Characterization of Arabidopsis eskimo1 reveals a metabolic link between xylan O-acetylation and aliphatic glucosinolate metabolism
Abstract
Glucuronoxylan is present mainly in the dicot of the secondary cell walls, often O-acetylated, which stabilizes cell structure by maintaining interaction with cellulose and other cell wall components. Some members of the Golgi localized Trichome Birefringence-Like (TBL) family function as xylan O-acetyl transferase (XOAT). The primary XOAT in the stem of Arabidopsis is ESKIMO1/TBL29, and its disruption results in decreased xylan acetylation, stunted plant growth, and collapsed xylem vessels. To elucidate the effect on metabolic reprogramming and identify the underlying cause of the stunted growth in eskimo1, we performed transcriptomic, targeted, and untargeted metabolome analysis, mainly in the inflorescence stem tissue. RNA sequencing analysis revealed that the genes involved in the biosynthesis, regulation, and transport of aliphatic glucosinolates (GSLs) were upregulated, whereas those responsible for indolic GSL metabolism were unaffected in the eskimo1 inflorescence stem. Consistently, aliphatic GSLs, such as 4-methylsulfinylbutyl (4MSOB), were increased in stem tissues and seeds. This shift in the profile of aliphatic GSLs in eskimo1 was further supported by the quantification of the soluble acetate, decrease in accumulation of GSL precursor, i.e., methionine, and increase in the level of jasmonic acid.
1 INTRODUCTION
Plant cell walls are reliable sources for the synthesis of biorefinery-based value-added chemicals because they contain energy-rich polysaccharides and non-polysaccharide components (Zoghlami & Paës, 2019). These components play a pivotal role in maintaining the cell structure throughout growth and development and provide a protective barrier against different kinds of stresses (Vaahtera et al., 2019; Wan et al., 2021). Primary cell wall components are deposited during plant growth, followed by secondary cell wall layer. The primary cell wall is a hydrated matrix that gives flexibility to the cell, contrary to the secondary cell wall, which is more rigid because of cellulose, xylan, and lignin (Gu & Rasmussen, 2022; Vaahtera et al., 2019). Xylan is the predominant hemicellulose in hardwood biomass, but a significant hurdle for processing the lignocellulosic biomass because of its structure and interaction with other components of the cell wall; however, relatively little is known about the genetic regulation and metabolic processes governing its biosynthesis (Rao et al., 2023; Isayenkov & Maathuis, 2019). Xylan is a polymer made up of repeating units of β-1,4 linked xylose residue, which is substituted with acetyl and methylated or non-methylated glucuronic acid and often attached with reducing end sequence (RES) of xylose, rhamnose, and galacturonic acid (Scheller & Ulvskov, 2010; Pauly et al., 2013; Rennie & Scheller, 2014; Chong et al., 2014). According to several studies, these substitution patterns on the xylan backbone are necessary for interaction with cellulose (Busse-Wicher et al., 2014; Grantham et al., 2017; Pereira et al., 2017). Compositional differences in lignin can also influence xylan-lignin interaction, although more studies are needed on how substitution can alter this interaction (Kang et al., 2019; Zhao et al., 2022).
The biosynthesis and modification of xylan depend on metabolic precursors derived from primary metabolism, such as acetyl-CoA, S-Adenosyl Methionine (SAM), and nucleotide sugars. These substrates are incorporated in the activated form in Golgi for the synthesis of xylan backbone, which is substituted with O-acetyl and methylated or non-methylated glucuronic acid (Wierzbicki et al., 2019). Almost 60% and 8% of Arabidopsis xylan is O-acetylated and glucuronylated, respectively (Lee et al., 2014). The C-2 position of xylose is more acetylated than the C-3 position. This substitution affects polymer properties, mainly xylan digestibility. Decreasing the xylan O-acetylation by modifying REDUCED WALL ACETYLATION (RWA) proteins or by expressing xylan de-esterifying enzymes can improve cellulose and xylan digestibility (Pawar et al., 2016, 2017, Rastogi et al., 2022). Therefore, it is important to understand the mechanism(s) controlling xylan acetylation to fine-tune its level for biotechnological applications.
Until recently, the mechanism of O-acetylation of plant cell wall polysaccharides was unknown. The number of Golgi localized protein families are involved in the acetylation process in plants, such as the REDUCED WALL ACETYLATION (RWA) (Manabe et al., 2013; Oliveira 2024), ALTERED XYLOGLUCAN 9 (AXY9) (Schultink et al., 2015), and the TRICHOME BIREFRINGENCE-LIKE (TBL); are identified both in monocot and dicot species (Pauly & Ramírez 2018). Recently, Golgi and plasma-membrane localized xylan acetyl esterases from the GDSL lipase esterase (GELP) family have been identified, suggesting that maintenance of polysaccharide O-acetylation is necessary (Zhang et al., 2017; Zhang et al., 2019b; Rastogi et al., 2022). Evidence also suggests that expressing fungal acetyl xylan esterase post-synthetically can alter the expression of Golgi localized RWA genes, suggesting a homeostasis response from plants because of alteration in polysaccharide O-acetylation (Pogorelko et al., 2011, 2013; Ratke et al., 2015). This response from the plants is probably because of a change in plant cell wall integrity and acetylation metabolism, which is of further interest to understanding the mechanism of polysaccharide acetylation. According to Xiong et al. (2013) and Lefebvre et al. (2011), Arabidopsis plants with the loss of function alleles of one of the members of the TBL family, eskimo1(tbl29-1 and tbl29-2), exhibited 50–60% reduction in xylan acetylation with collapsed xylem vessels and these mutants showed resilience to cold, drought, and salt stressors (Xiong et al., 2013; Lefebvre et al., 2011). The other tbl mutants of this family with reduction in O-acetylation do not show such defect in growth, probably because of a lesser degree of reduction in xylan acetylation compared to eskimo1/tbl29 (Yuan et al., 2016; Zhong et al., 2017, 2018; Stranne et al., 2018). But by substituting glucuronic acid moieties for the acetyl substituents in the eskimo1 mutant through the expression of a glucuronic acid transferase, the aberrant xylem phenotype was abolished, resulting in normal plant development (Xiong et al., 2015). According to these findings, balance substitution on xylan is necessary for xylem vessel integrity and maintaining overall plant growth. Biochemical evidence of purified ESKIMO1 (TBL29) demonstrated that ESKIMO1(TBL29) is a xylan-specific O-acetyltransferase (XOAT1) that catalyzes the addition of O-acetyl groups to the 2-position of xylose residue (Urbanowicz et al., 2014; Lunin et al., 2020). Previous findings suggest that while the other TBLs might be contributing more functionality and complexity to the acetylation pattern (Xiong et al., 2013; Yuan et al., 2013), ESKIMO1/TBL29 might be the dominant TBL that drives xylan acetylation mainly in the stem. The eskimo1 suppressor studies revealed that mutation in KAKTUS1 and MAX4 genes can restore the dwarf phenotype of the eskimo1 (Bensussan et al., 2015; Ramírez et al., 2018). KAKTUS1 controls the amounts of endoreduplication in cotyledons and hypocotyls and functions as ubiquitin E3 ligase that mediates the proteasomal degradation of cyclin-dependent kinase inhibitor KRP2 (El Refy et al., 2004; Xue et al., 2023). MAX4 plays a role in the synthesis of strigolactone (Wang et al., 2023). The double mutants eskimo1/kak1and eskimo1/max4 display wild-type-like growth and restored vessel morphology without restoring polysaccharide acetylation level (Bensussan et al., 2015; Ramírez et al., 2018). This suggests that cellular pathways other than xylan acetylation might have contributed to the stunted growth of eskimo1. To re-engineer xylan acetylation, it is important to understand cell wall acetylation metabolism and homeostasis response to alter its structure for various biotechnological applications. Therefore, we studied eskimo1 to unravel metabolic reprogramming upon disruption in xylan O-acetylation. Our study with the global transcriptome and metabolome analyses and quantification of phytohormones and amino acids revealed changes in both primary and specialized metabolism in eskimo1. More specifically, we found a decrease in methionine and an increase in jasmonic acid, which is probably related to a higher accumulation of aliphatic glucosinolates (GSLs) in eskimo1. All these changes may be linked to an increase in the accumulation of soluble acetate levels in the eskimo1 inflorescence stem.
