Exploring the mechanisms underlying recovery from freeze–thaw injury in Colobanthus quitensis: mechanistic insights via transcriptome profiling
Abstract
Antarctic plants face significant challenges due to exposure to freeze–thaw stress throughout their life cycle. The ability to recover from freeze–thaw injuries during post-thaw recovery (PTR) periods is a crucial skill for their survival and growth. However, no research, to the best our knowledge, has explored their recovery mechanisms at the cellular and molecular levels. To investigate the potential cellular mechanism during PTR periods, we focused on Colobanthus quitensis, one of solely two vascular plant species in the Antarctic Peninsula. Having determined the lethal temperature causing 50% cellular injury (LT50) under freezing to be −8.0°C, we subjected plants to sub-injurious (−7.0°C) and injurious (−9.0°C) freezing treatments. We then compared recovery abilities at these stress levels using physiological indicators such as ion-leakage, PSII quantum efficiency (Fv/Fm), and antioxidant enzyme activities. Comparative analysis indicated that plants exposed to −7.0°C progressively recovered during PTR periods, showing reduced ion-leakage and increased Fv/Fm, while those stressed at −9.0°C exhibited irrecoverable damage with lower antioxidant enzymes activities. To investigate the molecular basis of recovery, we examined transcriptome changes in tissues exposed to −7.0°C during PTR periods through GO and KEGG pathway enrichment analyses. These analyses identified six potential cellular events involved in the recovery process, including ionic & pH homeostasis, cell wall remodeling, protein repair, defense against potential microbial attacks, free radical scavenging, and DNA repair. Understanding the cellular and molecular mechanisms of recovery from freeze–thaw injuries enhances our knowledge on how Antarctic plants adapt to extreme environments, offering valuable insights into their survival strategies.
1 INTRODUCTION
Freezing temperatures are one of the major environmental constraints, affecting plant performance and limiting distribution of plant species. During a natural frost episode, plant cells can undergo freeze-desiccation (cell contraction) due to the extracellular freezing, followed by thaw-induced rehydration (cell expansion), namely freeze–thaw stress. These two stressful events can interactively cause various cellular dysfunctions including structural/functional perturbations in the plasma membrane, reduction of PSII quantum efficiency and oxidative damage to macromolecules e.g., fatty acids, proteins, nucleic acids, etc. due to excessive accumulation of reactive oxygen species (ROS) e.g., superoxide, singlet oxygen, hydrogen peroxide, etc. (Arora, 2018, Kendall and McKersie, 1989; Min et al., 2014; Mittler, 2002). Hence, the ability of recovery from such injuries is imperative to survive a frost event, which are considered as an integral component of freeze–thaw stress tolerance (FTST) (Chen et al., 2013).
Antarctica stands as the most frigid territory on Earth, with the vast majority of its land mass blanketed in ice. In fact, only less than 1% of its territory consists of ice-free lands found along the Antarctic Peninsula, also called the maritime Antarctic, in which one of two vascular plants, the Antarctic pearlwort Colobanthus quitensis (Kunth) Bartl., has successfully established a natural population (Cavieres et al., 2016). During the thawing season (Nov. 2019 ~ Mar. 2020), the mean ground surface temperature of this zone ranged from −8.4 to 10.4°C (Baptista et al., 2024). Our data also showed similar patterns for it during the spring and fall seasons spanning 2018 to 2020, suggesting frequent frost events, with sub-zero temperatures occasionally dropping below −7.0°C (Figure S1). Given the documented ability of C. quitensis to endure freezing temperatures ranging from −4.0 to −6.0°C (Bravo et al., 2001), these climatic conditions imply that C. quitensis constantly faces frequent freeze–thaw injuries, necessitating recovery to ensure its survival and growth, particularly during the spring and fall seasons. While numerous studies have been conducted on freezing tolerance of C. quitensis, no research, to our knowledge, exists on the evaluation of its ability to recover from freeze–thaw injuries.
Few studies have reported the cellular and molecular mechanism of post-thaw recovery (PTR). The freeze–thaw injury could be reversible during PTR periods, as evidenced by leaked ions being reabsorbed in moderately injured onion tissues, first reported by Palta et al., (1977). Later a proteomic study revealed that proteins involved in onion tissue recovery including ROS scavenging, cell wall reconstruction, protein repair and ion homeostasis, were accumulated during PTR periods (Chen et al., 2013). This laboratory complemented their findings through another study of PTRs wherein decrease in ion-leakage, restoration of PSII quantum efficiency and activation of antioxidant enzymes were observed in reversibly injured spinach leaves (Chen and Arora, 2014). Metabolome changes in Avena sativa crowns recovering from frost injury (Henson et al., 2014) and the expression of specific genes e.g. HSP, GST, COR, etc., potentially associated with recovery in Arabidopsis (Vyse et al., 2020) also provided meaningful insights into the mechanism of PTR. However, a comprehensive analysis of transcriptome changes underlying PTR could further elucidate the molecular mechanism involved in the tissue recovery process, particularly in the case of C. quitensis, which thrives in the harshest environment on Earth, Antarctica.
In the present study, our main goals are twofold: (1) to explore the hypothesis that tissues exposed to sub-injurious freeze–thaw stress are able to recover from freeze–thaw injuries during the PTR process, while those exposed to injurious freeze–thaw stress cannot recover, as quantified by various physiological parameters, i.e., ion-leakage, PSII quantum efficiency (Fv/Fm), and activities of antioxidant enzymes, i.e., superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX); and (2), to explore transcriptome changes in reversibly injured tissues to understand the molecular basis for tissue recovery from freeze–thaw injuries. Our findings provide not only a new insight into the PTR process in reversibly injured tissues, but also suggest some clues regarding how C. quitensis has managed to successfully thrive in the challenging environment of the Antarctic Peninsula.
2 MATERIALS AND METHODS
2.1 Plant material
Colobanthus quitensis (Kunth) Bartl. (Caryophyllaceae) was collected from the Barton Peninsula from near the Korean King Sejong Antarctic Station (62°14′29″ S; 58°44′18″ W) in January 2013. The details on the methods used for initial sampling, handling, and propagation were described by Cho et al., (2018). The collected samples were transferred to the laboratory of the Korea Polar Research Institute, and have been maintained via continuous propagation. Briefly, three individual plants were propagated in vitro by supplementing 0.5 × Murashige and Skoog (MS) medium containing 2% sucrose in plant culture dishes (91.3 mm diameter × 38.2 mm height), followed by an incubation in a growth chamber at 16/16°C (day/night) with 16 h photoperiod under average photosynthetically active radiation (PAR) of ~150 μmol m−2 S−1 at plant height supplied by incandescent and fluorescent lamps. Three weeks after planting, we removed the roots of the plants and defined the remaining aerial parts, including the stem and leaves, approximately 1.5 to 2 cm in length, as shoot segments. These shoot segments were used as the material for all subsequent experimental procedures.
2.2 Determination of freezing tolerance (LT50)
where % Leakage (T) stands for percent leakage total at each test temperature, while % Leakage(C) means average of percent leakage for UFC. Percent injury at each temperature treatment was used to calculate the LT50, a lethal temperature causing 50% of maximum injury, by generating a sigmoid curve fitting the Gompertz function, as per Lim et al., 1998. LT50 (°C), a mid-point between the minimum and maximum injury, was regarded as the FT for the shoot segment of C. quitensis. These measurements were repeated five times, each including five shoot segments per temperature per treatment. The data of percent injury from five biological replications were pooled to calculate the representative treatment means with standard errors across three independent experiments, and analyzed by least significant difference (LSD) test (α = 0.05).
