The key contribution of OsGHD7 in controlling flowering time, grain yield, and abiotic stress tolerance in photoperiod-insensitive rice
Abstract
Developing rice types with shorter life cycle without compromising yield is vital for sustainable agriculture, as it can significantly reduce water and fertilizer consumption while enabling early harvest. Despite recent advancements in identifying the genes associated with heading date, the intricate regulatory network governing this process remains largely unexplored. In rice, one such gene, GHD7 (QTL for grain-heading-date on chromosome 7), encodes a CCT (CONSTANS, CONSTANS-LIKE and TIMING OF CAB1) domain protein and plays a pivotal role in regulating flowering time and associated developmental processes. To gain insight into the role of OsGHD7 in improving yield, we have overexpressed OsGHD7 in the widely cultivated and photoperiod-insensitive rice variety IR64. This led to notable phenotypic changes in rice, including tiller number and grain number (66% increase), along with the promotion of early flowering (8–9 days preponement). Interestingly, these plants also exhibited enhanced tolerance to drought and salinity stress and showed better post-stress recovery. This study emphasizes the potential of manipulating the multifaceted genetic determinants of key traits to optimize rice productivity under changing climate conditions.
1 INTRODUCTION
Flowering time is one of the most important agronomic traits that is controlled by a complex network of genes and their interactions. Grain Heading Date7 (GHD7), which encodes a CCT (CONSTANS, CONSTANS-LIKE, and TIMING OF CHLOROPHYLL A/B BINDING1) domain protein, is a crucial regulator of the rice-specific flowering pathway (Weng et al., 2014). Additionally, it plays a significant role in determining the yield potential of rice (Xue et al., 2008). In rice, flowering time is regulated by two main pathways. The first pathway involves OsGI (GIGANTEA), which activates Hd1 (Heading date1). Hd1 then promotes flowering by activating Hd3a (Heading date 3a) under short-day conditions and delays flowering under long-day conditions (Hayama et al., 2003; Izawa et al., 2002; Tsuji et al., 2011, 2013; Valverde, 2011, Zhang et al., 2019). The second pathway involves Ehd1 (Early heading date 1), which controls the RFT1 gene (rice FT-like) to regulate flowering under both short-day and long-day conditions (Doi et al., 2004; Komiya et al., 2009; Tamaki et al., 2007; Vicentini et al., 2023). Under long-day conditions, OsGHD7 and OsPRR37 delay flowering by suppressing Ehd1 and Hd3a/RFT1, while under short-day conditions, Hd1 promotes early flowering. This regulation allows rice to adjust flowering based on day length. However, some rice varieties have become photoperiod-insensitive due to domestication, a trait useful for breeding rice adapted to various latitudes (Khush, 2001). While the role of GHD7 in photoperiod-sensitive rice varieties is well understood, its function in photoperiod-insensitive varieties remains largely unexplored.
Heading date genes also influence various agronomic traits. Rice varieties with functional GHD7, PRR37, DTH8, and a nonfunctional hd1 allele exhibit enhanced grain yield (Gao et al., 2014; Leng et al., 2020; Liu et al., 2020; Wei et al., 2010; Yan et al., 2011, 2013; Zhang et al., 2015). DTH8 overexpression in rice also promoted early flowering and enhanced agronomic traits by upregulating MONOCULM1, which positively regulates the tiller number and panicle branching (Mishra et al., 2022; Wei et al., 2010; Yan et al., 2011). In contrast, the combination of functional HD1 and Ehd1 reduces the number of primary branches and spikelets per panicle by enhancing the expression of RICE CENTRORADIALIS 1 (RCN1) and RCN2 while decreasing the expression of genes involved in panicle formation (Endo-Higashi & Izawa, 2011). The increased transcription of GHD7 or PRR37 promoted plant height and the number of spikelets per panicle (Liu et al., 2013, 2015; Sun et al., 2022; Weng et al., 2014; Yan et al., 2013). Song et al. (2018) also reported that Mother of Flowering Locus FT (OsMFT1) and TERMINAL FLOWER1 (TFL1) enhances number of spikelets per panicle and prolongs the heading date in rice through the inhibition of Ehd1, FZP, and SEPALLATA-like genes.
