Melatonin application enhances salt stress-induced decreases in minerals, betalains, and phenolic acids in beet (Beta vulgaris L.) cultivars
Abstract
Melatonin is a potentially active signaling molecule and plays a crucial role in regulating the growth and development of plants under stress conditions, alleviating oxidative damage, enhancing antioxidant defence mechanisms and regulating ion homeostasis. This study examined the effects of exogenous melatonin application on leaf biomass, ion concentrations, betalains, phenolic acid and endogenous melatonin contents comparing red beet (Beta vulgaris L. ‘Ruby Queen’ and ‘Scarlet Supreme’) and white beet (‘Rodeo’ and ‘Ansa’) cultivars under increasing salinity levels of 50, 150, and 250 mM NaCl. Exogenous melatonin increased salinity-induced reductions in fresh and dry weights and osmotic potential in leaves. Na+ concentrations rose significantly with increasing salinity, but cultivar-specific decreases were observed in K+ and Ca2+ concentrations. Additionally, melatonin application improved betalain, betanin and neobetanin contents induced by salt stress. Furthermore, melatonin application caused salt stress and cultivar-specific changes in phenolic acid contents e.g., ferulic acid, sinapic acid, or m-coumaric acid, in soluble free, ester- and glycoside-conjugated and cell wall-bound forms. In addition, antioxidant enzyme activities and compound contents increased significantly in the beets and were subsequently lowered in a cultivar-specific manner by salt stress + melatonin treatment. The current findings indicate that exogenous melatonin improved plant stress tolerance suppressing reactive oxygen species levels, increasing the antioxidant enzyme activities and compound contents and reducing the levels of Na+, maintaining an ionic homeostasis in the selected red and white sugar beet cultivars. It appears that melatonin application may help improve cultivar-specific salt tolerance by enhancing ion homeostasis and betalain and phenolic acid production levels in beets.
1 INTRODUCTION
Salinity is a global environmental factor that affects one billion hectares of land, limiting plant growth and crop yields (Zhao et al., 2021; Singh, 2021). Plants have developed various mechanisms to adapt to salt stress. These include maintaining water balance and closing stomata to ensure efficient water use, regulating mineral ion levels to compartmentalize them in cells, tissues, and organs, accumulating osmolytes as compatible solutes, enhancing antioxidant metabolism to scavenge free radicals, modulating hormones and maintaining efficient photosynthetic capacity (Chele et al., 2021). Under salt stress conditions, an excessive Na+ concentration can worsen oxidative stress by inducing the overproduction of reactive oxygen species (ROS), which act as cellular toxicants and disrupt the ion balance (e.g., Na+-influx and K+-efflux), thus leading to metabolic disturbances like oxidizing DNA, proteins, carbohydrates, lipids and enzymes, and to programmed cell death (Chele et al., 2021; Kesawat et al., 2023). Conversely, physiological levels of ROS are also involved in redox signaling, thus regulating plant development. In order to counteract this effect, a well-suited antioxidant system coupled with enzymatic and non-enzymatic antioxidants is essential for the prevention of cellular destruction (Kesawat et al., 2023).
Research has demonstrated that betalains, such as red-violet betacyanins and yellow-orange betaxanthins, as well as phenolic compounds, including phenolic acids, flavonoids i.e. anthocyanins, catechins, and lignans, can act as ROS scavengers to assist plants in responding to stress. They perform their function by neutralising the deleterious effects of ROS and maintaining ion homeostasis. As a potent osmotic substance, they regulate cell osmotic pressure to assist plants in adapting to harsh external environments (Li et al., 2019; Adhikary et al., 2020; Sadowska-Bartosz and Bartosz, 2021). Both red and white sugar beets are of high nutritional value due to their antioxidant and anti-inflammatory capacities. They exert positive effects on health and disease by removing excess ROS (Li et al., 2019). Furthermore, Caryophyllales species that produce betalains, such as Amaranthus, Portulaca oleracea, Alternanthera and Mesembryanthemum, have been observed to increase betalain concentrations in response to different stresses (Zhou et al., 2021). Studies have shown that red colouration in plants is associated with salt tolerance, as confirmed in Disphyma australe (Aizoaceae), one of the most frequently studied species. Additionally, D. australe produces betacyanin, which results in the existence of both ‘red’ and ‘green’ vegetative morphs. The red morphs have been identified as more salt-tolerant (Zhou et al., 2021).
Melatonin (Mel; N-acetyl-5-methoxy tryptamine) is an indolamine compound that regulates plant growth and development in vrious ways. It exerts a protective effect against abiotic stresses, including drought, salt, heavy metals, cold, chilling, high ambient temperature, herbicides and UV radiation (Zhang et al., 2021; Zeng et al., 2022; Ahmad et al., 2023a). It also acts as an antioxidant and is proficient in reducing ROS activity (Murch and Erland, 2021; Ahmad et al., 2023b). The radical scavenging capacity of this molecule is attributed to its ability to activate various antioxidant pathways, including the glutathione ascorbate cycle (AsA-GSH), peroxidases (PODs), superoxide dismutase (SOD), catalase (CAT) and ascorbate peroxidases (APX), as well as the production of non-enzymatic phenolic antioxidants such as flavonoids and phenolic acids (Muhammad et al., 2022; Kurt-Celebi et al., 2023). Numerous studies have confirmed that the exogenous application of melatonin substantially improves tolerance, alleviating abiotic and biotic stress-responsive decreases in growth, biomass accumulation, chlorophyll loss, photosynthetic inhibition and antioxidant activities in most plants, thus enhancing agricultural productivity (Adhikary et al., 2020; Zeng et al., 2022; Huang et al., 2022; Ahmad et al., 2023a, b). Several reports have shown that exogenous melatonin can induce the production of polyphenols, such as phenolic acids, flavonoids including anthocyanins, which can efficiently scavenge ROS and support the antioxidant defence system in coping with harsh environmental conditions (Zeng et al., 2022; Ahmad et al., 2023a).
