COI1-mediated jasmonic acid signalling regulates mycorrhizal colonisation intensity and is an indispensable component of mycorrhiza-induced resistance
Abstract
Hormonal signalling plays an elementary role in the regulation of plant-microbe interactions. Jasmonic acid (JA) signalling is one of the major regulators that decides the fate of these interactions in plants. However, the role of JA is not unanimous and varies from neutral to positive or negative regulation. In the present study, we targeted SlCOI1, a key gene in JA signalling, by silencing (using artificial miRNA approach) and overexpressing constitutively in tomato transgenic hairy roots (HR) system. We developed in-vitro colonisation of these hairy roots with model arbuscular mycorrhizal fungi (AMF) Rhizophagus irregularis. The colonised HR exhibited a stronger induction of PAMPs-triggered immunity (PTI) response and reactive oxygen species (ROS) homeostasis against Fusarium oxysporum f. sp. lycopersici (FOL). The ROS signalling key gene RBOH-B and the PTI response marker genes LRR22 and PTI5 were expressed at an earlier stage and at a higher amplitude in the colonised HR, providing evidence for mycorrhiza-induced resistance (MIR). Further, SlCOI1 silencing resulted in a higher degree of R. irregularis colonisation, while overexpression restricted its proliferation. Intriguingly, despite a higher degree of colonisation, the SlCOI1-silenced HR lines manifested a weakened MIR compared to its control colonised HR line. This univocally indicates an indispensable role of COI1-mediated JA signalling in MIR. This is one of the few studies conducted using in-vitro AMF-colonised HR system to understand how signalling events occurred during AMF colonisation and tripartite interactions, specifically MIR.
1 INTRODUCTION
Plants interact with both beneficial and pathogenic microbes throughout their life. These interactions are dynamic and involve various mechanisms on either side of the interacting partners (Vishwakarma et al. 2020). Plants deploy an array of signalling mediated through phytohormones, reactive oxygen species (ROS), etc., to govern these interactions (Fahad et al. 2015; Gupta et al. 2020b). Phytohormones, specifically salicylic acid (SA) and jasmonic acid (JA), play a pivotal role in regulating the entry and proliferation of microbes. The specificity of the phytohormonal signalling is determined by the nature of interacting microbes. For instance, SA signalling is activated in response to biotrophic fungi, while the necrotrophs are known to induce JA signalling (Kadam and Barvkar 2024). Regardless, the role of JA signalling in defence against necrotrophs remains controversial. For example, JA signalling is hijacked by Fusarium oxysporum, leading to severe wilting in Arabidopsis, while it remained unaffected in tomato–F. oxysporum interaction (Thatcher et al. 2009; Di et al. 2017). CORONATINE INSENSITIVE 1 (COI1) protein acts as the main regulatory switch of the JA signalling pathway. Elevated levels of JA-isoleucine, a biologically active conjugate of JA, initiate JA signalling by binding to COI1 (Li et al. 2004). The COI1-JA-isoleucine complex then brings about the proteolytic degradation of JA signalling repressor protein, JASMONATE ZINC-FINGER EXPRESSED IN INFLORESCENCE MERISTEM (ZIM)- ZINC-FINGER EXPRESSED IN INFLORESCENCE MERISTEM (ZIM)- DOMAIN (JAZ), by 26S proteasome (Katsir et al. 2008; Gupta et al. 2020a).
Complex signalling events are known to occur when plants come in contact with symbiotically associating microbes, like arbuscular mycorrhizal fungi (AMF). AMF exhibit symbiotic association with nearly 80% of land plants. They are obligate symbionts and solely depend on plants for carbon source. In return, AMF provides soil nutrients, mainly phosphorus, to the plants. Though plants benefit from this symbiotic relationship, they need to keep a check on AMF proliferation to avoid the overburden on photosynthetic activity resulting from an increased carbon demand of thriving AMF. Thus, at an early stage and even after the establishment of colonisation, plants recruit a complex cascade of phytohormone-signalling for regulation of AMF association. The phytohormone SA is known to increase at an early stage of colonisation but, at the later stages, is brought down to a basal level. On the other hand, JA synthesis and signalling remain upregulated at all stages of the colonisation (Herrera Medina et al. 2003; Pez-Ra′ez et al. 2010; Nair et al. 2015; Hajiboland and Ahammed 2024).
During plant–pathogen interactions, the pathogen-associated molecular patterns (PAMPs) are recognised by pattern-recognition receptors (PRRs) of plants (Li et al. 2010; Zipfel and Robatzek 2010). The PAMPs–PRRs interaction effectuates PAMPs-triggered immunity (PTI), a first step in the defence against pathogens. The activated plant receptors, for development of PTI response, initiate relay of signalling events like induction of calcium fluxes, phytohormonal signalling, and activation of mitogen-activated protein kinase (MAPK) cascades (Wang et al. 2022). The reactive oxygen species (ROS) wave is generated to systemically spread the PTI response across the plant (Zhang and Zhou 2010). The failure of ROS and PTI response compromises the plant immunity (Yuan et al. 2021; Yu et al. 2024). The association of AMF can influence these defence responses and it provides plants with an induced resistance against various pathogens, a phenomenon termed mycorrhiza-induced resistance (MIR). For example, tomato plants colonised with AMF Funneliformis mosseae and Rhizoglomus irregulare showed effective MIR against the fungal pathogen Botrytis cinerea (Hoz et al. 2021; Dejana et al. 2022). The foliar pathogen Alternaria solani, which causes early blight disease, displayed restricted proliferation in AMF-colonised tomato plants (Song et al. 2015). Similar reports of MIR from other crops like wheat and rice are registered against an array of pathogens (Fiorilli et al. 2018; Campo et al. 2020; Sanmartín et al. 2020). However, few studies also describe the negative effects of AMF, wherein AMF colonisation hampers plant immunity, making plants susceptible to pathogen attack (Simon et al. 2017; Bernaola et al. 2018).