2.3 Experimental design for post-thaw recovery
Experimental design for post-thaw recovery (PTR) was on the basis of protocols that were successfully employed for onion scales (Palta et al., 1977; Arora and Palta, 1991), spinach (Chen and Arora, 2014; Min et al., 2014), and Arabidopsis (Vyse et al., 2020). Guided by the resultant LT50, two temperature treatments, i.e., −7.0 and − 9.0°C, representing relatively moderate and severe stress levels respectively, were selected for the characterization of the recovery processes. Tissues were then frozen to these two selected temperatures and thawn as described above. Injury percent of stressed tissues was calculated by measuring the ion-leakage right after thaw (RAT), along with the corresponding controls (RATUFC). Two different sets of replicated freeze–thaw injured samples were then subjected to a recovery condition comprising 1d (PTR-1D) and 6d exposure (PTR-6D) to 4–5°C at ~50 μmol m−2 S−1 PAR (12/12-h; D/N) in the test tube where the samples were initially frozen. Corresponding UFCs were also treated with the same recovery conditions, hence resulting in PTRUFC-1D and PTRUFC-6D. Ion-leakage assays were also conducted for UFC-1D, UFC-6D, PTR-1D and PTR-6D as described above. This test was independently repeated four times, each with four to five replications per temperature. Data of percent injury from these experiments were pooled to calculate the representative treatments means with standard errors, followed by analysis of pair-wise differences using LSD test (α = 0.05). Additional RATUFC, RAT, PTRUFC and PTR samples, each with three biological replicates consisting of five to six technical replicates per treatment per temperature, were immediately frozen at −80°C following sample collection for Fv/Fm measurement, and later used for assays of antioxidant enzyme activity and transcriptomic study.
2.3.1 Fv/Fm measurements
The chlorophyll fluorescence parameter Fv/Fm, i.e. the maximal quantum yield of PSII, was measured with an Imaging-PAM (Heinz Walz GmbH). Briefly, shoot segments that had been exposed to a freeze–thaw procedure (i.e., −7.0 and − 9.0°C) as described above were dark-adapted for 20 mins at room temperature with ~20°C, followed by the estimation of Fv/Fm at the following settings: measuring light intensity, 10; damping, 5; gain 5; saturating pulse intensity, 7; duration of saturating pulse, 0.8 s; these settings ensured a Ft value close to ~0.1 in healthy dark-adapted samples. This experiment was independently conducted three times, each with five technical replicates per temperature per treatment (one shoot segment/technical replicate). Data were pooled to calculate the representative treatment means with standard errors and mean difference was analyzed by an LSD test (α = 0.05). A representative image of the quantitative estimate of Fv/Fm, as indicated by the color intensity, in the shoot segment is presented in this study.
2.3.2 Measurements of antioxidant enzyme activities
The activities of three antioxidant enzymes, i.e. CAT, APX, and SOD, were measured as detailed by Chen and Arora (2011). Briefly, ground frozen shoot segments (100 mg) were mixed with 1 mL of 100 mM potassium phosphate buffer (pH 7.0), followed by a centrifugation at 10 000 g for 20 min at 4°C. Resulting supernatants were recruited as the enzyme extract for CAT, APX, and SOD. The activity was estimated as described by Chen and Arora (2014). One unit of SOD is defined as the amount of enzymes necessitated for 50% inhibition of formazan formation at 560 nm; one unit of CAT activity is defined as the removal of 1 μM H2O2 within 60 s at 240 nm and one unit of APX activity is defined as the conversion of 1 μM ascorbic acid into mono-dehydroascorbate within 60 s at 290 nm. The protein concentration in the total enzyme extracts in 100 mg was determined via the Bradford method (Bradford, 1976) using the Bio-Rad protein assay reagent. This experiment was independently performed three times, each with three technical replicates per temperature per treatment. Data were pooled to calculate the representative means with standard errors. Mean difference was analyzed by LSD test (α = 0.05).
2.4 Transcriptome analysis
2.4.1 RNA extraction, library construction, and sequencing
Ground frozen shoot segments, previously stored at −80°C, were used to extract total RNA using the Plant RNeasy mini kit (Qiagen), followed by an assessment of the quality and quantity for RNA extracts using a Bioanalyzer (RIN >6; Agilent Technologies) and a Qubit RNA Broad-Range Assay Kit (Life Technologies), respectively. To construct the sequencing library, 2 μg of total RNA from each sample was used as the input for the TruSeq RNA sample prep kit v2 (Illumina) following the manufacturer's recommended method and were validated and then quantified using the Bioanalyzer and the library qPCR quantification method. These libraries were paired-end sequenced on the Illumina HiSeq 4000 platform. A total of 129 Gb of sequence data were generated (Q30 > 91%). The RNA-Seq data were deposited into the Sequence Read Archive under accession number SRR28638925 ~ SRR28638936.
2.4.2 Data preprocessing, gene quantification, and GO enrichment analysis
The data of raw RNA sequences were trimmed and filtered using Trimmomatic (Bolger et al., 2014) in order to remove low-quality bases and adapter sequences. The trimmed/filtered data were evaluated via FastQC (https://www.bioinformatics.babraham.ac.uk/projects/fastqc/), and the resultant reports were then merged with multiQC with default parameters. The preprocessed data were mapped to the C. quitensis reference genome (Min et al., 2024) using the Hisat v2-2.1.0 software with the following parameter: “—dta-cufflinks”. Subsequently, the data of the transcriptome were quantified using HTSeq with a parameter: “-S no” to calculate the transcript read counts. Raw read counts were normalized using the median of ratios method of DESeq2 in the R package (Love et al., 2014). We subsequently performed principal component analysis (PCA), followed by differentially expressed genes (DEGs) analysis. Genes with |log2foldchange| ≥ 1 and adjusted p-value (FDR) < 0.01 were assigned as DEGs. Both GO enrichment and KEGG pathway analyses for DEGs were conducted based on the functional annotation of the Arabidopsis genome (Araport 11, http://www.arabidopsis.org). The best match of BLASTP with filtering option, i.e., e-value cutoff = 1e−5, was used in order to implement the mapping for the best hit proteins. The clusterProfiler (Yu et al., 2012) was then used to conduct both analyses along with org.At.tair.db, i.e. the Arabidopsis annotation package. Simplification of GO terms was done by grouping similar terms based on their semantic similarity using the rrvgo package (Sayols, 2023) with the default threshold of 0.7. The simplified GO terms and enriched KEGG pathways were respectively visualized using ggplot2.
2.4.3 qPCR analysis
Total RNA was extracted from the aerial parts of plant samples, followed by purification using the RNeasy Plant Mini Kit (Qiagen) as described above. cDNA was synthesized from 2 μg of total RNA using Superscript III reverse transcriptase (Invitrogen). Gene-specific primers were designed based on the sequences of the contigs and are listed in Table S6. The TIM gene (chloroplast-like triosephosphate isomerase) was used as the internal reference control (Cho et al., 2018). qPCR was performed in biological triplicates using SYBR® Premix Ex TaqTM DNA polymerase (Takara) and the Mx3000P Real-Time PCR System (Stratagene). Mean differences between treatments were analyzed by t-test (p < 0.05). Pearson correlation analysis between the data of qPCR and Log2FC as per DEG was performed at p < 0.05.