2 MATERIALS AND METHODS
2.1 Plant growth conditions and genotyping by PCR
Arabidopsis thaliana Columbia-0 (Col-0) and eskimo1 (SALK-089531) were used for all experiments and grown under 16/8 h light/dark cycle conditions at 22°C for the desired time according to the experimental set-up. The homozygous was isolated for the eskimo1 mutant using Primers PCWL01 (left primer), PCWL02 (right primer), and PCWL29 (border primer) (Table S1).
2.2 Alcohol Insoluble Residue (AIR) preparation
Individual 6-week-old plants' primary stems were harvested, and the homogenized tissue was treated for 30 min at 70°C with 80% ethanol prepared in a 4 mM HEPES buffer (MB016, HiMedia, India). After centrifugation, the supernatant was discarded, and the pellet underwent three sequential washes using 800 μL each of 70% ethanol, chloroform: methanol (1:1), and followed by acetone. After acetone removal, the pellet was dried overnight. Further examination of the cell wall was done using this AIR.
2.3 Cell wall acetyl content analysis
Acetyl content was analyzed by incubating AIR in 1 M NaOH and neutralization using 1 M HCl. The mixture was centrifuged, and the supernatant was analyzed using the Megazyme K-ACET kit to determine acetic acid.
2.4 Cell wall composition
Xylose content: 2 mg of AIR sample was incubated with 100 μL of 1.3 M HCL in the dry bath at 100°C for 1 h. The sample mixture was neutralized with 100 μL of 1.3 M NaOH, and the final volume was made up to 1 mL by adding MilliQ water. The mixture was centrifuged at 15000 g for 10 min, and 50 μL supernatant was analysed using a Megazyme K-XYLOSE kit to quantify xylose content.
Lignin content: It was determined by incubating AIR samples with freshly prepared 25% acetyl bromide (135968, Sigma-Aldrich) in acetic acid at 50°C for 2 h. The supernatant was diluted with 2 M NaOH and freshly prepared hydroxylamine hydrochloride (159417, Sigma-Aldrich,) and the absorbance at 280 nm was used to measure the amount of lignin per gram of AIR (Foster et al., 2010).
2.5 Staining and imaging of Arabidopsis stem sections
Equivalent segments of plant inflorescence stems were used to prepare hand-cut sections and stained with Wiesner stain (Mitra & Loqué, 2014). The sections were washed two times with sterile water and imaged under a Nikon ECLIPSE Ci-L plus microscope under 10× and 40× magnification. We observed at least two sections per plant from five plants.
2.6 Measurement of endogenous acetic acid contents
300 mg of Arabidopsis frozen tissue in liquid N2 was homogenized and vortexed in five volumes (w/v) of cold (4°C) water for 6 min (1 min shaking, 1 min on ice). Following centrifugation at 15,000 g for 1 min at 4°C, the supernatants were collected and 100 μL from the supernatant containing acetic acid was quantified by the K-ACET method (Kim et al., 2017).
2.7 RNA sequencing analysis
RNA was extracted using the TRIZOL method (Shi & Bressan, 2006), and RNA sequencing was carried out using the Illumina NovaSeq 6000 platform with 30 M reads. The following criteria were used to choose the DEGs: FDR was less than 0.05 (for downregulated genes log2FC range from −0.7 to −5 and for upregulated, log2FC range from 0.7 to 6.0) in FPKM between two samples. The Differentially expressed genes (DEGs) were subjected to gene ontology analysis using WebGestalt (WEB-based GEneSeTAnaLysis Toolkit) and g: Profiler bio. Tools.
2.8 Quantitative PCR Analysis
The RNA was extracted, as explained above. 500 ng of RNA per 10 μL reaction was used for cDNA preparation using the iScriptTM cDNA Synthesis Kit (1708891, Bio-Rad). The fold change expression of the genes was calculated by normalizing using housekeeping genes GAPDH and ACTIN.
2.9 Untargeted metabolome and data analysis
Plant tissues were ground in a ball mill with liquid N2. The fine powder was suspended in 1 mL of ice-cooled methanol: water (4:1) by vortexing. The mixture was sonicated for 15 min in a water bath and then centrifuged at 11,000 g for 10 min at 4°C. A 700 μL aliquot of the supernatant was transferred into fresh tubes and dried in a speed vacuum. The sample was resuspended in 25 μL of a 3:17 methanol: water mixture, vortexed for a short while, then centrifuged for 10 min at 4°C at 13,000 g and injected for analysis using Thermo Scientific's Orbitrap Fusion mass spectrometer installed with a heated electrospray ion source (Naz et al., 2017; Choudhary et al., 2019; Kumar et al., 2020). In brief, mass resolution was maintained at 120,000 for MS1 mode and 30,000 for MS2 capture. The data acquisition mass range was performed between 60–900 Da. The UHPLC column used for separation was ACQUITY HSS T3 (Waters, 2.1 mm × 100 mm × 1.8 m). Data preprocessing and identification of metabolites have been using Prognosis QI for metabolomics. Further, statistical data analysis was done using MetaboAnalyst version 5.0 (https://www.metaboanalyst.ca). The group averages were used to display the metabolite intensities from Col-0 and eskimo1. The Kyoto Encyclopedia of Genes and Genomes (KEGG) was used as the backend knowledge base for the MetPA (Metabolomics Pathway Analysis), a part of the MetaboAnalyst suite.
2.10 Estimation of total glucosinolate content by spectrophotometric method
100 mg of tissue was homogenized and incubated in 1 mL of 80% methanol at ambient temperature overnight. This homogenate was centrifuged at 850 g for 4 min, and the supernatant was diluted with 80% methanol to make 2 mL. This extract of 100 μL was used for estimation. 3 mL of 2 mM sodium tetrachloropalladate (58.8 mg sodium tetrachloropalladate +170 μL concentrated HCl + 100 mL double distilled water) and 0.3 mL of double-distilled water were mixed and the absorbance was measured at 425 nm and 405 nm after 1 h of incubation at room temperature. The glucosinolate content was calculated using the following formula y = 1.40 + 118.86 × A425/405, and total glucosinolate was calculated. This protocol was modified from Mawlong et al., 2017.
2.11 Glucosinolate quantification using HPLC
Freshly harvested seeds, major inflorescence stems from 6-week-old plants, and rosette leaves from 3- or 6-week-old plants cultivated soil were collected for the glucosinolate analysis. Desulfoglucosinolate contents were measured using a method outlined in Shin et al. (2023). In short, samples were extracted using 50% methanol containing an internal standard of sinigrin (Sigma-Aldrich). After adding 200 μL of QAE Sephadex solution (Sigma-Aldrich) to 300 μL of extract, the mixture was kept at room temperature for 5 min. The beads were resuspended in 100 μL of MilliQ water containing sulfatase (Sigma-Aldrich) and incubated at 37°C for 6 h after being washed twice with 50% methanol and MilliQ water. The desulfoglucosinolates were analyzed using a ThermoFisher Scientific UltiMate 3000 HPLC system. The metabolites in 10 μL of the reaction mixture were separated on an AcclaimTM 120 C18 column (150 mm × 4.6 mm, 5 μm) using the mobile phase of solvent A (water) and solvent B (100% aceto-nitrile) with a gradient program for solvent B (2–12% over 10 min, 12–15% over 15 min, 15–25% over 17.5 min, and 95% for 2 min). The temperature of the column was 40°C, and the flow rate was 1 mL min−1. Based on reaction parameters and peak area at 229 nm, desulfoglucosinolate contents were calculated (Brown et al., 2003).