In light of these controversies, the impact of AMF colonisation on plant's defence as well as the role of JA signalling in plant–microbe interactions are not unanimous. Therefore, in the first part of this study, we developed an in-vitro symbiotic system between tomato hairy root (HR) and AMF Rhizophagus irregularis to explore MIR against a fungal pathogen, Fusarium oxysporum f. sp. lycopersici (FOL).
Subsequently, we overexpressed and silenced a JA signalling key gene, COI1, in tomato hairy root (HR) system to assess the impact of JA signalling on AMF colonisation. To corroborate the findings, we took advantage of the available tomato JA signalling mutant jai1-1, and developed a COI1 complementation HR line. The complementation of COI1 expression provided compelling evidence of the contribution of JA signalling to AMF colonisation. Moving ahead, we assessed ROS and PTI response to evaluate the vitality of JA signalling in MIR against FOL PAMPs using this in-vitro system.
2 MATERIALS AND METHODS
2.1 Establishment of tomato hairy root culture
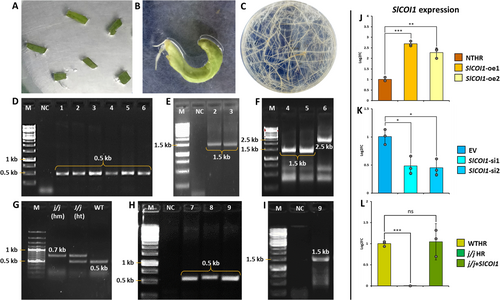
The hairy root cultures from tomato leaf explants were established as mentioned previously (Kadam and Barvkar 2024). In brief, the cotyledonary leaves of tomato (Solanum lycopersicum) cv. money maker were pre-cultured on half-strength Murashige and Skoog (MS media) for two days. Overnight grown culture from a single colony of Rhizobium rhizogenes (strain ARqua1) was used to grow secondary culture until the OD600 reached 0.4. Explants were co-cultivated with bacterial suspension for 15 min with intermittent shaking followed by blotting on sterile Whatman filter paper to remove excess bacterial load. These explants were incubated in darkness for three days on MS media containing 150 μM of acetosyringone. After three days, the explants were observed for bacterial over-growth and washed with cefotaxime (100 mg/L) and further maintained in the dark on half-strength MS media fortified with the same concentration of cefotaxime. The emergence of hairy roots was observed around 8–10 days. These roots were excised from explants once their length reached 2–3 cm and were then transferred to half-strength MS media containing 100 mg/L cefotaxime. Genomic DNA was extracted from the putative hairy root lines using the CTAB method and rolc (a bacterial gene integrated in plant genome) PCR-positive lines were treated as the confirmed hairy root lines (Figure 1, See Table 1 for primer sequences).
| Sr. no | Accession no. | Gene name | Gene function | Purpose | Primer sequence |
|---|---|---|---|---|---|
| 1 | Solyc05g052620.3 | SlCOI1 | JA signalling | Cloning pCambia 1301 + SlCOI1 cassette | S: 5′-ACTGCCATGGAGGAACGGAACTCAA-3′ |
| AS: 5′-ACTGCACGTGTCAGCGAGAAGGTAA-3′ | |||||
| Cloning pMDC32B-AtMIR390a-B/c-SlCOI1 cassette | S: 5′-TGTATCTCGTTGAGTTCCGTTCCTGATGAT GATCACATTCGTTATCTATTTTTTCAGGAACG GACCTCAACGAGA-3′ |
||||
AS: 5′-AATGTCTCGTTGAGGTCCGTTCCTGAAAA AATAGATAACGAATGTGATCATCATCAGGAA CGGAACTCAACGAGA-3′ |
|||||
| PCR confirmation of pCambia 1301 + SlCOI1 HR (1.5 KB) | S: 5′-GGAGAATGGGCACATGAA-3′ | ||||
| AS: 5′-CGATCTAGTAACATAGATGACAC-3′ | |||||
| PCR confirmation of pMDC32B-AtMIR390a-B/c-SlCOI1 HR (1.5 KB) | S: 5′-GTAAAACGACGGCCAG-3′ | ||||
| AS: 5′-GCAAGACCGGCAACAGGATT −3′ | |||||
| PCR confirmation of pMDC32B-AtMIR390a-B/c empty vector HR (2.5 KB) | S: 5′-CCACTGACGTAAGGGATG-3′ | ||||
| AS: 3′-CAGGAAACAGCTAGAC-3′ | |||||
| qPCR | S: 5′-CTGGTCGTGATCTCTTAGCG-3′ | ||||
| AS: 5′-CGGCAAGAGAATAGTAGGCA-3′ | |||||
| 2 | MT514512.1 | rolc | Oncogene from R. rhizogenes |
PCR confirmation of HR | S: 5′-CATTAGCCGATTGCAAACTTG-3′ |
| AS: 5′-ATGGCTGAAGACGACCTG-3′ | |||||
| 3 | Solyc06g051850 | PT4 | Mycorrhiza inducible tomato phosphate transporter | qPCR | S: 5′-GGCCCCAATTCAACCACA3′ |
| AS: 5′-ATTGCCCCTGCCTTACCAGA3′ | |||||
| 4 | Solyc02g077370 | PTI5 | PTI response | qPCR | S: 5′-CGTCCATTACAGTGCATAG-3′ |
| AS: 5′-GAACGTACCTAGCCATAC-3′ | |||||
| 5 | Solyc01g106620 | PR1 | SA signalling | qPCR | S: 5′-TGCTGTGAAGATGTGGGTTG-3′ |
| AS: 5′-CACCCGTTGTTGCACCTGA-3′ | |||||
| 6 | Solyc03g117980 | RBOB-B | ROS signalling | qPCR | S: 5′-AGGGAATGATAGAGCGTCG-3′ |
| AS: 5′-CATCGTCATTGGACTTGGC-3′ | |||||
| 7 | Solyc08g066210 | LRR22 | PTI response | qPCR | S: 5′-GGGAAGAAGAGAGTTTCCTTGAG-3′ |
| AS: 5′-AGTGCAGTCATGGTGCATATAA-3′ | |||||
| 8 | Solyc06g005060 | EFα1 | House keeping gene | qPCR | S: 5′-AAGCGTGGTTATGTTGCCTCA-3′ |
| AS: 5′-TGGGAAGTGTGGCAGTCAAG-3′ |
The SlCOI1 overexpression and silencing cassettes reported earlier (Kadam and Barvkar 2024) were used to generate transgenic hairy root events. To silence SlCOI1 expression, artificial miRNA approach was employed using pMDC32B-AtMIR390a-B/c vector, whereas the overexpression of SlCOI1 gene was achieved with the help of pCambia 1301 vector. The hairy roots emerging from different explants (and from different locations of the same explant) were considered as independent events. These events were confirmed by PCR using primers targeting a region within the T-DNA. The SlCOI1 expression in transgenic events was quantified by qPCR. For further studies involving SlCOI1 overexpression and silenced lines, two events with the highest (named as SlCOI1-oe1 & SlCOI1-oe2) and lowest (named as SlCOI1-si1 & SlCOI1-si2) expression of SlCOI1 were selected, respectively. Two more HR events, i.e. non-transgenic (NTHR) and empty vector (EV), were included as control for SlCOI1 overexpression and silenced lines, respectively.