Recent research findings have also suggested that genes responsible for flowering time may have a role in stress response. Overexpression of DTH8 facilitated the adaptation of rice plants to abiotic stresses, thereby mitigating the decrease in yield associated with these stresses (Mishra et al., 2022). Fang et al. (2019) demonstrated that overexpressing RFTL10 in rice leads to early flowering and improved drought tolerance. Furthermore, overexpressing WOX13 under a drought-inducible promoter in rice enhances drought resistance and early flowering by upregulating key stress and flowering genes (Minh-Thu et al., 2018). Moreover, GIGANTEA (GI) overexpressing plants were found to be more sensitive to salt, while gi mutants displayed increased tolerance and improved survival under conditions of salt stress, drought, oxidative stress (Grundy et al., 2015), and osmotic stress (Li et al., 2016). These findings suggest that the regulation of flowering transition is pivotal not only for the modulation of plant architecture plasticity but also for the adaptation to the surrounding environment.
Although OsGHD7 has been implicated in regulating heading date in photoperiodic-sensitive rice varieties and yield in previous studies, its role in regulating heading date in photoperiodic-insensitive rice varieties and ensuring grain yield under abiotic stresses remains relatively unexplored. Given the relevance of IR64 as a popular high-yielding mega rice variety, and considering the potential involvement of heading date genes in flowering, regulating yield and stress responses, we overexpressed OsGHD7 in rice (cv IR64) and observed that its overexpression leads to early flowering, increased yield and tolerance to drought and salinity stress. The early flowering and high-yielding trait of OsGHD7 overexpression lines were found to be linked with the alterations in the expression of specific genes associated with flowering/heading and yield. Additionally, OsGHD7 overexpressing lines also exhibited enhanced tolerance to drought and salinity stresses by maintaining efficient photosynthetic processes, improving overall physiological performance, and minimizing yield loss. Thus, this study explores the role of OsGHD7 in regulating flowering time in photoperiod-insensitive varieties and its impact on controlling grain yield under suboptimal conditions.
2 MATERIALS AND METHODS
2.1 Plant material and their growth condition
Seeds of Oryza sativa (cv. IR64) wild type (WT) and OsGHD7 overexpression (OsGHD7_Ox) lines were surface sterilized with 0.2% Bavistin (50% Carbendazim), thoroughly rinsed with distilled water and then grown in soil pots in the greenhouse under controlled conditions maintained at 28/23°C day/night temperature, 14/10 h day/night cycles and 70% relative humidity.
2.2 Construct preparation and generation of OsGHD7 overexpression lines
To generate OsGHD7 overexpression lines, the coding sequence of OsGHD7 (864 bp) was amplified from rice (IR64) flag leaf cDNA and was cloned into the TOPO-TA vector followed by sequencing. After sequencing, the full-length OsGHD7 gene was further amplified and cloned into the pRT101 shuttle vector. The whole cassette (CaMV35S promoter, OsGHD7 gene and the CaMV polyadenylation site) was amplified from pRT101 and subcloned into the pCAMBIA1302 plant expression vector. Cloning into pCAMBIA1302 was confirmed by colony PCR and restriction digestion using the HindIII restriction enzyme. The clone was further sequenced using vector-specific primers. Primers used in the cloning have been listed in Table S1. This overexpression construct was used for rice transformation through Agrobacterium-mediated transformation (Sahoo et al., 2011).
2.3 Screening of putative transgenic plants
Screening of putative transgenic plants was carried out through genomic DNA PCR. Genomic DNA was isolated from both WT and putative OsGHD7_Ox lines using CTAB method and quantification was done using NanoDrop One (Thermo Scientific). After quantification, an equal amount of genomic DNA was used for PCR using hygromycin gene-specific HPTF and HPTR primers.
2.4 Quantitative RT-PCR
To check the expression of OsGHD7 in various transgenic lines as well as the expression of flowering and yield-related genes in WT and OsGHD7_Ox lines, RNA was isolated from the topmost leaf of WT and transgenic plants grown under control conditions at the pre-flowering stage using TRIzol ® reagent (Life Technologies) in accordance with the manufacturer's protocol.. The first-strand cDNA was prepared using the Revert Aid First Strand cDNA Synthesis Kit (Thermo Scientific, K1622). Quantitative gene expression analysis was carried out using SYBR Premix Ex Taq (Applied Biosystems, USA) and amplified on a 7500 real-time PCR system (Applied Biosystems, USA) as per the procedure and reaction conditions defined in the manual. Primer Express 3.0 software was used to design primers, as listed in Table S1. The rice eEF1α gene was used as the endogenous control due to having the most stable expression across various tissues at different developmental stages in rice (Jain et al., 2006). The fold change (2−ddCt) in transcript level was calculated as described by Livak and Schmittgen (2001).
