Genome-wide identification and characterization of potato NRL gene family and functional analysis of StNRL-6 in response to Phytophthora infestans
Abstract
NPH3/RPT2-Like (NRL) proteins, as blue light receptor phototropin-interacting modules, regulate various aspects of physiological responses in light signaling. However, little information is available on NRL family members regulating plant immunity, especially concerning plants' late blight resistance to Phytophthora infestans. In this study, a systematical analysis of the potato NRL family was performed. In total, 35 StNRL genes were identified and phylogenetically classified into six subfamilies. Twelve StNRL genes were defined as seven pairs of segmental duplication, which was the major evolutionary driving force for StNRL expansion. Synteny analysis between the genomes of potato and Arabidopsis thaliana, tomato, and rice provided insights into evolutionary characteristics. Two StNRL family members, StNRL-6 and StNRL-7, interacted with the blue light photoreceptor Stphot1 and negatively regulated potato and Nicotiana benthamiana resistance against P. infestans. Moreover, the key motif RxSxS identified in the NRL family member is essential for StNRL-6 to interact with Stphot1 and enhance plant susceptibility to P. infestans. This comprehensive analysis of the StNRL family provides valuable information to elucidate key molecular mechanisms on how blue light regulates plant immunity.
1 INTRODUCTION
Light is a vital energy source for plants to survive. Plants use several families of photoreceptors to sense and respond to changes in light conditions, wavelength, intensity, and direction. Photoreceptors and associated signaling pathways allow plants to adjust and adapt to a changeable light environment. These photoreceptors comprise (1) red/far-red receptors phytochromes, (2) ultraviolet photoreceptor UVR8, and (3) cryptochromes (crys), phototropins (phots), and Kelch containing F-Box protein (KFB) subfamily involved blue light perception (De Wit et al., 2016).
Blue light receptor phototropins (phot1 and phot2) are plasma membrane-bound auto-phosphorylating serine/threonine kinases belonging to the AGCVIII (named after the cAMP-dependent protein kinase A, cGMP-dependent protein kinase G, and phospholipid-dependent protein kinase C) kinase family (Rademacher & Offringa, 2012). The N terminus of phots contains two light-sensing modules known as Light, Oxygen, or Voltage (LOV) domains (LOV1 and LOV2) (Christie et al., 2012). The LOV2 domain inhibits the kinase activity of phots in the absence of light. Following light perception, LOV2 undergoes a conformational change that activates the kinase domain and causes the receptor autophosphorylation on several serine residues (Hart & Gardner, 2021). There are two members of phots in land plants, including Arabidopsis: the highly photosensitive receptor phot1 and the less photosensitive receptor phot2, which redundantly regulate plant physiological responses such as phototropism, chloroplast movements, stomatal opening, leaf positioning, and leaf expansion (Christie 2007; Christie et al., 2015; Sakai et al., 2001).
The NON-PHOTOTROPIC HYPOCOTYL3 (NPH3)/ROOT PHOTOTROPISM2 (RPT2)-like family (NPH3/RPT2-Like; NRL) in Arabidopsis was named based on the founding members NPH3 and RPT2 (Motchoulski & Liscum, 1999; Sakai et al., 2000). As phots interacting proteins and signal transducers, NPH3 and RPT2 play roles in phototropin signaling. The NRL family proteins are composed of several conserved predicted structures, notably an N-terminal BTB/POZ (broad complex, tramtrack, bric-a-brac/pox virus and zinc finger) domain, a central NPH3 domain, and a C-terminal coiled-coil domain (Christie et al., 2018). NPH3 acts as the downstream transducer of phots and seems to be crucial for the redistribution of auxin (Fankhauser & Christie, 2015; Hohm et al., 2013; Legris & Boccaccini, 2020). Besides association with phot1, NPH3 also interacts with the NRL family member RPT2 (Haga et al., 2015). RPT2 stimulates the rebuilding of the phot1-NPH3 complex on the plasma membrane by regulating the phosphorylation status of NPH3 (Haga et al., 2015). In addition, RPT2 also redundantly mediates chloroplast responses with another NRL family member NCH1 (NRL PROTEIN FOR CHLOROPLAST MOVEMENT1, also called NRL31) (Suetsugu et al., 2016). Potato StNRL1, one of the NCH1 orthologous, has been reported to act as a susceptible factor to promote P. infestans, the late blight causal agent, colonization and be targeted by the P. infestans RxLR (Arg-any amino acid-Leu-Arg) effector Pi02860. StNRL1 promotes the proteasome-mediated degradation of the positive immune factor StSWAP70 and suppresses the PAMP INF1 (infestin1)-triggered cell death (He et al., 2018; Yang et al., 2016). StNRL1 interacts with Stphot1 and Stphot2, both of which negatively regulate plant immunity against P. infestans (Naqvi et al., 2022). Nevertheless, no more information is available about the NRL genes regulating plant immunity.
Late blight is the most devastating disease in potato caused by the oomycetes P. infestans. P. infestans secretes numerous RxLR effectors into plant cells by targeting the host's positive and negative regulatory factors to suppress immunity. On the one hand, some effectors hijack the host's positive immune regulators to suppress immune responses. On the other hand, other effectors utilize the negative immune factors to promote pathogen infection (He et al., 2020). These negative regulators are called “S” (susceptibility) factors. StNRL1 is the first reported NRL family “S” factor in potato that promotes P. infestans infection and suppresses INF1-triggered cell death (Yang et al., 2016). Blue light negatively regulates plant immunity against P. infestans (Naqvi et al., 2022). NRLs act downstream the phots to transduce the blue light signaling in regulating a wide range of phot-mediated responses and physiological activities (Christie et al., 2018). In this study, we focus on characterizing the potato NRL genes that play a role in regulating plant immunity against P. infestans. Understanding the nuanced functions of NRL proteins in regulating plant immunity not only comprehensively broadens their versatile roles but also unveils potential avenues for engineering plant disease resistance. Unraveling the molecular mechanisms by which NRL proteins integrate into the intricate net of defense signaling pathways promises novel insights into improving crop resilience against pathogens and environmental stresses. This study identified and characterized the potato NRL genes in the whole genome and examined the evolutionary relationships, conserved domains, expression profiles, and emphasized on the function of two family members. Our study lays the foundation to further elucidate the molecular mechanisms of potato NRL genes regulating immunity against P. infestans.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
Potato cultivar E3 (Solanum tuberosum cv. E3) plants were grown in a greenhouse at 22°C under 16 hours light/8 hours dark photoperiod conditions. Nicotiana benthamiana plants were grown in a growth chamber at 22°C with 16 hours light/8 hours dark photoperiod and 70% relative humidity.