SlCOI1 complementation HR line was developed using tomato COI1 mutant jai1-1. Seeds of the mutant were generously provided by Prof. Gregg Howe, Michigan State University, USA. The in-vitro-grown seedlings were screened for wild type (WT) and jai1-1 homozygous (j/j) genotypes by zygosity PCR as described by Li et al. (2004). The wild type HR line (WTHR) was developed from WT seedlings using ARqua1 strain devoid of any vector. On the other hand, the jai1-1 HR (j/j HR) and SlCOI1 complementation HR (j/j + SlCOI1) lines were developed using j/j mutant seedlings. To generate j/j HR line, ARqua1 without any inserted plasmid was used, while j/j + SlCOI1 HR line was developed using ARqua1 carrying pCambia 1301 + SlCOI1 cassette.
All transgenic events were maintained on hygromycin (20 mg/L) selection media till the 6th sub-culture and later were transferred to selection-free half-strength MS media. The non-transgenic events were maintained on selection-free media. At every sub-culture, PCR for rolc, and respective transgenic cassettes was carried out. The stable events were maintained as HR lines and used for further experimentation. The details of primers used for confirmational PCR are provided in Table 1.
2.2 Establishment of in-vitro symbiotic association between R. irregularis and tomato HR
The spores of R. irregularis (MUCL 57021) were shared by SOM Phytopharma (India), Ltd. The spores were surface-sterilised aseptically. Approximately 100 spores were washed with 90% ethanol, 2% calcium hypochlorite and 2% Chloramine-T. In between each wash, three water washes were incorporated to remove the residues of the reagents used. Finally, the sterilised spores were stored in an antibiotic solution (streptomycin 50 mg/L) till further use. A standard Modified Strullu and Romand (MSR) medium was used for the establishment of in-vitro symbiosis. The sterilised spores were inoculated on MSR medium under an inverted microscope (Olympus) and observed for five days to check fungal contamination. The non-contaminated spores were inoculated on fresh media plates near the growing tips of HR. Five root fragments (2–3 cm in length) were placed in each plate to increase the probability of colonisation. The colonisation was confirmed after five weeks by trypan blue staining and PCR amplification of mycorrhiza-inducible phosphate transporter PT4 using cDNA (Figure 2).

2.3 Measurement of extent of mycorrhizal colonisation
Roots were boiled in 10% KOH for 10 min and washed thrice with water followed by acid treatment of 2% HCl for 15 min. The acidified roots were stained with 0.5% trypan blue for 2 h, followed by three water washes prior to microscopy. To estimate the intensity of mycorrhizal colonisation (M%), 30 root segments (1 cm each) per plate were examined under light microscope at 10X magnification (Trouvelot et al. 1986). Three such plates were considered as three independent biological replicates.
2.4 Fungal crude extract treatment
The FOL culture (MTCC accession no. 10270) was procured from Microbial Type Culture Collection, Chandigarh, India. The spores were inoculated on potato dextrose agar at 28°C for a week. The spores from this plate were inoculated in potato dextrose broth and incubated at 28°C in a shaking incubator at 150 rpm for five days. This broth was then used to prepare the fungal crude extract following the protocol reported by Redkar et al. (2022). For this, the culture was passed through a four-layered autoclaved muslin cloth and the residual mycelia were re-suspended in autoclaved water (30% fresh weight/volume). The suspension was boiled in a water bath for 20 min at 100°C. The OD600 of the extract was adjusted to 0.5 with autoclaved water and stored at −20°C. The crude extract (reached at ambient temperature) was applied to the HR plates (approximately 10 mL/ plate) and tissue was harvested three days after the application. For time point assay, tissue was harvested 24 and 48 h post extract treatment.
2.5 Quantitative real-time PCR (qRT-PCR) assay for gene expression analysis
Total RNA was extracted from 100 mg tissue by TRizol (Sigma) method as per the manufacturer's instructions. cDNA was synthesised from DNase (Thermo) treated RNA (1 μg) by Thermo Revert Aid kit using Oligo-dT primers. The cDNA was diluted with nuclease-free water (NFW) in the ratio of 1:4 and used for qRT-PCR on CFX96 Real-Time System (Bio-Rad). In the 10 μL qRT-PCR reaction, 5 μL SYBR (Luna, Universal qPCR Master Mix, NEB), 0.1 μM forward and reverse primer each, and 1 μL cDNA were added. The final volume was adjusted to 10 μL with NFW. The two-step PCR amplification was set as-95°C/2 min, 40 cycles of 95°C/30 s and 55°C/30 s followed by melt curve analysis ramped from 60°C to 95°C. The expression of target genes was normalised against the expression of housekeeping gene, ELONGATION FACTOR α1 (EFα1). The relative expression was quantified using 2− ΔΔCt method and reported as log2 fold change (Livak and Schmittgen 2001). The gene expression analysis was carried out for three biological replicates, and the details of genes and their primers are provided in Table 1.