2.5 Evaluation of heading time and yield performance under control condition
OsGHD7_Ox lines and WT grown in pots were evaluated for phenology and yield component traits under greenhouse conditions. Various yield-related parameters such as number of tillers per plant, number of panicles per plant, number of grains per plant (total yield) and harvest index were recorded along with time of heading and maturity.
2.6 Stress treatment at the pre-flowering stage and assessment of various physiological and yield parameters
The WT and OsGHD7_Ox lines (approximately 70 days old) were subjected to salinity stress by irrigating with a mixture of salts (6.25 mM CaCl2, 15 mM MgSO4, 3.75 mM MgCl2, and 50 mM NaCl) to achieve electrical conductivity (EC) near to 10 dS m−1 (Singh & Flowers, 2010). Control plants were irrigated with water having EC below 1 dS m−1. The plant performance was consistently observed, and their morphological and physiological parameters were measured upon the appearance of stress symptoms, and yield-related parameters were recorded upon plant maturity. For drought stress treatment, WT and OsGHD7_Ox lines at the pre-flowering stage (approximately 70 days old) were not watered until soil moisture content reduced to 30% (in ~18 days) from the initial moisture level (~95%). Subsequently, the plants were recovered by re-watering, physiological parameters were recorded after 3 days of recovery, and all the parameters related to yield were measured upon plant maturity (Gupta et al., 2018). Various photosynthetic parameters such as Net Photosynthetic Rate (NPR), stomatal conductance, and chlorophyll fluorescence (Fv/Fm) were recorded using the LI-6400XT portable photosynthesis system (Model LI-6400, LI-COR Inc.). Greenness index (SPAD value) was measured using SPAD 502 plus chlorophyll meter (Konica Minolta Inc.).
2.7 Measurement of relative water content (RWC), electrolyte leakage (EL) and K+/Na+ ratio
Mature leaves of similar physiological age were used for the determination of RWC. The leaf samples were taken from both control and stress conditions and their fresh weight (FW) was recorded (Cole Parmer Symmetry Balance). Leaves were then kept in distilled water for 6 h at room temperature (25°C), and their turgid weight (TW) was measured. Thereafter, leaves were dried for 24 h at 80°C to check their dry weight (DW) (Reguera et al., 2013).
2.8 Analysis of grain quality using near-infrared reflectance spectroscopy (NIRS) scanning
Rice grain quality traits (protein, fat, fiber, ash, and others) were measured using a NIRSTM spectrometer (DS2500 Instruments, Wavelength: 400–2500 nm, USA). For assessing the quality parameters of OsGHD7_Ox transgenic lines and WT seeds, the calibrations developed by the DS2500 (FOSS) manufacturer were used with the default setting. The plastic cup was filled with seeds that could hold about 450–600 seeds, and excess samples were removed using the striker following company instructions. Each sample was scanned five times by repacking in reflectance mode using a rotating plastic cup. These observations were averaged per replication, and values were shown as percentages.
2.9 Statistical Analysis
Statistical analysis was performed, and bar graphs were generated using GraphPad Prism9 scientific software. The data was analyzed statistically by two-way ANOVA, and means were compared by Tukey's HSD post hoc analysis at p ≤ 0.05 for all experiments except for the data pertaining to the flowering time and yield analysis under control condition, transcript abundance of OsGHD7 as well as flowering and yield-related genes in both WT and OsGHD7_Ox lines. For these particular cases, one sample t-test was employed to facilitate the comparison between WT and OsGHD7_Ox lines. All the experiments were conducted in triplicate at T1 generation for all the 3 lines, with 3 plants for each line. Thus, a total of nine plants were analyzed for each transgenic line.
