biological processes, their biological role in apple plants under drought stress is not so clear. Previously, we identified a PRP protein, MdPRP6, from the apple genome and found that heterologous expression of MdPRP6 in tobacco plants enhances their tolerance to high-temperature stress (Zhang et al., 2021), and inhibition of MdPRP6 expression enhances the tolerance of apple plants to low nitrogen, salt, and natural drought stress (Zhang et al., 2022; Zhang et al., 2023a, 2023b, 2023c). Here, we performed experiments to investigate the biological function of MdPRP6 in long-term drought adaptation in apple plants. The results suggested that MdPRP6 negatively affects the long-term drought adaptation of apple plants, possibly by inhibiting both stomatal and non-stomatal water loss. The findings of this study will contribute to the development of high-quality germplasm with enhanced adaptation to abiotic stresses through genetic engineering. Additionally, MdPRP6 can serve as a key target for the synergistic regulation of drought adaptation in plants, providing a broader context for its potential role in improving crop drought resistance.
2 MATERIALS AND METHODS
2.1 Plant material and growth conditions
‘GL-3’ (Malus domestica) was used as the wild-type (WT) material in this study. Previously obtained WT and transgenic apple plants (MdPRP6-Ri1/2/3, Zhang et al., 2022) were grown on Murashige Skoog (MS) subculture medium, with the following formula: 4.43 g L−1 MS + 30 g L−1 sucrose +0.2 mg L−1 IAA + 0.3 mg L−1 6-BA +8 g L−1 agar; the pH of the medium was adjusted to around 5.8 with NaOH. Apple seedlings were placed at room temperature in a tissue culture room with a light cycle of 16 /8 h (light/dark) and propagated every 30 d. Healthy seedlings with uniform growth were placed on the rooting medium. The formula for the rooting medium was as follows: 2.22 g L−1 MS + 20 g L−1 sucrose +0.5 mg L−1 IAA + 0.5 mg L−1 IBA + 8 g L−1 agar (pH = 5.8). After approximately 45 d, the tissue-cultured seedlings grew roots. After opening the lids and training the seedlings one week in advance, they were transplanted into a plastic pot (10 × 10 × 10 cm) containing a mixture of organic matrix/vermiculite/perlite (3/1/1, v/v/v) and cultured in a light incubator (23°C, 16 /8 h of light cycle) for one month. Subsequently, the apple seedlings were transplanted into plastic pots (38 × 23 cm) containing a mixture with equal amounts of soil/sand/organic matter (v/v/v, 5/1/1) for long-term moderate drought treatment. All apple seedlings were placed in a semi-open greenhouse in the horticultural field of Northwest A&F University in Yangling, Shaanxi, China. After the apple seedlings reached a certain height, healthy and consistent seedlings were selected for drought treatment.
2.2 Long-term moderate drought treatment
The long-term moderate drought treatment was performed according to Jia et al. (2021) with slight modifications. The experimental materials used in this study were ‘GL-3’ and transgenic apple plants grown in a greenhouse for approximately 45 days, which were then randomly assigned to two experimental groups: a control group and a long-term drought treatment group while ensuring stable growth conditions for all plants. In the control group, the soil RWC was maintained at 75–85% of the maximum field capacity, while the long-term moderate drought treatment was applied by maintaining the soil RWC at 45–55% of the maximum field capacity throughout the treatment period. RWC measurements were taken every two days and recorded to ensure precise monitoring of soil moisture. The treatment lasted for 60 d, and physiological indicators were measured at 0, 20, 40, and 60 d. At each time point, plant samples were immediately frozen in liquid nitrogen and stored at −80°C for further analysis. Three independent biological replicates were performed for each experimental condition, with each replicate comprising at least 60 plants per strain.