3 RESULTS
3.1 LT50 of C. quitensis and its ability to recover during post-thaw periods
The freeze–thaw response curve ranging from −6 to −18°C for C. quitensis is shown in Figure 1A. Tissues were injured from ~1.2% (minimum) to ~73.2% (maximum) and − 8.2°C, i.e. a temperature causing ~36% injury, the mid-point between minimum and maximum, was determined as the LT50 of C. quitensis. Therefore, two temperatures, −7.0 and − 9.0°C, were selected as sub-injurious and injurious stress levels, respectively, to explore the hypothesis that tissues exposed to sub-injurious stress get recovered during PTR periods whereas those stressed at an injurious temperature result in a freeze–thaw injury that is irreversible. Tissues frozen at −7.0°C with ~23% injury at RAT, showed a significant reduction in their freeze–thaw injury at PTR-1D (~15%) and at PTR-6D (~8%), hence tissues stressed at −7.0°C were considered as reversibly injured (Figure 1B). In contrast, samples stressed at −9.0°C showed ~59% at RAT, ~60% at PTR-1D, followed by ~74% at PTR-6D, and therefore were determined as irreversibly injured (Figure 1B).
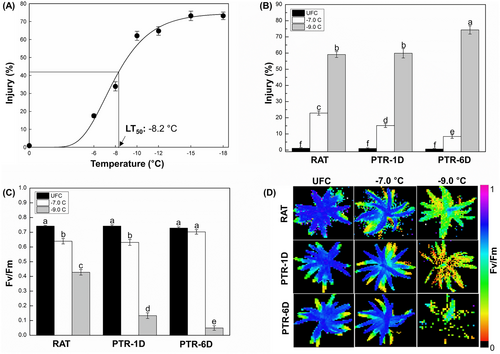
3.2 Post-thaw recovery of maximal quantum yield of PSII
The Fv/Fm, i.e. maximal quantum yield of PSII, in non-stressed tissues including RATUFC, UFC-1D, and UFC-6D was ~0.72, but decreased to 0.63 and 0.42 in response to −7.0 and − 9.0°C at RAT, respectively (Figure 1C). However, it progressively restored during PTR periods to ~0.65 at PTR-1D, followed by ~0.70 at PTR-6D at −7.0°C freezing stress, whereas tissues frozen at −9.0°C showed rapid reduction to ~0.13 at PTR-1D followed by ~0.05 at PTR-6D (Figure 1C). The visualization of Fv/Fm changes over the PTR period and is shown in Figure 1D. Non-stressed tissues e.g., RATUFC, PTRUFC-1D, and PTRUFC-6D appeared blue due to their higher Fv/Fm values (around 0.8), while stressed tissues with lower Fv/Fm values (closer to 0) appeared either red or were not visible. Tissues stressed at −7.0°C showed restoration of PSII quantum efficiency as evidenced by an increase in the distribution of blue color during PTR periods whereas those subjected to −9.0°C progressively compromised PSII quantum efficiency as shown by images where Fv/Fm intensity was turned from green at RAT to red or no visible color at PTR-6D (Figure 1D).
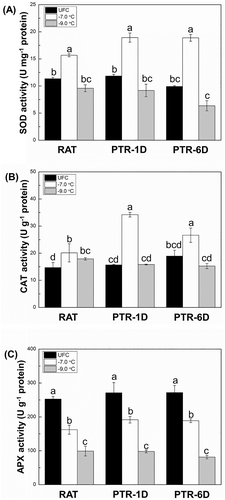
3.3 Antioxidant enzyme activities
The activities of three antioxidant enzymes on total protein extract basis are presented in Figure 2. The SOD and CAT activities in tissues stressed at −7.0°C had relatively higher levels compared to their corresponding UFCs at RAT as well as PTR periods, while those in samples subjected to −9.0°C showed no significant changes (Figure 2A, B). The APX activity in response to both stress levels remained consistently lower compared to corresponding UFCs at RAT and PTR periods (Figure 2C). In addition, activities of all three enzymes were more repressed at −9.0°C than at −7.0°C across all treatments.
3.4 Transcriptome sequencing
Physiological data confirmed that only tissues stressed at −7.0°C were able to recover from freeze–thaw injury after six days of thawing. Therefore, samples stressed at −7.0°C were chosen for the subsequent analysis. Our data also indicated PTR-6D treatment to be better repaired than PTR-1D. Hence, RAT was hereon compared with PTR-6D treatment for all transcriptome analysis to identify potential genes responsible for recovery. Accordingly, RNA-seq was conducted for four treatments, i.e., RATUFC, RAT (−7.0°C), PTRUFC-6D, PTR-6D (−7.0°C), each with three biological replicates per treatment, a total of 12 samples.
Principal component analysis (PCA) was performed to distinguish transcript phenotypes among RATUFC, RAT (−7.0°C), PTRUFC-6D, and PTR-6D (−7.0°C). PCA revealed a clear differentiation across the four treatments in which the two components accounted for 68% of the total variance (Figure 3A). The first principal component (PC1) explained 48% of the total variance of transcriptome data set, indicating a different response to freeze–thaw injured tissues and uninjured tissues. In contrast, the second principal component (PC2) accounted for 20% of total variance indicating a distinct response to time of post-thaw recovery.
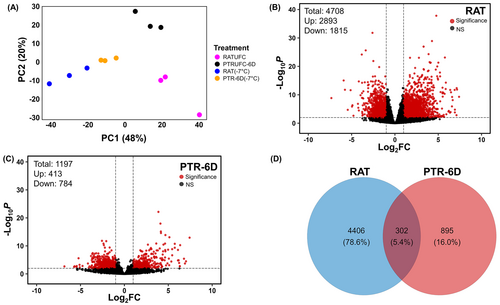
3.5 Differentially expressed gene (DEG) analysis
There were in total 4708 and 1197 DEGs generated from two different pairwise comparisons as follows: (1) RAT (−7.0°C vs. RATUFC; Figure 3B) and (2) PTR-6D (−7.0°C) vs. PTRUFC-6D (Figure 3C), respectively. These two different DEG sets are hereafter referred to as RAT and PTR-6D, respectively. A total of 302 DEGs were commonly identified between two pairwise comparisons, and 4406 and 895 DEGs were uniquely up- or downregulated in RAT and PTR-6D, respectively (Figure 3D).