2.12 Phytohormones Extraction and Analysis
25 mg of the lyophilized inflorescence stems of Col-0 and eskimo1 were mixed with 1 mL of methanol containing 40 ng ml−1 of D6-jasmonic acid (HPC Standards GmbH), 40 ng ml−1 D4-salicylic acid, 40 ng ml−1 D6-abscisic acid, and 8 ng ml−1 of jasmonic acid- [13C6] isoleucine conjugate as internal standards, vortexed for 2 min while keeping the sample at 4°C. The sample was then placed into a tube rotor at 4°C for 20 min with shaking, followed by centrifugation at 4°C and 12,000 g for 15 min. The supernatant was vacuum-dried and resuspended in 500 μL of methanol and analyzed by liquid chromatography-mass spectrometry (LC–MS/MS) (QTRAP 6500+, SCIEX) (Vadassery et al., 2012).
For the growth hormone estimation, the 25 mg of lyophilized inflorescence stem samples were treated with 1 mL of ice-cold extraction buffer (MeOH: H2O: HCOOH, 15: 4: 0.1) containing 25 ng of trans- [2H5] Zeatin and D2-indole-3-acetic acid (D2-IAA) as internal standards, vortexed for 8 min and centrifuged for 10 min at 12,000 g at 4°C. The supernatant was treated with 1 mL of MeOH and 0.1% formic acid and eluted using a C18 RP SPE column with 1 mL of ice-cold 0.1% HCOOH in acetonitrile. The eluted fraction was vacuum-dried, resuspended in 50 μL of 5% MeOH, and then analyzed by Liquid Chromatography-Mass Spectrometry (LC–MS/MS) (QTRAP 6500+, SCIEX) (Simura et al., 2018).
2.13 LC–MS/MS analysis of amino acids
The methanolic extract tissue was homogenized and incubated in 1 mL of 80% methanol at ambient temperature overnight. This homogenate was centrifuged at 850 g for 4 min. from 25 mg of lyophilized inflorescence stems from Col-0 and eskimo1 extraction were diluted in water with the 13C, 15N-labelled algal amino acid mix (Cambridge Isotope Laboratories, Inc.) at a concentration of 10 g of the mix per ml at a ratio of 1:20 (v:v). The amino acids were separated using A Zorbax Eclipse XDB-C18 column (50 X 4.6 mm, 1.8 µm, Agilent Technologies). was used with the mobile phase comprised solvent A (water, 0.1% formic acid) and solvent B (acetonitrile, 0.1% formic acid) with the following elution profile: 0–1 min, 3% B; 1–3.8 min, 3–50% B; 3.8–3.9 min 50–100% B, 3.9–5 min, 100% B; 5–5.1 min, 100–3% B, 5.1–7 min, 100% B with a flow rate of 1.1 mL min–1. The triple-quadruple-trap SCIEX 6500+ MS/MS was used for analysis using positive ion mode. Data collection and processing were performed using Sciex's Analyst 1.5 software. The corresponding 13C, 15N-labeled amino acid was used to measure the amount of each amino acid in the sample (Jander et al., 2004; Vadassery et al., 2014; Crocoll et al., 2016).
3 RESULTS
3.1 eskimo1/tbl29 shows stunted growth, collapsed xylem, less wall-bound acetate content, and elevated soluble acetate content
To understand the role of ESKIMO1/TBL29 in plant growth and cell wall formation, the morphological, anatomical, and cell wall composition analysis was performed on isolated homozygous eskimo1 mutant (SALK_089531), along with control plants Col-0. After two weeks, eskimo1 was distinguishable because of its dark green leaves as compared to the wild type (Figures S1A and S1B). Six-week-old eskimo1 was short in stem height and smaller in rosette size, as shown in Figure 1a. Anatomical studies on the cross-section of inflorescence stems revealed a collapsed xylem, corroborating past findings (Lefebvre et al., 2011; Bensussan et al., 2015) (Figure 1B). As ESKIMO1 is a xylan acetyltransferase (XOAT1) and mutation in XOAT1 affects polysaccharide acetylation (Lunin et al., 2020), the total cell wall acetyl content in eskimo1 was reduced by 33.3% as compared to wild type in 6-week-old inflorescence stem (Figure 1C). This decrease in wall-bound acetyl content is because of disruption in only xylan O-acetylation but not other acetylated polysaccharides (Xiong et al., 2013; Ramírez et al., 2018). However, the acetyl content in a six-week-old rosette leaf was comparable to the wild type because ESKIMO1 is expressed mainly in stem tissues (Xiong et al., 2013) (Figure 1C). The total xylose content coming mainly from xylan polysaccharides in the stem and leaf was similar to the wild-type (Figure 1D). Also, the total lignin content was similar in eskimo1 and Col-0 plants (Figure 1E). All these findings were corroborated with the past findings.
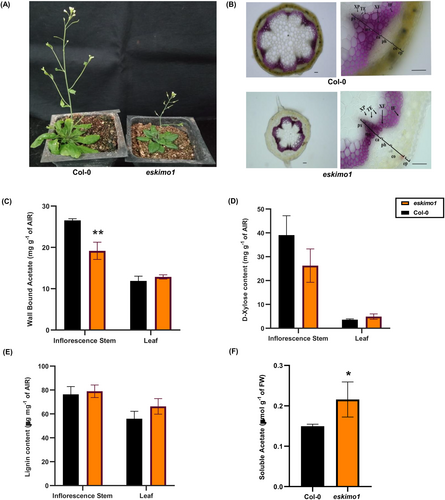
Characterization SALK_089531 mutant with insertion in TBL29/ESKIMO1 gene. The figure represents a comparison between Col-0 and eskimo1. (A) Morphology of six-week-old Col-0 and eskimo1 (B) Phloroglucinol-HCl (Wiesner) staining of 6-week Arabidopsis Col-0 and eskimo1 stem cross-sections, indicating different tissue and cell type: ep, epidermis; co, cortex; ph, phloem; ca, cambium; mx, metaxylem; px, protoxylem; XF, xylary fiber; XP, xylem parenchyma; TE, tracheary elements; IF. Interfascicular fiber. Panel A: 10X, Panel B: 40X. Scale bar = 50 μm. (C) The total wall-bound acetyl content was analyzed after saponification in alcohol insoluble residue (AIR) by K-ACET Megazyme kit. (D) The xylose content was measured after hydrolysis of AIR, and analysed by Megazyme K-Xylose kit. (E) The total lignin content is measured by acetyl bromoide soluble lignin in AIR. (F) The total endogenous acetic acid contents were analyzed in fresh weight of a 6-week-old inflorescence stem by K-ACET Megazyme kit. Data are represented as the mean ± standard deviation, n = 3–4. The asterisk represents significant differences using the Student's t-test at p ≤ 0.05*, p ≤ 0.005**.
Some earlier studies demonstrated that cytosolic acetic acid increases tolerance to drought stress by increasing jasmonate content while enriching histone H4 acetylation and stimulating the jasmonate signalling pathway in Arabidopsis (Kim et al., 2017; Rasheed et al., 2018). Depletion of cell wall acetyl ester in eskimo1 might cause accumulation of soluble acetic acid or acetyl coenzyme A (acetyl-CoA), which can enter into plant carbon metabolism, influencing different processes in plants. We measured the endogenous soluble acetic acid content in the inflorescence stem of the xylan hypoacetylated eskimo1. There was a significant increase in the soluble acetic acid in eskimo1 compared to wild-type stem (Figure 1F). Therefore, we further investigated the metabolic shift in eskimo1 to cover any potential cause for its stress-related traits or dwarfism.
