Halophyte-based crop managements induce biochemical, metabolomic and proteomic changes in tomato plants under saline conditions
Abstract
Halophytes display distinctive physiological mechanisms that enable their survival and growth under extreme saline conditions. This makes them potential candidates for their use in saline agriculture. In this research, tomato (Solanum lycopersium Mill.) was cultivated in moderately saline conditions under two different managements involving Arthrocaulon macrostachyum L., a salt accumulator shrub: intercropping, i.e., co-cultivation of tomato/halophyte; and crop rotation, in which tomato is grown where the halophyte was previously cultivated. The effect of these crop managements was evaluated in tomato plants in comparison with tomato in monoculture, with regards to physiological and biochemical variables and metabolomic and proteomic profiles.
Both halophyte-based managements reduced soil salinity. Crop rotation enhanced photosynthesis and protective mechanisms at the photosynthetic level. In addition, both crop managements altered the hormone profile and the antioxidant capacity, whereas a reactive oxygen species over-accumulation in leaf tissues indicated the establishment of a controlled mild oxidative stress. However, tomato production remained unchanged.
Metabolomic and proteomic approaches suggest complex interactions at the leaf level, driven by the influence of the halophyte. In this regard, an interplay of ROS/lipid-based signalling pathways is proposed. Moreover, improved photosynthesis under crop rotation was associated with accumulation of sugar metabolism-related compounds and photosynthesis-related proteins. Likewise, acylamino acid-releasing enzymes, a class of serine-proteases, remarkably increased under both halophyte-based managements, which may act to modulate the antioxidant capacity of tomato plants.
In summary, this work reveals common and distinctive patterns in tomato under intercropping and crop rotation conditions with the halophyte, supporting the use of A. macrostachyum in farming systems.
1 INTRODUCTION
Salinity is one of the most significant environmental challenges limiting plant productivity. Around 830 million hectares are affected by salinity worldwide (source: FAO 2021), covering 20% of global cultivable land (Acosta-Motos et al. 2017). The continuous growth of the population, especially in developing countries, and the need to increase food production are major challenges for the coming years. By the middle of this century, when the population will reach 9.7 billion, it is estimated that there will be less water resources available but also less arable land, mainly due to soil salinization and soil degradation (Ben Hamed et al. 2021). This situation is already forcing farmers to use groundwater, which contains salts, and inevitably to cultivate in areas affected by salinity. Thus, the use of salt-tolerant plants, such as halophytes, may provide an important alternative for many developing countries in order to produce food and fodder (Ben Hamed et al. 2021; Custódio et al. 2023).
Halophytes can complete their life cycle in the presence of high NaCl concentrations (300–500 mM) thanks to the development of adaptation mechanisms of morphological, anatomical and biochemical types (Acosta-Motos et al. 2017; Ben Hamed et al. 2021), the latter including ion homeostasis, osmolarity regulation and up-regulation of antioxidant defences (Ozgur et al. 2013; Bose et al. 2014).
Different investigators have used halophyte plants to improve the production of plants of agronomic interest using two crop management strategies: intercropping (co-cultivation of the halophyte with the crop plant of interest) and crop rotation (crop cultivation sequentially on the same plot where a halophyte has been previously grown). In that regard, intercropping has been successfully used to improve the production of watermelon, tomato and strawberry under moderate saline conditions (Albaho and Green 2000; Simpson et al. 2018; Karakas et al. 2021; Jurado-Mañogil et al. 2023; Jurado et al. 2024). On the other hand, halophyte-based crop rotation has been scarcely implemented (Rabhi et al. 2010; Barcia-Piedras et al. 2019). In addition, the introduction of halophytes into agricultural systems must be economically profitable for farmers, with halophyte desalting ability and biomass valorization being of prime importance in this regard (Ben Hamed et al. 2021; Hasnain et al. 2023).
Nowadays, different halophytes species are used as animal forage but also as food industry by-products and sources for medicinal and nutraceutical compounds. Moreover, halophytes such as Salicornia spp., Arthrocaulon macrostachyum L., Cakile maritima, and Capparis spinosa L., among others, contain edible parts appreciated in gourmet cuisine (Barreira et al. 2017; Hasnain et al. 2023). In this sense, halophytes are a good source of polyunsaturated fatty acids, antioxidants and minerals (Barreira et al. 2017). Therefore, there is still much scope for improving knowledge on halophyte integration (cultivation practices and possible product transformation/use) into a sustainable production system.
Tomato (Solanum lycopersium Mill.) is one of the most cultivated vegetable crops in the Mediterranean area. The global tomato production in 2021 was 189 million metric tons, with Mediterranean countries accounting for 20% of the total (source: FAOSTAT 2021). Tomato is classified as moderately sensitive to salinity, which could result in reduced crop yields in soils with an electrical conductivity (EC) over 2.5 dS m−1 (Hanson and May 2004). In this work, we have applied two crop management strategies, intercropping and crop rotation, between the halophyte A. macrostachyum L., a salt accumulator C3 shrub, and tomato plants in moderately saline conditions, analysing their effect on soil salinity. In tomato plants, we investigated the effect of these crop managements at physiological and biochemical levels (including Na+ and Cl− contents, chlorophyll fluorescence, antioxidant metabolism-related parameters, and hormone profile), and at metabolomic and proteomic levels. Finally, in tomato plants, fruit production and quality were evaluated.
2 MATERIALS AND METHODS
2.1 Plant material and sampling
Field trials were conducted in the greenhouse facilities at the Agricultural Demonstration Center “La Pilica” (Aguilas, Murcia, Spain) (37.416253, −1.592437) from 21st March 2022 to 20th July 2022. The irrigation water obtained from the desalination plant of Águilas/Guadalentín (Murcia, Spain) had an EC of 0.3–0.5 dS m−1. Additionally, starting on 4th April, 4.5 L 30 mM NaCl per plant was added every 14 days. At the onset of trial implementation, concentrations in soil of Na+ and Cl− were 2.94 and 1.64 g Kg−1, respectively.
Sixty-five days old tomato plants (var. Scatolone 2), provided by the farm association “Coáguilas SCL” (Águilas, Murcia, Spain), and four months old A. macrostachyum L. plants, obtained from a local plant nursery (“Viveros Muzalé”, Abanilla, Murcia, Spain), were used. Plots were arranged in a randomised block design with three replicates. The experimental design included three types of plots: tomato in monoculture (TM), tomato in mixed cultivation with halophyte (TH), and tomato under crop rotation (TR). Each plot consisted of a 10 m length row with 13 tomato plants. Additionally, 26 halophyte plants per plot were transplanted either simultaneously in TH – distributed at both sides of the tomato plant – or six months before tomato cultivation in TR. Two drippers per meter were arranged to provide ferti-irrigation according to the commercial production practices of “Coáguilas SCL”.