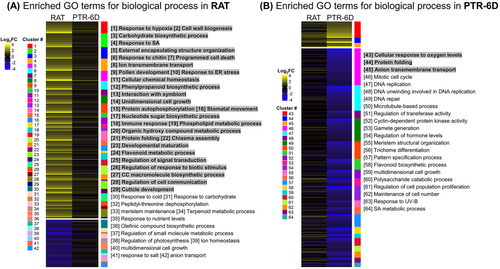
3.6 GO enrichment analysis and KEGG pathway analysis
To identify the biological processes enriched in either RAT or PTR-6D, GO enrichment analysis was performed using the uniquely up- and downregulated DEGs, this means that shared genes were not used for subsequent analysis. All parent GO-terms corresponding to each cluster for enriched (FDR p < 0.05) biological processes were displayed, along with the expression patterns of well characterized unique genes in Figure 4. For instance, 29 GO terms were, at RAT, enriched in the upregulated gene set (from 1 to 29), while an additional 13 GO terms were enriched in the downregulated gene set (from 30 to 42) (Figure 4A). The top five parent GO-terms for the upregulated gene set were ‘response to hypoxia’, ‘cell wall biogenesis’, ‘carbohydrate biosynthetic process’, ‘response to SA’, and ‘external encapsulating structure organization’, whereas those for the downregulated gene set were ‘response to cold’, ‘response to carbohydrate’, ‘peptidyl-threonine dephosphorylation’, ‘meristem maintenance’, and ‘terpenoid metabolic process’. For PTR-6D, a total of 22 GO terms were enriched, comprising three GO terms extracted from the upregulated gene set (from 43 to 45) and other 19 GO terms linked with the downregulated gene set (from 46 to 64) (Figure 4B). The top three GO terms related to the upregulated gene set were ‘cellular response to oxygen levels’, ‘protein folding’ and ‘anion transmembrane transport’, while those from the downregulated gene set were ‘mitotic cell cycle’, ‘DNA replication’, and ‘DNA unwinding involved in DNA replication’. The list of genes involved in each clustered biological process is shown in Table S1 for RAT and Table S2 for PTR-6D.
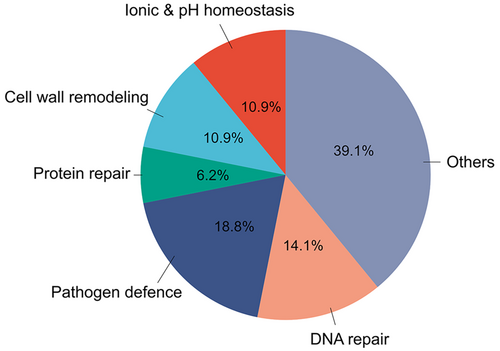
To better understand the recovery mechanism of C. quitensis underlying the transcriptional-level response to sub-injurious freeze–thaw stress, the specific parent GO-terms enriched at both RAT and PTR-6D were selected and grouped into six categories based on their biological functions in relation to potential cellular events for tissue recovery. These six functional categories are as follows: (1) ionic & pH homeostasis, (2) cell wall remodeling, (3) protein repair, (4) ABA-mediated defense mechanism against pathogen, (5) DNA repair, and (6) others (Figure 5; Table S3). The significance of these potential cellular events for tissue recovery was addressed in a section of the discussion below.
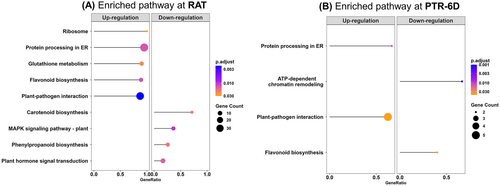
There are nine and four KEGG pathways (FDR-adjusted p < 0.05) enriched at RAT and PTR-6D, respectively (Figure 6). In RAT, five KEGG pathways including ‘ribosome’, ‘protein processing in endoplasmic reticulum (ER)’, ‘glutathione metabolism’, ‘flavonoid biosynthesis’, and ‘plant-pathogen interaction’ were uniquely upregulated, while four KEGG pathways consisting of ‘carotenoid biosynthesis’, ‘MAPK signaling pathway’, ‘phenylpropanoid biosynthesis’, and ‘plant hormone signal transduction’ were downregulated (Figure 6A). For PTR-6D, two KEGG pathways including ‘protein processing in ER’ and ‘plant-pathogen interaction’ were uniquely upregulated, whereas the other two KEGG pathways including ‘ATP-dependent chromatin remodeling’ and ‘flavonoid biosynthesis’ were downregulated (Figure 6B).
3.7 qPCR validation
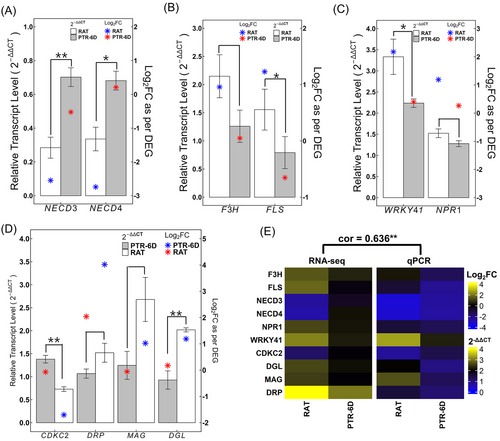
To validate the results of the Illumina sequencing analysis, ten known transcripts were selected for verification via qPCR. Details of these genes, along with their gene-specific primers, are provided in Table S6. The expression patterns obtained from both qPCR and DEG were significantly positively correlated (Pearson correlation coefficient = 0.636, p < 0.05) with those from the transcriptomes (Figure 7), confirming the reliability of our transcriptome data.
4 DISCUSSION
The ability of recovery from moderate freeze–thaw injuries is a vital component of a plant's ultimate survival strategy under a natural frost episode. However, there has been limited investigation on comprehensively understanding the recovery mechanisms during post-thaw periods. Few studies noting cellular events potentially associated with post-thaw recovery (PTR) have suggested various physiological and molecular explanations, ranging from reduction in ion-leakage, restoration of PSII quantum efficiency, activation of antioxidant properties, accumulation of proteins facilitating new cellular homeostasis to metabolic changes promoting for recovery during PTR periods (Arora and Palta, 1988, 1991; Chen et al., 2013; Chen and Arora, 2014; Henson et al., 2014; Vyse et al., 2020). These findings collectively provided preliminary mechanistic insight into plant recovery from moderate freeze–thaw injuries. To gain a more comprehensive understanding of the PTR process at the physiological and molecular level, here, we have evaluated the ability of recovery in C. quitensis determined through various physiological parameters of freeze–thaw injuries and conducted transcriptome profiling following an exposure of C. quitensis to moderate freeze–thaw stress to explore transcriptional regulations during PTR periods.
4.1 PTR ability of C. quitensis - a physiological evaluation through ion-leakage, Fv/Fm, and antioxidant enzyme activity
There is a widely accepted agreement that the functional perturbation of the plasma membrane is considered as a primary consequence of freeze–thaw injuries, evidenced by an increase in solute leakage from injured tissues (Arora and Palta, 1991; Steponkus, 1984; Uemura and Yoshida, 1986). Accordingly, a temperature-controlled, laboratory/ion-leakage-based freeze–thaw protocol is conventionally and routinely used to estimate FT (as LT50) of ‘excised tissues’ across diverse plant species. Based on this protocol, here, we initially determined the LT50 of C. quitensis shoot segments in order to select two stress levels, i.e., −7.0°C (a sub-injurious temperature) and − 9.0°C (an injurious temperature), which encompassed the LT50 of approximately −8.0°C (Figure 1A). Interestingly, however, the LT50 determined in this study was not consistent substantially with a previous report of LT50 for this species (approximately −5.0°C; Bravo et al., 2001). A closer examination revealed the difference between two studies. We estimated FTST by ensuring extracellular ice formation (ice-nucleation) at a given sub-zero temperature, while the other authors used silver iodide as a nucleating agent at room temperature prior to exposure to freezing stress (Bravo et al., 2001), likely resulting in different LT50 for the same species. Ice-nucleation at a given sub-zero temperature, usually at −1 or − 2°C, could play an important role in two key aspects: (1) there is a possibility for cells to be supercooled without ice-nucleation, potentially leading to intracellular freezing, i.e., cell rupture, irrespective of whether the tissue is hardy or not, and (2), if tissues supercool and do not experience any freezing during the test, plants remained undamaged leading investigators to over-estimate the FTST (Fuller and Wisniewski, 1998). Accordingly, two selected temperatures in our study represented physiologically relevant stress levels, one sub-injurious and the other relatively injurious (but not too injurious), respectively. The rationale for the selection of two stress levels was to test whether the tissues were able to recover from sub-injurious freeze–thaw injuries, but not from lethal stress.