2.6 Nitroblue tetrazolium (NBT) and 3,3′-Diamonibenzidine (DAB) staining
NBT staining assay was performed using 0.2% solution of NBT (Himedia) in sodium phosphate buffer (pH 7.5), whereas for DAB staining, 0.1% w/v DAB (Himedia) solution was prepared in water (pH 3.8). The staining protocol included incubation of root segments in NBT and DAB solutions for 1 hr. at 37°C in the dark. The roots were removed from the staining solution and placed on a tissue paper towel saturated with 60% glycerol. The stained roots were observed under a microscope (Nikon SMZ1270) attached with camera MIchrome 6 and photographed. The stain intensity was calculated by taking a mean of five root intensities quantified using the ImageJ software.
2.7 Statistical analysis
Three biological replicates were considered for all statistical analyses. One-tailed Student's t-test was applied to the groups and the values having p < 0.05 were considered significant (*p < 0.05, **p < 0.01, ***p < 0.001).
3 RESULTS
3.1 SlCOI1 silencing and overexpression significantly affected SlCOI1 levels in HR lines
The presence of rolc, a bacterial gene integrated in the plant genome, was checked in HR lines by PCR to determine positive lines. The rolc-positive HR lines were further checked for respective transgenic cassettes and evaluated for the expression of SlCOI1 gene by qPCR. The expression of SlCOI1 in silenced lines was significantly downregulated with transcript levels dropping to nearly half of those in EV control line. The SlCOI1 expression was reduced to 0.48 (±0.15) and 0.45 (±0.14) folds in SlCOI1-si1 and SlCOI1-si2 lines, respectively, compared to that in EV. On the other hand, SlCOI1 overexpression HR lines showed a two-fold elevation in SlCOI1 expression compared with NTHR control. This elevation was 2.69 (±0.11) and 2.27 (±0.24) folds higher in SlCOI1-oe1 and SlCOI1-oe2, respectively when compared to that in NTHR line (Figure 1G). Furthermore, complementation of jai1-1 mutant HR with SlCOI1 restored SlCOI1 levels in j/j + SlCOI1 HR and were equivalent, i.e. 1.04 (±0.31) folds, to those in WTHR line, whereas j/j mutant HR demonstrated nil expression of SlCOI1.
3.2 qPCR and trypan blue staining exhibited a unanimous correlation in SlCOI1 expression and the degree of colonisation
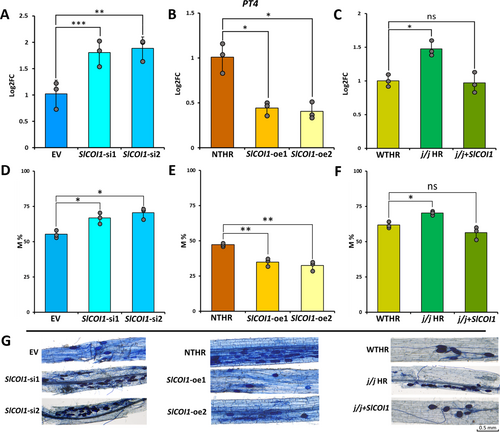
The extent of R. irregularis colonisation in HR was evaluated by quantifying the expression of the tomato PT4 marker gene. The expression of PT4 was higher in both si-SlCOI1 lines, indicating higher extent of colonisation in SlCOI1-silenced lines compared to EV. The expression was found to be 1.80 (± 0.24) and 1.88 (± 0.21) folds more in SlCOI1-si1 and SlCOI1-si2 lines, respectively, than that in EV control. On the contrary, both SlCOI1-overexpression lines exhibited a significant reduction in the expression of PT4. It was reduced to 0.44 (± 0.07) and 0.40 (± 0.09) folds in SlCOI1-oe1 and SlCOI1-oe2, respectively, in comparison to its expression in NTHR line, suggesting a lower degree of AMF colonisation. In the complementation experiment, the mutant j/j HR line had 1.47 (± 0.11) folds significant increase in the expression of PT4 than in WTHR control. On the contrary, the difference in PT4 levels between the complemented j/j + SlCOI1 line and WTHR line was insignificant, with a minimal lowering of PT4 expression to 0.97 (± 0.15) folds in j/j + SlCOI1 line.
The trypan blue staining technique is used for confirmation and quantification of AMF colonisation by staining AMF vesicles and arbuscucles. In the present study, the trypan blue staining results were congruous with the findings of qPCR data. It revealed that the intensity of colonisation (M%) diminished in both SlCOI1-oe1 and SlCOI1-oe2 lines but increased significantly in SlCOI1-silenced lines (Figure 3). The M% was found to be 47.11% (± 1.18), 34.887% (± 2.72) and 32.34% (± 3.39) in NTHR, SlCOI1-oe1, and SlCOI1-oe2 lines, respectively. In contrast, in SlCOI1-si1 and SlCOI1-si2 lines, it was recorded as 66.75% (±3.90), 70.37% (±4.04), respectively with 55.16% (2.68) in the EV line. Analogous to qPCR results of PT4, WTHR and j/j + SlCOI1 lines exhibited more or less similar intensity of colonisation, viz. 61.7% (± 2.14) and 56.32% (± 4.51), respectively while 70.25% (± 1,40) of M% was recorded in the j/j HR line (Figure 3). Further, the expression of SA signalling gene PR1 was also checked in the colonised NTHR and SlCOI1-overexpression lines. The PR1 transcript levels in NTHR, SlCOI1-oe1 and SlCOI1-oe2 lines were comparable to one another. Compared to PR1 levels in colonised NTHR line, SlCOI1-oe1 exhibited a marginal but insignificant 1.27 (±0.13) folds change, while SlCOI1-oe2 showed equivalent transcript level, i.e. 1.09 (±0.08) folds (Figure S1).
