Etiolation promotes protoplast transfection and genome editing efficiency
Abstract
In plants, DNA-free genome editing using preassembled clustered regularly interspaced short palindromic repeats (CRISPR)-ribonucleoprotein (RNP) has the advantage of avoiding transgene integration and limiting off-target effects. The efficiency of this gene editing strategy can vary, so optimization of protoplast transfection conditions is necessary to achieve maximum yield. In this study, we examined the effects of etiolation, or increased exposure to darkness during cultivation, on the transfection efficiency of protoplasts from lettuce and Chinese cabbage. Seedlings were grown under three different conditions: non-etiolated, etiolated, and de-etiolated. First, we tested PEG-mediated transfection after etiolation using a plasmid DNA for green fluorescent protein (GFP)-expression. Etiolated protoplasts had the highest percentage of GFP-expressing cells, with a 3.1-fold and 4.8-fold improvement in lettuce and Chinese cabbage, respectively, compared with non-etiolated protoplasts. We also assessed gene editing of endogenous genes after etiolation using CRISPR-RNP. Using targeted deep sequencing, we observed the highest editing efficiency in etiolated protoplasts from both plant species, for the LsPDS and LsFT genes in lettuce, this led to an 8.7-fold and 4.4-fold improvement compared with non-etiolated protoplasts, respectively. These results suggest that etiolation during seedling growth can improve transfection efficiency and DNA-free gene editing in protoplasts.
1 INTRODUCTION
Plant genome editing using the clustered regularly interspaced short palindromic repeats (CRISPR)-CRISPR-associated protein (Cas) system has become a powerful tool in agricultural breeding and biotechnology (Chen et al., 2019; Gao, 2021). This method utilizes Cas enzymes, such as Streptococcus pyogenes Cas9 (SpCas9), that are combined with complementary sequences of single guide RNA (sgRNA) to generate double strand breaks (DSBs) at the target sites in the genome (Jinek et al., 2012; Kim and Kim, 2014). DSBs are repaired either by non-homologous end joining (NHEJ) or by the homology-directed repair (HDR) system. The NHEJ system preferentially repairs breaks through small insertions or deletions (indels) of nucleotides, which can result in frameshifts and loss of gene expression in plants.
DNA-free methods for genome editing are also being developed. Some have used polyethylene glycol (PEG)-mediated transfection to deliver preassembled Cas9-sgRNA ribonucleoprotein (RNP) into protoplasts (Woo et al., 2015; Liu et al., 2022; Najafi et al., 2023). Another study introduced in vitro transcribed Cas9 mRNA-sgRNA dual RNAs into callus by particle bombardment (Zhang et al., 2016). These techniques can edit the target site quickly before being rapidly degraded, which can reduce off-target effects compared to plasmid-derived genome editing (Lawrenson et al., 2015). DNA-free genome editing may also serve as a method to perform genetic alterations in plants within current regulations for genetically modified organisms (GMOs) regulations (Zaidi et al., 2020).
Multiple studies have been performed to improve the RNP delivery into protoplasts through various physical or chemical disruptors, including PEG (Woo et al., 2015; Liu et al., 2022; Najafi et al., 2023), lipofectamine (Liu et al., 2020), nanoparticles (Lee et al., 2017) and electroporation (Subburaj and Agapito-Tenfen, 2023). However, studies of transfection efficiency in response to plant physiology or environment have been lacking. Changes in plant culture conditions have largely focused on protoplast yield (Lee et al., 2023; Panda et al., 2024). The aim of this study was to assess the impact of etiolation, or exposure to darkness during seedling cultivation, on protoplast transfection efficiency and genome editing in both lettuce and Chinese cabbage that have been widely applied in protoplast genome editing studies (Woo et al., 2015; Jeong et al., 2019). Although etiolation has been induced in some monocots to improve protoplast yield, this is the first study to investigate the relationship between culture conditions and the efficiency of transfection and gene editing (Hahn et al., 2020; Lee et al., 2023). We compared protoplasts isolated from etiolated, non-etiolated and de-etiolated seedlings and found etiolation improved transfection efficiency and genome editing in both species. Our findings suggest this strategy may be helpful for improving yield in protoplast-derived DNA-free genome editing.