The ion-leakage data indicated that tissues exposed to −7.0°C recovered significantly within one day of post-thawing, and their recovery was almost complete at PTR-6D (Figure 1B). Our protocol involved thawing frozen samples overnight for 14–16 hours prior to conducting ion-leakage assays to determine FT. Thus, the significant reduction of ion-leakage at PTR-1D suggests that the ‘right-after-thaw (RAT) injured state’ is considered as already somewhat recovered tissues. Indeed, it has been reported that a remarkable recovery measured by ion-leakage assays, was observed in spinach leaves and Arabidopsis within 24-h after freezing (Chen and Arora, 2014; Vyse et al., 2020). Presumably, various biological processes facilitating for recovery took place during overnight thawing. In fact, our transcriptome data supported this aspect, further discussed later. In contrast, tissues stressed at −9.0°C did not show any signs of repair since gradual increase in ion-leakage was observed during PTR periods (Figure 1B), indicating irreversibly injured membrane transport functions in the plasma membrane. One of such functional perturbations is associated with the loss of plasma membrane H+-ATPase activity (PMA), an active transport apparatus, which is frequently concomitant with enhanced ion-leakage in freeze–thaw injured tissues. Moreover, repair of PMA activity during PTR periods resulted in the re-uptake of leaked ions, hence, considered as one of the recovery processes (Arora and Palta, 1991). Therefore, it is reasonable that decrease in ion-leakage during PTR periods may be associated with a repair of PMA function against sub-injurious stress, further discussed under Section 4.2.1.
The compromised function of PSII caused by sub-injurious freeze–thaw stress was also restored after six days of thawing as assessed visually as well as by Fv/Fm measurement using chlorophyll fluorescence imaging, whereas tissues subjected to lethal freeze–thaw stress failed to recover (Figure 1C, D). This suggests that full repair of the photosynthetic apparatus might solely be feasible in moderately injured tissues. Indeed, PSII quantum efficiency, as quantified by Fv/Fm, in spinach leaves and Arabidopsis was recovered to the extent observed in their corresponding UFCs following six days of post-thawing, only as they were exposed to sub-zero temperatures warmer than their FT (LT50) threshold (Chen and Arora, 2014; Vyse et al., 2020). Conceivably, temperatures even slightly lower than the FT threshold tested in our study), caused complete disruption of the thylakoid membrane system, resulting in the failure of recovery for the compromised function of PSII. An altered balance of the oxidant-antioxidant system may possibly be another explanation for failure to recover compromised PSII quantum efficiency. It is well-known that freeze–thaw stress causes excessive cellular accumulation of ROS and resultant oxidative injury to PSII via the oxidation of the D1 protein, if not properly quenched by antioxidants (Arora, 2018; Augustyniak et al., 2020; Kendall and McKersie, 1989; Krieger-Liszkay et al., 2008; Li et al., 2018; Mittler, 2002). In the present study, moderately injured tissues had significantly higher antioxidant enzyme activities than injurious ones over the PTR periods (Figure 2). Higher activities of CAT and APX might efficiently scavenge H2O2, while SOD could eliminate accumulated O2•−. Unlike CAT, APX activity was consistently lower than that of the corresponding UFCs under sub-injurious stress. This contrasting behavior between CAT and APX suggests a differential response in the antioxidant system and CAT might be the primary scavenger of H2O2 in this species during recovery from freeze–thaw injuries. Therefore, it is tempting to assume, that D1 protein in moderately injured tissues might be less injured by ROS, facilitating the repair of compromised PSII quantum efficiency. This assumption could be supported by Chen and Arora (2014), reporting a gradual reduction in accumulated ROS (assessed by histochemical staining) alongside the recovery of antioxidant enzyme activities during the PTR periods, indicating a potential role of antioxidants in mitigating oxidative stress and promoting tissue recovery.
4.2 Transcriptome profiles in relation to recovery process
PCA revealed that the transcriptome differentially responded to both freeze–thaw stress and the duration of recovery time (Figure 3A). In fact, the expression profiles induced by the sub-injurious freeze–thaw stress are clearly different in timing and numbers of up-regulated and down-regulated genes, where RAT sample had ~4.2-fold DEGs relative to the PTR-6D sample (4708 DEGs in RAT vs. 1197 DEGs in PTR-6D; Figure 3B, C). This suggests that most repair processes were activated promptly after the initial freezing stress, likely during the overnight thawing phase, rather than during the post-thaw periods. Indeed, biological processes enriched in the RAT sample were more noticeable than in the PTR-6D sample, accounting for 69% and 13%, respectively, out of the total number of GO-terms for each treatment (compare Figure 4A and B). Similarly, the RAT sample exhibited more enriched KEGG pathways compared to the PTR-6D sample, with nine and four pathways, respectively (compare Figure 6A and B). In addition, we classified a total of 64 GO-terms related to biological processes enriched in RAT and PTR-6D samples into six functional categories. The relative distribution of each category across all GO-terms is shown in Figure 5, with more detailed information available in Table S3. The significance of these functional categories and the relevance of the enriched KEGG pathways to tissue recovery are discussed below.
4.2.1 Ionic and pH homeostasis
It has been frequently reported that freeze–thaw stress caused a water-soaking of tissues, presumably due to the leaked ions, leading to a reduction of the water potential gradient between intra- and extracellular spaces (Arora and Palta, 1988; Chen et al., 2013; Min et al., 2020). In fact, water-soaking results from the thaw water being driven from the cell to expanding ice crystals during extracellular freezing. This efflux of water has not yet been fully re-absorbed during thawing, hence, is mainly observed at RAT. Thus, it is not surprising that the GO term related to ‘response to hypoxia’ is enriched at RAT and genes involved in its processes were more up-regulated as compared to PTR-6D sample (Figure 4, cluster 1). This indicates that the degree of water-soaking may progressively be decreased via a flow back of leaked ions and water during PTR periods, thereby escaping from the hypoxia condition.