3.3 AMF-colonised HR demonstrated a higher accumulation of transcripts involved in PTI and ROS signalling
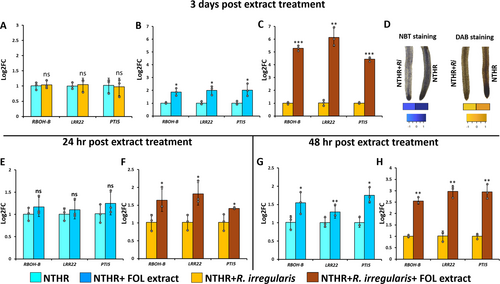
The expression of RBOH-B, a gene involved in ROS signalling, and two PTI marker genes, LRR22 and PTI5, was evaluated in AMF-colonised and non-colonised NTHR lines. The basal expression levels of these three genes, i.e. without FOL extract treatment, were comparable in the colonised and non-colonised NTHR lines with insignificant differences (Figure 4A). The expression of these genes was further evaluated three days after the fungal crude extract treatment. The treatment induced expression of all these genes in both colonised and non-colonised HR lines. However, the increment was much higher in AMF-colonised NTHR than that in non-colonised NTHR line when compared to their respective controls. The rise in the expression of RBOH-B, LRR22 and PTI5 genes was 1.86 (±0.28), 2.00 (±0.34) and 2.02 (±0.48) folds in the non-colonised HR line (Figure 4B), whereas that in AMF-colonised NTHR line was found to be 5.28 (±0.20), 6.15 (±0.71) and 4.43 (±0.16) folds, respectively (Figure 4C). This indicates that the AMF-colonised line demonstrated a better defence-response against fungal pathogen.
3.4 AMF-colonised and non-colonised HR lines showcased distinct NBT and DAB staining intensities after FOL treatment
The NBT and DAB staining intensities were assessed in FOL-treated AMF-colonised and non-colonised HR lines. The non-colonised HR was more intensely stained in both the staining assays in comparison to the colonised HR line. s The NBT stain (pixel) intensities were 128.66 (±4.58) in colonised HR versus 166.33(±4.16) in non-colonised HR line. DAB staining also showed a similar trend wherein the stain intensity in non-colonised HR line (257.11 ± 2.54) was much greater than that in the colonised HR line (176.44 ± 9.52) (Figure 4D). These results denote that the non-colonised HR line accumulated more intra-cellular ROS in response to FOL treatment than the level of ROS in AMF-colonised HR, suggesting a possibly activated ROS-scavenging mechanism in AMF-colonised HR line.
3.5 The time course assay manifested rapid induction of the expression of ROS signalling and PTI marker genes in colonised HR
Owing to the differential expression pattern of ROS signalling and PTI marker genes observed in the colonised and non-colonised HR lines, we performed a time course assay to assess the expression of RBOH-B, LRR22 and PTI5 genes in colonised and non-colonised NTHR at 24 hrs and 48 hrs post FOL treatment. Non-colonised NTHR exhibited slightly but insignificantly upregulated expression of RBOH-B (1.16 ± 0.21 folds), LRR22 (1.10 ± 0.20 folds), and PTI5 (1.24 ± 0.25 folds) 24 hrs after the treatment of fungal extract (Figure 4E). On the other hand, the colonised NTHR revealed more prominent elevation in gene expression after 24 hrs of FOL treatment. RBOH-B, LRR22 and PTI5 showed 1.64 (±0.31), 1.81 (±0.32), and 1.41 (±0.02) folds increase, respectively, compared to their corresponding controls post 24 hrs of fungal treatment (Figure 4F). Relative to the pattern observed at 24 hrs post FOL treatment, the PTI response increased at 48 hrs in both colonised and non-colonised NTHR. Yet again, it was more prominent in colonised NTHR than non-colonised NTHR. The non-colonised NTHR exhibited 1.54 (±0.29), 1.29 (±0.31) and 1.74 (±0.20) folds increase in the expression of RBOH-B, LRR22 and PTI5 genes, respectively (Figure 4G), whereas the colonised NTHR showed 2.55 (±0.13), 2.97 (±0.25) and 2.94 (±0.32) folds rise in expression, respectively (Figure 4H).
3.6 qPCR indicated SlCOI1 silencing resulted in weakened MIR in colonisedSlCOI1-silenced HR lines
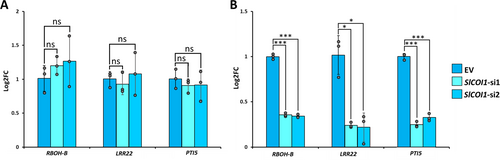
To evaluate the possible impact of SlCOI1 silencing on the basal expression of RBOH-B, LRR22, and PTI5 genes, we assessed their expression in EV and SlCOI1-silenced lines. qPCR results revealed that there was no significant difference in the expression of these genes between EV and SlCOI1-silenced lines (Figure 5A). Further, to assess the role of SlCOI1 in the colonisation-induced PTI response, the expression of these genes was checked in colonised EV, SlCOI1-si1, and SlCOI1-si2 lines post three days of fungal extract treatment. Compared to EV, the expression of RBOH-B was reduced to 0.34 (±0.01) folds, while the expression of PTI marker genes LRR22 and PTI5 was reduced to 0.21 (±0.16) and 0.32 (±0.03) folds in SlCOI1-si1 line. Similarly, in SlCOI1-si2 line, RBOH-B, LRR22, and PTI5 expression was reduced to 0.35 (±0.01), 0.24 (±0.02), and 0.25 (±0.03) folds, respectively, compared to that in EV. Taken together, SlCOI1 silencing resulted in a significant reduction in the mycorrhiza-induced PTI response of HR lines (Figure 5B).