3.2 The global transcriptome profiling of the eskimo1 stem reveals a high expression of aliphatic glucosinolate (GSLs) related genes
To comprehend the changes in the transcriptome of different metabolic pathways because of disruption in xylan acetylation, the RNA sequencing analysis was performed on six-week-old eskimo1 and the wild-type inflorescence stem. Several 2180 significantly differentially expressed genes (DEGs) were identified. As depicted on the volcano plot, 960 genes were downregulated, and 1220 were upregulated in eskimo1 (Figure 2A). These DEGs were submitted to gene ontology (GO) analysis using WebGestalt (https://www.webgestalt.org/) and g: Profiler for gene ontology (GO) analysis. GO comprises biological processes, cellular components, and molecular function, and all were enriched in eskimo1 (Figures S2A and S2B). Both the upregulated and downregulated DEGs in eskimo1 were found in cellular components that were first linked with the membrane and then the nucleus. The class of ion binding and protein binding genes were significantly annotated in upregulated and downregulated scenarios of eskimo1 (Figures S2A and S2B). The reference canonical pathways in KEGG were linked to the differentially expressed genes to further understand which pathways are affected. Figure 2B represents the top 5 enrichment routes of metabolism i.e., ribosomes, glucosinolate biosynthesis (GSL), pentose and glucoronate interconversion, 2-oxo-carboxylic acid, and monobactam biosynthesis (Figure 2B). We further investigated the GSL pathway since it was induced in the eskimo1.
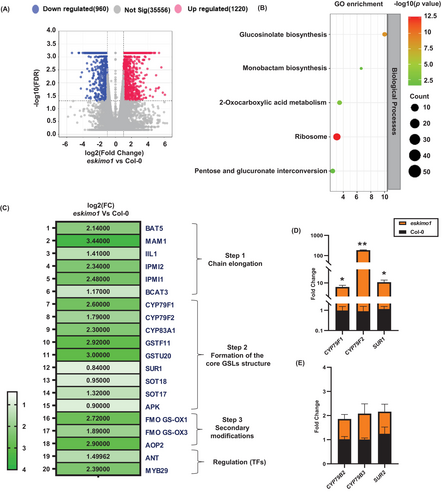
Transcriptome profiling of GSLs-related genes by RNA sequencing (A), (B) (C), and qPCR analysis (D) (E) in the inflorescence stem of eskimo1 comparing with wild-type. (A) Volcano plot representing functionally annotated differentially expressed genes. (B) GO enrichment representing differentially regulated genes in selected pathways (C) Heat map representing the gene expression of the biosynthetic and regulatory genes involved in aliphatic glucosinolate (GSL) (D) Real-time qPCR validation of selected genes involved in aliphatic GSLs biosynthesis and (E) indolic GSLs biosynthesis. Transcriptomic data is generated from two biological replicates, with FDR ≤0.05. q-PCR data are represented as the mean ± standard deviation, n = 3–4. The asterisk represents significant differences using the Student's t-test at p ≤ 0.05*, p ≤ 0.005**.
GSLs can be divided into three groups based on their amino acid precursors: aliphatic from Ala, Leu, Ile, Val, and Met; aromatic from Phe or Tyr; and indolic from Trp (Pfalz et al., 2009; Nguyen et al., 2020). Previous studies showed metabolic crosstalk between GSL biosynthesis and lignin biosynthesis, whereby GSLs intermediate metabolite can affect PAL function and phenylpropanoid metabolism (Kim et al., 2020, Shin et al., 2023, 2024, Hemm et al., 2003). Therefore, to understand whether the disruption of xylan O-acetylation in eskimo1 specifically affects GSL metabolism, we have examined the DEGs functioning in the GSLs pathway.
The GSL biosynthesis comprises three phases: side chain elongation, core structure formation, and secondary modification (Zhang et al., 2022). Of the 45 putative genes reported for GSLs, 35 were specifically associated with the aliphatic pathway (Sønderby, Burow, et al., 2010; Kitainda & Jez, 2021). Among these genes, 21 genes showed a noticeably elevated level in eskimo1 (Figure 2C). The genes belonging to GSL chain elongation, core structure, secondary modifications, and transcriptional regulation were differentially regulated in eskimo1.
BILE ACID TRANSPORTER 5 (BAT5), METHYLTHIOALKYLMALATE 1(MAM1), 3-ISOPROPYLMALATE DEHYDRATASE1 (IIL1), ISOPROPYLMALATE ISOMERASE (IPMI)1, IPMI2, and BRANCHED-CHAIN AMINOTRANSFERASE (BCAT5), which have a role in chain elongation, were upregulated in eskimo1 (Figure 2C). These genes are involved in side-chain extension activities, including deamination, condensation, isomerization, and oxidative decarboxylation (Schuster et al., 2006; Sønderby, Geu-Flores, et al., 2010; Kitainda & Jez 2021). Nine reported genes responsible for generating GSL core structures i.e., CYTOCHROME P450 (CYP) 79F1, CYP79F2, CYP83A1, GLUTATHIONE S TRANSFERASE (GSTF) 11, GSTF20, SUPERROOT1 (SUR1), SULFOTRANSFERASES (SOT) 18, SOT17 and APS KINASE (APK) were found upregulated in eskimo1transcriptome data (Figure 2C) (Chen et al., 2003; Sønderby, Burow, et al., 2010, Zhang et al., 2022). The results of RNA-sequencing gene expression were further validated by qRT-PCR analysis. CYP79F1, CYP79F2, and SUR1 were involved in aliphatic GSL biosynthesis and were upregulated in eskimo1 compared to the wild-type (Figure 2D). In comparison, expression of CYP79B2, CYP79B3, and SUR2 (CYP83B1) involved indolic GSLs biosynthesis and showed similar levels of expression in Col-0 and eskimo1 (Figure 2E). The expression of the genes functioning in secondary modifications (oxygenations, hydroxylations, alkenylations and benzoylations), such as FMOGS-OX1, FMOGS-OX3, and AOP2 were significantly higher in the eskimo1 stem compared to the control (Neal et al., 2010; Kong et al., 2016; Nugroho et al., 2020) (Figure 2C). GSL metabolism is under tight transcriptional control, which involves potential Feed-Forward Loops (FFLs). These FFLs involve important developmental regulators like AINTEGUMENTA (ANT), which can bind to MYB28 and MYB29 that can transactivate aliphatic glucosinolate biosynthetic genes (Elliott et al., 1996; Krizek et al., 2020; Li et al., 2018, Kliebenstein 2023). A combination of ANT and above MYBs can regulate both GSL synthesis and metabolite accumulation. Our results revealed that ANT and MYB29 were significantly upregulated in eskimo1 (Figure 2C), which may contribute to increased expression of aliphatic GLS biosynthesis genes. Overall, transcriptome data revealed a notable increase in the aliphatic GSL biosynthetic and regulatory genes in eskimo1 compared to Col-0 inflorescence stems.
3.3 Untargeted metabolome profiling of the eskimo1 stem reveals the alteration in both primary and secondary metabolites
Transcriptomic data showed that disruption in xylan acetylation in eskimo1 led to significant alteration in gene expression involved in several metabolic pathways. Therefore, we performed untargeted metabolomics on stem methanolic extracts of eskimo1 and wild type using the LC–MS. The principal component analysis (PCA) plot clearly showed the separation of Col-0 from eskimo1 (Figure 3A). Out of the 530 metabolites identified, 84 metabolites displayed significant levels of change (p value<0.05) between eskimo1 and Col-0. Compared to Col-0, eskimo1 had 54 additional elevated metabolites, whereas 30 metabolites were decreased in level (Figure 3B). Several metabolites involved in primary and secondary metabolism, such as hormones, amino acid, nitrogen, and fatty acid metabolism, were clustered among the significantly distinct metabolites (Figure 3C). The metabolite related to auxin and salicylic acid, methyl trans-cinnamic acid were significantly elevated among the group of phytohormones (Yalpani et al., 1993; Yang et al., 1999; Steenackers et al., 2017). Abscisic acid (ABA) responsive compounds (2-quinoline carboxylate, L-proline) (Hansen & Grossmann, 2000; Kaur et al., 2023), cytokinin responsive compounds (4-guanidinobutanoate) (Peach et al., 2021), jasmonic acid (JA) responsive compounds (C18:3 FA (linolenic acid), 2-hydroxyquinoline) (Borrego & Kolomiets, 2016), were all accumulated more in the eskimo1 stem (Figure 3C).