Beet or beetroot (Beta vulgaris L., fam: Amaranthaceae) is a herbaceous biennial or rarely, perennial plant that can grow up to 120 cm (max. 200 cm) in height. The cultivated forms are mostly biennial. The plant contains several cultivar groups, including the most important commercial crop, sugar beet or white beet, which supplies 35% of the world's table sugar. Red beet is another cultivar and is the most popular type due to its distinctive colour, which is caused by the presence of nitrogen-containing water-soluble pigments known as betalains. It is widely used as a natural food colourant labelled E-162 (Romeiras et al., 2016; Zhang et al., 2021). These plants are also a rich source of important nutrients, including carotenoids, nitrates, flavonoids, vitamins such as vitamin C and B6, folate, etc., and minerals such as K, Na, P, Ca, Mg, Cu, Fe, Zn and Mn. They confer health benefits through their antioxidant, anti-inflammatory, antimicrobial, antiviral, hepatoprotective, antidiabetic and anticancer properties. These benefits can be obtained through direct consumption or by using them as a raw or cooked ingredient in various industrial food products, such as purees, preserves, juices and baby food (Chen et al., 2021; Zhang et al. 2021).
Studies of beet response mechanisms under salt stress are particularly useful in terms of improving beet plantation policies under the threat of global climate changes. Melatonin is known to alleviate salt stress damage by regulating a series of physiological and biochemical processes (Zeng et al., 2022; Ahmad et al., 2023a, b). However, further information is needed regarding the improvement of beet growth under salt stress through the application of exogenous melatonin. Our hypotheses in the current study were therefore as follows: (i) increasing NaCl concentrations can enhance the salt stress tolerance of red (betalainic/cyanic) and white (non-betalainic/acyanic) beet cultivars, (ii) exogenous melatonin application can mitigate the damage caused by salt stress in cyanic/acyanic beets by improving betalain and phenolic acid synthesis and antioxidant systems and balancing ion homeostasis, and (iii) exogenous melatonin can contribute to the raising of endogenous melatonin levels to promote salinity tolerance in beets. The results will contribute to our understanding of the mechanism by which exogenous melatonin improves the tolerance of red and white sugar beets under salt stress. It will further contribute to the understanding of the response mechanisms of beets under salt stress in modulating betalain and phenolic acid synthesis in conjunction with ions and biomass.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
Seeds of extensively cultivated varieties of two red beet cultivars (Beta vulgaris var. crassa L. ‘Ruby Queen’ and B. vulgaris L. ‘Scarlet Supreme’) were provided by the Anderson Seed and Garden (Utah, USA). Seeds of two white sugar beet cultivars (Beta vulgaris L. ‘Rodeo’ and ‘Ansa’) were obtained from Beta Ziraat ve Ticaret A.Ş. and the Aegean Agricultural Research Institute (Menemen, İzmir, Türkiye).
The experiment was conducted using a completely randomised design with four replications. Beta vulgaris ‘Scarlet Supreme’ and Beta vulgaris var. crassa ‘Ruby Queen’ were used for sugar beet cultivation as red/cyanic cultivars and Beta vulgaris ‘Rodeo’ and ‘Ansa’ as white/acyanic cultivars. The NaCl concentrations (50, 150 and 250 mM) and melatonin application dose (50 μM) used in the present study were selected on the basis of previous research involving beets (Zhang et al., 2021; Liu et al., 2022) and various other plants (Mukherjee, 2018; Zeng et al., 2022; Ahmad et al., 2023a, b). Beet seeds of a consistent size were selected and sterilised by soaking them in 5% sodium hypochlorite (NaClO) for 10 min, and were then washed in sterile water 3–5 times until clean. The seeds were next soaked in sterile water for 24 hours in a 25°C incubator, after which the water was removed. The seeds were then placed in sterile Petri dishes and germinated in a growth chamber (25/20°C, day/night). The cultures were incubated in the dark in a 25°C controlled growth chamber, and pre-germination was carried out in distilled water for 24 hours. All seeds with the same growth features were next sown in four plastic nursery pots (10 length x 20 width x 20 height) filled with vermiculite and cultured with water in a greenhouse for one week. The beet seedlings with uniform growth size were then transferred to 0.5 L PVC pots in 1/2 Hoagland nutrient solution, and culturing continued for approximately six weeks. Melatonin was dissolved in a small amount of absolute ethanol, and 1/2 Hoagland nutrient solution was used to adjust the volume to the required concentration. The growth conditions of the beet seedlings were 25°C for 16 hours in the light and 20°C for 8 hours in the dark. The light intensity was between 300 and 350 μmol m−2 s−1, and the relative humidity was 60–70%. The beet seedlings were harvested after 12 days of treatment, at which time the plants had three expanded leaves were used all physiological measurements and biochemical analyses. After 12 days, the seedlings were randomly divided into eight groups. The seedlings with no salt (NaCl) formed the control group, plants treated with only 50 μM melatonin constituted the melatonin-treated group, plants exposed to 50, 150 and 250 mM NaCl represented the salt-treated groups and plants treated with 50, 150 and 250 mM NaCl +50 μM melatonin the salt stress + Mel groups. Three replicates were used for each treatment.
2.2 Determination of ion contents in beet leaves
Ion contents, namely Na+, Cl−, K+ and Ca2+, were quantified using the method described by Retka et al., (2010). Beet leaves were dried at 65°C, and 1 g of dried sample was then exposed to 10 mL nitric acid (HNO3) and 4 mL of purified H2O2 (Milli-Q system, Millipore). The samples were digested in a microwave from 50°C to 200°C (total digestion time 70 min). One milliliter of digested sample was diluted with ultrapure deionised water and then analysed using an inductively coupled plasma-mass spectrometer (Agilent 7700x ICP-MS).