2.2 Genome-wide identification of NRL genes in potato and tomato genomes
Thirty-three AtNRL protein sequences were downloaded from A. thaliana TAIR11 (https://www.arabidopsis.org/index.jsp), and a local BLAST search was conducted in the reference potato genome DM1-3 (PGSC, http://spuddb.uga.edu/index.shtml) and S. lycopersicum genome (ITAG4.0) downloaded from Phytozome (https://phytozome-next.jgi.doe.gov/), respectively by TBtools software (Chen et al., 2020). After removing redundant sequences, the candidate sequences were subsequently inspected with the Hidden Markov Model (HMM) profile of the NPH3 domain (PF03000) downloaded from the Pfam database (http://pfam.xfam.org/). All candidate sequences were reconfirmed through the NCBI Conserved Domain Database (Marchler-Bauer et al., 2011) and the Pfam database (Finn et al., 2016). Proteins containing the NPH3 domain were identified as the NRL family members. Different clades of family members were renamed based on their arrangement location on chromosomes of the potato genome. The online ExPASy server (https://www.expasy.org/) was used to predict the molecular weight (MW) and isoelectric point (pI) of the potato NRL proteins.
2.3 Phylogenetic analysis and classification of NRL gene family in potato
The multiple alignments of 100 NRL amino acids from potato, Arabidopsis, and tomato were conducted via the ClustalW with default parameters. A Maximum Likelihood (ML) phylogenetic tree was constructed in MEGA 11 software with the Jones-Taylor-Thornton (JTT) + Gamma Distributed (G) model. The phylogenetic tree was edited and visualized in iTOL (https://itol.embl.de/).
2.4 Conserved domain, motifs, and gene structures
The conserved domains were predicted by the NCBI Conserved Domain database, Pfam database, and HMMER database (https://www.ebi.ac.uk/Tools/hmmer/). The MEME online server (https://meme-suite.org/meme/tools/meme) was used to identify conserved motifs in NRL proteins with a maximum motif number of 10. The genomic DNA and CDS information for the potato NRL genes were downloaded from the PGSC database. TBtools (Chen et al., 2020) were used to display the conserved domains, motifs, and gene structures.
2.5 Chromosomal localization and synteny analysis
The chromosomal positions of StNRL genes were identified by TBtools (Chen et al., 2020) according to the gene location in the potato genome. To identify gene duplication, whole-genome protein sequences were aligned via BLASTP against themselves with an e-value of 1e-5. MCScanX Wrapper in TBtools (Chen et al., 2020) was used to classify the gene duplication patterns into tandem or segmental duplications. The gene duplications within the potato genome were plotted using Advanced Circos in TBtools (Chen et al., 2020). Syntenic blocks among potato, Arabidopsis, tomato, and rice were detected by One Step MCScanX in TBtools (Chen et al., 2020).
2.6 RNA-seq data analysis
The transcriptome data of the StNRL genes' expression in different tissues (leaves, petioles, shoots, whole mature flowers, stolons, whole tubers, roots) were downloaded from the PGSC (Potato Genome Sequencing Consortium, 2011). The normalized gene expression values FPKM (fragments per kilobase of exon per million fragments mapped) were transformed into log2(FPKM+1) form and heat maps were drawn by TBtools software (Chen et al., 2020).
2.7 Vector constructions
For N-terminal GFP or RFP fusing constructs, full-length genes were amplified and inserted into the pK7WGF2 or pK7WGR2 vectors by homologous recombination to generate corresponding constructs. Site mutagenesis was conducted to generate the serine point mutations at the N-terminus or the C-terminus of StNRL-6 using the site-directed mutagenesis and homologous recombination kit (Vazyme). Yeast vector pDEST series were generated by stepwise BP and LR reactions following the Gateway manual instructions. For the pGBKT7 and pGADT7 yeast vectors, the corresponding genes were cloned into the EcoRI and BamHI sites. The full length of StNRL-6 was cloned into the StuI site with the N-terminal HA tag. The resulting construct was used for the potato transformation and Agrobacterium-mediated transient expression. RNAi construct with about 200 bp of StNRL-6 specific region was cloned into the XhoI site of the pHELLSGATE8 vector followed by cloning the reverse sequence into the XbaI site. Primer sequences are shown in Table S2.
2.8 Yeast two-hybrid
For testing interactions between Stphots and StNRLs, the Clontech yeast system was used. DNA-binding domain “bait” fusions and activation domain “prey” fusions were generated by homologous recombination into the pGBKT7 or the pGADT7 vectors, respectively. These constructs were co-transformed into the Saccharomyces cerevisiae strain AH109 following the manufacturer's protocols of the Yeast Protocols Handbook (Clontech). The pairwise combinations of constructs or the control empty vectors were tested for protein–protein interactions using reporter gene assays, namely the ability to grow on media missing leucine, tryptophan, histidine, and adenine (-LWHA).
For screening of StNRL-6 interaction proteins, the Y2H screen was conducted in S. cerevisiae strain MAV203 using the Invitrogen ProQuest system following the user manual of the ProQuest Two-Hybrid System. DNA binding domain “bait” fusions and activation domain “prey” fusions were generated by LR reaction into the pDEST32 or the pDEST22 vectors, respectively. Interactions were confirmed by yeast growth on the selective medium lacking tryptophan, leucine, and histidine (-TLH) and YPDA medium with X-gal.
2.9 Agrobacterium-mediated transient expression and virus-induced gene silencing (VIGS)
Agrobacterium tumefaciens strain GV3101 was transformed with constructs and grown in the yeast extract beef (YEB) medium with appropriate antibiotics and shaking at 28°C overnight. The cultures were centrifuged at 4000 rpm to collect the bacterial pellets, which were then resuspended in MMA infiltration buffer (10 mM MES, 10 mM MgCl2, and 200 μM acetosyringone, pH = 5.6). The bacterial optical densities were adjusted to get the final OD600 at 0.5 for protein extraction, and 0.1 for P. infestans infection assay and subcellular localization. For cell death assays, the number of infiltrating sites in which cell death occurred (>50%) was counted and measured by the percentage of total inoculation sites per plant.
The orthologous genes in N. benthamiana for StNRL-6 and StNRL-7 were identified in the Sol Genomics Network (https://vigs.solgenomics.net/). For VIGS constructs, about 300 bp gene-specific fragment was selected for homologous recombination between the EcoRI and BamHI sites in pTRV2 vector in the sense orientation (Liu et al., 2002). Agrobacterium cultures containing a mixture of pTRV1 and each pTRV2 construct were pressure-infiltrated into the two largest leaves of four-leaf stage N. benthamiana at a final OD600 = 0.3. The pTRV2 vector expressing GFP was used as a control. After 3 weeks, the silencing levels in leaves were tested by quantitative reverse transcription PCR (qRT-PCR).