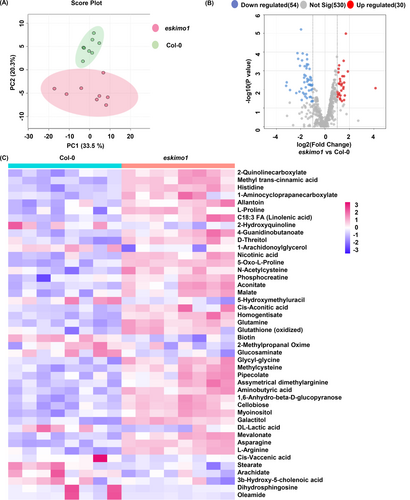
Untargeted metabolomics profiling of 6-week-old wild type and eskimo1 inflorescence stem. (A) Soluble metabolites isolated in methanol and run on LC–MS. Plot generated using relative intensities by the principal component analysis showing separation between Col-0 Vs eskimo1. (B) Volcano plots show significantly changed metabolic features between Col-0 and eskimo-1. Red dots indicate significantly increased metabolites and blue dots indicate significantly decreased metabolites with an adjusted value ≤0.05. (C) Heatmap presentation of significant annotated metabolites of Col-0 and eskimo1 based on relative intensities showing the extent of metabolite accumulation. Data are represented with biological replicates (8) with p-value ≤0.05.
Additionally, metabolites related to drought responses, such as nicotinic acid, 5-oxo-L-proline (Khan et al., 2019; Meng et al., 2019; Ahmad et al., 2021), and ROS scavenger (N-acetylcysteine and 5-oxo-L-proline) (Khan et al., 2019, He et al., 2022) were elevated (Figure 3C). Also, primary metabolites induced during the stress response were increased in eskimo1 stem, which included phosphocreatine, aconitate, malate, 4-guanidinobutanoate, cis-aconitic acid, homogentisate, glutamine, 5-oxo-L-proline, and glutathione (oxidized) (Farrés et al., 2002; Moeder et al., 2007; Mhamdi et al., 2010; Hasanuzzaman et al., 2014; Zhao, Luo, et al., 2018; Meng et al., 2019).
However, eskimo1 had lower levels of 5-hydroxymethyl-uracil, biotin, 2-methyl propanal oxime, and glucosaminate (Figure 3C) (Chu et al., 2010; Jørgensen et al., 2011; Wang et al., 2020; Torres et al., 2023).
Nitrogen metabolism-related amino acids (asparagine and L-arginine) and other amino acids accumulation were significantly up in eskimo1, which indicates the highly stressed condition of the cell (Baslam et al., 2020; Lin et al., 2021). Metabolites that are involved in amino acids catabolism and intermediates, i.e., methyl cysteine, carnitine, pipecolic acid, citramalate, and methylated arginine (asymmetrical-dimethylarginine), were significantly higher in mutant as compared to wild-type (Figure 3C) (Rébeillé et al., 2006; De Kraker, et al., 2007; Návarová et al., 2012; Rippa et al., 2012; Hu et al., 2019).
The levels of the metabolite, galactitol, and myoinositol, which are induced by elicitors during pathogen infection such as cell wall-related substances,1,6-anhydro-β-D-glucopyranose, and cellobiose, all were increased in eskimo1 (Eckardt 2010; Souza et al., 2017). Histidine, aminobutyric acid and other plant immune metabolites (pipecolic acid, and galactitol) levels were significantly higher (Seo et al., 2016; Deng et al., 2020).
Interestingly, the fatty acid and lipid derivatives (cis-vaccenic acid, stearate, arachidate, 3b-hydroxy-5-cholenoic acid, dihydrosphingosine, 1-arachidonoylglycerol, oleamide) were downregulated significantly in eskimo1 (Coursol et al., 2005; Barthet 2008; Teh & Ramli, 2011; Cheng et al., 2013; Kuang et al., 2016) (Figure 3C).
Untargeted metabolomics provided the details on numerous changes in metabolites of several pathways, including hormones and primary metabolism, and also represented the cellular stress condition in the eskimo1 stem.
3.4 The eskimo1 stem has an elevated level of GSLs and associated catabolites
We further examined GSL-related metabolites in untargeted metabolome (with significant p-value ≤0.05) and transcriptome with (FDR ≤0.05) for the genes involved in GSL catabolism and transport in eskimo1 inflorescence stem. eskimo1 showed the decreased level of aliphatic GSLs catabolites iberin(3-methylsulfinylpropyl isothiocyanate), sulforaphane (4-methylsulfinylbutyl isothiocyanate), alyssin (1-isothiocyanate-5-(methylsulfinyl)-pentane) (Figure 4A) (Katz et al., 2020; Malhotra et al., 2023). Also, among the catabolites, indolic GSLs catabolite (indole-3-carbinol) was upregulated in eskimo1, which is also an antagonist of auxin (Figure 4A) (Katz et al., 2015). The differential catabolites were also correlated with the myrosinase enzyme gene expression in our transcriptome data (Figure 4A and Data S1).
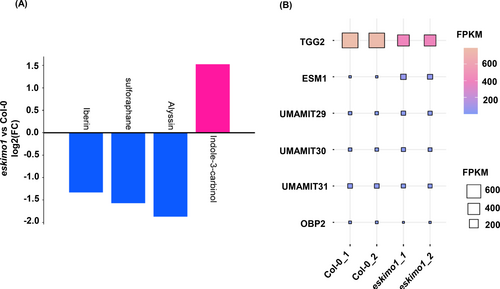
GSLs catabolites profiling and GSLs related genes expression in 6-week-old eskimo1 inflorescence stem. (A) Fold change of glucosinolate-related catabolites was calculated from untargeted metabolomics using peak intensities. Data represents eight biological replicates with p-value ≤0.05. (B) The fragments per kilobase of transcript per million fragments calculated from RNA sequencing analysis for the myrosinase genes (TGG2, ESM1), transporters genes (UMAMIT29, 30, 31), and the gene of a positive regulator of indolic GSLs pathway (OBP2) with FDR ≤0.05.
Myrosinase, which is also known as THIOGLUCOSIDEGLUCOHYDROLASES (TGG); TGG1 and TGG2 can primarily degrade aliphatic but not indolic GSLs (Barth & Jander, 2006; Ahuja et al., 2016; Zhang et al., 2019a). TGG2 was downregulated in eskimo1 as compared to Col-0 (Figure 4B and Data S1). But our findings also show an upregulation of EPITHOSPECIFIER MODIFIER (ESM1), which is also involved in GSLs hydrolysis, especially isothiocyanates formation (Zhang et al., 2006) (Figure 4B and Data S1).
The transport of GSLs inside organs such as leaves depends on the plasma membrane-localized GLS transporters NPF2.10/GTR1 and NPF2.11/GTR2 (Nambiar et al., 2021). For instance, gtr1 gtr2 double mutants exhibit severe impairments in GLS accumulation in seeds, roots, and along leaf margins (Andersen & Halkier, 2014; Xu et al., 2019). According to our RNA sequencing analysis of the eskimo1 inflorescence stem, the expression of GTRs was the same as in wild-type Data (S1). USUALLY MULTIPLE ACIDS MOVE IN AND OUT TRANSPORTERS 29 (USMAMIT 29), UMAMIT30, and UMAMIT31 are necessary for exporting GSLs from the site of synthesis within the reproductive organ to seeds in Arabidopsis thaliana. While UMAMIT31-expressing oocytes favoured indolic GSLs over aliphatic GSLs, UMAMIT29- and UMAMIT30-expressing oocytes accumulated both kinds of GSLs (Meyer et al., 2023; Xu et al., 2023). With a significant FDR value of less than 0.05, our transcriptome data showed upregulation of the UMAMIT29- and UMAMIT30 and downregulation of UMAMIT31 (Figure 4B and Data S1), suggesting the preferred transport of aliphatic GSLs over indolic in eskimo1. DOF transcription factor AtDof1.1 (OBP2) is an inducer of indolic GSLs and its expression is triggered by injury, MeJA, and insect feeding, and it is notably expressed in the phloem of leaves and other organs (Skirycz et al., 2006). In our data, OBP2 gene expression was downregulated (Figure 4B and Data S1), again indicating the unchanged indolic GSLs content in eskimo1.