2 MATERIALS AND METHODS
2.1 Plant materials
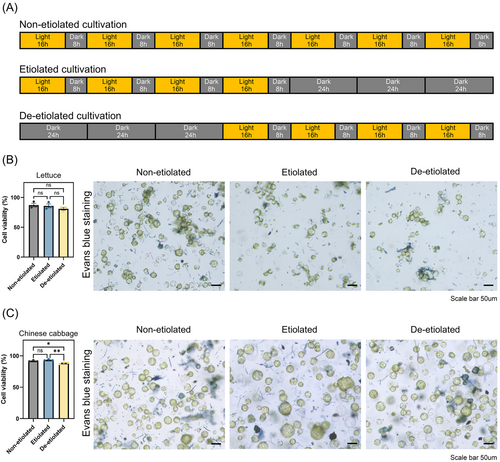
Lettuce (Lactuca sativa cv. Cheongchima) and Chinese cabbage (Brassica rapa cv. Seoul) seeds were surface sterilized in 70% ethanol for 30 s and in a 0.4% hypochlorite solution for 15 min and were washed three times in distilled water. Then, the seeds were germinated on 0.5X Murashige and Skoog (MS) medium supplemented with 2% sucrose and 0.7% plant agar under conditions of 16 h light and 8 h dark at 25°C for seven days (non-etiolation). Etiolated seedlings were cultivated under conditions of four days of light and three days of darkness. De-etiolated seedlings were cultivated under conditions of three days of darkness and four days of light.
2.2 Cas9 protein and guide RNAs
A plasmid (pET28a-Cas9-His, Addgene no. 98158) encoding the Cas9 protein with a C-terminal His purification tag was transformed into BL21 Star (DE3)-competent E.coli cells and purified using Ni-NTA agarose as previously described (Kim et al., 2021). Guide RNAs of lettuce phytoene desaturase (LsPDS, NC_056626.2), lettuce flowering locus T (LsFT, AB602323.1), and Chinese cabbage eukaryotic translation initiation factor (iso) 4E (BreIF(iso)4E, Bra035531) were designed using Cas-designer (Park et al., 2015). DNA templates for guide RNA (gRNA) were prepared by PCR using Q5 High-fidelity DNA Polymerase (Table S1). Guide RNAs were synthesized by in vitro transcription using the T7 RNA polymerase (New England Biolabs) according to the manufacturer's protocol (Figure S1). The synthetic guide RNAs were purified using a RNA purification kit (Riboclear, GeneAll).
2.3 Protoplast isolation
Protoplasts isolation was performed as described previously (Kang et al., 2021). Cotyledons from 7-day-old lettuce and Chinese cabbage seedlings were cut into 1 cm long pieces and digested in an enzyme solution (1% Viscozyme, 0.5% Celluclast, 0.5% Pectinex, 3 mM MES, 9% mannitol and CPW salts, pH 5.8). They were incubated at 25°C with rotation at 25 rpm for 2.5 h in the dark. The digested protoplast mixture was filtered using a 100 μm nylon mesh and washed with an equal volume of W5 solution (154 mM NaCl, 125 mM CaCl2, 5 mM KCl, 5 mM glucose and 1.5 mM MES, pH 5.6). The protoplasts were isolated on a sucrose gradient (21%) by swing-out centrifugation at 100 g for 7 min. The intact protoplasts were incubated in W5 solution for 1 h at 4°C before protoplast morphology was assessed by a viability test or cells were transfected.