2.3 Observation of leaf stomata and epidermal wax
The methods for observing leaf stomata and epidermal wax were based on the methods of Jia et al. (2021), with the specific procedures as follows. At the end of the treatment, six mature leaves were randomly selected from the same position in both the control and treatment groups, avoiding the main vein. Small leaf segments (3 × 3 mm) were excised, fixed in 4% glutaraldehyde phosphate buffer, and then vacuum infiltrated to ensure complete fixation. The samples were stored at 4°C for stomatal observation. For scanning electron microscopy (SEM), samples were washed with phosphate buffer, dehydrated in an ethanol gradient (30–100%), and then infiltrated with isoamyl acetate. After supercritical CO2 drying, the samples were gold-coated. Stomatal observations were performed using a scanning electron microscope (JSM-6360LV; JEOL Ltd.) at 300× and 3000× magnification. Stomatal density and aperture parameters were analyzed using ImageJ software.
After the treatment, six mature leaves from the same position in both the control and treatment groups were randomly selected, fixed with paper clips onto cardboard, and dried in an oven at 37°C. Small leaf blocks (6 × 6 mm) were excised and glued to the sample holder with conductive adhesive. After vacuum treatment and gold sputtering, the samples were observed using a field-emission scanning electron microscope (JSM-6360LV; JEOL Ltd.) to examine the morphology of wax crystals in the tissue. The leaf wax content was determined according to the method described by Jiang et al. (2021). Mature leaves from the same position of each genotype and treatment group were completely immersed in 25 mL of chloroform and gently shaken for 1 minute. After removal, 20 μL of C24 alkane (1 μg μL−1) was added as an internal standard. The extracted wax mixture was filtered through filter paper into a clean, dry glass tube. The chloroform was evaporated in a fume hood at room temperature, and 1 mL of chloroform was added to re-dissolve the wax. The solution was transferred to a brown injection vial, evaporated to dryness with nitrogen, and re-dissolved with 30 μL pyridine. Subsequently, 30 μL of BSTFA derivatizing agent was added, and the mixture was heated at 70°C for 60 min. After nitrogen blow-drying, 1 mL chloroform was added to re-dissolve the sample, which was then filtered through a 0.45 μm organic filter membrane for gas chromatography analysis.
2.4 Physiological indicator measurement
On a sunny morning (9:00–11:00 AM), 10 plants from the control group and 10 plants from the treatment group were selected. The CIRAS-3 portable photosynthesis system (CIRAS) was used to measure the net photosynthetic rate (Pn), stomatal conductance (Gs), intercellular CO2 concentration (Ci), and transpiration rate (Tr) of mature leaves. The Chlorophyll Fluorescence Imaging System (IMAGINATION-PAM) was used to detect chlorophyll fluorescence and related parameters. RWC was determined based on the methods of Jia et al. (2021). Nitroblue tetrazolium (NBT) and 3,3-diaminobenzidine (DAB) were used to stain the leaves of various strains in the control and treatment groups to qualitatively detect the accumulation of superoxide radical (O2−) and hydrogen peroxide (H2O2) in the leaves (Xiang et al., 2024). The content of MDA, H₂O₂, and O₂−, as well as the activity of superoxide dismutase (SOD), peroxidase (POD), and catalase (CAT), were measured using corresponding detection kits (Suzhou Comin Biotechnology Co., Ltd.).
2.5 Determination of growth indicators and long-term water use efficiency
The methods of determining the growth indicators and water use efficiency were described by Jing et al. (2024). Briefly, at 0 and 60 d of the long-term drought treatment, 10 plants were randomly selected from each line in both the treatment and control groups. Plant height was measured from the base of the stem to the tip of the shoot, and stem thickness was recorded at the thickest part of the stem base. Subsequently, 10 whole plants from each variety were excavated, and the soil around the roots and dust on the leaves were gently rinsed with tap water and blot-dried with filter paper. The fresh weight of each plant was then recorded. After measuring the fresh weight, plants were placed in an envelope and dried in an oven at 65°C for several days until a constant weight was achieved. The dry weight of each plant was then determined. Biomass accumulation was calculated as the difference in dry weight between the 0 and 60 d time points. The instantaneous water use efficiency (WUEI) was calculated as the ratio of the net photosynthetic rate to the transpiration rate. Long-term water use efficiency (WUEL) was calculated following the method of Ehdaie (1995): WUEL = (dry weight at 60 d - dry weight at 0 d) / total water consumption during the treatment period.