All these data suggested that catabolites of aliphatic GSLs and the expression level of myrosinase TGG2 were reduced while the expression of aliphatic GSLs transporters was enhanced in the eskimo1 inflorescence stem. To quantify the concentration of total GSLs, we used the modified spectrophotometric method (Mawlong et al., 2017) (Figure S3A) and showed an increase in the total GSLs in the inflorescence stem compared to Col-0 (Figure S3B).
3.5 The targeted evaluation reveals that the eskimo1 stem has a higher level of aliphatic GSL
In Arabidopsis, the composition and content of GSLs vary widely among issues and developmental stages (Petersen et al., 2002). The Arabidopsis siliques and seeds are GSL-rich and can accumulate diverse GSLs, while roots have the lowest quantity of GSLs (Hooshmand & Fomsgaard, 2021). Therefore, we measured the levels of an aliphatic GSL, 4-methylsulphinylbutyl (4MSOB), and an indolic GSL, indolyl-3-methyl glucosinolate (I3M) by the targeted metabolomic approach. eskimo1inflorescence stems had more accumulation of 4MSOB than Col-0, but the I3M level was comparable to wild type (Figure 5A). Similarly, the concentration of 4MSOB was higher in eskimo1 mature seeds (Figure 5B). The changed level of aliphatic GSLs in seeds and inflorescence stems from eskimo1 compared to Col-0 indicated a shift in the synthesis, transport, and storage profiles of GSLs in eskimo1, particularly with regard to aliphatic GSLs. Interestingly, the level of 4MSOB in 3-week-old rosette leaves of eskimo1 was similar to the wild type, whereas the concentration of the primary indolic GSLs, I3M, was reduced (Figure 5C).
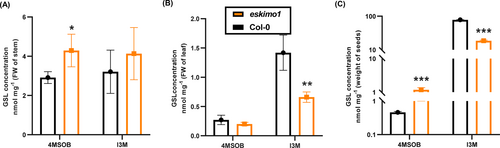
GSLs quantification in different tissue types between wild type and eskimo1. (A) 6-week-old stem. (B) 3-week-old rosette leaf and (C) mature seeds. The concentration of GSLs was measured using respective standards. 4MOSB - 4-methylsulfinylbutyl, I3M - indolyl-3-methyl GSLs. Data are represented as the mean of biological replicates (n = 4) with standard deviation. The asterisk represents significant differences using the Student's t-test at p ≤ 0.05*, p ≤ 0.005** p ≤ 0.0005***.
As stated previously, eskimo1 showed similar growth as compared to the wild-type up to two weeks after germination and after that started showing visible stress symptoms (Figures S1a and S1b). The expression analysis of 16-day-old seedlings and rosette leaves from 24-day-old plants revealed distinct expression patterns in eskimo1. Both young 16-days-old seedlings and 24-days-old rosette leaves did not exhibit any variation in the expression of the aliphatic GSLs genes (Figures S4A and S4B), while rosette leaves of 24-days-old displayed a low fold change for the indolic GSLs genes in eskimo1 as compared to wild-type, but 16-days-old seedlings did not show any change in indolic GSLs expression (Figure S4C and S4D). The GSL profiles in the rosette leaves are correlated with the qPCR expression data (Figure 5B, S4D).
3.6 The eskimo1 stem exhibits an elevated amount of jasmonic acid and other phytohormones
Both defence and growth hormones are involved in the GSL metabolism (Mitreiter & Gigolashvili, 2021). Plants can incorporate a wide range of inputs from both internal and external environments to change their metabolic processes (Valentino et al., 2020), and these interactions can affect the GSL metabolism (Khan et al., 2019; Sontowski et al., 2019). Although the transcriptional machinery is at the center of regulating GSL production and can incorporate a wide range of input, a large portion of this information is communicated by plant hormones (Moore et al., 2022). Jasmonic acid (JA) and salicylic acid (SA), can control the production of GSLs (Brader et al., 2001; Yang et al., 2019; Zhang et al., 2020; Wang et al., 2021). We examined the expression of SA and JA responsive genes, i.e., PLANT DEFENSIN TYPE 1 (PDF1), LOW-MOLECULAR-WEIGHT-CYSTEINE-RICH 69(LCR69), NON EXPRESSOR of PATHOGEN RELATED 3(NPR3), PR4, PR5, and LIPOXYGENASE 4 (LOX4) and found all except PR5 were upregulated in eskimo1 (Figure 6A, Figure S5A). LC–MS/MS measurement of JA and its conjugate JA-Isolecucine (JA-Ile) and its biosynthetic precursor 12-oxyphytodienoic acid (OPDA) showed elevated levels of JA in eskimo1 (Figure 6B). Also, the SA levels were upregulated in eskimo1 (Figure 6C).
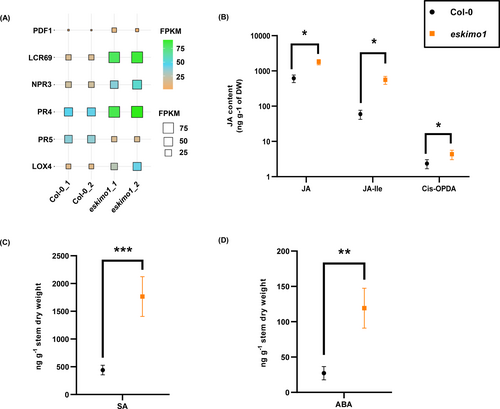
Expression profiling of JA and SA marker genes and absolute quantification of phytohormones in 6-week-old in wild type and eskimo1 inflorescence stem. (A) The expression levels (FPKM, fragments per kilobase of transcript per million fragments) are presented for the JA and SA marker genes (PDF1, LCR69, NPR3, PR4, PR5, and LOX4) in Col-0 and eskimo1 inflorescence stem. Data are represented with biological replicates (2) with FDR ≤0.05. (B) Quantification of defense phytohormones related to GSLs by LC–MS/MS, JA, jasmonate-isoleucine, JA precursor, cis-OPDA in eskimo1 inflorescence stem with comparison to Col-0. (C) SA. (D) ABA in eskimo1 inflorescence stem with comparison to Col-0. Data are represented as the mean ± standard deviation, n = 3–4. Asterisk represents significant differences using Student's t-test at p ≤ 0.05*, p ≤ 0.005** p ≤ 0.0005***.
The ABA level is upregulated under high salt, waterlogging, cold, high temperature, and drought conditions (Zhao et al., 2021). These abiotic stressors boost ABA supply to guard cells, and ABA also indirectly induces the hydrolysis of GSLs by myrosinases (Wittstock & Burow, 2010; Liu et al., 2022). It is already shown that eskimo1 leaves had significantly higher ABA contents (Lefebvre et al., 2011). Additionally, from our data, ABA measurement showed more accumulation in the eskimo1 stem than Col-0 (Figure 6D).
Gibberellins (GA) are known to modulate the JA-SA balance in plant immunological signalling, and it is also growth-promoting hormones (Denancé et al., 2013; De Bruyne et al., 2014). Both the DELLA subfamily member RGL3 gene and the GID1C, which encodes a gibberellin (GA) receptor involved in signal transduction (Zentella et al., 2007; Fuentes et al., 2012), were downregulated (Figure S5B). The GIBBERELLIN 2-OXIDASE 6 (GA2OX6), can inactivate the GA production (Wiesen et al., 2016), and its expression was increased in eskimo1 (Figure S5B). These expression data suggested the possibility of a decrease in GA level contrary to an increase in JA level of eskimo1 as compared to wild type plants.