For the different analyses, fully expanded tomato leaves of the third and fourth nodes from the apex of the main stem were used. Samples were taken before fruit harvesting (June 2022, 68 days after NaCl application started); except for Na+ and Cl− levels determination, these samples (leaves, roots and soil) were taken at the end of the experiment (121 days after planting, DAP). For additional analyses of antioxidant metabolism, hormones and -omics, plant samples were snap-frozen in liquid nitrogen and stored at −80°C until use. Tomato fruits were harvested at 86, 105 and 121 DAP.
2.2 Na+ and Cl− content
Soil samples were taken at 20 to 30 cm depth and at a distance of 20 cm from the closest halophyte and tomato plants by using an auger (5 cm diameter) and dried at room temperature for 48 h. Plant material was properly washed with tap water, followed by three washes with distilled water. Then, plant material was dried at 60°C for 4 days, and the dry material was ground into powder using a mill. Leaf and root powder, as well as soil samples, were filtered through a sieve. Na+ and Cl− contents were analyzed as previously described (Jurado et al. 2024).
2.3 Chlorophyll Fluorescence determination
Chlorophyll fluorescence measurements were performed in dark-adapted leaves between 9:00 and 11:00 hours (GMT), using a portable modulated chlorophyll fluorimeter (FMS2, Hansatech Instruments). The chlorophyll fluorescence parameters [maximum quantum efficiency of PSII photochemistry (Fv/Fm), quantum efficiency of PSII [Y(PSII)], photochemical quenching coefficient (qP), non-photochemical quenching (NPQ) and its coefficient (qN), and electron transport rate (ETR)] were determined as described in Jurado et al. (2024).
2.4 Antioxidant metabolism-related parameters
Lipid peroxidation was estimated by determining the concentration of thiobarbituric acid-reactive substances (TBARS), as previously described (Cantabella et al. 2017). Superoxide anion radical (O2.-) and hydrogen peroxide (H2O2) accumulation were performed by incubating tomato leaves with 0.1 mg mL−1 nitroblue tetrazolium and 0.1 mg mL−1 3,3′-diaminobenzidine, respectively (Hernandez et al. 2001). Then, chlorophyll was removed by incubating with 70% ethanol at 65°C and photographs were taken with an Olympus BX40 microscope (Olympus Medical Systems Corp.). Enzymatic antioxidants were determined in leaf samples as previously described (Cantabella et al. 2017; Jurado et al. 2024).
2.5 Hormone analysis
Plant hormone analysis was performed using 30 mg of lyophilized leaf material at the Plant Hormones Quantification Platform (IBMCP, Valencia, Spain). The hormones extraction, analysis [using a Q-Exactive mass spectrometer (Orbitrap detector; ThermoFisher Scientific)] and quantification were performed as previously described (Hernández et al. 2021a, 2021b).
2.6 Metabolomic approach and data analysis
Four samples of TM, five samples of TH and five samples of TR were analysed using a metabolomic approach. The sample extraction (70 mg of freeze-dried material with 50% methanol in a ratio 1/20, w/v) and non-target metabolomics analyses (by an ultra-performance liquid chromatography–quadrupole-time-of-flight mass spectrometry) were performed as previously described (Jurado-Mañogil et al. 2023; Barba-Espín et al. 2024).
Metabolomics data were analysed using the MetaboAnalyst 5.0 software (https://www.metaboanalyst.ca) with data subjected to the following normalization: logarithmic (base 10) transformation and Pareto scaling. Then, a Principal Component Analysis (PCA) and a clustering dendrogram analysis were conducted. Putative metabolite identification was done on the top 25 variable features (m/z) found by a heatmap analysis (t-test/ANOVA; distance measure, Euclidean; clustering algorithm, Ward), with the identification based on their MS/MS spectra and using the Human Metabolome database (http://www.hmdb.ca/). In addition, a pairwise comparison by Volcano Plot analysis [fold change ≥2 and p-value ≤0.05) was also performed. Finally, the Mummichog algorithm (Li et al. 2013) was used in order to decipher the biological meaning of pairwise metabolomic comparisons (Jurado-Mañogil et al. 2023; Barba-Espín et al. 2024).
2.7 Shotgun proteomics
Twenty milligrams of freeze-dried leaf sample were extracted in 1.5 mL acetone containing 10% trichloroacetic acid (w/v) using a Retch mill, followed by sonication for 10 min and incubation overnight at −20°C. After centrifugation (15,000 g, 5 min), the supernatant was discarded, and the resulting pellet dried under air before resuspension in 800 μL SDS buffer (2% sodium dodecyl sulfate, w/v; 30% sucrose, w/v; 5% beta-mercaptoethanol, v/v; 5 mM EDTA, 100 mM TRIS, pH 8) for 15 min at 25 °C using an orbital shaker (800 rpm). Upon addition of 400 μL phenol (TRIS saturated), the samples were vortexed and centrifuged (15,000 g, 10 min). The phenolic phase was transferred to a new tube and precipitated overnight with 100 mM ammonium acetate (1.6 mL) dissolved in methanol. The supernatant was removed following centrifugation (5 min, 15,000 g, 4 °C), and the pellet was washed with 80% acetone. The dried pellet was resuspended for 60 min in 200 μL 8 M urea dissolved in 100 mM NH4HCO3 at 25°C using an orbital shaker (600 rpm). Total protein concentration was determined with Bradford assay, and aliquots containing 100 μg protein were transferred to Low Protein Binding tubes. Following cysteine alkylation, the samples were diluted with 50 mM NH4HCO3 containing 2.5% acetonitrile and digested with 1 μg trypsin at 29°C overnight. The samples were desalted using C18 columns and aliquots corresponding to 2.5 μg total peptides were analysed by nanoflow C18 reverse-phase liquid chromatography using a 15 cm column (Zorbax, Agilent Technologies), a Dionex Ultimate 3000 RSLC nano-UPLC system (Thermo Fisher Scientific) and Orbitrap Fusion Lumos Tribrid Mass Spectrometer (Thermo Fisher Scientific) as described previously (Hallmark et al. 2020). The resulting spectra were searched against the reference S. lycopersicum (cv. Heinz 1706) database using Proteome Discoverer 2.4 with the Sequest HT (Thermo Fisher Scientific) and MS Amanda (Dorfer et al. 2014) search engines using the following parameters: max two missed cleavage sites; modifications-carbamidomethyl (Cys) and up to three dynamic modifications including Met oxidation, Asn/Gln deamidation, N-terminal acetylation; MS1 tolerance-5 ppm (MS Amanda), 10 ppm (Sequest), MS2 tolerance—0.02 Da (MS Amanda), 0.1 Da (Sequest). Proteins with at least two unique peptides were considered for quantitative analysis.