The instrument analysis conditions for the determination of Na+, Ca2+ and K+ were optimized according to the characteristics of each respective element. The plasma power of the instrument was set in the range of 1500 Watts for Na+ and K+. Argon (Ar) gas flow rate was set at 10 L min−1 and auxiliary gas flow rate was set at 0.2 L min−1 to stabilize the plasma. The nebuliser pressure was maintained at 0.6 L min−1 to enhance atomization efficiency and minimize the occurrence of interferences. Optimal functionality was designated at wavelengths, 589.0, 422.7 and 766.5 nm for Na+, Ca2+ and K+, respectively. The calibration curve was generated using standard solutions for Na+, Ca2+ and K+, ranged from 0 to10 ppm. In contrast, the Ar gas flow rate was set at approximately 14 L min−1 and the auxiliary gas flow rate was set at 1.8 L min−1 for chlorine (Cl−) determination. The nebuliser pressure was maintained at 0.73 L min−1 in order to optimise atomisation efficiency and minimize the occurrence of interferences. The calibration curve was generated using standard solutions (range; 0–250 ppm for Cl−).
2.3 Determination of antioxidant enzyme activity and antioxidant compound content
Fresh samples (0.5 g) treated with liquid nitrogen were ground in 5 mL of 50 mM precooled phosphate buffer (pH 7.0) including 5 mM ascorbic acid. The homogenate was centrifuged at 27 670 g for 30 min at 4°C. The activities of antioxidant enzymes were estimated with the extracted supernatants. The activities of peroxidase (POD; E.C. 1.11.1.7), catalase (CAT; E.C. 1.11.1.6), superoxide dismutase (SOD; E.C. 1.15.1.1), ascorbate peroxidase (APX; EC 1.11.1.11), glutathione reductase (GR; EC 1.6.4.2), dehydroascorbate reductase (DHAR; E.C. 1.8.5.1) and monodehydroascorbate reductase (MDHAR; E.C. 1.6.5.4) were estimated using the methods described by Kurt-Celebi et al., (2023) based on fresh weight (FW). The reduced glutathione (GSH) content was determined using a glutathione colorimetric detection kit (703.002, Cayman Chemical) and calculated as the difference between nmol GSSG g−1 FW and the total nmol GSH g−1 FW.
2.4 Extraction and determination of total betalain (TB) content in beet leaves
Lyophilised plant material was ground to a fine powder, after which 300 mg was homogenised using 3 mL of purified water (Milli-Q system, Millipore) for 1 min. The homogenate was centrifuged for 10 min (12 298 g), and the resulting clear supernatant was collected. The insoluble part (for red beet only) was extracted in triplicates with 3 mL double distilled water in three additional steps. The red and white beet extracts were finally combined and immediately used for further analyses.
2.5 UHPLC–MS (Ultra-High Performance Liquid Chromatography-Mass Spectrometry) analysis of betalains in beet leaves
A Thermo Scientific Dionex UltiMate 3000 Series UHPLC+ device (Thermo Scientific) was used for chromatographic analysis. A Gemini C18 (150 × 4.6 mm 3 μm; Phenomenex, Torrance) analytical column was employed. The following gradient (Nemzer et al., 2011) was used for the separation of analytes: 3% (v/v) A at 0 min; gradient to 16% A at 17 min; gradient to 50% A at 30 min ([A] acetonitrile; [B] 1% formic acid (Merck) in double distilled and purified water (Milli-Q system). The injection volume was 10 μL, and a flow rate of 0.5 mL min−1 was maintained. Detection was generally performed at λ = 538 nm with a UV–Vis detector or a diode array detection (DAD) system at 505, 480, and 310 nm. The column temperature was kept at 35°C.
All compounds were identified by an HPLC-Finnigan MS detector and an LCQ Deca XP MAX (Thermo Finnigan) instrument with an electrospray interface (ESI) operating in positive ion mode. The analyses were carried out using full scan data-dependent MSn scanning from m/z 110 to 1500. Column and chromatographic conditions were identical to those used for the HPLC-DAD analyses. The injection volume was 10 μL, and the flow rate was maintained at 0.5 mL min−1. The capillary temperature was 250°C, the sheath gas and auxiliary gas were 60 and 15 arbitrary units, respectively, the source voltage was 3 kV for negative ionization and 4 kV for positive ionization and normalised collision energy was between 20% and 35%. Spectral data were elaborated using the Excalibur software (Thermo Scientific). The identification of compounds was confirmed by fragmentation, comparison of retention times and compound spectra and previous reports. The relative peak area of the betalains was compared among the four beet cultivars, and individual betacyanins and their derivatives were calculated from relative HPLC peak areas (for LC–MS extracted ions) and expressed as percentage (%) values of the total peak area, since commercial standards were not available. Betanin/isobetanin was also calculated. Betalain profiles were categorised into three main groups - betanin (BTs), isobetanin (IBTs) and neobetanins (NBTs), including the sum of their derivatives.
2.6 Determination of endogenous melatonin content in beet leaves
The extraction and determination of melatonin was performed using the Stürtz et al. (2011) method with slight modifications in our extraction and analysis conditions. The leaf samples were ground in liquid nitrogen, homogenised in 20 mL of methanol, and coarsely ground using a pestle and mortar. The homogenate was then shaken in an ultrasonic bath for 30 min. The resulting homogenate was centrifuged at 12 000 g for 10 min. After centrifugation, the supernatant was evaporated to dryness under a vacuum using a rotary evaporator under reduced pressure at 40°C. The dried extract was finally suspended in 1 mL methanol (LC–MS grade) by ultrasound (20 sec) and cleared by centrifugation (10 min, 18 840 g).