4 DISCUSSION
4.1 The transgenic HR lines and in-vitro symbiosis system provide a platform to understand the concordant role of COI1-mediated JA signalling in symbiosis and induced resistance
In their life span, plants are in continuous interactions with different types of microbes. The plant–microbe interactions are governed at molecular, metabolite and cellular levels in plants. Ca2+ spiking and hormonal signalling are the earliest events that occur during these interactions (Ruth et al. 2021; Bhar et al. 2023). In general, Ca2+ spiking ignites downstream signalling, bringing the presence of microbes to light. For example, Ca2+ spiking activates the SYM pathway to establish a symbiotic association between AMF and plants (Genre et al. 2012), the fate of which is further decided by hormonal signalling. As stated earlier, JA has always emerged as a major player in hormonal signalling, and its crosstalk with other phytohormones is an essential component of plant-microbe interaction (Aerts et al. 2021). Though essential for plant–microbe interactions, JA signalling may also hamper the growth of plants. Hence, JA is under tight regulation in resting conditions, during which it is in the suppressed form modulated by the JAZ repressors. A key component of this system is the COI1 protein, which acts as a master switch to initiate the JA signalling (Turner et al. 2002). Thus, in the present study, we targeted SlCOI1 to understand its regulatory role (and specifically the nature of the regulation; i.e., positive or negative) in plant–microbe interactions. The overexpression and silencing of this gene provided information about the role of COI1-mediated JA signalling, firstly, in mycorrhizal symbiotic association and, secondly, in plant defence, specifically MIR.
MIR is reported to be influenced by different soil types (specifically rhizospheric soil) and surrounding environmental conditions (Santoyo et al. 2021). It is proposed that AMF modulates the rhizospheric microbiome, which directly restricts the growth of pathogenic microbes (Offre et al. 2007) or indirectly by promoting the growth of bacterial population in the rhizosphere having an antagonistic correlation with soil pathogens (Hodge and Storer 2014). Thus, MIR is considered a variable priming defence rather than a sophisticated, robust molecular response and, most importantly, it is attributed to the consortium of the rhizosphere in lieu of AMF alone. In the present study, the standalone role of AMF in plant defence was evaluated using an in-vitro symbiotic system.
4.2 COI1-mediated JA signalling regulates the extent of R. irregularis colonisation
The AMF symbiosis and its association with JA synthesis and signalling is studied in a variety of plant systems. In barley, the endogenous JA level was elevated in AMF-colonised plants, which further facilitated colonisation (Hause et al. 2019). But, in the case of rice JA biosynthesis-deficient mutant photomorphogenesis 2 (cpm2), there was no effect on the extent of AMF colonisation, suggesting no regulatory impact of JA level on AMF colonisation (Gutjahr et al. 2015). Similarly, JA was found to play a neutral role in the interaction between AMF and Nicotiana (Riedel et al. 2008). On the other hand, JA signalling played a positive regulatory role in Medicago. Repeated wounding triggered JA signalling, which is reported to increase AMF colonisation and its functionality in terms of active transport of phosphate to the plants (Landgraf et al. 2012). The promotionary, negative or neutral roles of JA perception and synthesis are mostly verified with the ‘loss of function’ mutants. However, the impact of constitutive JA signalling on AMF colonisation is not yet reported. By means of overexpression and silencing of SlCOI1 in tomato HR, in this study, we attempted to corroborate the role of COI1-mediated JA signalling in AMF colonisation depicted using JA signalling mutants.
In the present study, the SlCOI1-silenced HR lines showed a high degree of colonisation, which probably suggests that the COI1-mediated JA signalling works as a negative regulator of AMF colonisation (Figure 3A,D). Nonetheless, the SlCOI1-overexpressed lines were not completely resistant to AMF colonisation. However, R. irregularis colonisation intensity was reduced to half in SlCOI1-overexpressed lines (Figure 3B,E). This impact of SlCOI1 overexpression is exactly reciprocal to that of SlCOI1-silencing, underscoring the regulatory importance of COI1-mediated JA signalling. Consistent results indicating JA-mediated negative regulation of AMF colonisation were reported by Herrera-Medina et al. (2008) in tomato JA signalling mutant jai1-1, in which JA perception was completely absent. The jai1-1 mutant exhibited a higher percentage of AMF colonisation (Herrera-Medina et al. 2008). However, the difference in colonisation degree was found to be insignificant between the wild type and jai1-1 mutant plants at the later time point. This implies that, in the absence of JA signalling, the restriction of colonisation is governed by other factors at later stages. One such factor could be SA signalling and the SA signalling-mediated systemic acquired resistance (SAR) (Foo et al. 2013). The constitutive SA synthesis was associated with reduced AMF colonisation, while the transgenic lines with hampered SA synthesis demonstrated prominent AMF proliferation in tobacco. This suggests that SA signalling may negatively regulate AMF colonisation in tobacco (Herrera Medina et al. 2003). Though SA and JA interactions are known to be antagonistic, a synergistic interplay between the two was recently evidenced (Mur et al. 2006; Yamada et al. 2012; Tamaoki et al. 2013). For example, SA receptors NPR3 and NPR4 directly activated JA synthesis and signalling in tobacco, developing a synergistic crosstalk between these two phytohormones (Liu et al. 2016). Hence, to test a possible role of SA signalling in restricting the proliferation of R. irregularis in SlCOI1-overexpressed lines, we evaluated the expression of the SA signalling marker gene PR1. The difference between its expression in colonised SlCOI1-overexpressed and NTHR lines was found to be insignificant (Figure S1). Thus, the restricted proliferation of R. irregularis in the SlCOI1-overexpressed lines can be linked to the COI1-mediated JA signalling. To further corroborate these findings, we assessed the intensity of colonisation in COI1-complemented jai1-1 HR line, and it was found to be equivalent to the wild type line (Figure 3C, F). This provides strong evidence for the restrictions imposed by the COI1-mediated JA signalling on AMF colonisation.