2.6 Data analysis
All data were analyzed using IBM SPSS statistical software (version 20; SPSS Inc.). Statistical analysis was conducted using one-way ANOVA, Tukey's multiple range test, or t-test, and the threshold for statistical significance was p < 0.05.
3 RESULTS
3.1 Phenotypic analysis of MdPRP6 transgenic apple plants under long-term drought stress
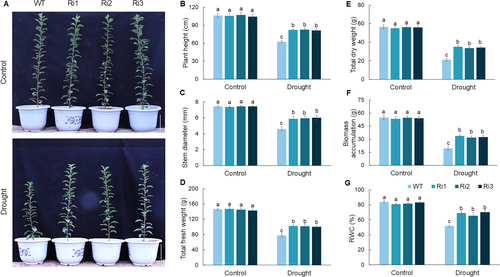
To investigate the function of MdPRP6 in response to long-term drought stress in apple, WT and MdPRP6-Ri plants with consistent growth status were selected for 60-d long-term drought treatment. After 60 d of a normal water supply, the growth of WT and MdPRP6-Ri plants was normal, and no significant differences in their phenotype and growth indicators were observed (Figure 1). After 60 d of long-term drought treatment, the growth of MdPRP6-Ri plants was significantly improved compared with that of WT plants (Figure 1A), and the height, stem diameter, dry and fresh weight, and biomass accumulation were greater in MdPRP6-Ri plants than in WT plants (Figure 1B–F). The RWC of MdPRP6-Ri plants was significantly higher than that of WT plants (Figure 1G). The above results indicate that MdPRP6 negatively regulates the adaptability of apple plants to long-term drought stress.
3.2 Analysis of the photosynthetic parameters of MdPRP6 transgenic apple plants under long-term drought stress
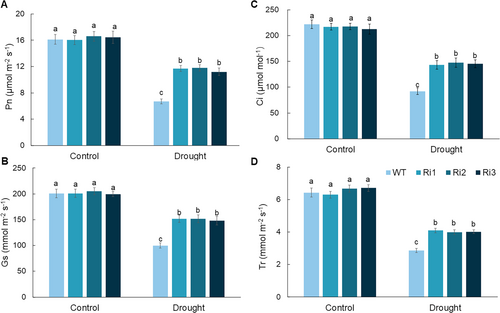
Photosynthesis is an effective pathway for dry matter accumulation, and it directly affects plant growth and biomass production (Zhang et al., 2023a). Figure 2A–D illustrates the effect of drought stress on various photosynthetic parameters in both WT and MdPRP6-Ri apple plants and highlights the enhanced photosynthetic capacity of MdPRP6-Ri plants under stress conditions. Under control conditions, Pn, Gs, Ci, and Tr were similar across both genotypes, and no significant differences were observed. However, WT plants experienced a marked reduction in all measured parameters under drought stress. Specifically, the values of Pn decreased significantly in WT plants to approximately 6 μmol m−2 s−1, whereas it in MdPRP6-Ri plants was maintained at higher levels, between 8 to 10 μmol m−2 s−1. Similarly, the values of Gs, Ci, and Tr also decreased more substantially in WT plants compared with them in MdPRP6-Ri plants. For example, the values of Gs in WT plants decreased more significantly under drought stress, to approximately 100 mmol m−2 s−1, whereas it in MdPRP6-Ri plants ranged from 100 to 150 mmol m−2 s−1. A similar pattern was observed for Ci and Tr, with WT plants showing greater declines in both parameters under drought conditions. These results suggest that the photosynthetic efficiency of MdPRP6-Ri plants is maintained to a greater degree under drought stress compared with that of WT plants, and this contributes to their better overall growth compared with WT plants under long-term drought stress.