2.4 Protoplast transfection
Lettuce and Chinese cabbage protoplasts (2.5×105 cells) in MMG solution (4 mM MES, 0.4 M mannitol, and 15 mM MgCl2) were transfected with a GFP-expression plasmid (Addgene no. 91043, 30 μg) or preassembled RNP (Woo et al., 2015; 30 μg of Cas9 protein and 80 μg of gRNA) using a polyethylene glycol solution (PEG 4000, 40% (w/v), 0.2 M mannitol and 0.1 M CaCl2). After a 20 min incubation at room temperature, the PEG-protoplast mixture was washed three times with each equal volume of W5 solution with gentle inversion. Protoplasts were harvested by swing-out centrifugation at 100 g for 5 min and then resuspended in WI solution (4 mM MES, 0.5 M mannitol, and 20 mM KCl) and incubated at 25°C for 72 h in the dark (Yoo et al., 2007). Protoplast transfection efficiency was measured by GFP fluorescence using microscopy (Axiovert A1, Zeiss, GFP: 400–650 nm).
2.5 Targeted deep sequencing
Genomic DNA was extracted from RNP (Cas9 + gRNA) transfected protoplasts using the DNeasy Plant Mini Kit (QIAGEN). Each target region was amplified using the Phusion Plus PCR master mixture with the targeted deep sequencing primers (Table S1). Three rounds of PCR were performed, first, nested PCR, second, 2nd PCR and third, index PCR, to generate index sequencing added amplicons. Equal amounts of the DNA libraries were pooled and sequenced using the Illumina iSeq100 platform. The paired-end sequencing files were analyzed by the Cas-analyzer (Park et al., 2017).
2.6 Protoplast viability
After protoplast isolation, lettuce and Chinese cabbage protoplasts in W5 solution were harvested by centrifugation at 100 g for 5 min. Protoplasts were resuspended using Evans blue dye solution (0.02%) and incubated at room temperature for 10 min. The unstained (live) and stained (dead) protoplasts were observed on a hemocytometer (Marienfeld, 0650030) under a light microscope (Zeiss AxioVert A1, Axiocam 705 color).
2.7 Statistical analysis
GraphPad Prism 10 software was used to analyze the data. All experiments were conducted with three independent replicates. All numerical values are presented as mean ± s.e.m., and compared using a one-way ANOVA analysis followed by a Turkey's multiple comparisons test.
3 RESULTS
3.1 Determination of protoplast viability after transfection
First, we assessed the impact of etiolation on the viability of the isolated protoplasts from lettuce and Chinese cabbage cotyledons using Evans blue solution. To establish the efficient protoplast-derived genome editing, we isolated protoplasts from cotyledons from three different conditions: non-etiolation (control), etiolation and de-etiolation (Figure 1A). We confirmed that chloroplasts were decreased in protoplasts from cotyledons by increasing the period of darkness exposure in the etiolation and de-etiolation groups compared to the control group (Figure 1A, Figure S2). We observed lettuce protoplasts from non-etiolated, etiolated, and de-etiolated seedlings were 87.2, 85.9 and 81.0% viable, respectively (Figure 1B). In lettuce an increased exposure to darkness during cultivation did not appear to substantially impact protoplast viability. When we examined protoplasts from Chinese cabbage, we found that their viability did not differ meaningfully between non-etiolated and etiolated conditions (91.8% and 93.3%), but was lower in de-etiolated protoplasts (88.4%, Figure 1C). These results suggest that etiolated protoplasts remained intact and healthy after isolation from cotyledons, but de-etiolated protoplasts from Chinese cabbage were less robust.