2.8 Tomato fruit production and quality
Mature fruits from ten plants per crop management were harvested, and the number of fruits and mean fruit weight per plant were determined. The data of the three harvests – 86, 105 and 121 DAP – were added to calculate the total production per plant. The juice of twenty-five representative fruits from each crop management and harvest time point was analyzed in a PAL-BX/ACID refractometer (Atago Co. Ltd.) in order to determine the total soluble solid (TSS, expressed as°Bx) and the acidity (expressed as citric acid equivalents).
2.9 Statistical analysis
Except where indicated, at least five biological replicates per treatment were analyzed. Data were subjected to an analysis of variance (ANOVA), followed (when applicable) by Tukey's Multiple Range Test (p ≤ 0.05), using SPSS® for Windows (IBM Statistics, version 27).
3 RESULTS AND DISCUSSION
Salt-tolerant plants, such as halophytes, can be a feasible alternative for agriculture production in soils affected by salinity and water scarcity and may also provide economic revenue to the farmers. Crop management approaches using halophytes, such as those proposed in this work, aim for salinity alleviation in soils where salt limits crop production (Simpson et al. 2018). In this work, we used A. macrostachyum, whose desalting capacity and nutritional benefits for human health have been proven (Barreira et al. 2017; Barcia-Piedras et al. 2019).
3.1 Na+ and Cl− content and EC analysis
At the end of the experiment, samples of leaves and roots of tomato plants and soil were taken, and the levels of Na+ and Cl− were determined. TH produced a significant decline in soil Na+ content by 35%, whereas a significant decrease in Cl− occurred in TR (Table 1). The observed changes in Na+ and Cl− led to a decrease in the electrical conductivity of the soil, especially in TH samples (3.95, 2.90 and 3.10 dS m−1 for TM, TH and TR soils, respectively). This observed soil desalting capacity of A. macrostachyum is in agreement with that reported by other authors (Barcia-Piedras et al. 2019). Regarding plant tissues, the analysis of variance indicates that crop management significantly affected Na+ contents in roots and Cl− levels in leaves and roots. In this sense, the presence of the halophyte plants (TH) provoked a 25% decline in tomato root Na+, whereas leaf Na+ remained statistically unchanged (Table 1); together with the lower soil Na+ in TH, it can be suggested an enhanced Na+ accumulation in the halophyte (data not shown). In tomato leaves, TR contained 44% more Cl− than the other crop managements, while both TH and TR increased root Cl−, especially in TR plants, with a content 62% higher than in TM roots (Table 1). This behaviour for Na+ and Cl− is in agreement with that observed in tomato var. “Sargento” when cultivated under intercropping with A. macrostachyum; however, crop rotation led to contrasting results (Jurado et al. 2024). It is likely that the observed accumulation of Cl− in TH and TR plants contributes to cell osmotic adjustment, improving plant water uptake under saline conditions (Cui et al. 2020; Munns et al. 2020).
| Leaf | Na+ [g Kg−1] | Cl− [g Kg−1] |
|---|---|---|
| TM | 3.02 ± 0.26 | 9.35 ± 0.69b |
| TH | 3.44 ± 0.26 | 9.40 ± 0.28b |
| TR | 3.19 ± 0.40 | 13.49 ± 0.18a |
| F-values | 0.60 | 4.56* |
| Root | ||
| TM | 7.61 ± 0.50a | 12.49 ± 0.15b |
| TH | 5.80 ± 0.18b | 18.44 ± 0.15 a |
| TR | 7.81 ± 0.36a | 20.22 ± 0.13 a |
| F-values | 13.62** | 8.04** |
| Soil† | ||
| TM | 4.80 ± 0.47a (1.60) | 5.48 ± 0.03a (3.34) |
| TH | 3.11 ± 0.47b (1.06) | 4.57 ± 0.57ab (2.79) |
| TR | 3.63 ± 0.32ab (1.23) | 3.40 ± 0.22b (2.07) |
| F-values | 4.20* | 6.70* |
- Data represent the mean values ± SE of at least 5 different biological samples. Different letters indicate significant differences according the Tukey's Multiple Range Test (p ≤ 0.05). F-values significant at 99% (**) and 95% (*) level of probability. TM: tomato in monoculture; TH: tomato in intercropping; TR: tomato under crop rotation. † In soil data, the increase (fold change) respect to initial contents at the onset of the assay is shown in brackets.
3.2 Chlorophyll fluorescence
Fv/Fm value in dark-adapted leaves relates to the potential of quantum PSII efficiency, which is being used as an indicator of proper photosynthesis performance. Higher plants present values around 0.80–0.83, whereas lower values can be associated with stress-induced photo-inhibition (Maxwell and Johnson 2000). Although in TR, a slight decrease in the Fv/Fm compared with the other crop managements was observed, under our experimental conditions, Fv/Fm values in the three crop managements were above 0.8 (Supplemental Figure 1), which indicates that soil salinity is not affecting photosynthesis significantly.
Increased photochemical quenching values and ETR are related to the proper performance of the photosynthetic machinery. On the other hand, non-photochemical quenching is linked to the safe dissipation of excess light energy (Maxwell and Johnson 2000), which has also been associated with salt stress response (Acosta-Motos et al. 2015a, 2015b). In this work, crop rotation led to increases in the photochemical [qP, Y(II)] and non-photochemical (qN, NPQ) quenching parameters, as well as in ETR values, with respect to TM. On the other hand, TH plants only showed significant changes with respect to ETR, the values being intermediate between those observed for TM and TR (Supplemental Figure 1). These results are in contrast with those observed in the tomato var. “Sargento”, in which both intercropping and crop rotation with the halophyte increased photochemical and/or non-photochemical parameters (Jurado-Mañogil et al. 2023; Jurado et al. 2024). These contrasting results suggest that both the experimental conditions and the tomato variety used may influence photosynthesis performance.
3.3 Antioxidant metabolism
The extent of lipid peroxidation, as well as the activity of some antioxidant enzymes, were analyzed in leaves. Both halophyte-based crop managements increased monodehydroascorbate reductase (MDHAR) activity compared to TM plants, reaching this increase 2-fold in TR plants (Supplemental Figure 2). In this regard, overexpression of a MDHAR gene from the halophyte Avicennia marina led to increased MDHAR activity, which in turn enhanced salt tolerance in transgenic tobacco plants (Kavitha et al. 2010). According to the sum of the MDHAR and dehydroascorbate (DHAR) activities (data not shown), TR leaves presented a higher ascorbate-recycling activity than the other crop management. Moreover, the contribution of the MDHAR activity to the sum MDHAR + DHAR was above 70% for both halophyte crop managements. This response implies that ascorbate is predominantly recycled by the MDHAR pathway, which is much more efficient energetically than the DHAR pathway. In this regard, it can be suggested that TR plants developed an improved ascorbate recycling ability, as reported recently on the tomato var. “Sargento” (Jurado et al. 2024). In addition, MDHAR activity has been linked to salinity tolerance in other plant species (Acosta-Motos et al. 2015a, 2015b; Cantabella et al. 2017).