An Acquity UHPLC system with a binary solvent manager, column manager and FLR fluorescence detector (Waters Corporation) was used for chromatographic analysis. An Acquity UPLC HSS T3 (2.1 × 100 mm 1.8 μm; Waters Corporation) analytical column was used for that purpose. The column temperature was kept at 35°C. The following linear gradient constituting of different ratios of fromic acid, acetonitrile and pure water (Merck, >99% and Milli-Q system, Millipore) was used for the separation of analytes: 17% (v/v) B at 0 min; 27% B at 3 min; 100% B at 3.4 min; 100% B at 7.3 min; 17% B at 7.35 min ([A] 0.1% formic acid in double distilled water; [B] 0.1% formic acid in acetonitrile). The total run time was 8 min, the injection volume was 7.5 μL, and a flow rate of 0.5 mL min−1 was maintained. Detection was performed at an excitation wavelength λ = 290 nm and emission wavelength λ = 335 nm.
The melatonin retention window (2–4 min, typical retention time = 3.2 min) was additionally analysed using a Xevo QTof MS/MS instrument (Waters/Micromass UK Ltd) with an ESI ion probe operating in positive ion mode. Mass spectra were recorded from m/z 50 to 600, and MS/MS data were taken for the ion m/z = 233 (collision energy = 15 eV; characteristic daughter ion at m/z = 174). The desolvation gas flow (nitrogen) was 800 L h−1 and the desolvation temperature was 450°C. The source voltage was 3 kV. Spectral data were recorded and analysed using Masslynx 4.1 software (Waters Corporation). The identification of compounds was confirmed by fragmentation and comparison. The concentration of melatonin was measured by integration of the fluorescence detector signal.
2.7 Extraction and HPLC-DAD (High Performance Liquid Chromatography-Diode Array Detector) determination of phenolic acids (PHAs)
Crude methanolic extracts were fractionated into four extracts consisting of soluble free (SF), ester-conjugated (SEC), glycoside-conjugated (SGC), and cell wall-bound (CWB) forms in line with the protocol employed by Ayaz et al., (2005). The PHA analysis conditions consisted of a validated method recently published by us (Colak et al., 2021; Bouafia et al., 2023). In brief, an HPLC system consisting of an Agilent 1100 series instrument (Palo Alto) equipped with a quaternary HPLC pump, micro vacuum degasser (MVD), thermostated column compartment (TCC), DAD detector and standard micro and preparative autosampler was used in the separation and quantification of PHAs liberated from the leaf samples. The chromatograms were monitored and integrated using an Agilent Chem Station software. An Agilent Zorbax Eclipse XDB-C18 column (4.6 mm × 250 mm, 5-μm particle size) thermostated at 35 ± 1°C was employed during the analysis. The DAD signals for each compound were selected according to their spectra obtained from the Agilent Chem Station software. Phenolic acid standards were of analytical grade (>99%): p-hydroxybenzoic acid (p-HBA), m-hydroxybenzoic acid (m-HBA) and vanillic acid (VaA), gallic acid (GaA), protocatechuic acid (PA), caffeic acid (CaA), syringic acid (SyA), gentisic acid (GeA), p-coumaric acid (p-CoA), ferulic acid (FeA), sinapic acid (SiA), m-coumaric acid (m-CoA), o-coumaric acid (o-CoA) and salicylic acid (SaA) and were purchased from Sigma-Aldrich Fine Chemicals. Appropriate wavelengths were selected: 214 nm for p-HBA, m-HBA and VaA, 280 nm for GaA, PA, CaA and SyA, and 325 nm for GeA, p-CoA, FeA, SiA, m-CoA, o-CoA, and SaA. A gradient elution system with two different mobile phases [(A - UV-pure H2O (Milli-Q system, Millipore) with 0.2% HCOOH (Merck, >99%)] and B - 50% methanol with 0.2% HCOOH (Fluka Chemie, >99%) wıth a flow rate of 0.5 mL min−1 was used as follows: 0–10 min (15–35% B), 10–20 min (35–55% B), 20 °C 30 min (55–65% B), 30–40 min (65–85% B), 40–45 min (85–95% B), 45–50 min (95–75% B) and 50–60 min (75–35% B). Injection was performed in 5 μL volumes.
2.8 Statistical analysis
All extraction and analysis values are shown as mean ± standard deviation (SD; n = 3). The results were subjected to one-way analysis of variance (ANOVA), and differences between means were determined using the Duncan test at the 0.05 significance level. Principal component analysis (PCA) was conducted in order to interpret the difference in all analysed compounds among beet cultivars. The major component score values of the variables/observations are evaluated in Table 2.
3 RESULTS
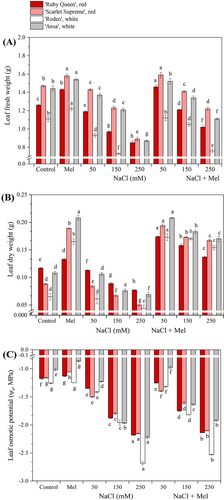
3.1 Melatonin improved leaf biomass and osmotic potential (ψs) in red and white beets under salt stress
The external morphologies of the red and white sugar beet seedlings grown under melatonin application and salt stress alone and melatonin + salt stress conditions are shown in Figure 1. The leaf growth of the beets was suppressed by salt stress alone and enhanced by meatonin + salt stress treatments. Exogenous melatonin application increased the fresh and dry weights of the tested beet cultivars in a dose-dependent manner compared to the control plants (Figure 2A, B). The biomass weights of the beet cultivars, which were suppressed by salt stress, were significantly enhanced when exogenous melatonin application was combined with salinity in the same manner. Furthermore, the application of melatonin alone resulted in a slight increase in leaf water potential (ψs) in the beets (Figure 2C), but this increase was not statistically significant. On the other hand, salinity significantly promoted the leaf water potential in the same manner. However, when melatonin application was combined with salt stress, it reduced the salt stress-induced enhancement of the water potential. The results indicate a significant, strong, and negative correlation range between the salinity in the beets and the leaf ψs (r = −0.834 to −0.922, p < 0.05) as well as the fresh weight (range; r = −0.783 to 0.847, p < 0.05). However, no correlation found between salinity and dry weight.