3 RESULTS
3.1 Generation and confirmation of putative GHD7 overexpression (OsGHD7_Ox) lines
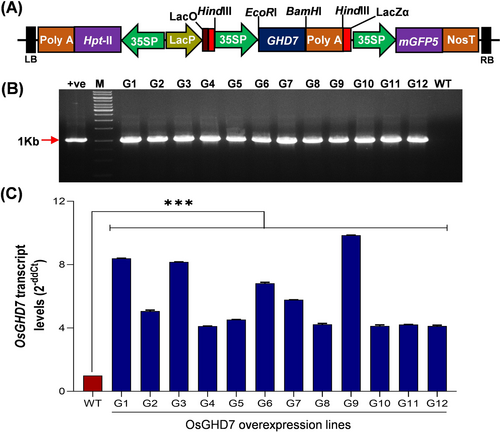
To raise the OsGHD7_Ox lines, we checked the tissue-specific and developmental stage-specific expression of OsGHD7 using the Genevestigator database. OsGHD7 was observed to be expressed at all the developmental stages of rice, but tissue-specific expression was found to be highest in the flag leaf tissue (Figure S1A, B). The coding sequence of OsGHD7 (864 bp) was amplified from the flag leaf-specific cDNA and cloned into the plant expression vector pCAMBIA1302 (Figure 1A). Rice was transformed with the overexpression construct, and putative transgenic plants were screened by tissue PCR using hygromycin gene (Hpt-II) specific primers, giving a 1 Kb amplicon (Figure 1B). The positive lines (G1 to G12) were further tested for the transcript level of OsGHD7 using quantitative real-time PCR, which showed a 4-10-fold increase in transcript levels in transgenic lines compared to WT (Figure 1C). The lines showing relatively higher overexpression of OsGHD7 (G1, G3 and G9) were selected for further analysis.
3.2 OsGHD7_Ox lines show early flowering and enhanced grain yield
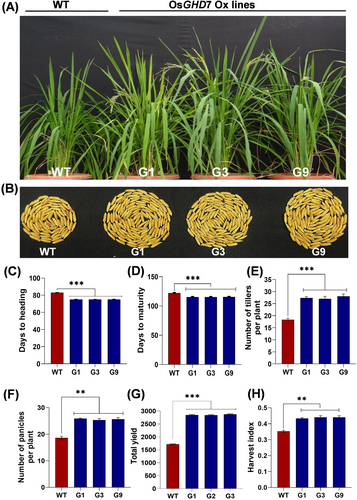
To assess the role of OsGHD7 overexpression in influencing the flowering time and grain yield in rice, both WT and OsGHD7_Ox lines at T1 generation (G1.1, G3.9 and G9.2) were grown in earthen pots under controlled greenhouse conditions. For brevity's sake, these lines are being referred to as G1, G3 and G9 in the following text. It was observed that the OsGHD7_Ox lines showed higher biomass and early flowering (Figure 2A). These lines also showed an increased number of grains per panicle as compared to WT plants (Figure 2B). The OsGHD7_Ox lines exhibited early flowering, approximately 8 days before the WT plants. This finding was supported by the notable differences in the days of heading (~83 days for WT and ~75 days for overexpression lines) and days of maturity (~122 days for WT and ~114 days for overexpression lines) (Figure 2C, D). We further investigated the influence of OsGHD7 overexpression on the rice grain yield components. The OsGHD7_Ox lines displayed around 49% more tillers, 37% more panicles, 66% higher total yield, and 25% more harvest index compared to the WT (Figure 2E-H). These findings show that higher expression of OsGHD7 in IR64 rice resulted in early flowering and higher yield under controlled environmental conditions.
3.3 OsGHD7_Ox lines have differential expression of flowering and yield-related genes
To delineate the underlying mechanism of early flowering and increased yield, we examined the expression patterns of genes involved in relevant pathways in both OsGHD7 overexpression T1 generation lines (G1, G3 and G9) and WT under control conditions. We investigated the impact of OsGHD7 overexpression on the expression of florigen genes OsRFT1 and OsHd3a, as well as other genes associated with flowering and yield, using qRT-PCR in WT and OsGHD7_Ox lines. Expression analysis was done in the samples harvested at the pre-flowering stage. Our results revealed a 2-fold higher transcript levels of OsRFT1 and OsHd3a genes and ~2.5-fold increase in OsMADS51 transcript levels in OsGHD7_Ox lines compared to the WT (Figure 3A-C). These findings suggest that the early flowering phenotype observed in OsGHD7_Ox lines may be attributed to the upregulation of OsRFT1, OsHd3a, and OsMADS51 genes.