2.10 Co-immunoprecipitation and Western Blot
For co-immunoprecipitation (Co-IP), vectors expressing GFP-Stphot1, GFP-Stphot2, GFP-StNRL-6, RFP-StNRL-6, RFP-StNRL-7, RFP-StNRL-10, RFP-StNRLS50AS52A, RFP-StNRL-6S600AS602A under the CaMV 35S promoter and the control vector RFP-EV were co-expressed with different combinations in N. benthamiana leaves. Samples were collected two days post-infiltration and extracted using GTEN extraction buffer [10% v/v glycerol, 25 mM Tris–HCl (pH = 7.5), 1 mM EDTA, 150 mM NaCl] with 10 mM dithiothreitol (DTT), 1 mM phenylmethyl sulphonyl fluoride (PMSF), protease inhibitor cocktail, and 0.2% Nonidet P-40. To immunoprecipitate the GFP-tagged proteins, protein extracts were centrifuged to collect the supernatant liquid, which was then incubated with the GFP agarose beads (KTSM, China) for 2 hours at 4°C on a thermomixer. Beads were washed three times in GTEN-based wash buffer supplemented with protease inhibitor and 1 mM PMSF before resuspending in 2 × SDS loading buffer (100 mM Tris–HCl, 4% SDS, 20% glycerol, 0.2% bromophenol blue and 200 mM DTT). The samples were then separated on the Bis-Tris PAGE gel (polyacrylamide gel electrophoresis) and transferred onto a PVDF membrane (BioRad). Membranes were stained with Ponceau solution to normalize the amount of loading proteins. Monoclonal GFP antibody (Bioyeargene) and RFP antibody (Chromotek) were used at 1:5000. Anti-mouse polyclonal secondary antibody (Bioyeargene) was used at 1:5000. Immunoblots were visualized by ChemDoc™ XRS+ gel imaging system (Bio-Rad).
2.11 Confocal image for bimolecular fluorescence complementation (BiFC)
The Agrobacteria containing the constructs of YC-Stphot1 and YN-StNRL-6 or YN-StNRL-7 were co-infiltrated into the N. benthamiana leaves. YC-EV and YN-EV were set as the controls. After 48 hours post infiltration, the leaves were collected for confocal imaging observation using Leica SP8 with 514 nm as excitation and 530–575 nm as emission.
2.12 P. infestans infection assay
P. infestans isolates 88069 and HB09-14-2 were grown on the rye agar medium (Andriani et al., 2021) at 19°C in a dark growth chamber. Two-week-old cultures on plates were used for the collection of sporangia. The plates were flooded with 3 mL water and scraped to release sporangia. The sporangia suspension was filtered through a 300-micron nylon filter mesh to remove the mycelium, and then concentrated through a 1200-micron nylon filter mesh. Sporangia numbers were counted under the biological microscope and adjusted to 200,000 sporangia per mL. For inoculation, 10 μL droplets were inoculated onto the abaxial side of the detached N. benthamiana or potato leaves on moist tissue in sealed boxes. Disease lesions were measured at 4–8 days post inoculation (dpi) according to the disease symptoms. The lesion diameters served as a metric for quantifying the lesion sizes on N. benthamiana leaves as reported by Wang et al. (2015). Similarly, the lesion areas were employed to measure the dimension of the lesions on potato leaves as described (Vleeshouwers et al., 1999).
2.13 Plant genomic DNA extraction and expression analysis by qRT-PCR
Plant genomic DNA extraction was conducted following the instructions of the Plant Genomic DNA kit (TIANGEN). For total RNA extraction, plant total RNA was isolated using the Plant Total RNA Kit (Zomanbio), and cDNA was synthesized from 1 μg RNA following the instructions of the All-In-One 5 × RT MasterMix kit (Applied Biological Materials). qRT-PCR was performed on a QuantStudio™ 6 Flex Real-Time PCR system (Thermo Fisher Scientific) using Blastaq 2 × qPCR Mastermix (Applied Biological Materials) according to the manufacturer's protocols. StACTIN and NbACTIN were used as the internal controls in potato and N. benthamiana, respectively. All primer sequences are listed in Table S2.
3 RESULTS
3.1 Identification and characterization of the NRL family genes in potato
To identify the whole NRL family genes in potato, the 33 NRL protein sequences of A. thaliana were used to perform a BLASTP alignment in the genome database of potato DM1-3 v6.1. Pfam and NCBI conserved domain searches were used to check the domains of potato candidate NRL proteins. Finally, 36 candidate genes were originally obtained and 1 predicted gene (Soltu.DM.02G027880.2) without the NPH3 domain was removed. Totally, 35 NRL genes were selected and renamed with a unified nomenclature in accordance with the gene location on the chromosomes (Table S1). Thirty-five NRL genes are unevenly distributed on 11 chromosomes (chr) across the potato genome except for chr12 (Table S1 and Figure 1). Chr02 contains eight StNRL genes, the number of which is the highest among all chromosomes. Chr01, chr07, and chr09 have four StNRL genes each, while chr03, chr06, and chr10 have three StNRL genes each. Chr05 and chr11 harbor two StNRL genes, and chr04 and chr08 carry a single StNRL gene (Table S1 and Figure 1).
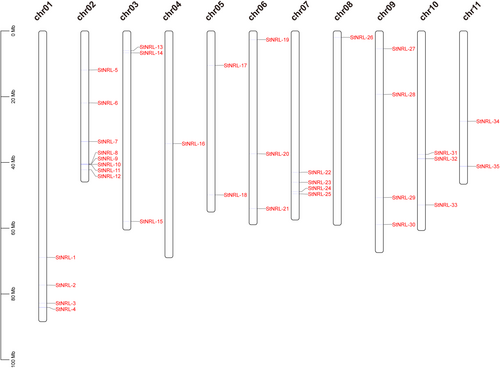
The chromosome (chr) numbers were indicated above each schematic chromosome (black vertical cylinders). The NRL genes were color-coded with red. The left scale shows the length of each potato chromosome in megabase (Mb).
Among the 35 potato NRL family members, StNRL-32 is the longest protein, consisting of 782 amino acids, while StNRL-20 is comprised of the shortest 102 amino acids (Table S1). The predicted isoelectric points (pI) and molecular weights (MW) of StNRLs range from 5.13–9.2 and 11.50–86.71 kilodalton (kDa), respectively (Table S1).