K+ has previously been nominated as the major cation of the ionic pool leaked from freeze–thaw injured tissues and such K+-leakage could be attenuated by the activation of PMA, i.e., be responsible for the regulation of K+ influx/H+ efflux (Arora and Palta, 1991; Palta et al., 1977). Moreover, Min et al. (2021) reported that the percentage of injury, measured by the ion-leakage assay, was quantitatively correlated with K+ leakage in spinach leaves. Accordingly, recovery of PMA activity may be one of the essential processes for the restoration of altered ion-homeostasis. In the present data, the GO-terms related to ‘ion-homeostasis’ and ‘ion-transmembrane transport’ were simultaneously enriched in the down-regulated and up-regulated gene sets of RAT samples, respectively (Figure 4A, clusters 8, 39), suggesting that the restoration of ion-homeostasis likely commenced during overnight thawing, possibly through the activation of various ion-channel proteins, such as PMA. Notably, our data indicate that the gene expression of HA1 (i.e., plasma membrane H+-ATPase; proton pump) in the RAT sample was approximately 2.42 times higher than in its corresponding UFC sample. (Table S2) suggesting an upregulation of K+ influx through the expulsion of protons from the cell. This upregulation of HA1 could conceivably be associated with tissue recovery by facilitating the uptake of K+ and thawn water, resulting in the reduction of ion-leakage as well as the degree of water-soaking, which alleviate in turn, hypoxia conditions during PTR periods. Indeed, the restoration of PMA activity during tissue recovery has been reported by measuring decreased ion-leakage/water-soaking (Arora and Palta, 1991; Chen et al., 2013).
Maintaining pH homeostasis is crucial for tissue recovery as required for the proper function of various cellular processes including enzymatic reactions, protein functions and metabolic activities. Three cellular events have been implicated to explain the lowering of cytosolic pH in freeze–thaw injured tissues: (1) inhibition of PMA activity preventing the transport of H+ ions across the plasma membrane (Arora and Palta, 1991), (2) accumulation of lactic acid due to water-soaking of tissues experiencing anaerobic conditions/respiration or hypoxia (Min et al., 2020; Roberts et al., 1984), and (3) demixing of vacuolar content including H+ ions due to an injured tonoplast including the dysfunction of vacuolar H+-ATPase (V-ATPase; Yoshida et al., 1999). Restoration of PMA activity and reduction of hypoxia conditions during PTR periods potentially contributed to the stabilization of intracellular pH balance. As implicated by a third potential explanation of cytoplasmic acidification, repair of V-ATPase may accelerate cytosolic pH homeostasis. Indeed, our data showed that transcripts for two subunits consisting of V-ATPase, VHA-A/VHA-E3, were more upregulated at RAT than at PTR-6D (Table S1), postulating their potential involvement in the mechanism for repairing the disturbed cytoplasmic pH via the direct H+ influx across the tonoplast membrane during PTR periods, a proposal worth further investigation.
4.2.2 Cell wall remodeling
The plasma membrane is known to be closely pressed against the cell wall (CW) through physical connections, referred as Hechtian strands which have been proposed to be involved in signaling and CW synthesis (Arora, 2018; Chen et al., 2013; Codjoe et al., 2022; Roberts, 1990; Seifert and Blaukopf, 2010). It has been reported that freeze-contraction and subsequent thaw-expansion caused mechanical stress on the CW, thereby compromising its structural integrity, including reduction of CW thickness, decreased levels of CW polysaccharides and loosing connection between PM and CW (Rajashekar and Lafta, 1996; Kubacka-Zebalska and Kacperska, 1999; Stefanowska et al., 1999). Thus, the restoration of the normal structure and function, so called CW remodeling, may be one of the core processes for tissue recovery. Interestingly, cell wall remodeling-related biological processes were enriched at RAT samples including ‘cell wall biogenesis’, ‘carbohydrate biosynthetic process’, ‘external encapsulating structure organization’, ‘nucleotide-sugar biosynthetic process’, ‘cellular component macromolecule biosynthetic process’, ‘cuticle development’, and cell wall modification involved in ‘multidimensional cell growth’ (Figure 4A, clusters 2, 3, 5, 17, 27, 29, 40). Zooming in on the transcriptional profiles involved in these biological processes revealed that transcripts for CW composition and biosynthesis & assembly of the CW were upregulated, such as cellulose biosynthesis (CESA1, CESA9; Scheible et al., 2001), xyloglucan backbone synthesis (CSLC5, CSLC12; Cocuron et al., 2007), construction and remodeling of cellulose/xyloglucan crosslinks (XTH8, XTH15, XTH26, XTH30; Rose et al., 2002), and cellulose microfibril organization (COB, COBL7; Roudier et al., 2002, 2005). The relatively lower abundance of expansin expression including EXPA10, EXPA13, EXPB3 at PTR-6D compared to RAT indicates that the process of CW remodeling is almost complete after six days of post-thaw, as these proteins responsible for loosening the connections between cellulose microfibrils and other cell wall components are low in abundance (Choi et al., 2006; Li et al., 2002).
4.2.3 Protein repair mechanism during PTR periods
Freeze-induced cell contraction and the resulting cytoplasm concentration can cause the aggregation of proteins and disrupting the efficiency of the protein-processing machinery (Chen et al., 2013). Our data supports this previous report, as the GO-term ‘response to endoplasmic reticulum (ER) stress’ was enriched at RAT (Figure 4A, cluster 10). This observation indicates that freeze–thaw stress can reduce the efficiency of the ER for protein folding via the accumulation of unfolded polypeptides (Malhotra and Kaufman, 2009; Naidoo, 2009). Hence, it is necessary to restore proteostasis for tissue recovery during PTR periods. In fact, our data showed that GO-terms related to ‘protein autophosphorylation’, known as a type of post-translational modification of proteins, and ‘protein folding’ (Figure 4A, cluster 15, 21) were activated at RAT. This suggests that denatured/misfolded proteins are repaired to restore their normal functions during PTR periods. This suggestion could be reinforced by two KEGG pathways, i.e. ‘ribosome’ and ‘protein processing in ER’ enriched at RAT, of which the latter remained upregulated even up to six days of post-thaw (Figure 6A, B). In ‘protein processing in ER’ pathways, heat shock protein (HSP) genes were upregulated at both RAT and PTR-6D, including HSP17.4, HSP 70, HSP 81–2, HSP 90.1, BIP2 i.e., HSP70 protein of ER, and cpHSC 70–1 (Table S4, S5). The role of HSPs as molecular chaperones is well-established, encompassing the regulation of protein folding, accumulation, assembly, translocation, and degradation, especially under stress conditions including freeze–thaw stress (Maruyama et al., 2015; Sabehat et al., 1998; Vyse et al., 2020). Moreover, it has been observed that HSPs play a vital role in mitigating ER stress, especially by BIP (Cho and Kanehara, 2017; Maruyama et al., 2015; Ron and Walter, 2007). Potentially, the upregulation of HSPs during the PTR period could help alleviate ER stress, thereby facilitating the repair of denatured or misfolded proteins to restore their functionality.
Five PDI-like genes were upregulated specifically at RAT including PDIL1-1, PDIL1-2, PDIL1-4, PDIL1-6, and PDIL2-2 (Table S4). PDIs, also known as ER-localized proteins, are responsible for catalyzing disulfide bond formations to ensure protein conformation and function as ER chaperones (Gruber et al., 2007; Gupta and Tuteja, 2011). Furthermore, two DERLINs were also upregulated at RAT including DER1 and DER2.2 (Table S4). It has been reported that DERLINs are involved in ER-associated degradation (ERAD) facilitating the elimination of misfolded or unwanted proteins out of the ER, but the mechanism by which they contribute to the ERAD remains unclear (Fanata et al., 2013). Based on their functions in the ER, it is likely that both genes are involved in the stabilization of ER environments to improve the protein folding machinery during PTR periods.