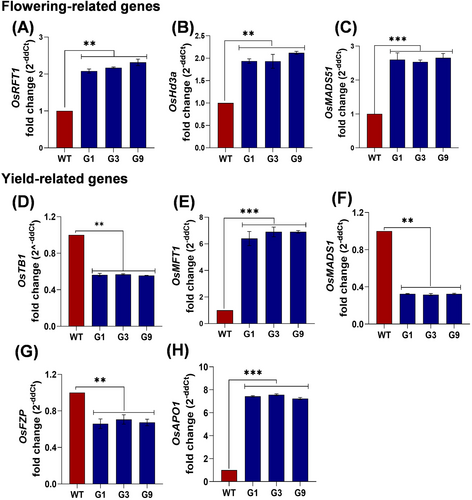
Further, we explored the expression pattern of genes involved in pathways associated with yield. A 2-fold decrease in OsTB1, >6-fold increase in OsMFT1, ~3-fold decrease in OsMADS1, ~1-fold decrease in OsFZP and >7-fold increase in OsAPO1 transcript levels in OsGHD7_Ox lines was observed as compared to WT (Figure 3D-H). These results suggest that the enhanced yield observed in OsGHD7 transgenic lines may be attributed to the upregulation of OsMFT1, OsAPO1 and the downregulation of OsTB1, OsMADS1, and OsFZP genes.
3.4 OsGHD7 overexpression improves drought stress tolerance in transgenic rice plants
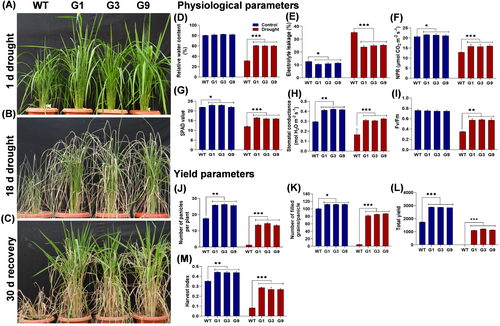
The drought stress tolerance potential of OsGHD7_Ox lines was evaluated by withholding irrigation till soil moisture content was reduced to 30% at the pre-flowering stage (Figure 4A). The most noticeable visible changes, such as leaf rolling and leaf drooping, were observed in both WT and overexpression lines after 18 days of drought stress (Figure 4B). Following this, the plants were recovered by rewatering. The overexpression lines were able to recover quickly, while the WT plants were not able to recover to the same extent (Figure 4C). One of the most important parameters for determining the severity of drought stress is relative water content (RWC) (Figure 4D). At 3 days post-recovery, RWC was found to be lower in both WT and transgenic lines, however, the reduction in RWC was observed to be up to 49% in WT, whereas, in overexpression lines, the reduction in RWC was recorded only up to 22% (Figure 4D). Under stress conditions, the electrolyte leakage in WT plants was 32% as compared to 25% noted in the transgenic lines (Figure 4E). Under drought stress, WT plants showed 38% reduction in net photosynthetic rate (NPR), 45% reduction in chlorophyll content (SPAD value), 44% reduction in stomatal conductance, and 54% reduction in Fv/Fm as compared to the control (Figure 4F-I). Whereas, OsGHD7_Ox lines showed 26% reduction in net photosynthetic rate (NPR), 29% reduction in chlorophyll content (SPAD value), 25% reduction in stomatal conductance, and 23% reduction in Fv/Fm as compared to the control (Figure 4F-I). Reduction in all yield-related parameters was observed in both WT and OsGHD7_Ox lines, however, the severity of reduction was higher in WT than in overexpression lines. In WT plants, under drought recovery, the reduction in the number of panicles per plant, number of filled grains per panicle, total yield, and harvest index was 90, 94, 99, and 70%, respectively, compared to the OsGHD7_Ox lines (Figure 4J-M). Under drought recovery till maturity, OsGHD7_Ox lines produced 1176 seeds per plant, while the WT was able to produce only 6–7 seeds per plant (Figure 4K). This indicates that the WT shows a yield penalty of 99% while the OsGHD7 transgenic plants show 33% yield penalty when compared to the WT grown under normal (non-stress) conditions. It is inferred that OsGHD7 overexpression in rice contributes significantly towards the improvement of physiology and yield in rice under drought stress.