3.2 Phylogenetic analysis of the StNRL family
To figure out the classification and evolutionary relationships of the StNRL gene family, an interspecific phylogenetic tree was conducted based on the multiple sequence alignment of 100 NRL protein sequences, including 35 NRLs in potato, 33 in Arabidopsis, and 32 in S. lycoperscium. All the 100 NRL proteins were classified into six groups similar to the Arabidopsis NRL gene family (Figure 2). The potato NRLs were more closely related to the tomato NRLs), suggesting that NRLs were conserved during the evolution of Solanaceae plant lineages. The clades RPT2/NCH1 and NPH3 were named after the two founding members of the Arabidopsis NRL family, respectively. The NPY clade contained 8 NPYs in Arabidopsis, namely NRL5, NRL15, NRL26, NRL20, NRL6, NRL30, NRL21, and NRL7. Among the six clades, the RPT2/NCH1 clade contained 24 members, which was the biggest group. The NPY clade ranked second with 21 members (Figure 2).
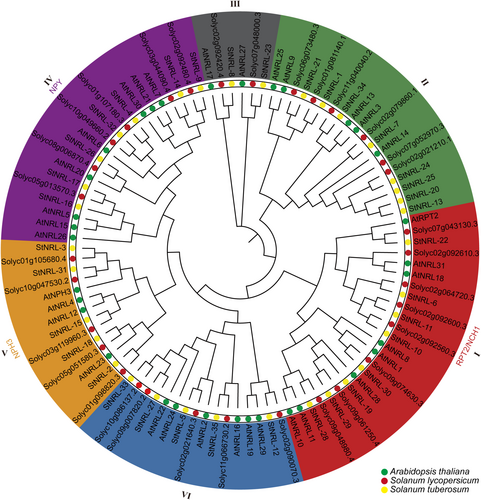
All NRL protein sequences from A. thaliana, S. tuberosum, and S. lycopersicum were aligned by ClustalW, and the Maximum Likelihood phylogenetic tree was generated using MEGA11 software with full-length amino acid sequences of 100 NRLs. The green, yellow, and red circles indicate the NRL genes from A. thaliana, S. tuberosum, and S. lycoperscium, respectively. Different colors indicate the different sub-families (I-VI).
3.3 Structure and conserved domains, motifs of StNRLs
The NRL family was defined by the presence of the NPH3 domain (Pedmale et al., 2010). Members of the NRL family typically exhibit two other conserved structures, namely the BTB domain and the coiled-coil domain. In Arabidopsis, 2 members lack the BTB domain and 10 are devoid of the coiled-coil domain (Pedmale et al., 2010). Among the 35 potato NRL family members, 6 of them lack the BTB domain, and 12 lack the coiled-coil domain (Figure 3).
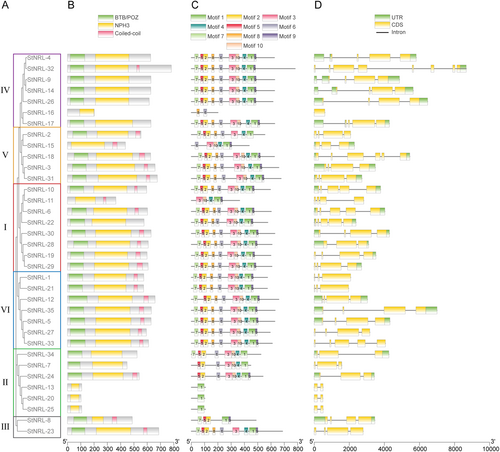
(A) Phylogenetic tree of the 35 potato NRL proteins. (B) Conserved domains analysis of potato NRL proteins. The green, yellow, and red boxes on the top indicate the BTB/POZ, NPH3, and Coiled-coil domains, respectively. The scale bar below the conserved domains represents amino acid numbers. (C) Predicted motifs in 35 potato NRL proteins. Boxes with numbers (1–10) in the center represent different motifs. The boxes with different colors indicate different motifs (with number) on the top. The scale bar below the predicted motifs represents amino acid numbers. (D) Gene structure analysis illustrates the intron/exon/UTR configurations of potato NRL genes. UTR, untranslated region; CDS, coding sequence. The green, yellow boxes and black straight lines indicate the UTR, CDS, and intron respectively displayed on the top. The scale bar below the gene structures represents nucleotide numbers.
To further reveal the structural and functional diversification of the 35 StNRLs, 10 predicted consensus motifs were detected via the online website MEME. Motifs in NRLs from different clades showed variability (Figure 3). Twenty-three family members contained all 10 motifs in the same order, mainly from clade I, IV, V, and VI. StNRL-13, StNRL-20, and StNRL-25 possessed the least motifs due to their short length in the amino acids. StNRL-16 from clade IV harbored only motif 6 and motif 8, while the rest of the members in this clade contained all 10 motifs (Figure 3). Nevertheless, in the same clade, most motifs showed high similarity, indicating the conserved function of NRLs.
The diversity of the gene structure is crucial in implying the evolutionary lineages of the gene family. Potato NRL family genes contained 1 to 8 exons, among which StNRL-32 ranked first and StNRL-16 ranked last (Figure 3). More than half of the StNRL genes (18) had 4 exons. StNRL-16 was the only one without any intron. Most of the phylogenetically close StNRL genes shared the same exon number and position (Figure 3).
3.4 Synteny analysis of potato NRL genes
To explore the genomic evolution of the NRL gene family in potato, the syntenic relationships of the StNRL genes were analyzed. Gene duplication mainly occurred throughout the evolution of plant genomes by tandem duplication and segmental duplication. There were no tandem duplication gene pairs detected in the potato NRL gene family. Collinearity analysis showed that among the 35 potato NRL genes, 7 pairs of segmental duplication genes were identified (4–9, 4–32, 6–10, 7–24, 9–14, 19–29, and 27–33) (Figure S1, Table S3). Every pair of these segmental genes were in the same clade and acted as the closest paralogs. This might indicate these pairs of genes came from potato genome duplication events during the evolution. The Ka/Ks ratios were calculated to investigate the potential selective pressure of the 7 pairs of segmental duplication genes in potato. The results showed that all of the 7 pairs of segmental duplication genes had Ka/Ks less than one (Table S3). These results suggested that the NRL gene family may have suffered intense purification selection pressure during evolution and segmental duplication events might be a major driving force behind this evolution.