Taken together, in HR culture of tomato, the symbiotic association of AMF R. irregularis is predominantly governed by COI1-mediated JA signalling and the results suggest its negative regulatory role in the proliferation of AMF.
4.3 AMF symbiosis brings about prompt and profound PTI response
4.3.1 Strong PTI response and oxidative stress alleviation: witness to MIR in R. irregularis-associated HR
In plant defence, whether it is induced systemic resistance (ISR) or SAR, PTI serves as the first step to initiate the defence response. Therefore, we evaluated the PTI response in R. irregularis-colonised and non-colonised HR lines subjected to FOL by quantifying the expression of PTI marker genes LRR22, PTI5, and RBOH-B. The LRR22 gene is one of the membrane-bound PRRs that senses PAMPs and initiates the downstream signalling to establish PTI. Its expression is thus used to study the PTI response in an ample number of studies (Kim et al. 2009; Nguyen et al. 2010; Li et al. 2014). Once PAMPs are perceived, a ROS wave is induced by oxidases located on the plasma membrane; RBOH gene family are hence good candidates to study the ROS-signalling mechanism. Thus, we quantified the expression of RBOH-B gene (Li et al. 2015). Another PTI marker gene, PTI5, modulates ROS homeostasis and hormonal signalling in plant defence (TANG et al. 2022). The expression of these three genes revealed an increase in both colonised and non-colonised HR lines. The induction of RBOH-B, LRR22, and PTI5 genes in both HR lines provided evidence that the fungal crude extract was capable of inducing the PTI response in HR. Further, the amplitude of relative expression of these genes was higher in R. irregularis-colonised HR, hinting at a more prominent PTI response against fungal PAMPs in the extract (Figure 4). Though PTI is thought to be an initial basal defence against adopted pathogens, its significance in the development of a complete defence response cannot be overlooked. For example, Arabidopsis cerk1 mutant, which lacks the PRR receptor CERK1, was highly susceptible to the fungal pathogen Alternaria brassicicola (Miya et al. 2007). This mutant was also found to be highly susceptible to bacterial pathogen Pseudomonas syringae (Gimenez-Ibanez et al. 2009). Moreover, PTI and more sophisticated effector-triggered immunity (ETI) are reported to have overlapping mechanisms and are considered a continuum rather than two distinct modules of plant immunity (Thomma et al. 2011; Naveed et al. 2020). Hence, the evident PTI response revealed in the present study underscores the capability of AMF-colonised roots to further induce a complete defence cascade.
The accumulation of ROS in the preliminary stages of defence is normalised later by plants through the complex process of ROS homeostasis. Over-accumulation of ROS causes oxidative stress in plants, which can lead to cell damage and cell death through ROS-activated cell death-inducing signalling events (Tripathy and Oelmüller 2012). Our results demonstrated that the non-colonised NTHR lines were under higher oxidative stress, as depicted by NBT and DAB staining (Figure 4D). On the contrary, the R. irregularis-colonised NTHR lines showed alleviated levels of ROS, indicating an improved efficiency of AMF-colonised NTHR lines in recruiting ROS-scavenging system. Many species of necrotrophic fungi, such as Fusarium spp., strategically secrete phytotoxins like fusaric acids (FA). FA induces the in planta accumulation of ROS, which ultimately leads to cell death (Iqbal et al. 2024). Thus, the superiority of R. irregularis-colonised NTHR in alleviating oxidative stress, as displayed in NBT and DAB staining assay, advocates the effectiveness of the MIR in managing the oxidative stress induced during the defence against fungal pathogens.
4.3.2 R. irregularis-colonised roots generate a rapid PTI response
Though the colonised roots expressed a prominent PTI response, the non-colonised roots were also able to generate a similar response but at a lower magnitude three days after FOL treatment. We further carried out a time-course assay to compare the promptness of R. irregularis-colonised and non-colonised roots in perceiving PAMPs and ultimately initiating the PTI response. As depicted by qPCR results, the colonised roots were quicker to detect PAMPs from crude extract, hence initiating the ROS burst and finally inducing PTI response earliest (24 h). On the other hand, the non-colonised roots failed to show a significant PTI response at this time point and exhibited a delay in generating ROS signalling cascade and PTI response of significant magnitude (Figure 4E). At 48 h, the non-colonised HR managed to initiate these defence responses, whereas the colonised roots amplified the defence response at much higher scale at this time point (Figure 4F).
The delay in defence response can lead to compromised immunity, resulting to the uncontrolled spread of pathogens and severe disease symptoms. In Arabidopsis quadruple mutant dde2 ein2 pad4 sid2, the defence response to P. syringae was slow, consequently making the mutant more susceptible to P. syringae (Tsuda et al. 2009; Mine et al. 2018). Pathogens sometimes secret elicitors to slow down the PTI response. For instance, bacterium Xylella fastidiosa secrets lipopolysaccharides O-antigen, which delays the PTI response, allowing the bacteria to invade the grapevine aggressively (Rapicavoli et al. 2018). Thus, it is intriguing to postulate that the early induction of PTI response in mycorrhizal NTHR can contribute to the MIR observed against a range of pathogens.