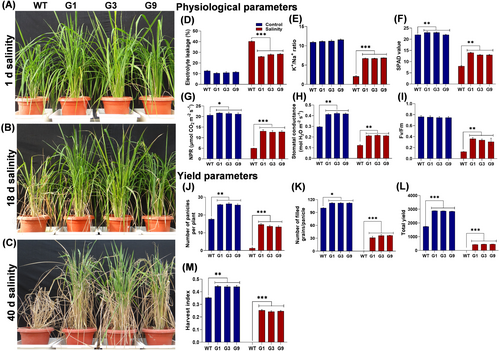
3.5 OsGHD7_Ox lines show improved physiology and yield under salinity stress
The OsGHD7_Ox lines were subjected to salinity stress at the pre-flowering stage to analyze the role of this gene in conferring salinity tolerance (Figure 5A). Under salinity stress, WT plants were severely affected (Figure 5B, C), whereas the OsGHD7_Ox lines sustained growth even after 40 days of stress (Figure 5B, C). Under salinity stress, WT plants showed 28% increase in electrolyte leakage, 81% reduction in K+/Na+ ratio, 64% reduction in chlorophyll content (SPAD value), 75% reduction in net photosynthetic rate (NPR), 58% reduction in stomatal conductance, and 84% reduction in Fv/Fm as compared to the control (Figure 5D-I). On the other hand, OsGHD7_Ox lines showed 16% increase in electrolyte leakage, a 40% reduction in K+/Na+ ratio, a 41% reduction in chlorophyll content (SPAD value), 40% reduction in net photosynthetic rate (NPR), a 48% reduction in stomatal conductance, and 55% reduction in Fv/Fm as compared to the control (Figure 5D-I). A higher K+/Na+ ratio in OsGHD7_Ox lines contributes towards growth and cellular homeostasis (Figure 5E). We also evaluated the yield performance of OsGHD7_Ox lines as compared to the WT under salinity stress. A 90% decrease in the number of panicles per plant and a 100% decrease in filled grains per panicle, total grains per plant and harvest index was observed in the WT as compared to the OsGHD7_Ox lines (Figure 5J-M). Under salinity stress, OsGHD7_Ox lines produced 448 seeds per plant, while the WT was unable to produce any viable seed (Figure 5K). This indicates that the WT shows a yield penalty of 100% while the OsGHD7 transgenic plants show a 74% yield penalty when compared to the WT grown under normal (non-stress) conditions, highlighting the contribution of OsGHD7 in reducing the yield gap under salinity stress conditions.
4 DISCUSSION
Flowering time plays a crucial role in determining rice yield and adaptability to various environmental conditions. In this context, the present study reveals that OsGHD7 promotes early flowering in photoperiod-insensitive rice variety IR64, and is also associated with enhanced grain yield and improved tolerance to both drought and high salinity. While previous research on OsGHD7 highlights its crucial role in delaying flowering under long-day conditions in a photoperiod-sensitive rice (Zheng et al., 2019) and across diverse field locations (Wang et al., 2020), our findings highlight its unique effects in inducing early flowering in a photoperiod-insensitive rice IR64. These insights underscore the potential of OsGHD7 in promoting early flowering and mitigating yield loss under adverse climatic conditions, thereby supporting more sustainable rice production.
The effect of OsGHD7 on rice flowering time varies with genetic background and photoperiodic conditions. Rice, being a short-day plant, shows flowering under short-day conditions and delays flowering under long-day conditions (Zong et al., 2021). However, some genotypes, such as IR64, have become photoperiod-insensitive specifically due to loss of functional Hd1 and Ehd1 alleles (Wei et al., 2010, 2016). In IR64, this loss is due to a 1.9 Kb insertion in Hd1 and a nucleotide substitution in Ehd1 (Doi et al., 2004). These photoperiod-insensitive varieties are often grown as early-season rice at various altitudes (Wei et al., 2010).
In this study, we found that OsGHD7 overexpression significantly increased the transcript levels of OsRFT1 and OsHd3a (Figure 3A, B), which are crucial for the transition of the vegetative shoot apical meristem to the reproductive meristem (Komiya et al., 2009). We have also observed an increase in OsMADS51 transcript levels in OsGHD7_Ox lines (Figure 3C). OsMADS51, a short-day dependent flowering promoter, accelerates flowering by one to two weeks when overexpressed, while its mutants flower two weeks later under short-day conditions (Kim et al., 2007). Additionally, overexpression of OsGHD7 increases OsMFT1 (Mother of FT and TFL1) expression (Figure 3E), consistent with earlier findings that GHD7 positively regulates OsMFT1, a gene involved in flowering time regulation (Wang et al., 2014). This upregulation may also lead to an early onset of flowering, as also seen in Arabidopsis (Yoo et al., 2004). These results confirm that OsGHD7 regulates heading date and promotes early flowering in the IR64 background by modulating the expression of key genes in the flowering. This highlights the complex genetic interactions that control flowering time in rice.