3.3 Chlorophyll fluorescence parameters of MdPRP6 transgenic apple plants under long-term drought stress
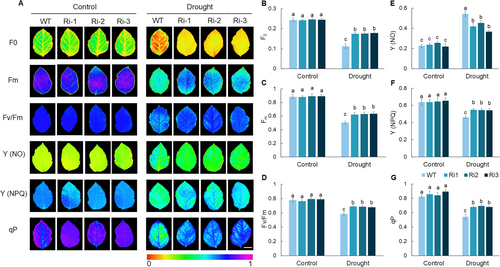
Chlorophyll fluorescence parameters are frequently used to evaluate the photosynthetic mechanisms and physiological status of plants (Bhagooli et al., 2021). Under well-watered conditions, chlorophyll fluorescence parameters did not significantly differ between MdPRP6-Ri and WT plants. However, following 60 d of prolonged drought stress, a marked divergence in fluorescence signals was observed between WT and MdPRP6-Ri plants (Figure 3). The fluorescence origin (F0) and fluorescence maximum (Fm) were significantly higher for MdPRP6-Ri plants than for WT plants (Figure 3B and C). Fv/Fm is the photochemical production capacity of PSII, which reflects the efficiency of light energy conversion in the PSII reaction center. Under a normal water supply, the Fv/Fm values of WT and Ri plants were both around 0.8, and no significant differences between them were observed. After 60 d of long-term drought treatment, the Fv/Fm values were significantly higher in MdPRP6-Ri plants than in WT plants (Figure 3D). Y (NO) is an important indicator of light damage, and higher values indicate more severe damage to plants (Wang et al., 2024). After 60 d of long-term drought treatment, the Y (NO) value of MdPRP6-Ri plants was significantly lower than that of WT plants (Figure 3E). Under long-term drought stress, the coefficient of photochemical quenching (qP) and the quantum yield of regulated energy dissipation Y (NPQ) was higher in MdPRP6-Ri plants than in WT plants (Figure 3F and G). The above results indicate that the photosynthetic system of MdPRP6-Ri plants experienced less damage than that of WT plants, which contributes to their higher photosynthetic efficiency under long-term drought stress.
3.4 ROS accumulation and scavenging of MdPRP6-Ri apple plants under long-term drought treatment
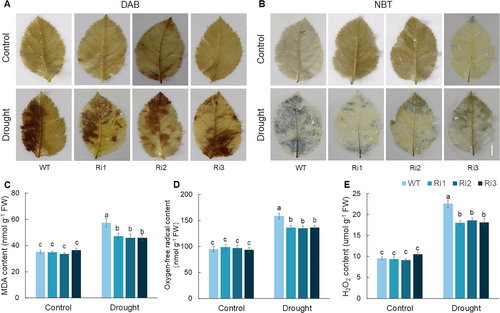
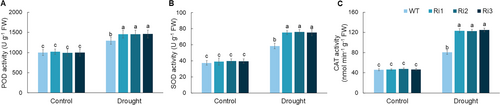
Analysis of the accumulation of ROS in WT and MdPRP6-Ri plants under long-term drought revealed that the colour of MdPRP6-Ri plants was lighter than that of WT plants after staining with DAB and NBT (Figure 4A and B). The content of MDA was significantly higher in WT plants than in MdPRP6-Ri plants (Figure 4C). The content of H2O2 and O2− was significantly higher in WT plants than in MdPRP6-Ri plants (Figure 4D and E), indicating that WT plants experienced more severe oxidative damage. The antioxidant enzyme system can regulate ROS homeostasis in plants. Under a normal water supply, no differences in POD, SOD, and CAT activities were observed between WT and MdPRP6-Ri plants. After 60 d of long-term drought treatment, the POD, SOD, and CAT activities of all plants were significantly increased, but the increases in the activities of these enzymes were greater in transgenic plants than in WT plants (Figure 5). In sum, the above results indicate that ROS accumulation was reduced and antioxidant enzyme activities were enhanced in MdPRP6-Ri plants than in WT plants, which reflects the improved ability of MdPRP6-Ri plants to adapt to long-term drought stress.