4.4 COI1-mediated JA signalling is indispensable to initiate the PTI component of MIR
The major plant hormones (i.e., JA, SA, ethylene, and abscisic acid) contribute to the PTI response depending on the pathogen system to which plants are exposed. In Arabidopsis, bacterial PAMPs flg22 treatment triggered SA production, whereas JA synthesis remained unaffected (Tsuda1 et al. 2008; Nomura et al. 2012). The induction of SA signalling was found to take place in the initial stages against a wide range of PAMPs, whereas JA perception was induced at later stages (Halim et al. 2009). We, thus, wondered if the expedited PTI response observed in R. irregularis-colonised NTHR against fungal PAMPs requires COI1-mediated JA signalling? To answer this question, we repeated FOL treatment experiment in colonised SlCOI1-silenced lines. In the experiment discussed earlier, the R. irregularis-associated NTHR revealed a strong PTI response. But, in colonised SlCOI1-silenced lines, the same response was lower than that in colonised EV line (Figure 5B). Previously, we have reported that SlCOI1 is essential to initiate strong ROS and total PTI response in HR system against FOL PAMPs (Kadam and Barvkar 2024). In continuation to that, here, in the case of induced resistance in colonised plants, SlCOI1 was found to play a crucial role in MIR. The expression of ROS signalling gene RBOH-B and both PTI marker genes was lower in SlCOI1-si lines than in EV line. Interestingly, the silencing of SlCOI1 led to a higher intensity of R. irregularis colonisation compared to EV. Previously, it was reported that the mycorrhiza-induced resistance largely depends on the degree of colonisation (Khaosaad et al. 2007; Vierheilig et al. 2008). Yet, in our study, the PTI response was found to be more evident in colonised EV than in SlCOI1-si lines, regardless of the higher intensity of colonisation in SlCOI1-silenced lines. This indicates that, in the HR system, PTI response like ROS signalling requires COI1-mediated JA signalling at first. The induction in COI1 expression brings about degradation of JAZ repressor protein, which ultimately activates the defence response coordinating transcription factors (TFs) from MYC TF family (Lorenzo et al. 2004). MYC TFs are involved in the regulation of various biotic stresses and several members of the family, like MYC2, are reported to play an essential role in such induced resistance (Pozo et al. 2004; Marta-marina et al. 2021). Thus, it is probable that the loss of COI1-mediated JA signalling resulted into compromised or failed activation of downstream induced defence signalling cascade.
Overall, the COI1-mediated JA signalling plays a crucial role in plant-microbe interactions. It is found to negatively regulate the AMF spread inside the roots and is indispensable to initiate the PTI aspect of MIR.
5 CONCLUSION
Plants employ a range of hormonal signalling pathways to precisely interact with a variety of microbes. The main discrimination plants are required to do is between the beneficial and pathogenic microbes. Interestingly, JA plays an important role in both beneficial and detrimental interactions. Yet, there are controversies on the nature of regulation that JA signalling implements. JA plays a mixed (viz., promotionary, negative or a neutral) regulatory role. In the present study, the in-vitro symbiotic association was developed between the model AMF, R. irregularis and tomato HR. The in-vitro system offered an advantage in understanding the sole contribution of R. irregularis in induced resistance. As mentioned earlier, PTI is an initial defence response generated by plants upon recognition of PAMPs from pathogens by the plant receptors. The colonised HR lines were found to be more efficient in inducing the expression of RBOH-B gene that contributes to ROS signalling, a crucial step in PTI as well as ETI. These colonised HR lines were also capable of inducing a prompt and prominent PTI response against fungal PAMPs. Moreover, these lines effectively scavenged the oxidative stress induced during the PTI response. The concurrent delay in PTI response in non-colonised HR may lead to the development of severe disease symptoms. The transgenic SlCOI1-silenced and overexpressed HR lines provided insights into the role of COI1-mediated JA signalling in the establishment and progression of AMF colonisation and it was found to be a negative regulator of further spread of AMF. COI1 has emerged as a major signalling regulator and AMF-growth-modulator in tomato. Intriguingly, irrespective of the higher degree of AMF colonisation, the SlCOI1-silenced lines were unable to induce a strong PTI response. This indicates that in MIR against FOL, the role of COI1-mediated JA signalling is indispensable in the HR system.
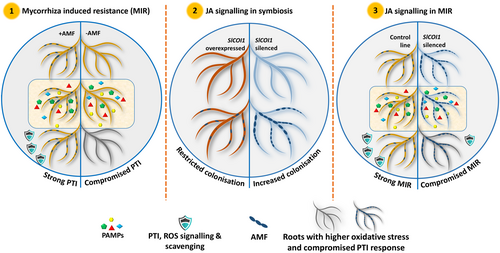
The findings in the HR system can vary when compared to the whole plant system. For example, 12-oxophytodienoic acid (OPDA) is reported to activate JA signalling independently of COI1 (Bosch et al. 2014). Thus, it is possible that OPDA from shoots can compensate the silencing effect of COI1 in the intact plant. However, the HR system provides an excellent opportunity to work precisely on targeted genes and microbes. The present study advocates the importance of mycorrhiza in elevating the plant defence response against fungal pathogens like FOL and the role of JA signalling in plant-microbe interaction. The study puts forth evidence for a univocal role of JA signalling, specifically mediated by COI1, in MIR. The in-vitro system used in this work provides a good model system to study the plant-microbe tripartite interactions between plant roots, beneficial, and pathogenic microbes. It will be also interesting to conduct a similar study with transgenic SA, and SA-JA signalling mutant HR lines to shed light on its role and crosstalk in MIR. In future, the findings of the present study can benefit the design of similar experiments in the whole plant for a better understanding of the dynamic role played by JA signalling in plant-microbe interactions. The outcomes of the study are presented in Figure 6.
AUTHOR CONTRIBUTIONS
SBK performed all the experiments and drafted the manuscript with inputs from VTB. VTB conceived the project, designed the study, acquired funding, revised the manuscript and supervised the research work.
ACKNOWLEDGEMENTS
Authors acknowledge Dr. Anupama A. Pable, Department of Microbiology, Savitribai Phule Pune University for extending lab facility and useful discussion. Authors also thank Prof. Sujata Bhargava for extending her insightful suggestions and support. We would like to thank Dr. Venkatesh Devanur, AgriLife and SOM Phytopharma (India) Ltd. for gifting the spores of R. irregularis. SBK acknowledges the research fellowship from the project sanctioned by Board of Research in Nuclear Science (BRNS) (Sanction number: 55/14/18/2020-BRNS/10362), India to VTB.
Open Research
DATA AVAILABILITY STATEMENT
Data will be made available on request.