It has been reported in the literature previously that photoperiod-insensitive varieties experience a short, fixed period of photoperiod sensitivity that is unaffected by daylength, leading to early maturation (Wei et al., 2008). These varieties begin panicle initiation immediately after completing the basic vegetative phase (Sakaguchi et al., 2024). In OsGHD7_Ox lines, the basic vegetative phase is shorter as these lines showed early flowering as compared to WT plants. This may be attributed to the increased expression of OsHd3a, OsRFT1, and OsMADS51 genes (Figure 3A-C).
The present study also elucidates the regulatory role of OsGHD7 in key agronomic traits in rice, including tiller number, panicle number, and total grain yield. In OsGHD7 overexpressing lines, the modulation of tiller branching and panicle number is mediated via the phytochrome B-TEOSINTE BRANCHED1 (TB1) pathway (Weng et al., 2014). Specifically, OsGHD7 overexpression results in reduced expression of OsTB1 (Figure 3D), a known negative regulator of lateral branching in rice. Consequently, this reduction promotes increased tiller branching and panicle number, ultimately contributing to enhanced yield potential. Moreover, OsGHD7 overexpression upregulates the expression of OsMFT1, which in turn suppresses the expression of OsMADS1 and OsFZP genes (Figure 3F,G), consistent with findings by Song et al. (2018). OsFZP plays a crucial role in the transition from panicle branching to spikelet formation, and its repression correlates with increased lateral branches and spikelets (Bai et al., 2016; Komatsu et al., 2003). Similarly, OsMADS1 has been documented to cause reduced branching and smaller panicles, as observed by Wang et al. (2017). Thus, the OsMFT1-mediated repression of OsFZP and OsMADS1 contributes to increased branches and spikelets per panicle, positively impacting yield potential. Furthermore, OsGHD7 overexpression is associated with enhanced expression of OsAPO1 (Figure 3H), a gene known to regulate panicle size and grain number by influencing the precocious conversion of inflorescence meristems to spikelet meristems, as described by Ikeda et al. (2005, 2007) and Ikeda-Kawakatsu et al. (2012). Bai et al. (2016) reported that FZP is a major negative regulator of APO2. Consequently, the downregulation of FZP in OsGHD7_Ox lines could result in increased transcript levels of APO1. This is significant because APO1 and APO2 work together to regulate floral meristem determinacy (Ikeda-Kawakatsu et al., 2012). Thus, OsGHD7 may interact with different proteins to form distinct complexes, which then regulate different sets of target genes. These collective findings underscore the complexity of the regulatory network governed by OsGHD7 overexpression, which modulates key genes involved in branching and panicle development, ultimately contributing to enhanced yield potential in rice.