4.2.4 Inhibition of ABA biosynthesis may upregulate pathogenesis-related (PR) genes during PTR periods
Several studies have reported that tissue ABA levels increase under various abiotic stresses including cold, and its accumulation is involved in improved stress tolerance via ABA-dependent signaling pathway (Wang et al., 2019; Xiong et al., 2002; Xin and Browse, 2000, and references in Vishwakarma et al. (2017)). In a similar context, the process of cold acclimation, induced FT, is well known to include ABA biosynthesis of tissues exposed to low but non-freezing temperature (Gusta et al., 2005; Xin and Browse, 2000), whereas an ABA-deficient mutant (aba) of Arabidopsis thaliana lost its capacity to enhance FT during cold acclimation (Heino et al., 1990). In contrast to these reports, our data indicated that ABA biosynthesis was inhibited after exposure to sub-injurious freeze–thaw stress (RAT), even though it was not directly measured. This is suggested by the downregulation of the GO term ‘terpenoid metabolic process’ (Figure 4A, cluster 34) and three enriched KEGG pathways: ‘carotenoid biosynthesis,’ ‘MAPK signaling,’ and ‘plant hormone signal transduction’ (Figure 6A). ABA belongs to the class of isoprenoids synthesized from either MVA (mevalonate)- or MEP (called non-mevalonate)-pathway, of which the latter is known to be involved in ABA synthesis through the pathway of carotenoid biosynthesis (Nambara and Marion-Poll, 2005). Our data showed that the three important transcripts involved in ABA synthesis ZEP, NECD3, and NECD4 were down-regulated within the carotenoid biosynthesis pathway at RAT (Table S4). The former gene is responsible for the conversion of zeaxanthin to violaxanthin, and the other two genes catalyze the cleavage of xanthoxin, the C15 precursor of ABA (Danquah et al., 2014). The downregulation of NECD3 and NECD4 was further validated through qPCR, confirming the suppression of these key ABA biosynthesis genes at RAT (Figure 7A). Taken together, a reduced terpenoid metabolic pathway and a down-regulated carotenoid biosynthesis pathway may result in decreased precursors required for ABA synthesis, which, in turn, could lead to the down-regulation of ABA-inducible ‘MAPK signaling’ (Danquah et al., 2014) as well as ‘plant hormone transduction’, evidenced by the down regulation of ABA-related genes including HAB1, HAI2, and HAI3 (Table S4).
Collectively, the observation outlined above led us to hypothesize that ABA signaling is inhibited during PTR periods. The specific mechanisms underlying this inhibition are beyond the scope of this study. However, based on evidence from the literature, we propose two potential explanations related to tissue recovery as follows: (1) the activation of guard cell PMA and (2) the establishment of defense mechanisms against pathogens. ABA accumulation is known to inhibit the activation of guard cell PMA, resulting in th depolarization of the guard cell plasma membrane, thereby inducing stomatal closing (Kim et al., 2010; Schroeder et al., 2001). Conceivably, inhibited ABA signaling during PTR periods, may potentially be linked to the activation of guard cell PMA, allowing for photosynthesis via stomatal opening, as supported by recovery of PSII quantum efficiency (Figure 1C, D). This assumption is partially supported by Merlot et al. (2007), who reported that the dominant ost2-1 and ost2-2 mutants, characterized by impaired stomatal closure due to a loss of function, exhibited continuous activation of PMA, leading to persistent stomatal opening and, consequently ABA insensitivity. Moreover, it has been reported that compromised function of stomal closing in the dominant ost2 is associated with ABA-inhibition of PMA (Shimazaki et al., 1986).
GO-terms related to pathogen-related defense mechanisms including ‘response to SA’, ‘response to chitin’, ‘programmed cell death’, biological process involved in interaction with symbiont’ ‘regulation of response to biotic stimulus' and ‘SA metabolic process' (Figure 4A, clusters 4, 6, 7, 13, 18, 26) and the KEGG pathway of ‘plant-pathogen interaction’ (Figure 6A) were both upregulated at RAT. Accordingly, it is tempting to assume that freeze–thaw injured tissues developed SA-mediated defense mechanisms via a down-regulated ABA signaling, thereby facilitating for tissue recovery since such tissues could be considered as an ideal locus for pathogen infection due to leakage of cell contents like salts, sugars, proteins, etc. (Arora, 2018). This assumption could be further reinforced by a study of Zabala et al. (2009) reporting that ABA could suppress SA-mediated defense mechanisms. SA is a well-known signaling molecule to mediate such defense mechanisms including the accumulation of secondary metabolites to combat pathogens (Raskin, 1992; Vicente and Plasencia, 2011). Among such metabolites, flavonoids are frequently implicated due to their antioxidant property (Dixon and Paiva, 1995; Gondor et al., 2016; Lu et al., 2017). Our data also showed that the GO-term related to ‘flavonoid metabolic process' (Figure 4A, cluster 24) and the KEGG pathway of ‘flavonoid biosynthesis' (Figure 6A) were upregulated at RAT, potentially linked to an SA-mediated response in order to establish pathogen-related defense mechanisms. Both SA and flavonoids are phenylpropanoid derivatives, synthesized from phenylalanine via the shikimic acid pathway, where shikimic acid is a key intermediate within the broader phenylpropanoid pathway (Falcone Ferreyra et al., 2012; Gondor et al., 2016). Therefore, it is somewhat intriguing that the KEGG pathway of phenylpropanoid biosynthesis was down-regulated at RAT (Figure 6A), but flavonoid biosynthesis was upregulated, as further validated by qPCR of two key genes involved in flavonoids—flavanone 3-hydroxylase (F3H) and flavanol synthase (FLS; Figure 7B). Supposedly, down-regulation of phenylpropanoid at RAT could be a reflection of significant consumption of phenylpropanoid derivatives for either SA or flavonoid biosynthesis. This assumption aligns with Vogt (2010), noting that the phenylpropanoid pathway intermediates could be diverted towards the biosynthesis of stress-related compounds including flavonoids, particularly under sub-optimal conditions or plant stress. Additionally, in their review on secondary metabolism Dixon et al., (2002) mentioned that the metabolic flux can shift towards specific branches of secondary metabolism based on environmental conditions or stress, thereby limiting the pool of intermediates available for other pathways. This shifting or metabolic flux helps plants to prioritize certain stress-response metabolites, leading to the consumption of shared precursors.
At PTR-6D, the down-regulation of ‘flavonoid biosynthesis’ and ‘SA metabolic process,’ as shown by GO terms (Figure 4B, clusters 58 and 64) and KEGG pathway (Figure 6B), suggests that pathogen-related defense mechanisms are almost, but not fully, resolved after six days of post-thaw. This is supported by the continued upregulation of the ‘pathogen-interaction’ KEGG pathway (Figure 6B). Consistent with this, our qPCR data showed that two crucial components of plant immune responses mediated by SA signaling—non-expressor of pathogenesis-related gene 1 (NPR1) and the WRKY41 transcription factor—were kept upregulated at PTR-6D. As expected it was not to the extent observed at RAT (Figure 7C), reinforcing the notion that pathogen defense mechanisms remain active even after six days of post-thawing.