On the other hand, TR produced a remarkable increase in catalase (CAT) activity of about 66% in relation to the other crop managements (Supplemental Figure 2), suggesting a more efficient elimination of the H2O2 produced by photorespiration. The involvement of photorespiration in the salt-tolerance response of higher plants has been suggested by different authors (Corpas et al. 1993; Acosta-Motos et al. 2015a, 2015b). In addition, a correlation between CAT activity and photosynthesis has been described since the increase in CAT activity reduced the photorespiratory loss of CO2 (Brisson et al. 1998). Thus, the increase in CAT activity in TR plants could be related to the observed increase in the photochemical and non-photochemical quenching parameters (Supplemental Figure 1). For the rest of the antioxidant enzymes activities analyzed – ascorbate peroxidase (APX), glutathione reductase, superoxide dismutase (SOD) and peroxidase (POX) – no significant differences were found among crop managements, neither for the levels of lipid peroxidation (data not shown).
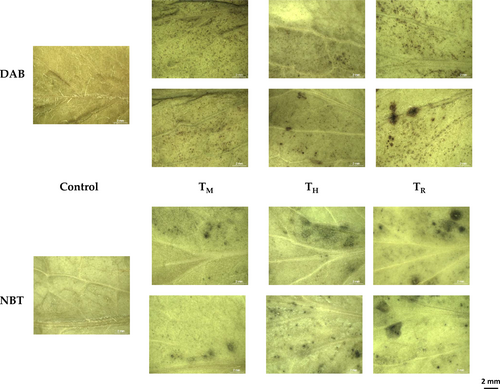
Concerning histochemical staining (Figure 1), O2.- and H2O2 over-accumulation in TH and TR leaf tissues suggests the establishment of mild oxidative stress in tomato plants induced by halophyte-based crop management. The oxidative stress could favour adaptive responses in tomato plants under saline conditions. These accumulation patterns may correlate with the higher CAT and MDHAR activities observed in TR and in TH and TR, respectively, and may support the occurrence of a controlled mild oxidative stress in the halophyte-mediated crop managements, as previously reported in the tomato var. “Sargento” (Jurado-Mañogil et al. 2023; Jurado et al. 2024). In this sense, it may be suggested that the modulation of the tomato plant physiology can be mediated by allelopathic interactions between the tomato plant and the halophyte, driven by one or more biochemicals produced by the halophyte that influence the physiology of the tomato plant. In this regard, the allelopathic potential of A. macrostachyum has been reported (Mohamed et al. 2020). In crop rotation management, allelopathy has been widely documented in weed control, by which allelopathic crops release allelochemicals through roots or via the decomposition of crop residue to suppress weeds and other pests (Khamare et al. 2022). In addition, it can be argued that one of the defence mechanisms associated with salinity tolerance is improved antioxidant properties. Moreover, a strong correlation between the antioxidant activity of plants and their potential allelopathic activity has been found (Aniya et al. 2022). In this context, we may hypothesize that the interaction of halophyte/tomato is driven by the stimulation of the antioxidant system.
3.4 Hormone profile
The introduction of halophytes in the crop system modified the levels of some plant hormones in tomato leaves. Regarding the stress hormones, intercropping slightly increased salicylic acid (SA) content, whereas crop rotation increased jasmonic acid (JA) levels by 50% and 43% compared to monoculture and intercropping, respectively (Figure 2A). It is well known that SA plays an important role in the response to abiotic stresses such as drought, chilling and saline stress (Takatsuji and Jiang 2014; Janda et al. 2020). The application of exogenous SA improved ion homeostasis and photosynthesis rate and decreased H2O2 accumulation in leaves of salt-stressed Egletes viscosa plants (Batista et al. 2019). However, the information about the effect of salinity on JA levels is scarce (Acosta-Motos et al. 2016). The basal levels of JA in shoots and roots were found to be much higher in a salt-tolerant tomato variety than in a salt-sensitive variety, both in the absence and in the presence of NaCl stress (Pedranzani et al. 2003). In addition, the exogenous application of JA or methyljasmonate to barley plants before salinization improved growth and photosynthetic performance as well as the chlorophyll fluorescence parameters qP and Y(II) (Tsonev et al. 1998; Sirhindi et al. 2020). In the present work, we also observed a correlation between JA levels (Figure 2A) and photosynthesis performance (Figure S1) in TR plants. On the other hand, the presence of the halophyte increased SA contents in TH plants regarding TR management (Figure 2A). It seems that SA and JA have antagonistic interactions, and the SA/JA ratio has been suggested as a salt stress marker (Gupta et al. 2000; Acosta-Motos et al. 2016). A concomitant increase in SA/JA ratio along with salinity levels was reported in myrtle plants (Acosta-Motos et al. 2016). In this work, crop rotation led to a lower SA/JA ratio in tomato leaves (data not shown), which could be related to a better plant performance in TR than in the other crop managements.
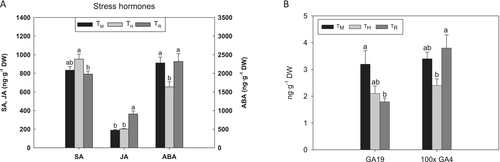
On the other hand, abscisic acid (ABA) levels significantly decreased in TH leaves in relation to the other crop managements (Figure 2A). ABA is an important player in the response of the plant to stresses, acting as a signalling molecule allowing plants to cope with salinity (Keskin et al. 2010). The drop in ABA in TH plants was accompanied by an increase in SA. This opposite behaviour between ABA and SA has been previously reported in Brassica napus under stress conditions (Park et al. 2021). In addition, treatments with SA reduced ABA levels in salt-stressed maize plants (Elhakem 2020).
Regarding gibberellins, under our experimental conditions, we detected GA19, a GA1 precursor, and GA4, the levels of the latter being much lower than those of GA19 (Figure 2B). Compared with the stress hormones, the GA contents were very low (Figure 2). The GA19 content decreased in TH and TR in relation to TM, whereas no important changes were observed for GA4 (Figure 2B). It has been reported that the Arabidopsis mutant line ga1-3, which shows low GA contents, displayed enhanced survival under salt stress (Achard et al. 2006). As a consequence, a correlation between lower GAs levels and better plant performance of tomato plants under saline conditions can be suggested. Taking together ABA and GAs levels, leaves of TR plants displayed a higher ABA/GAs ratio than the other crop management (data not shown). In this sense, it has been suggested that enhanced salinity tolerance is related to increased ABA/GAs ratio (Colebrook et al. 2014).