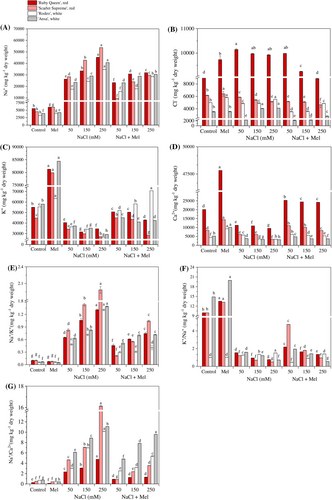
3.2 Melatonin regulated ion homeostasis in leaves in red and white beets under salt stress
The changes in ion contents and ratios (Na+, Cl−, K+ and Ca2+, Na+/K+, K+/Na+ and Na+/Ca2+) in red and white beets under experimental conditions are shown in Figure 3. As expected, salinity resulted in cultivar-specific increases in Na+ concentrations and decreases in K+ and Ca2+ concentrations in the beets (Figure 3A, C, D). However, the combination of exogenous melatonin application with salt stress resulted in a significant decrease (p < 0.05) in Na+ concentrations in the leaves (Figure 3A), while K+ and Ca2+ concentrations increased significantly compared to the control plants (Figure 3C, D). Furthermore, the cultivar-specific increase in Cl− concentrations induced by melatonin was ameliorated when combined with salinity (Figure 3B). These results indicate a strong negative correlation (r = −0.777 to −0.874, p < 0.05) between increasing salinity and decreasing K+ concentrations, as well as between increasing salinity and decreasing Ca2+ concentrations (r = −0.713 to −0.772, p < 0.05) and the K+/Na+ ratio (r = −0.881 to −0.998). Analysis showed that the leaf water potential (leaf ψs) was negatively correlated with the Na+ concentration in the leaves (r = −0.834 to −0.922, p < 0.05), as well as with leaf fresh weight (r = −0.782 to −0.847, p < 0.05). Additionally, the leaf ψs was strongly positively correlated with the Na+/K+ ratio (r = 0.868 to 0.976, p < 0.05) and Na+/Ca2+ ratio (r = 0.797 to 0.929, p < 0.05; Figure 3E, F, G).
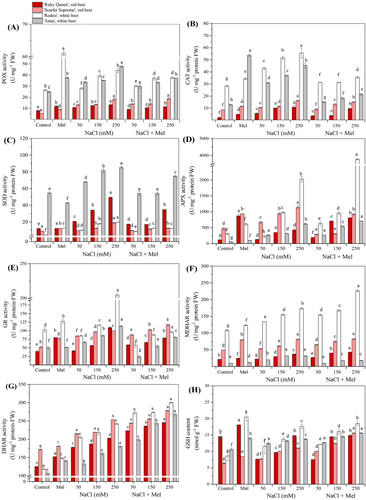
3.3 Melatonin stimulated enzymatic and non-enzymatic antioxidant activity in leaves in red and white beets under salt stress
As shown in Figure 4, antioxidant enzyme activities (A - G) and GSH content (H) in the four tested beet leaves were cultivar-specific. In general, melatonin application alone significantly (p < 0.05) enhanced the activity of the studied enzymes and the GSH contents in comparison to the control plants, particularly in the white beets, with the exception of APX activity. In addition, salt stress alone induced a significant (p < 0.05) increase in POX and CAT activities in the white beets (‘Rodeo’ and ‘Ansa’, respectively), and in SOD activity in the red ‘Ruby Queen’ and the white ‘Ansa’. Salt stress alone and salt stress + melatonin treatment significantly (p < 0.05) subsequently increased the activities of APX, MDHAR and DHAR in particular in the red ‘Scarlet Supreme’ and the white ‘Rodeo’ (Figure 4D, F, G). GR activity (Figure 4E) increased in all the tested beets, particularly in the white ‘Rodeo,’ while being reduced by salt stress + melatonin application. In the tested beet leaves, significant (p < 0.05) increases in GSH contents were induced by melatonin application and salt stress alone. These results demonstrated that the addition of melatonin in the salt stress-growth medium significantly mitigated the salt stress-induced increases in the antioxidant enzyme activities and GSH contents in the beets. The results of enzyme activities and GSH contents were cultivar-specific and significantly high and strong correlated in ‘Ruby Queen’ (r = 0.714–0.860), ‘Scarlet Supreme’ (r = 0.721–0.964), ‘Rodeo’ (r = 0.712–0.933) and ‘Ansa’ (r = 0.710–0.934).
3.4 Melatonin application increased betalain contents in leaves in red and white beets under salt stress
Chromatographic, spectrophotometric and mass spectrometric data of the analysed betacyanins and their derivatives in leaves in the tested beet cultivars are presented in Table 1. Since betalain profiles are often subject to change during the purification process and further pigment degradation is possible, the samples were analysed directly after water extraction. The UV–Vis spectra of some betanin derivatives were unclear due to their low content. However, mass spectra and chromatographic results enabled them to be identified (Table 1). All compounds identified in the present analysis have previously been described in extracts from different beetroot parts and cultivars (Beta vulgaris L. spp. vulgaris; Slatnar et al., 2015). The 13 betacyanins in the present study, together with their derivatives, were isolated, quantified and listed according to their retention times (Table 1).