3.2 Improvement in protoplast transfection efficiency with etiolation
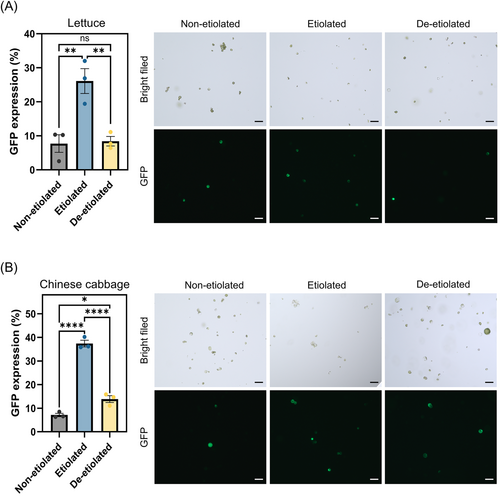
To investigate the impact of etiolation on transfection efficiency, we delivered a plasmid DNA encoding GFP expression to each type of protoplast by PEG-mediated transfection and measured the transfection efficiency. In lettuce protoplasts, the highest frequency of GFP expression was observed in etiolated protoplasts (32.0%) compared to control and de-etiolated protoplasts (10.3 and 11.1%), respectively (Figure 2A). In Chinese cabbage protoplasts, etiolated protoplasts were most efficient with an average of 40.7% cells expressing GFP, whereas control and de-etiolated protoplast expressed GFP in 8.5% and 15.8% of cells, respectively (Figure 2B). These data indicate that etiolation induced the highest protoplast transfection in both species, with a 3.1 and 2.9-fold improvement compared with the non-etiolated and de-etiolated protoplasts in lettuce, and 4.8 and 2.6-fold improvement in Chinese cabbage protoplasts. By comparison, de-etiolation did not meaningfully impact transfection efficiency in lettuce compared to the control group.
3.3 The effect of etiolation on genome editing efficiency in protoplasts
Based on these results, we hypothesized that etiolation would also enhance genome editing in protoplasts by Cas9 and gRNA-ribonucleoprotein (RNP) complexes. To test this, we selected two endogenous genes from lettuce, lettuce phytoene desaturase (LsPDS) and lettuce flowering locus T (LsFT), and one gene from Chinese cabbage, eukaryotic translation initiation factor (iso) 4E (BreIF(iso)4E), for analysis and designed guide RNAs for each target gene. We transfected the RNP complex into non-etiolated, etiolated, and de-etiolated protoplasts via PEG-mediated delivery and harvested at 72 h of incubation. Genomic DNA was extracted from transfected protoplasts and analyzed by overall indel frequency, both insertions and deletions, and editing patterns at the target sites (Figure 3). In lettuce protoplasts, the efficiency of genome editing for both genes was improved by etiolation compared to the non-etiolated or de-etiolated groups. Targeted amplicon sequencing demonstrated that etiolated protoplasts showed the highest editing efficiencies, with overall indel frequencies ranging from 13.6 to 14.9%, compared with non-etiolated (1.3–2.2%) and de-etiolated (3.2–3.8%) protoplasts when using the LsPDS targeted RNP (Figure 3A, Figure S3). Indel frequencies also demonstrated that etiolated protoplast had the highest percentage of genome editing compared with the other protoplasts, resulting in an average 8.7 and 4.1-fold improvement to editing compared with non-etiolated and de-etiolated protoplasts. For LsFT gene editing, etiolated samples had the highest overall indel frequency (24.0–25.8%) compared with other samples, resulting in an average 4.4-fold improvement compared with non-etiolated protoplasts (indel frequencies 5.0–5.6%), and a 2.3-fold improvement compared with de-etiolated protoplasts (indel frequencies 9.9–11.2%; Figure 3B, Figure S3).
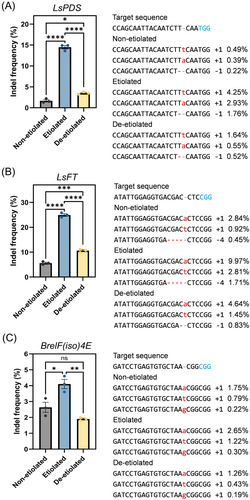
We also investigated non-etiolated, etiolated, and de-etiolated Chinese cabbage protoplasts and BreIF(iso)4E as target. We observed that, again, etiolated protoplasts had the highest editing efficiency (3.6–4.6%), which was approximately a 2.2-fold improvement compared to non-etiolated protoplasts (indel frequencies 1.8–2.0%) and 1.6-fold improvement compared with de-etiolated protoplast (indel frequencies 2.1–3.2%; Figure 3C, Figure S4). Interestingly, all genome editing insertions or deletion patterns were similar at each target region for non-etiolated, etiolated, and de-etiolated protoplast transfections.