3.5 Metabolomic approach
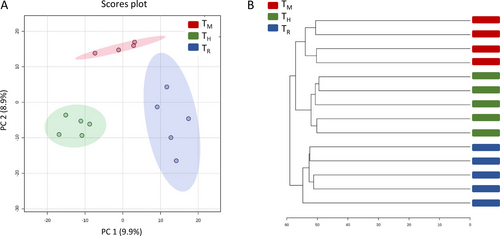
The metabolomic fingerprints in tomato leaf samples taken under different crop management were studied. PCA and clustering dendrogram analysis provided a clear segregation among group samples (TM, TH and TR) (Figure 3). The latter analysis revealed two main clusters, TM and TH samples, being grouped in one cluster (Figure 3b). These results were somewhat different to those previously reported in the tomato var. “Sargento” under intercropping conditions, in which no segregation was found between monoculture and intercropped tomato samples, although it must be taken into account that in that study, samples from crop rotation were not included (Jurado-Mañogil et al. 2023).
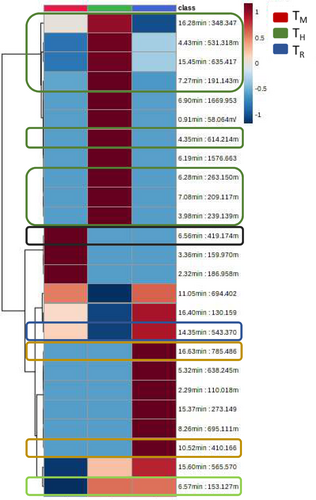
The MS/MS spectra identification of the top 25 variable mass features displayed by the heatmap analysis (Figure 4) revealed that most of the putatively identified metabolites (a total of 13) were lipid and lipid-related metabolites (Table 2). In TH, the accumulation of several fatty acyl and acyl-lipids was observed (Table 2). In plants, acyl-lipids act mainly as membrane components, carbon and energy storage forms, and signalling transduction players (Li-Beisson et al. 2013; Cahoon and Li-Beisson 2020). Likewise, as plant cuticle components, acyl-lipids play important roles in plant water loss prevention and under environmental stress conditions, thus also being important components of plant adaptation mechanisms to the surrounding environment (Li-Beisson et al. 2013; Cahoon and Li-Beisson 2020). On the other hand, a metabolite identified as diacylglycerol, which is involved in plant lipid metabolism and membrane remodelling (Dong et al. 2012), was found to be downregulated by TH conditions (Table 2). Previously, we described a decrease in diacylglycerol in A. macrostachyum plants grown under intercropping conditions but not in tomato plants (Jurado-Mañogil et al. 2023). Intercropping-induced changes in lipid composition differ depending on the tomato cultivar studied, which may affect lipid metabolism and lipid-based signalling in different manners (Dong et al. 2012; Ruelland et al. 2015; Pohl and Jovanovic 2019); this may converge in reactive oxygen species (ROS)- and lipid-based signalling pathways interplay (Ruelland et al. 2015), as suggested by the induction of moderate oxidative stress in the tomato var. “Sargento” (Jurado-Mañogil et al. 2023) and in the present study.
| m/z | Putative Identification (chemical class) | Molecular formula | Adduct | ΔPPM |
|---|---|---|---|---|
| Upregulated by TH | ||||
| 348.3466 | (15Z)-tetracosenoate (fatty acyl) | C24H45O2 | M + H-H2O | 20 |
| 531.3180 | Kukoamine D (catechol) | C28H42N4O6 | M + H | 1 |
| 635.4161 | Soyasapogenol B 3-O-b-D-glucuronide (triterpene saponin) | C36H58O9 | M + H | 1 |
| 191.1430 | trideca-5,8,11-trienoic acid (fatty acyl) | C13H20O2 | M + H-H2O | 3 |
| 614.2148 | 4-Hydroxyestrone-2-S-glutathione (fatty acyl) | C28H37N3O9S | M + Na | 1 |
| 263.1484 | Phaseolic acid (keto acid) | C12H22O6 | M + H | 2 |
| 209.1165 | Dodeca-2,4-dienedioic acid (fatty acid) | C12H18O4 | M + H-H2O | 6 |
| 239.1386 | 1,2-Dehydro-alpha-cyperone (sesquiterpenoid) | C15H20O | M + Na | 9 |
| Downregulated by TH | ||||
| 543.3698 | Diacylglycerol (glycerolipid) | C33H52O7 | M + H-H2O | 2 |
| Upregulated by TM | ||||
| 419.1737 | Lirioresinol A (furanoid lignan) | C22H26O8 | M + H | 9 |
| Upregulated by TR | ||||
| 785.4842 | Oxidized phosphatidic acid (fatty acid) | C45H69O9P | M + H | 11 |
| 410.1682 | Linustatin (cyanogenic glycoside) | C16H27NO11 | M + H | 6 |
| Upregulated by TH and TR | ||||
| 153.1269 | cis-Sabinol (monoterpenoid) | C10H16O | M + H | 3 |
Among the lipid-related metabolites, TH and TR leaves accumulated some terpene and terpenoid compounds (Table 2). These classes of compounds are present in all living organisms, with plants exhibiting an unusually high number (Pichersky and Raguso 2018). In plants, terpenoids have myriad functions, playing key roles in biotic and abiotic interactions and in the modulation of ROS signalling (Pichersky and Raguso 2018; Boncan et al. 2020). In addition, terpenes and terpenoids have many implications for human health and in different industries (pharmaceutical, food, chemical and biofuel production). Skin UV protection, as well as anti-tumour, -inflammatory and -microbial effects, are among the medicinal and nutraceutical properties attributed to terpenes/terpenoids (Jahangeer et al. 2021). It is well reported that moderate stresses that do not compromise plant cell integrity and functionality increase the content of plant specialized secondary metabolites (da Silva Magedans et al. 2021). In the present study, tomato plants were subjected to moderate salt stress, so the observed increase in terpenes/terpenoids may be related to salt-mediated induction of the secondary metabolism rather than an effect of crop management. In contrast, in a previous study, the intercropping between the tomato var. “Sargento” and the halophyte led to a decrease in the content of terpenes/terpenoids (Jurado-Mañogil et al. 2023). Moreover, an alteration of antioxidant enzyme activity levels (Figure S2) and ROS accumulation (Figure 1) by both halophyte-based crop managements suggests the occurrence of a controlled moderate oxidative stress in tomato plants. Taking into account that, under stress conditions, terpenoids may contribute to ROS signalling by modulating membrane physicochemical properties (Blande et al. 2014; Sewelam et al. 2016; Boncan et al. 2020), a correlation between terpenes/terpenoids and oxidative signalling induced by the crop managements can be suggested.