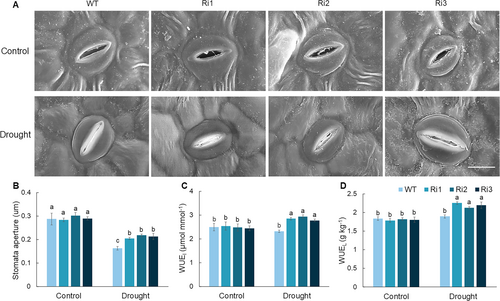
3.5 Effect of MdPRP6 on the stomatal movement of apple plants under long-term drought stress
Stomata are crucial determinants of plant drought tolerance and WUE. When exposed to drought stress, plant stomata are adaptively regulated to minimize water loss and enhance survival (Vives-Peris et al., 2024). To investigate whether MdPRP6-Ri affects plant stomatal characteristics, the stomatal aperture of plants under prolonged drought conditions was analyzed. Under a normal water supply, no significant differences were observed in the stomatal characteristics of WT and MdPRP6-Ri plants. Following long-term drought treatment, the stomatal aperture of both plants was reduced to minimize water transpiration, and the stomatal aperture of MdPRP6-Ri plants only slightly decreased (Figure 6A and B). We then calculated the WUE of different plants under long-term drought stress, and the results showed that the suppression of MdPRP6 significantly promoted the WUEI and WUEL of apple plants under long-term drought conditions (Figure 6C and D). The above results indicate that MdPRP6-Ri can regulate stomatal aperture under long-term drought and maintain WUE.
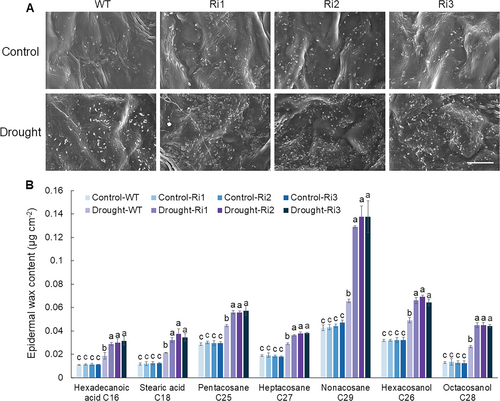
3.6 Effect of MdPRP6-Ri on wax formation in apple plants under long-term drought conditions
The primary function of plant epidermal wax is to reduce non-stomatal water loss in plants (Wen et al., 2023). To investigate whether MdPRP6-Ri affects the wax content of apple plants under long-term drought stress, the wax morphology of transgenic and WT plant leaves after long-term drought treatment was examined. Under a normal water supply, no significant difference in the ultrastructure of wax crystals on the leaf epidermis was observed between WT and MdPRP6-Ri plants. After long-term drought treatment, the wax crystal structure of the leaf epidermis in all plants increased significantly, and the increase in wax crystals was significantly higher in MdPRP6-Ri plants than in WT plants (Figure 7A). The content of seven wax monomers in the leaves of WT and transgenic plants under long-term drought treatment was analyzed, including Hexadecanoic acid C16, Stearic acid C18, Pentacosane C25, Heptacosane C27, Nonacosane C29, Hexacosanol C26, and Octacosanol C28, was determined. Under a normal water supply, no significant differences in the content of these seven wax monomers between WT and MdPRP6-Ri plants were observed. After being exposed to long-term drought conditions, the wax monomer content measured in the leaves of each strain significantly increased, and the increase was more significant in MdPRP6-Ri plants (Figure 7B). The above results indicate that suppression of MdPRP6 can promote the accumulation of wax components in apple leaves under long-term drought conditions, thereby reducing water loss and enhancing the adaptability of plants to long-term drought.