| Peak no. | Compound | ʎmax | m/z [M + H]+ | m/z from MS/MS of [M + H]+ | Beta vulgaris | |||
|---|---|---|---|---|---|---|---|---|
| ‘Ruby Queen’ | ‘Scarlet Supreme’ | ‘Rodeo’ | ‘Ansa’ | |||||
| 1 | Betanin | 536 | 551 | 389 | + | + | + | + |
| 2 | 17-Decarboxy-neobetanin | 444 | 505 | 343, 297 | + | + | + | + |
| 3 | 2,17-bidecarboxy-neobetanin | 462 | 461 | 299 | + | + | + | + |
| 4 | Isobetanin (IBN) | 536 | 551 | 389 | + | + | + | + |
| 5 | 2-Decarboxy-neobetanin | 490 | 505 | 343, 297 | + | + | + | + |
| 6 | Isomer-2,17-bicarboxy-neobetanin | 461 | 461 | 299 | + | + | + | + |
| 7 | 2,17-Bidecarboxy-2,3-dehydro-neobetanin | 462 | 461 | 299 | + | + | + | + |
| 8 | 2′-O-Glucosyl-betanin | 535 | 713 | 551, 389 | + | + | + | + |
| 9 | Neobetanin | 464 | 549 | 387 | + | + | + | + |
| 10 | 2′-O-Glucosyl-isobetanin | 535 | 713 | 551, 389 | + | + | + | + |
| 11 | 2-Decarboxy-2,3-dehydro-neobetanin | 420 | 503 | 341 | + | + | + | + |
| 12 | 6-O-Feruloyl-2’-O-glucosyl-betalanin | -a | 889 | 713, 551 | + | + | + | + |
| 13 | 6-O-Feruloyl-betanin/isobetanin | -a | 727 | 551 | + | + | + | + |
- a ʎmax was not determined due to co-elution with another compound or low concentration.
Exogenous melatonin application alone increased the total betalain content (Figure 5A) in the leaves of the four tested beet cultivars, especially in the red beets, ‘Ruby Queen’ and ‘Scarlet Supreme’. When combined with salt stress treatments, exogenous melatonin application significantly (p < 0.05) enhanced the total betalain contents, particularly in leaves in the two red beets, in a dose-dependent manner. The sum of individual major betalains included betanins (BTs; betanin, 2-O-glucosyl-betanin, 6-O-feruloyl-2’-O-glucosyl-betalanin), isobetanins (IBTs; isobetanin, 2-O-glucosyl-isobetanin, 6-O-feruoyl-betanin/isobetanin) and neobetanins (NBTs; neobetanin, 17-decarboxy-neobetanin, 2,17-bidecarboxy-neobetanin, 2-decarboxy-neobetanin, isomer-2,7-bicarboxy-neobetanin, 2,17-bidecarboxy-2). This study analysed the betalain content of various beet cultivars, including derivatives such as 3-dehydro-neobetanin and 2-decarboxy-2,3-dehydro-neobetanin. Results showed that exogenous melatonin application and salt stress, both alone and in combination, significantly increased the betanin, isobetanin and neobetanin contents compared to the control plants. Betanins and neobetanins emerged as the major contributors to betalains in red beets, particularly in ‘Ruby Queen’ (Figure 5B), followed by ‘Scarlet Supreme’ (Figure 5C). However, the major betalains in the white beets were NBTs, in contrast to the red beets which exhibited higher concentrations of betanin, particularly in ‘Rodeo’ compared to ‘Ansa’ (Figure 5D, E). The red beets generally had higher betanin contents than the white beets, owing to their high betacyanin content. The major betalains in ‘Ruby Queen’ possessed the highest betanin contents (r = 0.744–0.918), while ‘Scarlet Supreme’ and ‘Ansa’ had the highest neobetanin content (r = 0.731–0.879), and ‘Rodeo’ the highest isobetanin content (r = 0.769–0.818). All these correlations were significant (p < 0.05).
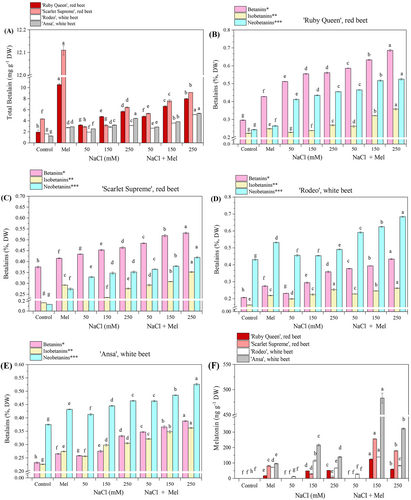
*sum of betanin and its derivatives (BTs); betanin, 2-O-glucosyl-betanin, 6-O-feruloyl-2’-O-glucosyl-betalanin.
**sum of isobetanin and its derivatives (IBTs); isobetanin, 2-O-glucosyl-isobetanin, 6-O-feruoyl-betanin/isobetanin.
***sum of neobetanin and its derivatives (NBs); neobetanin, 17-decarboxy-neobetanin, 2,17-bidecarboxy-neobetanin, 2-decarboxy-neobetanin, isomer-2,7-bicarboxy-neobetanin, 2,17-bidecarboxy-2.3-dehydro-neobetanin, 2-decarboxy-2,3-dehydro-neobetanin.
3.5 Exogenous melatonin stimulated endogenous melatonin content in the leaves of red and white beets under salt stress
The levels of endogenous melatonin content in the studied red and white beet cultivars are shown in Figure 5F. The melatonin levels were cultivar-specific and varied significantly under the experimental conditions. No endogenous melatonin was detected in the leaves of the control plants throughout the beet growth period. However, higher levels of exogenous melatonin application induced notable endogenous melatonin levels in all tested beets. Under salinity conditions, endogenous levels of melatonin were enhanced at higher salt concentrations (150 and 250 mM NaCl). This effect was even more pronounced when salt stress was combined with melatonin application. At the lowest salinity treatment (50 mM NaCl) with melatonin application, a detectable level of melatonin was only observed in ‘Rodeo’ (white beet) compared to the control plants (Figure 5F). This study found no correlation between increasing salinity and dose-dependent induction of endogenous melatonin levels.