Kukoamine accumulated in TH plants. Kukoamines are dihydrocaffeic acid derivatives of polyamines (putrescine, spermidine and spermine). These specialized secondary metabolites have been found in plant species of the Solanaceae family, including tomato, and have attracted attention due to their bioactive properties (Li et al. 2015). Different metabolomics studies in plants have revealed that the biosynthesis of polyamines, as well as other compounds such as sugars, aminoacids, organic acids and phytohormones, are commonly altered (Rajkumari et al. 2023). Moreover, it has been reported that the maintenance of polyamines biosynthesis under salinity conditions contributes to increasing the antioxidant capability as well as to the protection of the photosynthetic machinery from oxidative damage (Ikbal et al. 2014).
The Volcano Plot analysis showed a similar fluctuation in the number of significantly affected metabolites by both intercropping (34 downregulated and 44 upregulated) and crop rotation (33 downregulated and 41 upregulated) conditions compared with plants in monoculture (Figures S3a,b). However, a greater number of significant differential mass features were observed when TH and TR were compared, with 65 metabolites downregulated and 95 upregulated in the comparison intercropping/crop rotation (Figure S3c).
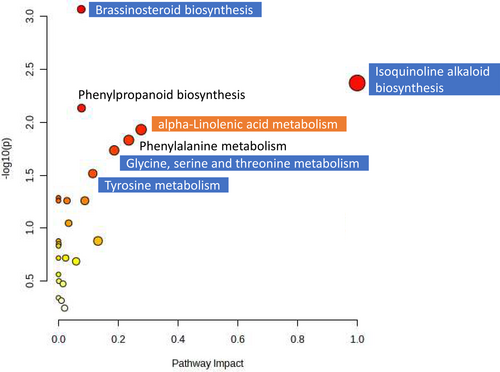
The Mummichog algorithm (Li et al. 2013) allowed the identification of metabolic pathways altered by halophyte-mediated crop management. Compared with control plants, in TH plants, different amino acids-related pathways were affected (Figure 5 and Table S1), whereas the crop rotation led to an alteration of some amino acids metabolism pathways and to an increase in the abundance of different compounds related to sugar metabolism (Figure 6 and Table S2). The alteration of sugar metabolism in plants has been described as a common response under different stress conditions, affecting the production of secondary metabolites (Boonchaisri et al. 2021). Taking into account that photosynthesis and CO2 fixation are closely related to sugar metabolism, the increase in some compounds related to sugar metabolism in TR may be linked to the increased levels of both photochemical [qP, Y(II)] and non-photochemical (qN, NPQ) quenching parameters (Figure S1). A similar response in terms of better photosynthesis performance and the induction of sugar metabolism was found in the tomato var. “Sargento” under intercropping conditions (Jurado-Mañogil et al. 2023).
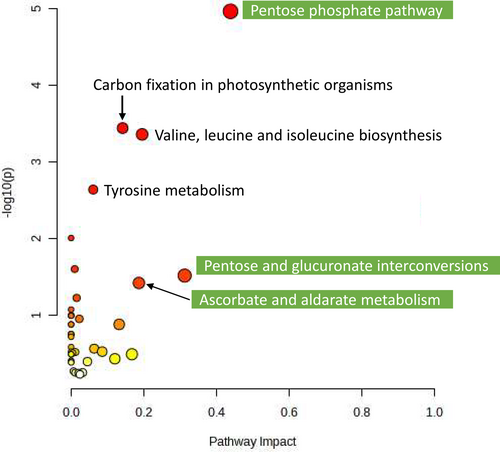
Different sugars accumulate during salinity in both the early and late phases of the stress response, acting as osmolytes or as energy suppliers (Rajkumari et al. 2023). An increase in glucose, as well as its conversion to other organic compounds, has been described as an important trait in salt-tolerant rice genotypes (Ma et al. 2018). Moreover, carbohydrate accumulation affected the activity of different H2O2-related antioxidant enzymes such as SOD, POX and CAT activities in rice (Rahman et al. 2017). In this sense, in TR plants, the increase in the abundance of sugar metabolism-related compounds was accompanied by altered levels of antioxidant enzymes, including a strong increase in CAT activity (Figure S2). In addition, in the tomato var. “Sargento”, both intercropping and crop rotation conditions increased the activity of the H2O2-scavenging enzyme APX (Jurado et al. 2024), also displaying in intercropped tomato plants a stimulation of sugar metabolism (Jurado-Mañogil et al. 2023).
Among the amino acid-related pathways, “Tyrosine metabolism” was affected in both TH and TR plants (Figures 5 and 6; Tables S1 and S2). It is important to note that key compounds for plant survival (including tocopherols, plastoquinone, and ubiquinone), as well as a large variety of plant metabolites (including isoquinoline alkaloids), are tyrosine-derived compounds (Xu et al. 2020). In transgenic tomato plants expressing a stilbene-synthase gene, “Tyrosine metabolism” was also affected in both leaves and fruits (Barba-Espín et al. 2024). Changes in amino acid accumulation have also been reported in different rice genotypes in response to salt stress, with increases in specific amino acids linked with both cellular signalling and structural processes (Rajkumari et al. 2023). In addition, the “alpha-linolenic acid metabolism”, involved in the biosynthesis of the stress-related phytohormone jasmonic acid, was also stimulated in TH plants (Figures 5 and 7; Tables S1 and S3), although an increase in JA was only observed in TR leaves (Figure 2). In a salt-tolerant rice variety, an accumulation of JA was also observed under salt stress conditions, this response being correlated with impaired root growth and reduced sodium translocation to shoots as well as with an increased antioxidant capacity (Rajkumari et al. 2023).
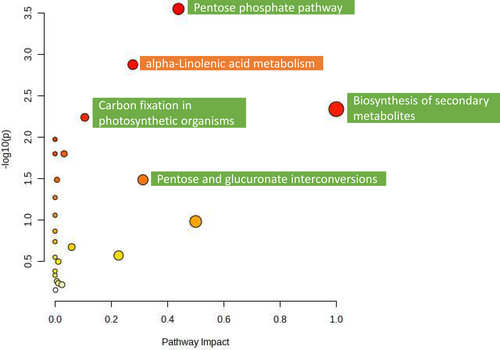
The comparison between TH and TR plants allowed the identification of similar affected pathways to those observed in the crop management pairwise comparison with TM, since a stimulation of “alpha-Linolenic acid metabolism” in TH and of sugar metabolism in TR was observed (Figure 7 and Table S3).