4 DISCUSSION
Drought is a global issue that restricts plant growth and reduces crop yield. Long-term drought stress can inhibit plant growth and development, leading to reductions in crop quality and yield (Barnabás et al., 2008; Iglesias et al., 2011; Thabet et al., 2020; Sattar et al., 2020; Ishimaru et al., 2022). Many functional genes, for example, autophagy-related genes, improve the WUE of plants under drought stress (Jia et al., 2021) AtMC3, which encodes a metacaspase in Arabidopsis, plays a crucial role in enhancing drought tolerance by regulating programmed cell death and maintaining cellular functions (Pitsili et al., 2023). PRPs are cell wall structural proteins that play important roles in the response of different species to various types of stress (Li et al., 2016; Mellacheruvu et al., 2016; Chen et al., 2022). In our previous study on the PRP gene family within the apple genome, we observed differential expression patterns of MdPRP genes across various tissues and under different stress conditions. Specifically, MdPRP6 exhibited high expression levels in all tissues, with particularly elevated expression in the leaves, where its transcript levels were more than 100 times higher than those in the roots. The expression profile of MdPRP6 was distinct from that of other MdPRP genes. Further investigation revealed that heterologous expression of MdPRP6 in tobacco plants enhanced the functionality of cell membranes and improved the plant's response to environmental stimuli, thereby increasing adaptation to heat stress. To explore its role in adaptation to long-term drought stress, we utilized MdPRP6-Ri transgenic plants, which were previously generated, and subjected them to prolonged drought conditions. Under these stress conditions, MdPRP6-Ri plants exhibited enhanced growth compared to WT plants. Specifically, MdPRP6-Ri plants showed greater plant height, stem diameter, dry and fresh weight, biomass accumulation, and RWC than WT plants (Figure 1). These results suggest that MdPRP6 plays a negative role in the adaptation of apple plants to long-term drought stress.
Photosynthesis provides carbon and energy necessary for normal plant growth, and photosynthetic efficiency directly affects biomass accumulation during plant growth (Yamori et al., 2016). Drought stress severely impacts the photosynthesis and chlorophyll fluorescence parameters of key food crops and economic crops (Elsalahy et al., 2022; Grieco et al., 2020; Jensen et al., 2024; Li et al., 2018; Radzikowska et al., 2022). Under drought stress, photosynthesis is often impaired due to the closure of stomata (Sala et al., 2021). However, stomatal closure is also a common physiological response to drought, as it helps minimize water loss through transpiration. So, plants trigger different biological responses only to survive drought adversity. The smaller stomatal apertures have been shown to improve tolerance to short-term drought stress by reducing water loss and maintaining larger stomatal openings can be much more beneficial under prolonged drought conditions (Jia et al., 2021; Jiang et al., 2022; Xu et al., 2008; Yavas et al., 2023; Zhao et al., 2021). In this work, MdPRP6-Ri transgenic apple plants exhibited significantly larger stomatal openings compared to WT plants under long-term drought stress (Figure 6). This larger stomatal aperture likely played a key role in alleviating the photosynthetic limitations typically associated with stomatal closure under drought conditions, thereby enabling MdPRP6-Ri plants to maintain higher rates of photosynthesis, and supporting better growth and biomass accumulation under drought stress. Water use efficiency (WUE), which reflects the balance between carbon assimilation and water loss, serves as a key indicator of plant adaptation to drought stress (Benjamin et al., 2024). Plants with higher WUE means that they accumulated higher biomass with less water consumption. Here, we found that MdPRP6-Ri plants accumulated higher biomass under long-term drought stress, and they indeed also had significantly higher WUEI and WUEL compared to WT plants (Figure 6C and D). This improvement in WUE is likely to benefit from the stronger photosynthesis capacity of the Ri plants. These results suggest that silencing MdPRP6 improves drought tolerance by enhancing the plant's ability to regulate stomatal aperture and thus better manage the trade-off between water conservation and photosynthetic efficiency, which finally promotes the WUE of apple plants under the prolonged period of water scarcity.