3.5 Synteny and evolutionary analyses of potato and other plant NRL genes
To further explore the evolutionary and phylogenetic mechanisms of the potato NRL gene family, a multiple comparative synteny analysis with three representative plant species was conducted. The three representative plant species contained two dicots (Arabidopsis and tomato) and one monocot (rice) (Figure 4). A total of 17 StNRL genes showed a syntenic relationship with genes in Arabidopsis, followed by Solanum lycopersicum (29), and Oryza sativa (12). The numbers of StNRL orthologous genes in A. thaliana, S. lycopersicum, and O. sativa were 21, 29, and 11, respectively. The results revealed that 25 orthologous gene pairs between potato and Arabidopsis displayed a syntenic relationship, followed by S. lycopersicum (39), and O. sativa (13) (Figure 4, Table S4). Among all the orthologous gene pairs, StNRL-9 and StNRL-14 in the syntenic analysis of potato and Arabidopsis and StNRL-4 and StNRL-9 in the syntenic analysis of potato and tomato were identified to be associated with three syntenic gene pairs. There were more syntenic gene pairs identified between potato and dicots than those between potato and rice. However, there were two syntenic gene pairs (StNRL-28) just available between potato and rice (Table S4).
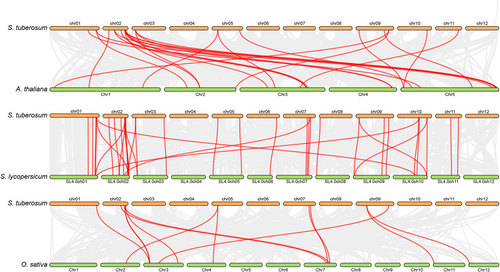
The gray lines in the background show all collinear blocks within potato and Arabidopsis genomes, while the red lines indicate the collinear blocks of the NRL genes. The species names with the prefixes ‘S. tuberosum’, ‘A. thaliana’, ‘S. lycopersicum’, and ‘O. sativa’ indicate Solanum tuberosum, Arabidopsis thaliana, Solanum lycopersicum, and Oryza sativa, respectively.
3.6 Expression profiles of StNRL genes in different tissues
To examine the possible roles of NRL genes in the development and growth of potato, the expression profiles of the StNRL genes in different potato tissues were extracted from the public RNA-Seq data. According to the expression profiles, the 35 genes were divided into 2 major groups. The first group contained 7 genes (StNRL-10, StNRL-11, StNRL-22, StNRL-3, StNRL-5, StNRL-14, and StNRL-26) which were highly expressed in all potato tissues, especially StNRL-10, StNRL-11, and StNRL-22 (Figure 5). Eight genes (StNRL-13, StNRL-16, StNRL-18, StNRL-21, StNRL-23, StNRL-27, StNRL-28, and StNRL-33) showed very low expression level in all the 7 potato tissues (Figure 5). Some family members showed a tissue-specific expression pattern. For example, the expression level of StNRL-30 in tubers was much higher than that in other tissues, suggesting that this gene may be necessary for tuber development. StNRL-10 and StNRL-22 were highly expressed in all tissues, especially in shoots and roots.
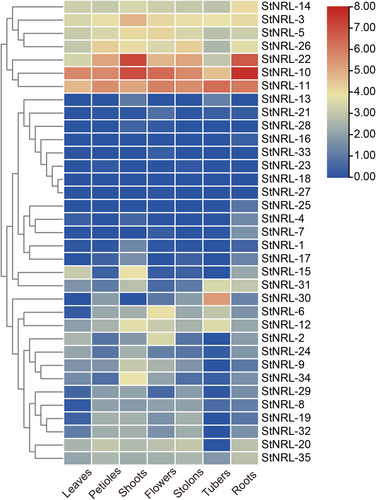
Expression profiles of StNRL genes in leaves, petioles, shoots, flowers, stolons, tubers, and roots based on PFKM values. The color scale with red and blue represents high and low expression, respectively.
3.7 StNRL-6 and StNRL-7 interact with Stphot1
NRL family members have been reported to involve plant phototropin (phot) signaling and act in concert with upstream phototropins to regulate various light-mediated physiological responses (Christie et al., 2018). Potato phototropin1 (phot1) activates and interacts with the downstream StNRL-10 (formerly called StNRL1), which acts as a susceptibility factor to suppress INF1-triggered immunity and promote P. infestans colonization in N. benthamiana (Yang et al., 2016). StNRL-10 promotes the proteasome-mediated degradation of the positive immune regulator nucleotide exchange factor StSWAP70 (He et al., 2018). Yeast-two-hybrid assay was used to explore the modes of interaction between NRLs and phots in potato. Among the pairs of NRLs and phots, StNRL-10 interacts with Stphot1 and Stphot2 (Figure 6), which was also reported in Co-IP assay (Naqvi et al., 2022). Two newly identified NRL family members, StNRL-6 and StNRL-7, interact with Stphot1 but not with Stphot2 (Figure 6).
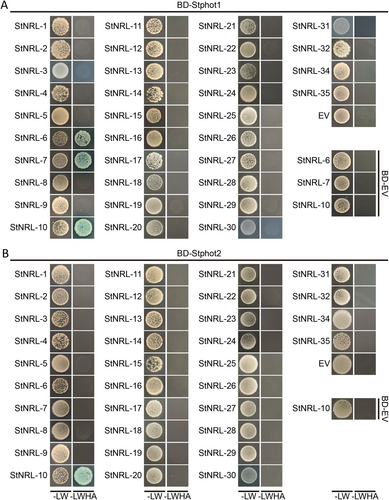
Potato NRL genes were fused to the activation domain to generate the bait constructs, and phototropins were fused to the DNA-binding domain to generate the prey constructs. Interactions were determined by the growth of yeast co-transformed into AD-StNRLs and BD-Stphot1 (A) or BD-Stphot2 (B) on the medium lacking Leu, Trp (-LW) and Leu, Trp, His, and Ade (-LWHA) supplemented with x-α-gal.
To further confirm the interactions between StNRL-6 and StNRL-7 with Stphot1, Co-IP assay, and BiFC assay were conducted in N. benthamiana. Co-IP confirmed the specific association of RFP-StNRL-6 and RFP-StNRL-7 with GFP-Stphot1 but not with GFP-Stphot2 (Figure 7). BiFC assay revealed that YFP fluorescence signals mainly observed in the plasma membrane in the combination of StNRL-6 or StNRL-7 with Stphot1 (Figure 7). These results proved the interactions of StNRL-6, StNRL-7 with Stphot1 in both yeast and in planta.