3.6 Proteomic approach
We queried the effect of the halophyte-based crop management systems on global protein abundances using a label-free quantitative shotgun proteomics approach. More than 2,600 proteins characterized by at least two unique peptides were identified and subjected to statistical analysis. Both halophyte-based crop management systems had a significant impact on the plant proteome in comparison to monoculture conditions (Figure 8). While in the metabolomic analysis TM and TH samples match within the same cluster (Figure 3A), the proteomic analysis revealed that TH and TR proteome profiles were closely related, with similar patterns compared to TM (Figure 8B). In TH plants 65 proteins accumulated (FC >2, p ≤ 0.05), whereas the abundances of 27 proteins decreased (FC >2, p ≤ 0.05) compared to plants grown in monoculture. Crop rotation resulted in the over-accumulation of 62 and a decrease of 55 proteins (FC >2, p ≤ 0.05). Most of them were similarly affected by both halophyte-based crop management practices (Figure 8B). Among the proteins with increased abundance, Solyc09g083120.3.1 accumulated 6.2- and 9.2-fold in TH and TR, respectively (Tables 3 and 4). Intriguingly, its Arabidopsis ortholog ACYLAMINO ACID-RELEASING ENZYME (AARE) plays an important role in maintaining the cytoplasmic antioxidant system (Nakai et al. 2012). The accumulation of this protein could be related to the increase in MDHAR and the accumulation of ROS observed in TH and TR, as well as to the high CAT activity recorded in TR leaves (Figure S2). Moreover, the suggested occurrence of controlled mild oxidative stress in tomato plants induced by halophyte-based crop management may trigger the accumulation of AARE.

| Accession | Description | Fold change | p value | Arabidopsis homolog (s) |
|---|---|---|---|---|
| Upregulated by TH | ||||
| Solyc07g007120.3.1 | Class II knotted-like homeodomain protein | 100.00 | 1.20E-03 | AT5G25220 |
| Solyc03g118640.3.1 | Ketose-bisphosphate aldolase class-II family protein | 7.11 | 7.18E-03 | AT1G18270 |
| Solyc01g111700.2.1 | 26S proteasome non-ATPase regulatory subunit 3 | 6.63 | 6.69E-03 | AT1G20200 AT1G75990 |
| Solyc09g083120.3.1 | Acylamino-acid-releasing enzyme | 6.24 | 6.96E-03 | AT4G14570 |
| Solyc11g065600.2.1 | Xyloglucan endotransglucosylase-hydrolase 4 | 5.70 | 1.39E-03 | AT5G13870 |
| Solyc02g072105.1.1 | Dihydroorotase | 4.99 | 6.93E-03 | AT4G22930 |
| Solyc01g007920.2.1 | Isochorismatase-like | 4.89 | 1.56E-02 | AT5G23230 |
| Solyc02g068540.3.1 | Leucine-rich repeat receptor protein kinase EMS1-like | 4.83 | 1.45E-02 | AT2G34930 |
| Solyc05g052140.4.1 | ATP synthase subunit delta’, mitochondrial | 4.68 | 4.52E-03 | AT5G47030 |
| Solyc01g111520.3.1 | Calcium-dependent lipid-binding (CaLB domain) family protein | 4.50 | 5.38E-03 | AT2G20990 AT1G20080 |
| Downregulated by TH | ||||
| Solyc05g013720.3.1 | Alpha-galactosidase | 0.11 | 3.78E-02 | AT3G26380 |
| Solyc05g012620.4.1 | uvrB/uvrC motif-containing protein | 0.13 | 6.40E-03 | AT2G03390 |
| Solyc10g076290.2.1 | F-box/LRR-repeat protein 3 | 0.14 | 1.40E-02 | AT5G01720 |
| Solyc08g016670.3.1 | Calcyclin-binding protein-like | 0.19 | 1.42E-02 | AT1G30070 |
| Solyc05g055430.5.1 | RAP release 2, galactose-binding-like domain protein, putative | 0.22 | 2.56E-03 | AT5G04440 |
| Solyc09g090570.2.1 | Proton gradient regulation 5 | 0.25 | 2.86E-02 | AT2G05620 |
| Solyc01g111480.2.1 | Acetylornithine aminotransferase | 0.26 | 4.05E-02 | NA |
| Solyc12g009060.2.1 | Vacuolar sorting-associated protein 2-like | 0.26 | 5.77E-03 | AT2G06530 |
| Solyc04g015750.3.1 | CobN/magnesium chelatase | 0.32 | 4.08E-02 | AT5G13630 |
| Solyc09g084470.3.1 | Proteinase inhibitor.1 | 0.34 | 3.00E-02 | AT3G46860 AT2G38900 |
| Accession | Description | Fold change | p value | Arabidopsis homolog (s) |
|---|---|---|---|---|
| Upregulated by TR | ||||
| Solyc01g111400.5.1 | Subtilisin-like protease-like protein | 22.93 | 4.67E-02 | AT4G34980 |
| Solyc03g118640.3.1 | Ketose-bisphosphate aldolase class-II family protein | 19.25 | 2.75E-07 | AT1G18270 |
| Solyc09g083120.3.1 | Acylamino-acid-releasing enzyme | 9.16 | 5.47E-08 | AT4G14570 |
| Solyc04g007960.4.1 | Caleosin | 7.60 | 5.80E-05 | AT1G70680 AT1G23240 AT1G70670 |
| Solyc01g111700.2.1 | 26S proteasome non-ATPase regulatory subunit 3 | 7.54 | 5.34E-06 | AT1G20200 |
| Solyc03g083430.3.1 | Splicing factor SF3a60 homolog | 6.88 | 1.51E-05 | AT5G06160 |
| Solyc05g052140.4.1 | ATP synthase subunit delta | 6.63 | 7.74E-10 | AT5G47030 |
| Solyc09g075700.1.1 | Alpha/beta-Hydrolases superfamily protein | 6.49 | 3.84E-02 | AT3G48690 |
| Solyc03g082440.1.1 | Seven transmembrane receptor | 5.59 | 1.10E-06 | AT5G42090 |
| Solyc03g034180.3.1 | 14–3-3-like protein | 5.46 | 4.16E-03 | AT5G38480 |
| Downregulated by TR | ||||
| Solyc10g045390.2.1 | Histone acetyltransferase GCN5 | 0.01 | 1.58E-01 | AT3G54610 |
| Solyc06g071470.4.1 | Peroxisomal membrane protein PEX14 | 0.01 | 1.26E-02 | AT5G62810 |
| Solyc04g072030.1.1 | Dynamin-related protein 4C | 0.01 | 2.32E-02 | AT1G60500 |
| Solyc04g081440.3.1 | Beta-fructofuranosidase | 0.01 | 2.12E-02 | AT4G34860 |
| Solyc09g065755.1.1 | Histone H2A | 0.10 | 4.14E-02 | AT3G54560 |
| Solyc05g013720.3.1 | Alpha-galactosidase | 0.12 | 2.03E-02 | AT3G26380 |
| Solyc01g106360.3.1 | Fatty acid amide hydrolase | 0.14 | 3.29E-02 | AT5G64440 |
| Solyc08g080110.3.1 | FAM63A-like protein | 0.17 | 4.02E-02 | AT4G11860 |
| Solyc12g095800.2.1 | Chaperone protein dnaJ 10 | 0.18 | 4.39E-02 | AT1G21080 AT1G76700 |
| Solyc12g011270.2.1 | Ethylene-responsive elongation factor EF-Ts precursor | 0.20 | 1.51E-02 | AT4G11120 |
Regarding proteins with decreased expression in both crop managements, we found the F-box protein Solyc10g076290.2.1, whose levels in TH and TR were repressed 7.2 and 3.7-fold, respectively (Table 3; File S1). Their two Arabidopsis orthologs, RAE1 and RAH1, regulate the stability of the C2H2-type zinc finger transcription factor STOP1 via ubiquitin-directed degradation (Fang et al. 2021). STOP1 is an important regulator of proton and aluminium rhizotoxicity and is also required for root system architecture alterations in response to nitrate deficiency (Tian et al. 2021; Tokizawa et al. 2023). In Arabidopsis plants subjected to salt stress (200 mM NaCl), within the leaf transcriptome analysis, the STOP1 gene was upregulated (Alotaibi and Abulfaraj 2023); thus, the downregulation of Solyc10g076290.2.1 by TH and TR suggests an amelioration of salt stress by the halophyte-based crop management.