3.6 Melatonin increased phenolic acid contents (PHAs) in the leaves of red and white beets under salt stress
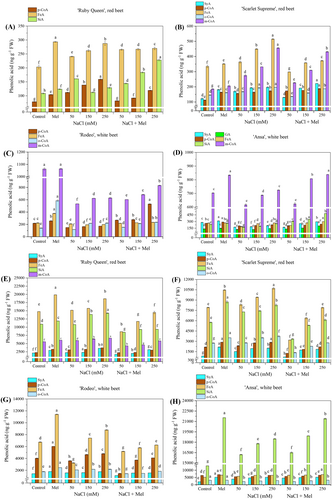
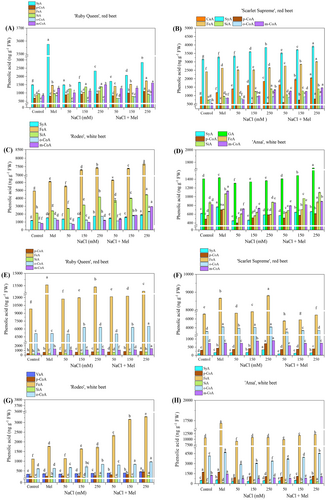
The types and contents of phenolic acids in the studied beet cultivars are shown in Figures 6 and 7. These were all cultivar-specific, the contents increasing or decreasing in a dose-dependent manner in most cases. The phenolic acids were present in the form of soluble free (SF), ester-conjugated (SEC), glycoside-conjugated (SGC), and cell wall-bound (CWB) forms. The contents of the acids were significantly stimulated (p < 0.05) by exogenous melatonin application in comparison to the control plants. Generally, the PHA content increased in response to increasing salt stress treatments in a dose-dependent manner. However, when exogenous melatonin was applied in combination with salt stress treatments, the increase in PHA content was lower compared to the control plants and to some extent, melatonin application alone. The primary phenolic acid in the SF form found in red beets was ferulic acid (FeA) in both ‘Scarlet Supreme’ and ‘Ruby Queen’ varieties. High m-coumaric acid (m-CoA) contents were observed in the white beets ‘Ansa’ and ‘Rodeo’ (Figure 6A-D). In the red beets, the predominant PHA in SEC form was FeA, followed by sinapic acid (SiA), while in the white beets, it was p-coumaric acid (p-CA; Figure 6E-H). The addition of melatonin to salt-stressed plants reduced the salt stress-induced enhancement in PHA contents (including remaining PHAs) compared to control plants treated with melatonin alone. Under increasing salt stress, an accumulation of PHAs was observed in its glycoside-conjugated form (SGC; Figure 7A-D). The predominant PHAs in red beets were syringic acid (SyA), followed by FeA or m-CoA. In white beets, the predominant PHAs were SiA, followed by SyA or GA and m-CoA (Figure 7A-D). The addition of melatonin in salt stress-growth medium depleted the significant salt stress-induced rise in cell wall-bound (CWB) form phenolic acids, particularly the major FeA and o-CoA contents, in all the tested cultivars (Figure 6E-H).
3.7 Principal component analysis (PCA) of physiological traits, ions, betalains and phenolic acids
A detailed PCA of the dataset based on Jolliffe's (2022) criterion was adopted for the selection of the number of principal components (PCs), with a minimum accumulated variance [Var(X)] of 70%. Comprehensive comparison of these PCA blots (range; Var(X); 74.83–88.51%) shows that basic physiological traits are highly associated (average Var(X): 84.96%) and correlated with increasing salinity (Na+) or ion concentrations/ratios (Table 2). Additionally, the table includes PCs, comparing the activities of antioxidant enzymes and antioxidant compound, betalains, and phenolic acids contents in soluble free (SF), ester-conjugated (SEC), glycoside-conjugated (SGC) and cell wall-bound forms (CWB) (Table 2). The PC results in Table 2 were consistent with the trait-by-trait analyses for exogenous melatonin application, increasing salinity, and the combination thereof (melatonin + salinity) that explain relatively high variation and associations between observations and variables. The correlation was mostly dose-dependent and significant (p < 0.05), either positively or negatively highly and strongly correlated, or moderately or highly correlated. The PCs score results show that melatonin application alone (Mel) or combined with salt stress, particularly at 150 and 250 mM NaCl treatments (e.g., S150 + Mel and S250 + Mel) share common characteristics capable of mitigating the adverse effect of salt stress in the tested beet leaves, in either a cultivar-specific or dose-dependent manner.
| Physiological traits (average Var(X): 84.96%)* | Phenolic acids (average Var(X): 77.86%)* | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ions | antioxidants** | Betalains | soluble free (SF) | soluble ester-conjugated (SEC) | soluble glycoside-conjugated (SGC) | cell wall-bound (CWB) | ||||||||
| PC1 and PC2 (87.57%) | PC1 and PC2 (78.79%) | PC1 and PC2 (88.51%) | PC1 and PC2 (76.31%) | PC1 and PC2 (75.31% | PC1 and PC2 (84.99%) | PC1 and PC2 (74.83%) | ||||||||
| Score labels | PC1 (74.35%) | PC2 (13.22%) | PC1 (57.22%) | PC2 (21.57%) | PC1 (6 1.86%) | PC2 (26.65%) | PC1 (53.12%) | PC2 (22.19%) | PC1 (47.42%) | PC2 (28.64%) | PC1 (49.50%) | PC2 (35.94%) | PC1 (49.25%) | PC2 (25.58%) |
| Control | 4.38 | −2.93 | −7.10 | −2.92 | 6.34 | −4.33 | 3.52 | −3.25 | 2.96 | 0.14 | −5.95 | −3.24 | 4.23 | −3.98 |
| Mel | 8.30 | −0.78 | −3.78 | 0.76 | 8.45 | 1.33 | 7.58 | 2.53 | 3.72 | 7.84 | −6.40 | 4.97 | 7.12 | 1.27 |
| S50 | −0.96 | −0.89 | −2.03 | −2.33 | −0.08 | −2.73 | −1.05 | −3.44 | −0.51 | −1.43 | −1.75 | −4.74 | −0.45 | −3.61 |
| S150 | −4.55 | −1.27 | 2.87 | −3.24 | −3.9 | −2.93 | −4.42 | −1.63 | −4.46 | −0.44 | 2.76 | −4.22 | −3.90 | −2.74 |
| S250 | −7.92 | −1.64 | 8.32 | −2.94 | −7.5 | −2.80 | −7.13 | 0.40 | −7.69 | 0.61 | 6.25 | −2.70 | −7.41 | −0.85 |
| S50 + Mel | 2.90 | 2.14 | −4.02 | 2.94 | 2.0 | 3.09 | 3.09 | −1.73 | 5.82 | −4.26 | −2.33 | 1.48 | 3.00 | 1.37 |
| S150 + Mel | 0.34 | 2.39 | 0.31 | 3.70 | −0.97 | 3.75 | 0.60 | 1.94 | 1.95 | −1.91 | 1.54 | 3.08 | 0.12 | 2.91 |
| S250 + Mel | −2.49 | 2.95 | 5.44 | 4.03 | −4.21 | 4.62 | −2.21 | 5.19 | −1.78 | −0.54 | 5.87 | 5.36 | −2.71 | 5.61 |
- Abbreviations: S; NaCl (mM), SF; soluble free, SEC; soluble ester-conjugated, SGC; soluble glycoside-conjugated, CWB; cell wall-bound, Mel; melatonin.