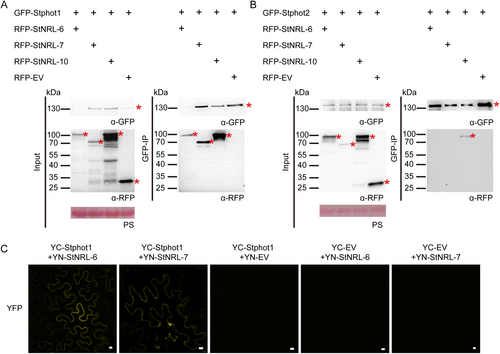
(A) and (B) Co-IP assays showing the interactions between 3 potato NRLs with phototropins. GFP-tagged Stphot1 or Stphot2 were co-expressed with RFP-tagged StNRL-6, StNRL-7, StNRL-10, or empty vector in N. benthamiana by agroinfiltration, respectively. Samples were collected after 48 hours post infiltration. Total proteins were extracted, followed by IP with GFP-trap beads. Anti-GFP and anti-RFP antibodies were used to detect target protein bands. Protein loading is indicated by Ponceau stain (PS). * indicates target protein band. + indicates expression of constructs in N. benthamiana leaves. The predicted protein sizes of GFP-Stphot1, GFP-Stphot2, RFP-StNRL-6, RFP-StNRL-7, RFP-StNRL-10 are 141.6 kDa, 133.5 kDa, 93.7 kDa, 78 kDa, and 93.1 kDa, respectively. (C) BiFC assays confirming the interactions of StNRL-6 and StNRL-7 with Stphot1. YC-Stphot1 or YC-EV was transiently co-expressed with YN-StNRL-6, YN-StNRL-7, or YN-EV in N. benthamiana leaves. The combinations of YN-StNRL-6 or YN-StNRL-7 with YC-Stphot1 result in YFP fluorescence signals. Bars = 10 μm.
3.8 StNRL-6 and StNRL-7 promote P. infestans colonization in potato and N. benthamiana
To investigate the possible roles of StNRL-6 and StNRL-7 in regulating potato late blight resistance, the expression pattern of the two genes was tested 0, 24, 36, 48, and 60 hours after P. infestans infection. The RT-qPCR results revealed that both NRL genes responded to P. infestans induction. StNRL-6 showed a decreased expression pattern (Figure S2). On the contrary, StNRL-7 demonstrated an increased expression pattern during the infection. StWRKY8 was set as a positive control induced by P. infestans and it indeed showed an increased expression.
To further confirm the function of the two NRL genes, transient expression assay and VIGS assay in N. benthamiana were conducted. N. benthamiana leaves expressing HA-StNRL-6 or HA-StNRL-7 showed significantly larger lesions compared with the control HA-GFP when challenged with P. infestans isolate 88069 (Figure 8A). In contrast, silencing of the corresponding orthologous NbNRL-6 and NbNRL-7 reduced the colonization of P. infestans and showed stunted growth compared with the control TRV2-GFP (Figures 8B and S3). Further, stable transgenic potato lines were generated by transforming the constructs 35S::HA-StNRL-6 (for overexpression; OE) or pHellgate8-StNRL-6 (for RNA-interfering; RNAi) into the potato cultivar E3. When transgenic potato leaves were challenged with P. infestans isolate HB09-14-2 inoculation, OE-lines sustained larger disease lesions and RNAi-lines showed smaller disease lesions compared to the wild type (Figures 8C-F and S4). These results above indicate that StNRL-6 and StNRL-7 act as a “S” factor to promote late blight disease development.
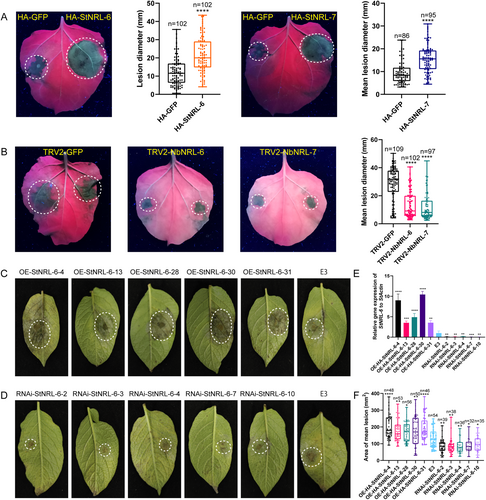
(A) Transient expression of both HA-StNRL-6 and HA-StNRL-7 in N. benthamiana leaves enhanced P. infestans colonization compared to the HA-GFP control. Box plots on the right of representative leaves showing disease lesion diameters on the areas transiently expressing HA-StNRL-6 or HA-StNRL-7 at 5 dpi with P. infestans isolate 88069 (Welch's t-test, **** p < 0.0001). (B) VIGS NbNRL-6 or NbNRL-7 in N. benthamiana plants reduced P. infestans colonization compared to the TRV2-GFP control plants. Box plots on the right show disease lesion diameters on the plants silencing NbNRL-6 or NbNRL-7 at 6 dpi with P. infestans isolate 88069 (one-way ANOVA with Dunnett's multiple comparisons test, **** p < 0.0001). (C) and (D) Representative images of P. infestans isolate HB09-14-2 inoculation on leaves of OE-StNRL-6 and RNAi-StNRL-6 potato lines. (E) Relative expression level of StNRL-6 in OE-StNRL-6 and RNAi-StNRL-6 transgenic lines by qRT-PCR. (F) Box plots showing disease lesion areas on the leaves of OE-StNRL-6 or RNAi-StNRL-6 transgenic potato lines. Overexpressing StNRL-6 enhanced lesion areas compared to the wild-type E3 control, while RNA-interfering StNRL-6 lines showed decreased P. infestans lesion areas compared to the E3 control. Significant differences were determined by one-way ANOVA with Dunnett's multiple comparisons test in (E) and (F) (* p < 0.05, ** p < 0.01, *** p < 0.001, **** p < 0.0001). Error bars represent mean ± SD. In (A), (B), and (F), the middle line represents the median value in the analyzed data in each box plot, while the upper and lower lines represent the quartiles of each respective half. The lower and upper extremes represent the minimum and maximum values in the data set, respectively. The data distribution is depicted as individual small points.
3.9 StNRL-6 and StNRL-7 did not suppress INF1-triggered cell death
StNRL-10 was the first potato NRL protein that was found to interact with P. infestans RxLR effector Pi02860 and promote P. infestans virulence (Yang et al., 2016). Pi02860 and StNRL-10 both suppressed plant PTI by attenuating cell death triggered by INF1 (Yang et al., 2016). Same as StNRL-10, the newly identified StNRL-6 and StNRL-7 interact with Stphot1 and function as “S” factors. To explore their effect on the INF1-triggered cell death, HA-StNRL-6 or HA-StNRL-7 was co-expressed with INF1 in N. benthamiana leaves. Expression of HA-StNRL-10 or GFP-Avr3a obviously suppressed INF1-triggered cell death (Figure S5). However, neither HA-StNRL-6 nor HA-StNRL-7 expression affected INF1-mediated cell death. This result suggests that the function of StNRL-6 and StNRL-7 are different from StNRL-10 in regulating plant immune pathways, though they both function as “S” factors.