Abiotic stresses are major environmental constraints for grain yield (Singla-Pareek et al., 2003). The detrimental impact of these stresses at the reproductive stage is reflected in the stress-associated symptoms like decreased tillering, lesser panicle branching, a significant drop in spikelet numbers, and an increase in the prevalence of unfilled grains (Cramer et al., 2011; Munns & Tester, 2008; Tripathi et al., 2012). These effects stem from several stress-related physiological processes, decrease in photosynthetic efficiency, reduced relative water content, shortened vegetative phase and perturbed source-sink balance (Munns & Tester, 2008; Reguera et al., 2013; Werner et al., 2008). Our results showing early flowering and enhanced yield in OsGHD7Ox lines prompted us to investigate if overexpressing OsGHD7 in IR64 could reduce yield penalty under drought and salinity stress conditions. We observed that transgenic rice plants overexpressing OsGHD7 exhibited increased tolerance to drought and salinity stress compared to the WT plants. This was evidenced by higher chlorophyll content (Figure 4G, 5G), relative water content (Figure 4D), and reduced membrane damage (Figure 4E, 5D) in the transgenic lines. Furthermore, overexpression of OsGHD7 enabled maintenance of the K+/Na+ ratio at a certain threshold, further enhancing tolerance to salinity (Figure 5E). Our results are also consistent with indicative findings of some previous studies, which have shown the role of flowering genes in mediating stress responses. For instance, early flowering and increased drought tolerance was observed in RFTL10 overexpressing lines of rice, which was primarily due to the upregulation of transcription factors, including OsDREB2A, bZIP23, and SNAC1 in transgenic lines (Fang et al., 2019). Similarly, WOX13 overexpression lines of rice were drought tolerant and flowered early due to the upregulation of drought stress and flowering-related genes such as OsDREB1A, OsDREB1F, Hd3a, and MADS14 (Minh-Thu et al., 2018). DTH8 overexpression lines of rice were also found to be associated with early flowering and, drought and salinity stress tolerance (Mishra et al., 2022). In line with these findings, OsGHD7_Ox lines were also observed to have a better ability to cope with drought and salt stress (Figure 4, 5). Moreover, these lines had better physiological parameters (RWC, EL, K+/Na+ content) (Figures 4D,E, 5D,E), photosynthetic parameters (Chlorophyll content, NPR, stomatal conductance, Fv/Fm) (Figure 4F-I, 5F-I) and root biomass (Figure S2) under drought and salinity stresses which further contributed to minimize the yield penalty (Figure 4J-M, 5J-M) under these stresses. Moreover, the NIRS analysis of seeds of WT and OsGHD7_Ox lines revealed a significant increase in the protein content and branched-chain amino acids, such as isoleucine, leucine, and valine in transgenic lines (Table S3). These amino acids contribute to stress tolerance by functioning as compatible osmolytes or alternative energy sources (Shim et al., 2023). These results demonstrated the importance of OsGHD7 in conferring stress tolerance and highlights its potential for improving crop resilience under adverse environmental conditions.
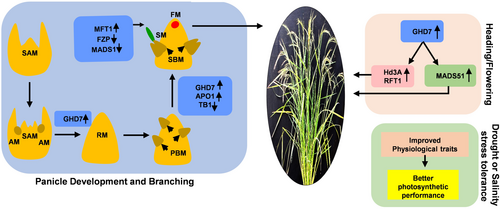
5 CONCLUSION
Overexpression of OsGHD7 in rice leads to a complex modulation of gene expression that influences both flowering and yield. The OsGHD7 overexpression induces the expression of key flowering-related genes such as Hd3a, RFT1, and MADS51, which are essential for transitioning from vegetative to the reproductive phase, a process critical for panicle initiation and flowering time regulation (Figure 6). Concurrently, the OsGHD7 overexpression induces OsMFT1 expression, which in turn reduces the expression level of FZP and MADS1. This reduction in the level of FZP and MADS1 led to prolonged branch meristem differentiation, resulting in an increase in branches and spikelets. FZP acts as a major negative regulator of the APO gene, which is crucial for floral meristem determinacy. The reduced expression of FZP in OsGHD7 overexpressing lines results in elevated levels of APO1. APO1 regulate panicle size and grain number by influencing the precocious conversion of inflorescence meristems to spikelet meristems in rice (Figure 6). Furthermore, OsGHD7 overexpression leads to a decrease in OsTB1 expression, a gene known to negatively regulate lateral branching, which leads to increased lateral branching. Moreover, under stress conditions, rice plants overexpressing OsGHD7 exhibit improved biochemical and physiological traits, including enhanced photosynthetic performance (Figure 6). This results in reduced yield penalties compared to WT plants, demonstrating that OsGHD7_Ox not only optimizes flowering and yield but also enhances stress resilience in rice.
AUTHORS CONTRIBUTION
Conceptualization, SLS-P. Data curation, MM, RSR and JB. Formal analysis, MM, RSR, and RNB. Resources, SLS-P. and AP. Supervision, SLS-P. Writing-original draft, MM and RSR. Writing-Review & editing, MM, RSR, RNB, AP, and SLS-P. All authors have read and agreed to the published version of the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the core funds of ICGEB, New Delhi, India to S.L.S.-P.
FUNDING INFORMATION
This work was supported by the funds received from core grant support of International Centre for Genetic Engineering and Biotechnology (ICGEB) Core Funds, New Delhi.
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
The data reported in this study are contained within the manuscript and there is no associated data available.