4.2.5 Glutathione metabolism
Membrane damage caused by ROS, i.e., lipid peroxidation, could be one of the reasons for an increase in ion-leakage of freeze–thaw injured tissues, which could be mitigated by antioxidants. Notably, glutathione peroxidase (GPX) and glutathione S-transferase (GST), both of which are involved in glutathione metabolism, are capable of effectively eliminating by-products of lipid membrane peroxidation (Bela et al., 2015; Hayes and McLellan, 1999; Ketterer and Meyer, 1989). In the present study, transcripts involved in glutathione metabolism were upregulated at RAT, including glutathione peroxidase (GPX6, GPX8) and glutathione S-transferase (GSTU25, ERD9, GSTF8; Figure 6A; Table S4). They potentially contribute to the recovery of membrane damage, as evident by reducing ion-leakage during PTR periods, through the inhibition of lipid peroxidation. GST is also known to be involved in the DNA repair process by removing thymidine hydroperoxide, a by-product of DNA oxidation (Hayes and McLellan, 1999; Ketterer and Meyer, 1989).
4.2.6 DNA repair
Freeze–thaw stress is known to compromise the nucleic acid integrity of plant tissues, as manifested by an increase in RNA oxidation due to oxidative stress (Jaikumar et al., 2020). It has been also noted that oxidative stress under stress conditions triggers DNA damage, necessitating sufficient time for DNA repair by arresting the cell cycle (Barzilai and Yamamoto, 2004; Nisa et al., 2019; Shimotohno et al., 2021). Indeed, a perturbation of the cell cycle by delaying the entry into mitosis has been documented in tissues exposed to various abiotic stresses (references in Qi and Zhang, 2020). Above observations collectively let us to hypothesize that the cell cycle becomes arrested/inhibited, while DNA repair is increased for the restoration of a compromised genome integrity during PTR periods. Based on GO enrichment analysis, no evidence to satisfy our hypothesis was found at PTR since GO-terms related to cell cycle and DNA repair including ‘mitotic cell cycle’, ‘DNA replication’, ‘DNA unwinding involved in DNA replication’, ‘DNA repair’, ‘regulation of cyclin-dependent protein kinase activity’, ‘microtubule-based process’, ‘regulation of cell population proliferation’, and ‘maintenance of cell number’ were intriguingly down-regulated (Figure 4B, clusters 46, 47, 48, 49, 50, 52, 61, 62). No direct evidence exists, as to why both cell cycle and DNA repair were simultaneously down-regulated at PTR-6D. Speculatively, the repair of DNA damage had been completed before six days of post-thawing, e.g. between RAT and PTR-5D, and therefore no need exists for its further upregulation. Supporting this hypothesis, our qPCR data revealed that genes involved in the cell cycle, such as cyclin-dependent kinase group C2 (CDKC2), were downregulated at RAT, while genes involved in DNA repair, including DNA/RNA polymerases superfamily protein (DRP), DNA glycosylase superfamily protein (DGL), and DNA 3-methyladenine glycosylase (MAG), were upregulated (Figure 7D). This suggests that the cell cycle is halted to initiate DNA repair processes at RAT. Further evidence comes from the down-regulation of the ATP-dependent chromatin remodeling pathway at PTR-6D (Figure 6B). Given that chromatin must be properly packaged after DNA repair to ensure genomic stability and function (Probst and Mittelsten Scheid, 2015), the down-regulation of this pathway at PTR-6D suggests that DNA repair was nearly complete at this stage, necessitating chromatin remodeling for proper genome function.
5 CONCLUSIONS
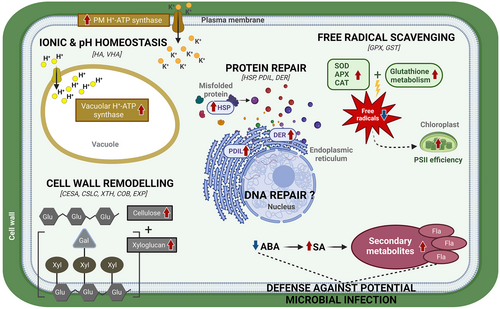
As extrapolated by the daily air temperature data of Barton Peninsula (Figure S1), C. quitensis could be damaged by freeze–thaw stress, thus requiring recovery from injury to ensure its survival and growth in Maritime Antarctica. Based on our previous discussion, a hypothetical model is suggested to illustrate potential cellular/molecular events in C. quitensis during the recovery from freeze–thaw injuries (Figure 8). This recovery process involves several key mechanisms. The restoration of ionic and pH homeostasis is facilitated by the upregulation of HA (i.e. plasma membrane H+-ATPase) and VHA (i.e. vacuolar H+-ATPase), which help re-establish the ion balance and stabilize cytoplasmic pH. Simultaneously, cell wall remodeling, supported by the upregulation of key biosynthetic and structural protein genes (e.g., CESA, CSLC, COB, EXP, XTH), contributes to the repair and strengthening of damaged walls. Protein repair is enabled by molecular chaperones such as HSPs and ER-localized chaperones like PDIL and DER, ensuring the restoration of proteostasis. Additionally, defense against potential microbial attacks is supported by SA-induced pathways and the synthesis of secondary metabolites, including flavonoids. Antioxidant enzymes actively scavenge free radicals to mitigate oxidative damage, while glutathione metabolism, as indicated by the upregulation of GPX and GST, aids in repairing lipid membranes. Finally, DNA repair mechanisms, indicated by ATP-dependent chromatin remodeling, further ensure cellular recovery and survival during post-thaw periods. Taken together, these coordinated mechanisms underscore the resilience of C. quitensis and its ability to adapt to the harsh freeze–thaw cycles of Maritime Antarctica and genes related to these mechanisms will likely serve as primary targets for future functional studies investigating this plant's responses to climate change.
AUTHOR CONTRIBUTIONS
K.M., J.L. and Hs.L. conceived the idea. K.M. and Hs.L. designed experiments;. K.M., J.-H.J. and S.S. performed the experiments. K.M., S.S., Hd.L and Hs.L. analyzed the data. K.M. wrote the paper with contributions from S.S., J.-H.J., Hd.L, J.L., J.H.L. and Hs.L.
ACKNOWLEDGEMENTS
We thank Dr. Seung Chul Shin for his valuable advice for bioinformatic analysis. This work was supported by a Korea Polar Research Institute grant funded by the Ministry of Oceans and Fisheries (KOPRI PE24130). This research was also supported by the project titled “Development of potential antibiotic compounds using polar organism resources (20200610, PM24030)”, funded by the Ministry of Oceans and Fisheries, Korea.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are openly available in GenBank of NCBI at https://www.ncbi.nlm.nih.gov. The BioProject, Bio-Sample, and SRA numbers are PRJNA1099400, SAMN40937327, SAMN40937328, SAMN40937329, SAMN40937330, SRR28638925, SRR28638926, SRR28638927, SRR28638928, SRR28638929, SRR28638930, SRR28638931, SRR28638932, SRR28638933, SRR28638934, SRR28638935, and SRR28638936 respectively.