3.10 C-terminal RxSxS motif is important for the susceptibility of StNRL-6
NPH3 and other NRL family members (such as RPT2, DOT3, NPY1, and NRL1) in Arabidopsis are substrates of phot1 (Sullivan et al., 2021). The conserved C-terminal consensus motif RxSxS of NPH3 is the phosphorylation site by phot1 and is necessary to promote phototropism and petiole positioning in Arabidopsis (Reuter et al., 2021; Sullivan et al., 2021). This conserved C-terminal motif sequence in RPT2 is also important for its role in phot-mediated chloroplast accumulation movement, leaf positioning, and phototropism (Waksman et al., 2023). In the potato NRL family, 13 members contain the conserved C-terminal RxSxS motif (Figure S6). StNRL-6 has two RxSxS motifs in both the C-terminus (598–602, RTSFS) and the N-terminus (48–52, RNSIS). To test whether the conserved RxSxS motif is important for StNRL-6 to promote P. infestans colonization, the two key serine residues in the RxSxS motif were mutated into alanine to generate two different StNRL-6 mutations. N. benthamiana leaves expressing HA-StNRL-6 exhibited larger lesions, while the N-terminal mutation HA-StNRL-6S50AS52A still maintained the ability to produce larger lesions (Figure 9A). However, the lesions on the leaves expressing the C-terminal mutation HA-StNRL-6S600AS602A were similar to that of the control HA-GFP, indicating that the serine residues in the C-terminal RxSxS motif were important for StNRL-6 to negatively regulate late blight resistance.
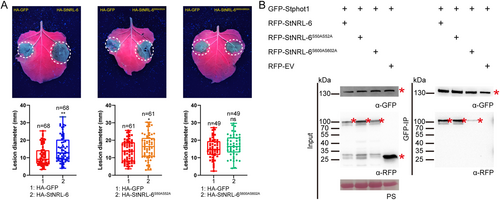
(A) The C-terminus but not the N-terminus of StNRL-6 attenuated the susceptibility when inoculated with P. infestans isolate 88069. Each construct was transiently expressed in N. benthamiana leaves by agroinfiltration. Box plots under representative leaves showing P. infestans lesion diameters on the areas transiently expressing the control and mutated constructs (Welch's t-test, * p < 0.05, ** p < 0.01). Error bars represent mean ± SD. In each box plot, the middle line represents the median value in the analyzed data, while the upper and lower lines represent the quartiles of each respective half. The lower and upper extremes correspond to the minimum and maximum values within the data set, respectively. The individual data distribution is shown as small points. Error bars represent mean ± SD. (B) Co-IP assays showing the interactions between GFP-Stphot1 and RFP-StNRL-6 or the two mutations RFP-StNRL-6S50AS52A and RFP-StNRL-6S600AS602A. GFP-tagged Stphot1 was co-expressed with RFP-tagged StNRL-6, the N-terminal, and the C-terminal RxSxS mutations of StNRL-6, or empty vector in N. benthamiana by agroinfiltration, respectively. Samples were collected after 48 hours post infiltration. Total proteins were extracted, followed by IP with GFP-trap beads. Anti-GFP and anti-RFP antibodies were used to detect target protein bands. Protein loading is indicated by PS. * indicates target protein band. + indicates expression of constructs in N. benthamiana leaves. The predicted protein sizes of RFP-StNRL-6 and its derived mutations are 93.7 kDa.
To determine whether the C-terminal RxSxS motif affects the interaction between StNRL-6 and Stphot1, RFP-StNRL-6S50AS52A and RFP-StNRL-6S600AS602A were co-expressed with GFP-Stphot1 in N. benthamiana. Co-IP results showed that the N-terminal and C-terminal mutation of StNRL-6 still interacted with Stphot1. However, the interaction between Stphot1 and StNRL-6S600AS602A was much weaker than that of Stphot1 with the wild-type StNRL-6 or with the N-terminal mutation StNRL-6S50AS52A (Figure 9B). Collectively, the above results underscored the vital role of the C-terminal RxSxS motif of StNRL-6 in the interaction with Stphot1 and immune regulation.
4 DISCUSSION
NRL proteins are unique in plants and have been identified to play various roles in plant physiological response regulation, especially reported in Arabidopsis (Christie et al., 2018). However, there is little attention on NRL proteins regulating plant immunity. Information about NRL genes in potato is also limited. In this study, 35 members of StNRL genes were identified in S. tuberosum genome and classified into six subfamilies based on the phylogenetic analysis (Figure 2). Systematic bioinformatics analyses, including chromosome distributions, phylogenetic relations, protein domains, motifs, gene structures, gene duplication, synteny relationships, and expression pattern, were performed. Further, deep analyses focused on the interaction mode and functional identification. The analyses and results in this study provide fundamental information for further characterization of StNRL genes function.
A previous study identified 33 NRL genes in A. thaliana (Pedmale et al., 2010). In this study, we identified 35 NRL genes in potato and 32 NRL genes in tomato. Phylogenetic analysis of NRL genes from potato, tomato and Arabidopsis revealed a closer relationship within the Solanaceae species than with Brassicaceae species (Figure 2). Gene duplication is one of the driving forces of evolution involved in the formation and functional differentiation of new genes. Tandem duplication and segmental duplication play a major role in the expansion of a gene family. In the potato NRL gene family, there are 7 pairs of segmental duplication but no tandem duplication (Figure S1). These results support the hypothesis that segmental duplications occur far more frequently than tandem duplications (Ahmad et al., 2020). Synteny analyses showed that there was less collinearity observed between potato and rice NRL genes compared to the other two dicot species Arabidopsis and tomato (Figure 4). This indicated that the dicotyledonous species potato and the monocotyledonous species rice separated earlier during the evolution.
The NRL gene family typically contains three conserved domains. The BTB domain mainly interacts with the CUL3 to form an E3 ligase (Figueroa et al., 2005; Gingerich et al., 2005) and the C-terminal coiled-coil mainly mediates the interaction between proteins (Inada et al., 2004). Two family members lack the BTB domain in the Arabidopsis, namely AtNRL4 and AtNRL12 (Christie et al., 2018; Pedmale et al., 2010). The homologous gene LsNRL4 in lettuce lacks the BTB domain and plays an important role in controlling chlorophyll content, chloroplast development, and secondary cell wall development (An et al., 2022). In potato, six family members do not contain the BTB domain (Figure 3). In addition to functioning as a substrate adapter with CUL3 to form an E3 ligase, the BTB domain of NPH3 in Arabidopsis also forms a heterodimer in yeast (Roberts et al., 2011). For these family members lacking the BTB domain, they may not interact with CUL3 to form an E3 ligase and may hence function differently from others.