In contrast to proteins whose abundances either increased or decreased in both crop management practices, several proteins were only affected in one of the conditions relative to plants in monoculture (File S1). For example, the levels of cryptochrome 1b (Solyc12g057040.2.1) increased 2.2 times in TR compared to TM, whereas its abundance was not affected significantly under intercropping conditions (File S1). The orthologs of cryptochrome 1b in Arabidopsis are the blue light photoreceptors CRY1 and CRY2, which are crucial players in plant developmental and stress programs. In Arabidopsis, the overexpression of CRY genes confers hypersensitivity to ABA and salinity (Xu et al. 2009; Zhou et al. 2018), whereas the cry1 mutant displayed more salt-stress tolerance than wild type plants (D'Amico-Damião and Carvalho 2018). Similarly, the levels of the chloroplastic protease Do-like 8 (Solyc02g067360.3.1) increased under crop rotation (FC 4.6), but its abundance in the presence of halophytes was only 1.4-fold higher relative to TM (File S1). Its Arabidopsis ortholog DEG PROTEASE 8 is involved in photosystem II repair through cleavage of photo-damaged D1 protein (Kato et al. 2012). The increase in those proteins in TR plants correlated with the recorded enhanced photosynthesis performance (Figure S1).
3.7 Production and quality
Concerning fruit production and quality, no major differences were observed in crop management. Tomato production was calculated as the aggregate of the three consecutive commercial harvests performed. The kg of tomatoes per plant was statistically equivalent for the different crop management (Figure S4A). However, the number of tomatoes per plant increased by over 15% in TR with respect to TM plants, whereas in TH plants, this variable remained comparable to TM and TR plants (Figure S4B). This is in contrast with previous studies in which intercropping with halophytes enhanced production in different crop species such as watermelon (Simpson et al. 2018), tomato (Zuccarini 2008; Jurado et al. 2024), pepper (Colla et al. 2006) and strawberry (Karakas et al. 2021). In this study, tomato, a moderately tolerant species to salinity, was cultivated under salinity conditions that did not have a major impact on production. Thus, it can be argued that the degree of soil salinity and the crop sensitivity to salinity are determinants of fruit yield of crops intercropped with halophytes. Finally, in relation to fruit quality, no changes in total soluble solids were registered, while a slight increase in acidity in fruits grown under crop rotation conditions was observed (Figure S4C – D).
4 CONCLUSIONS
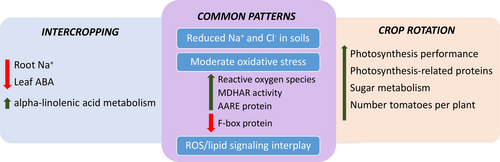
Halophyte-based crop management may become a relevant component of farming systems, favouring cash crop productivity in saline environments while providing a high added-value product. Figure 9 summarizes the main changes observed in the tomato plants under the halophyte-based crop management conditions in comparison to tomato plants grown in monoculture. Both halophyte-based management strategies reduced the levels of Na+ and Cl− in soil, whereas solely intercropping conditions led to a decline in tomato root Na+. Crop rotation enhanced photosynthesis and protective mechanisms at the photosynthetic level. In addition, both crop managements affected the hormone profile and the antioxidant capacity of tomato leaves, whereas a reactive oxygen species over-accumulation in leaf tissues suggests the establishment of a controlled moderate oxidative stress in leaves. Metabolomic and proteomic analyses indicate intricate relationships occurring at the leaf level, influenced by the presence of the halophyte. These interactions suggest a connection between ROS/lipid-based signalling pathways. Additionally, enhanced photosynthesis due to crop rotation was linked to the accumulation of metabolites associated with sugar metabolism and proteins related to photosynthesis. This work brings novelty to the interactions between halophyte and tomato in co-cultivation strategies and supports the use of A. macrostachyum in farming systems.
AUTHOR CONTRIBUTIONS
Conceptualization, G.B.-E., J.A.H., and P.D.-V.; methodology, G.B.-E., C.J.-M., Z.P., P.I.K. and P.D.-V; formal analysis, G.B.-E., C.J.-M., J.A.H., and P.D.-V.; investigation, G.B.-E., J.A.H., and P.D.-V.; data curation, G.B.-E., P.I.K., P.D-V.; writing—original draft preparation, G.B.-E., C.J.-M., P.I.K., J.A.H., and P.D.-V.; writing—review and editing, G.B.-E., J.A.H., and P.D.-V.
ACKNOWLEDGEMENTS
This work was supported by the HaloFarms project (PCI2020-111977; MCIN/AEI/10.13039/501100011033; PRTR) within the EU Program for Research and Innovation solutions in the Mediterranean region (PRIMA S2 2019). The authors wish to thank Spanish National Research Council (CSIC) for its support through the ILINK23006 action (2023 I-LINK Programme) and COOPB20631 action (2021 I-COOP Programme). We thank Martin Cerny for assistance with mass spectrometry analysis.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
The datasets analysed during the current study are available from the corresponding author on request.