Auxin has also been investigated for its impact on GSLs in plants determined the accumulation of total GSLs by treating hairy root cultures of Brassica oleracea with various concentrations of indole-3-acetic acid (IAA) (Zhao 2010, Kim, et al., 2013). GSLs have emerged as a critical link between auxin and ABA signals mediated by drought and stomatal aperture (Salehin et al., 2019). CYP79F1and CYP79F2 control function in an early step in the aliphatic GSL pathway, and defects of these genes result in dwarfism and altered growth and development (Hansen et al., 2001; Reintanz et al., 2001; Tantikanjana et al., 2001; Chen et al., 2003; Tantikanjana et al., 2004; Chen et al., 2012). Also, the perturbed contents of auxin and cytokinins may be responsible for their altered morphology (Reintanz et al., 2001; Tantikanjana et al., 2004; Chen et al., 2012; Garrido et al., 2020). Therefore, we measured the levels of IAA and cytokinin in eskimo1 compared to the wild-type (Figure S5C). IAA and trans-Zeatin (tZ) levels were enhanced in eskimo1 (Figure S5D). Overall, we found a significant alteration in hormone levels, especially JA, using transcriptomic and metabolic studies, which could be a possible change in GSL metabolism in eskimo1inflorescence stems.
3.7 eskimo1 displays a decreased concentration of Free Met but higher levels of other amino acids
The concentrations of transport activities of amino acids across membranes are the signatures of stress or the generation of secondary metabolites for defence, stress, or growth (Moormann et al., 2022). Methionine (Met) is the precursor to the aliphatic GSLs in Arabidopsis, which go through a three-step chain-elongation cycle. In Arabidopsis, there can be up to six rounds of elongation, each of which results in the net addition of one methylene group (Textor et al., 2004, 2007). Previous research on specific GSL mutants showed that levels of free Met and Met-related metabolites increased concurrently with a decrease in aliphatic GSLs (Schuster et al., 2006; Knill et al., 2008; Chen et al., 2012). Since S-adenosylmethionine (SAM) and S-methylmethionine (SMM) are the two main derivatives of Met, it appeared likely that at least one of these metabolites may also be present in higher levels in the mutants of aliphatic GSLs.
HOMOCYSTEINE METHYLTRANSFERASE (HMT) catalyzes the synthesis of two molecules of Met from homocysteine and SMM, while METHIONINE METHYLTRANSFERASE (MMT) catalyzes the synthesis of Met from SMM using AdoMet (Zhao, Chen, et al., 2018). The gene expression of HMT3 and MTO3 (AdoMet synthase) was higher in eskimo1 (Figure 7A) (Goto et al., 2002). The gene expression of CYSTATHIONINE Β-LYASE (CBL), which is involved in the Met synthesis, was also elevated in eskimo1 than wild type (Wang et al., 2022). The O-ACETYL-SERINE(THIOL)LYASE (OASA1), is a crucial enzyme in Cys biosynthesis for the fixing inorganic sulfide and further for the Met synthesis, which was also elevated in transcriptome data of the eskimo1 stem (Figure 7A, Wirtz et al., 2004). When we measured the Met level, it was substantially lower (0.00135 μmol mg−1) in eskimo1 than Col-0 (0.00204 μmol mg−1) (Figure 7B). We then examined the amino acids (Thr, Ile, Asp, Asn, Lys, Ser) that are involved in the same Met biosynthesis, belongs to the aspartate family (Wang et al., 2018). Asp is a key site from which the salvage pathway produces homoserine and homocysteine, and Asp was increased along Thr, Ile, Asn, Lys, and Ser in eskimo1 (Figure S6A). Also, shikimate pathway-derived amino acids, i.e., Phe, Trp, and Tyr, were estimated, and Tyr showed significantly higher levels in the eskimo1 than the Col-0 stem (Figure S6B). The levels of Phe and Trp were similar in wild type and eskimo1. Some of the Met chain-elongation pathway enzymes in Arabidopsis also synthesized branched-chain amino acids (Knill et al., 2008). The stem of eskimo1 had considerably higher amounts of Val (Figure S6B). Also, Ala, Pro, Glu, His, Arg, and Gln levels in the eskimo1 mutant were significantly increased, indicating the highly stressed condition in the eskimo1 (Figure S6B).
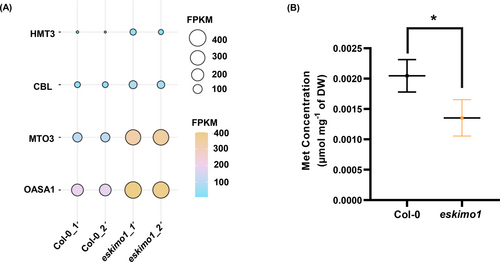
Expression profiling of key genes of Met metabolism and quantification of Met in 6-week-old eskimo1 inflorescence stem. (A) The expression levels (FPKM, fragments per kilobase of transcript per million fragments) are presented for the Met biosynthetic genes. Data are represented with biological replicates (2) with FDR ≤0.05. (B) Quantification of Met is done by LC–MS/MS after methanolic extraction. Data are represented as the mean ± standard deviation, n = 3–4. The asterisk represents significant differences using the Student's t-test at p ≤ 0.05**.
Overall, a lower level of Met in eskimo1 further correlated with the effect on aliphatic GSLs and differential level of other amino acids correlated with stress-related phenotype in eskimo1.
4 DISCUSSION
Mutation in ESKIMO1/TBL29 led to aberrant growth phenotypes and a reduction in xylan O-acetylation in the stem, but the other TBL mutants do not show any such growth phenotype (Yuan et al., 2016; Zhong et al., 2017, 2018; Stranne et al., 2018; Wierzbicki et al., 2019). Since xylan acetylation is an important determinant for lignocellulosic biomass properties, we characterized eskimo1 by transcriptomic, untargeted, and targeted metabolomic approaches.
In the eskimo1 stem, change has been observed in both central and specialized metabolism, primarily in the metabolism of amino acids, fatty acids, phytohormones, soluble acetate, and GSLs, indicating major reprogramming of metabolic pathways (Figure S8). We have shown that increased GSL production is correlated with xylan hypo-acetylation. The mutant of xylan O-acetyltransferase, ESKIMO1, exhibited elevated soluble acetate, which could be either because of disruption in polysaccharide acetylation and/or a consequence of phenotype or chemotype (Figure 1F). Subsequently, acetate is also involved in the process of synthesizing acetyl-CoA. Therefore, the elevated acetate level may also indirectly indicate an increase in acetyl-CoA in the cytosol when acetate is converted to acetyl-CoA by an enzyme called acetyl-coenzyme A synthetase (ACS). The fast utilization of this important C2 metabolite occurs in both catabolic (such as mitochondrial respiration) and anabolic (such as lipid and flavonoid production) metabolism processes (Fatland et al., 2005; Lin & Oliver, 2008; Chen et al., 2017; Jardine & McDowell, 2023). The ATP Citrate Lyase (ACL) is the key enzyme that converts citrate to acetyl-CoA in the cytosol (Verschueren et al., 2019), and its downregulation by RNAi approach can significantly affect fatty acid, starch, flavonoid, and cell wall biosynthesis (Fatland et al., 2005). Further analysis of the ACLA-RNAi line revealed a decrease in xylan acetylation, collapsed xylem vessel, and aberrant growth (Zhong et al., 2020). The two subunits of ACL, ACLA1 and ACLA2, were upregulated, as demonstrated by RNA sequencing and qPCR (Figures S7A and S7B). Additionally, the gene of the cytosolic member of ACETYL-CoA CARBOXYLASE (ACCase), ACC1, which catalyzes the first committed step of the fatty acid synthesis, the carboxylation of Acetyl-CoA to malonyl-CoA (Sasaki & Nagano, 2004), was downregulated (Figures S6A and S6B). This suggests that acetyl-CoA in the cytosol may not be converted into malonyl-CoA to facilitate the synthesis of flavonoids and fatty acids since fatty acid metabolites were down in the untargeted metabolome data (Figure 3C and Figure S8). However, this assumption can be further confirmed by analyzing metabolites of the cytosolic acetyl-CoA pathway.