NRL family members usually act in concert with phototropins and function as modules to regulate various aspects of light signaling processes. Based on the involvement of NRL proteins, these physiological responses are separated into two categories. In the NRL-dependent category, NRL proteins participate in phototropism, petiole positioning, leaf expansion, and chloroplast accumulation (Inoue et al., 2008; Motchoulski & Liscum, 1999; Sakai et al., 2000; Suetsugu et al., 2016). In potato, StNRL-10 is the only reported NRL family member. StNRL-10 interacts with phototropins and regulates plant immunity against P. infestans (Naqvi et al., 2022). In this study, only 4 pairs of positive interactions were identified (Figure 6). In Arabidopsis, NPH3, RPT2, and NCH1 were reported to interact with phot1 (Inada et al., 2004; Motchoulski & Liscum, 1999; Suetsugu et al., 2016). However, the corresponding orthologous proteins StNRL-3 and StNRL-22 did not interact with Stphot1 at least in yeast. The different results may be due to the nuclear yeast system. NRL proteins, such as NPH3 and RPT2, are plasma membrane-localized proteins. Other protein interaction systems should be applied to determine their interactions.
Among the 7 pairs of intraspecific duplication events, StNRL-6 and StNRL-10 show high similarity to each other, indicating that the two segmentally duplicated genes might have similar functions. Interestingly, StNRL-6 and StNRL-10 both interact with Stphot1, while StNRL-10 also interacts with Stphot2 (Figures 6 and 7). StNRL-10 acts as a “S” factor, which negatively regulates late blight resistance (He et al., 2018; Naqvi et al., 2022), similar to what we observed with StNRL-6 (Figure 8). What's more, INF1-triggered cell death can be suppressed by StNRL-10 but not by StNRL-6 (Figure S5). These results suggested that the two genes may come from genome duplication, but suffer functional differentiation. Y2H assay showed the interaction between StNRL-6 and StNRL-10 (Figure S7). StNRL-10 can form a homodimer (He et al., 2018). Here, we found that StNRL-6 also can form a homodimer and a heterodimer (Figure S7). It has been reported that StNRL-10 relies on the two conserved residues in the BTB domain to form dimerization (He et al., 2018). Substance interaction and ubiquitination are lost when the conserved pocket residues in the BTB domain are mutated (Genschik et al., 2013; Melnick et al., 2002). Since StNRL-6 and StNRL-10 contain the BTB domain, they probably form dimers based on the BTB domain. However, StNRL-7, which also contains the BTB domain in the N-terminal, did not interact with StNRL-6 (Figure S7), indicating that the key amino acid residues decided the dimerization in the NRL proteins. Whether the heterodimer or the homodimer is necessary and how the two types of dimerizations regulate plant immunity during the interaction with P. infestans still need to be further verified.
NPH3 and RPT2 are the founding members of the NRL gene family and are well-characterized in Arabidopsis. NPH3 and other NRL family members are the substrates of upstream kinase phot1. The conserved C-terminal sequence is important not only for binding with 14–3-3 proteins but also for phosphorylation by phot1 (Reuter et al., 2021; Sullivan et al., 2021; Waksman et al., 2023). The phosphorylation of the C-terminal RxSxS motif and subsequent 14–3-3 binding is important for the function of RPT2 and NPH3 (Reuter et al., 2021; Sullivan et al., 2021; Waksman et al., 2023). When the RxSxS phosphorylation site in NPH3 was mutated, the ability of NPH3 to promote phototropism in etiolated seedlings was diminished (Reuter et al., 2021; Sullivan et al., 2021), while the transgenic Arabidopsis seedlings expressing the RPT2 RxSxS mutant showed reduced phototropism (Waksman et al., 2023). These illustrated that the phosphorylation by phot1 at the C-terminal RxSxS site and concomitant binding with 14–3-3 were necessary for the full ability of phototropic responsiveness. In this study, we identified 13 potato NRLs among the 35 members containing the C-terminal RxSxS motifs. Besides the C-terminal RxSxS motif, StNRL-6 also has an N-terminal RxSxS motif. Mutation of the C-terminal RxSxS motif but not the N-terminal RxSxS motif made a significant difference in the disease lesion development on N. benthamiana leaves when challenged with P. infestans. This indicated that the conserved C-terminal RxSxS motif was important for StNRL-6 to regulate plant immunity. The C-terminal RxSxS motif of StNRL-6 is probably the phosphorylation site by Stphot1. Mutation of this site caused a much weaker interaction intensity (Figure 9). The Y2H also found the putative interaction proteins 14–3-3 isoforms (data not shown), which supported that the C-terminal RxSxS motif was vital in late blight resistance regulation and 14–3-3 binding. The C-terminal RxSxS motif is conserved in the NRL family, which suggests that phosphorylation, 14–3-3 binding, and even function for regulating of plant immunity may be universal for the NRL proteins.
5 CONCLUSIONS
In this study, the potato NRL family genes were comprehensively characterized for the first time. Thirty-five NRL family genes were identified by genome-wide analyses in potato. Their chromosomal localizations, phylogenetic relationships, gene structure, conserved domains, motifs, gene duplication, syntenic relationships, and expression patterns were analyzed. Phylogenetic analysis showed that 35 NRL genes belonged to 6 subgroups. Seven segmental duplication events were identified to play a major role in the expansion of potato NRL family genes. Interspecific syntenic relationship analysis between NRL genes from potato, tomato, rice, and Arabidopsis provides valuable clues for the evolutionary characteristics of the potato NRL genes. Moreover, we confirmed the interaction modes between Stphots and StNRLs and identified two phototropin-receptor-interacting NRL proteins. Furthermore, we demonstrated that two StNRL proteins acted as “S” factors and negatively regulated late blight resistance. Conserved motif RxSxS in C-terminal of StNRL-6 was further investigated on its effect on protein interaction and plant immunity regulation. Our results provided valuable information for further comprehensive functional dissection of NRL genes in potato.
AUTHOR CONTRIBUTIONS
Qin He, Zhendong Tian, and Dong Cheng conceived and designed this research. Dong Cheng carried out most of the experiments, conducted the bioinformatic analyses, analyzed the results, and wrote the manuscript. Huishan Qiu helped in co-immunoprecipitation assay. Tianyu Lin and Jiahui Nie helped in P. infestans infection assay. Lang Liu collected the potato leaves samples with P. infestants infection at the indicated time course. Dan Zhou participated in vectors construction and plants cultivation. All authors read and approved the final manuscript. We thank all members in the potato group at Huazhong Agricultural University for their support during this project.
ACKNOWLEDGMENTS
This research was supported by the National Natural Science Foundation of China (grant Nos. 32072409, 32072121, 32372172). We sincerely appreciate the generous help and valuable support from Prof. Paul Birch (University of Dundee, UK).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study will be made available from the corresponding author upon reasonable request.