Wax is primarily composed of ultra-long chain fatty acids and their derivatives, which aid in reducing non-stomatal water loss and epidermal permeability (Jetter et al., 2006). The wax content is closely associated with plant drought tolerance (Singh et al., 2018). The structure of leaves and roots of plants that inhabit deserts differs from that of plants that inhabit humid areas; for example, the wax content of desert-adapted plants is greater than that of plants adapted to humid environments (Hetherington and Woodward 2003; Iqbal et al., 2013). The increase in epidermal wax can reduce the loss of non-stomatal water and enhance the drought tolerance of plants (Zhu et al., 2013). Thus, plants can enhance drought stress by promoting wax synthesis (Guo et al., 2016; Xue et al., 2017). The wax content of transgenic apple plants that interfere with the expression of MdGH3.6 is increased, and this contributes to their higher drought tolerance and WUE compared with WT plants (Jiang et al., 2021). In poplar trees, overexpression of PeSHN1 enhances drought tolerance and WUE by increasing the wax content and altering wax monomer components (Meng et al., 2019). After long-term drought treatment, the wax content of MdPRP6-Ri plants was significantly higher than that of WT plants; the content of nonacosane C29 was significantly higher in transgenic lines than in WT plants (Figure 7). Nonacosane C29 is a significant component of plant epidermal waxes, and it acts as a hydrophobic barrier on the leaf surface, thereby reducing the rate of water loss and enhancing the WUE of plants under drought conditions. Guo et al. (2022) showed that the accumulation of C29 in the epidermal leads to the formation of a denser and more effective barrier against water loss. This is also supported by the findings of Xue et al. (2017), showing that the enhanced hydrophobicity provided by C29 and other long-chain hydrocarbons significantly reduces epidermal transpiration, which helps maintain the water balance during periods of limited water availability. Thus, a higher wax content, especially a higher C29 content, on the surface of MdPRP6-Ri transgenic leaves effectively reduces water loss due to non-stomatal factors, compensating for water loss from larger stomatal openings and enhancing plant adaptation to drought stress.
In conclusion, we investigated the role of MdPRP6 in the response of apple to long-term drought stress and found that the silencing of MdPRP6 significantly enhances drought adaptation in apple plants. Compared with WT plants, MdPRP6-Ri plants exhibited superior growth characteristics, higher photosynthetic efficiency, and lower ROS accumulation under drought conditions. The enhanced drought adaptation of MdPRP6-Ri plants is associated with increased antioxidant enzyme activity, larger stomatal openings, and elevated wax content on the leaf surfaces. The wax component nonacosane C29 plays a key role in improving the water retention of MdPRP6-Ri plants. Overall, our findings suggest that MdPRP6 effectively regulates the response to short-term and long-term drought stress and that MdPRP6 could be an important target in apple genetic breeding programs.
AUTHOR CONTRIBUTIONS
Data curation, Benzhou Zhao, Xiaoli Zhang and Xiaoqing Gong; Formal analysis, Qianwei Liu and Lin Luo; Funding acquisition, Xiaoqing Gong and Fengwang Ma; Methodology, Qianwei Liu and Xiaoli Zhang; Supervision, Xiaoqing Gong and Fengwang Ma; Writing-original draft, Benzhou Zhao and Xiaoqing Gong; Writing revising and editing, Benzhou Zhao, Hui Zhou, and Xiaoqing Gong.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (32372648, 32472667), the Natural Science Basic Research Program of Shaanxi (2023-JC-YB-170), and the earmarked fund for the China Agricultural Research System (CARS-27). The authors are grateful to Dr. Zhihong Zhang from Shenyang Agricultural University for providing tissue-cultured GL-3 plants and Dr. Jing Zhang from the Horticulture Science Research Center of our college for providing professional technical assistance in this work.
CONFLICT OF INTEREST STATEMENT
The authors declare that there are no conflicts of interest.
DATA AVAILABLILITY STATEMENT
Data sharing is not applicable to this article as all new created data is already contained within this article.