According to transcriptome analyses, eskimo1 has a notably higher expression of 21 aliphatic GSL synthesis genes than Col-0 (Figure 2C). It is possible that changes in acetyl-CoA metabolism and soluble acetate level due to the wall hypoacetylation may affect the synthesis of aliphatic GSLs. RNA sequencing data also showed that MYB29 and ANT, two transcription factors exclusive to aliphatic GSLs, were elevated (Figure 2C). ANT is responsible for the patterning and coordination of growth (Xiong et al., 2019; Krizeket al., 2020). ANT binds to the promoters of multiple aliphatic GSLs TFs (MYB28/29) and downstream biosynthetic genes, suggesting the presence of a developmental or metabolic FFL. This confirms that there is an ANT-MYB28/29 FFL and that the various biosynthetic genes have different structural variations (Kliebenstein, 2023). Transcriptome results revealed that the expressions of MYB29 and ANT were elevated, possibly for the ANT-MYB28/29 FFL. MYB28 can regulate genes involved in the biosynthesis of aliphatic GSLs, including those involved in the production of core structures (CYP79F1, CYP79F2, and CYP83A1) and side-chain elongation (MAM1 and MAM3) (Gigolashvilirt et al., 2007). The transcriptome data showed that MYB28 expression was also increased in eskimo1 (log2FC - 0.75, p-value ≤0.05) (Supplementary file1).
The targeted metabolomics revealed that the eskimo1 stem had elevated levels of aliphatic GSL such as 4MSOB, which was associated with the accumulation of JA-responsive metabolites (C18:3 FA (linolenic acid), 2-hydroxyquinoline) by untargeted metabolome (Figure 5A and 3C) (Guo et al., 2013; Steindal et al., 2015). eskimo1 underwent an alteration in the synthesis, transport, and storage profiles of GSLs, especially aliphatic GSLs, as evidenced by the increased amount of aliphatic GSLs (4MSOB) in seeds and unchanged level in 3-week-old leaf by targeted metabolome (Figure 5). This alteration in GSL metabolism could be the possible reason that eskimo1 is tolerant to multiple environmental stresses. Isothiocyanates produced from aliphatic GSLs offer improved resistance to generalist insects, whereas nitriles are mainly effective against specialized insects (Burow et al., 2006; Mumm et al., 2008). In untargeted metabolome data, the three aliphatic GSLs catabolites isothiocyanates were down (Figure 4A), suggesting that induction in the aliphatic GSLs is probably because of internal signals such as cytosolic acetyl content or xylan hypoacetylation and not a stress response.
The two most commonly used elicitors i.e., JA and MeJA, can induce GSL accumulation, which was upregulated in eskimo1 (Figure 7b) (Sasaki-Sekimoto et al., 2005, Denoux et al., 2008). For JA to regulate GSL, the COI1/JAZ/MYC2 pathways mediated by jasmonate-L-isoleucine (JA-ILE) must be active. When endogenous JA is below the threshold concentration, JAZ binds to MYC and inhibits its transcription, inhibiting the expression of genes sensitive to JA (Major et al., 2017). Under stress conditions, the amount of JA increases and isomerizes into JA-ILE, which may stimulate the interaction between COI1 and JAZ, release MYC, and induce JA-responsive genes. When MYC is released, the R2R3-MYB transcription factor expression then triggers the activation of GSL synthesis genes (MYB, CYP79, CYP83, AOP2, FMOGS-OX5, and others) (Cao et al., 2016; Zhai & Li 2019; Raza et al., 2021). It appears that aberration in xylan acetylation could be recognized as a stress signal in eskimo1, which can further affect JA and, consequently, GSL biosynthesis.
Studies on certain GSLs mutants, e.g. bcat3 and cyp79f1/f2 where the levels of aliphatic GSLs were dropped, showed that the level of free Met and Met-related metabolites were enhanced (Knill et al., 2008; Chen et al., 2012). In the RNA sequencing data of eskimo1 inflorescence stem, the gene expression of the Met synthesis enzymes HMT3, MTO3 and CBL (Zhao, Chen et al., 2018; Goto et al., 2002; Wang et al., 2022) were upregulated (Figure 7b) but total Met level was significantly less in eskimo1 (Figure 7b) which suggests that it might have consumed for aliphatic GSLs synthesis. This again suggests the eskimo1 hypo-acetylated inflorescence stem is the site of aliphatic GSLs biosynthesis.
An earlier work by Faria-Blanc et al., 2018, examined the stem transcriptome of several xylan mutants to determine whether abnormal xylan synthesis can trigger a signalling cascade for maintaining the cell wall integrity. The xylan mutants irx9, irx10, irx14, and gux1/gux2 displayed varying numbers of important genes impacted. However, irx9 presented the highest number of transcripts, comprising genes related to the cell wall polysaccharides biosynthesis, UDP-sugars, SAM, acetyl-CoA pathways and receptor-like kinases. Interestingly, neither mutant displayed differential regulation in GSL gene expression (Faria-Blanc et al., 2018). This further confirms that the effect on aliphatic GSL metabolism is unique to eskimo1 through a possible mechanism explained earlier.
In conclusion, our research showed that xylan hypo-acetylation in the eskimo1 inflorescence stem might be the reason for the elevated soluble acetate level, which can subsequently induce transcription regulation of JA biosynthesis, which can induce JA production. This has resulted in the upregulation of aliphatic GSL gene expressions and the cellular uptake of free Met to aliphatic GSL synthesis and formation of GSL catabolites i.e., isothiocyanates (Figure 4A and Figure 8). This complex interplay between cell wall acetylation, GSLs, and hormone biosynthesis can be exploited further to generate plant varieties with distinct characteristics for biotechnological applications.
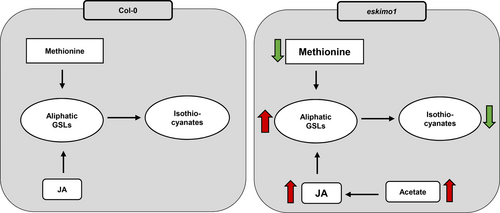
Proposed model explaining changes in different metabolites in eskimo1 as compared to wild-type. The xylan hypo-acetylation in the eskimo1 inflorescence stem may cause increased soluble acetate that can induce the synthesis of the phytohormones JA. JA can further induce aliphatic GSL accumulation and reduce methionine levels (indirectly). Lower levels of isothiocyanates could be because of major reprogramming in GSL metabolism.
Accession Numbers
ESKIMO1 (AT3G55990); CYP79F1 (AT1G16410); CYP79F2 (AT1G16400); SUR1 (AT2G20610); CYP79B2 (AT4G39950); CYP79B3 (AT2G22330); SUR2 (AT4G31500); BAT5 (AT4G12030); CYP83A1 (AT4G13770); APK1 (AT2G14750); FMO-GS-OX1 (AT1G65860); MYB29 (AT5G07690); GSTF9 (AT2G30860); GGP1 (AT4G30530); CYP81F2 (AT5G57220), PR1 (AT2G14610); PR4 (AT3G04720); PDF1.2 (AT5G44420); LOX2 (AT3G45140); ACLA1 (AT1G10670); ACLA2 (AT1G60810); ACLA3 (AT1G09430); ACC1 (AT1G36160).
AUTHOR CONTRIBUTIONS
DS designed and performed most of the experiments. HZ and JK performed glucosinolate analysis. YK and SK performed the analysis of the untargeted metabolites. DS and PP wrote the manuscript with inputs from the authors. PP conceptualized, designed, and secured funding for the project. All authors read and agree to publish the manuscript.
ACKNOWLEDGEMENTS
This work was supported by RCB core, DST-INSPIRE Faculty program to PP, and the National Science Foundation Division of Integrative Organismal Systems-CAREER-2142898 to JK. Untargeted metabolomics was performed at THSTI-Faridabad. Amino acid and hormone analysis was performed at the Metabolomics Facility, National Institute of Plant Genome Research (NIPGR), New Delhi. We would like to acknowledge Dr. Prasad Abnave (National Centre for Cell Sciences, India) for the discussion on omics analysis.
FUNDING INFORMATION
This work was supported by RCB core, DST-INSPIRE Faculty program to PP, and the National Science Foundation Division of Integrative Organismal Systems-CAREER-2142898 to JK.
CONFLICT OF INTEREST STATEMENT
The authors declare no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All the relevant data will be found within the manuscript and its supporting materials.