4 DISCUSSION
The CRISPR-Cas system is a powerful method to perform precise editing of endogenous genes, but further improvement is needed to increase its efficiency in plants. In the past, etiolation has been used for high-yield isolation of protoplasts (Lee et al., 2023; Panda et al., 2024), but this method has not been widely used to enhance protoplast transfection efficiency. Previous studies have focused on physical (Subburaj and Agapito-Tenfen, 2023) and chemical methods (Liu et al., 2020; Lee et al., 2017) to increase transfection efficiency. Here, we examined etiolation as a strategy to improve genome editing in lettuce and Chinese cabbage seedlings and found up to an 8.7-fold improvement in efficiency compared to non-etiolated and de-etiolated protoplasts.
Given the interest in GMO-free agriculture, the development of DNA-free genome editing strategies such as CRISPR-RNP are becoming increasingly important for molecular breeding in plants (Kawall et al., 2020). One straightforward way to improve yield is to alter the environment to enhance the initial genome editing efficiency. We found etiolation increased transfection and gene editing efficiencies in lettuce and Chinese cabbage protoplasts without decreasing cell viability. This study focused on protoplasts from cotyledons, but it is likely that this cultivation strategy would be effective in other plant tissues as well, i.e. leaves and callus. Further studies are also warranted to determine the mechanism by which etiolation leads to improved transfection efficiency.
Notably, skotomorphogenesis induced by etiolation leads to structural and biochemical changes within cells, including chloroplasts (Armarego-Marriott et al., 2020). In this study, we found that the efficiency of transfection and genome editing in etiolated protoplasts from skotomorphogenic plants was higher compared to non-etiolated and de-etiolated protoplasts from photomorphogenic plants in light conditions. These differences suggest changes within the chloroplasts, specifically the transition from chloroplast to etio-chloroplast, and changes in the cell membrane that have been observed by others may facilitate gene editing in etiolated protoplasts (Sheen, 1991; Solymosi and Schoefs, 2010). Further research is needed to characterize etiolation-dependent intracellular changes in protoplasts and their impact on transfection efficiency. A recent study found that under fully etiolated conditions, protoplast transfection efficiency was high in monocot plants, and decreased in dicot plants (Panda et al., 2024). Therefore, the benefit to inducing skotomorphogenesis may depend on the type of plant used.
In conclusion, we have found etiolation promotes more efficient transfection and genome editing in lettuce and Chinese cabbage protoplasts. This simple cultivation strategy may serve as a valuable method to improve genome editing across a variety of CRISPR systems and crops.
AUTHOR CONTRIBUTIONS
Y. K., E. L. and B.-C. K. designed the study. Y.K. and E. L. performed the experiments. B.-C. K. wrote the manuscript and supervised the project.
ACKNOWLEDGEMENTS
We thank Allison Williams (BioSerendipity, LLC) for editorial assistance.
FUNDING INFORMATION
This work was supported by the National Research Foundation of Korea (NRF) grant funded by the Korea government (MSIT) (No. RS-2022-NR072144).
Open Research
DATA AVAILABILITY STATEMENT
All data supporting the findings of this research are available in the article, supplementary figures and tables, or from the corresponding author upon reasonable request. Sequence data are present in NCBI (https://www.ncbi.nlm.nih.gov/) or Brassica database (http://brassicadb.cn) under following accession number: LsPDS (NC_056626.2), LsFT (AB602323.1), and BreIF(iso)4E (Bra035531).