- * explains variances, comparing measured physiological traits with ions, antioxidants, betalains, and phenolic acids using PCA.
- ** antioxidant enzyme activities and antioxidant compound content in Figure 4.
4 DISCUSSION
Biomass is a commonly used growth indicator for evaluating the salinity tolerance of crops. It includes the weight of shoots, roots, and leaves, as well as the length and diameter of shoots and roots (Hao et al., 2021; Zhang et al., 2021). In general, the fresh and dry weights of crops decrease under salt stress. The extent of that decrease depends on the type of crop plant, such as tomato, sunflower, wheat, rice, maize, and sugar beet, and the level of salinity to which they are exposed. Previous studies have shown varying degrees of reduction in different crops (Wu et al., 2013; Wang et al., 2017; Hao et al., 2021). No decrease in biomass was observed in halophytes, such as Salicornia, until the NaCl level exceeded 400 mM (Hao et al., 2021). Similarly, salt-tolerant glycophytes, such as sugar beet, can maintain good growth status even under low salt concentrations, for example at 70 mM NaCl (Zhang et al., 2021). Research has demonstrated that the application of melatonin can increase the biomass of plants growing under salt stress conditions and alleviate the negative effects of salinity, which can lead to a decrease in biomass (Zhang et al., 2021; Hao et al., 2021). In this study, the fresh and dry weights of the tested beet cultivars decreased due to salt stress, but this decrease was significantly improved when melatonin was applied in a dose-dependent manner. Similar to our own findings, Zhang et al., (2021) also observed significant increases in fresh and dry weights of sugar beet under salt stress (600 mM NaCl) when comparing different forms of melatonin application.
Plants possess a protective mechanism against abiotic stress conditions, which involves maintaining a good osmotic potential. This is achieved by accumulating and compartmentalising osmotic regulatory substances, such as toxic ions (e.g. Na+) and/or compatible solutes (K+, sugars, proline, betaine/betalains, GB, etc.; Sharma et al., 2019). Melatonin has been widely reported to modulate osmolyte synthesis under abiotic stresses (Zeng et al., 2022; Rajora et al., 2022; Ahmad et al., 2023a, b). The application of melatonin combined with an increase in salinity resulted in a significant increase in leaf osmotic potential (Ψleaf), which was reduced by salt stress treatment alone. Similar results were reported by Wu et al., (2013) and Wang et al., (2017) for sugar beets under salt stress alone. Consistent with a previous study on sugar beet by Zhang et al., (2021), this present study also confirms that the application of melatonin combined with salt stress promotes the leaf water potential of red and white beet cultivars. These results suggest that melatonin application may improve plant water status by enhancing osmotic adjustment, which is a general response to osmotic stress induced by both salt and abiotic stresses.
Plants' adaptation to salinity stress involves maintaining a balance between Na+, K+, and Ca2+ to regulate intercellular ion homeostasis (Hao et al., 2021). In this study, salinity was found to increase Na+ concentrations in the leaves of both red and white beets, while K+ and Ca2+ concentrations decreased in a dose- and cultivar-specific manner. Wu et al., (2013) and Wang et al., (2017) previously reported an increase in Na+ concentrations and a decrease in K+ concentrations in sugar beets induced by salt stress. Studies have shown that exogenous melatonin plays a crucial role in regulating ion homeostasis and alleviating ion toxicity in crops (Zhang et al., 2021; Zeng et al., 2022). Consistent with previous findings, the current study found that the application of exogenous melatonin in combination with salt stress resulted in a significant reduction in Na+ concentrations, while increasing K+ and Ca2+ concentrations in beets. This is in line with the results reported by Zhang et al., (2021), which suggest that melatonin may promote Na+ compartmentalisation to alleviate toxicity in sugar beets caused by salt stress. Consistent with previous reports, the current study also found that low Na+/K+ and Na+/Ca2+ ratios are associated with salinity tolerance in plants (Hao et al., 2021). This study showed that increasing salinity induced K+ efflux, resulting in increased Na+/K+ and Na+/Ca2+ ratios and a reduced K+/Na+ ratio. However, melatonin application combined with salt stress improved these ratios, leading to low Na+/K+ and Na+/Ca2+ ratios. These reports and findings suggest that exogenous melatonin can improve the tolerance of red and white beets under salinity conditions in a cultivar-specific manner.
Studies have shown that endogenous melatonin production is highly responsive to abiotic stresses. Many crop plants have been reported to exhibit enhanced melatonin levels under various abiotic stressors, such as drought, saltwater logging, chilling, heavy metals, light and ionising radiation with gamma rays (Zeng et al., 2022). In such cases, plant tissues are vulnerable to oxidative damage, resulting in the overproduction of reactive oxygen species (ROS). High concentrations of melatonin act as efficient ROS scavengers (Sati et al., 2023). This study found that combining the existing melatonin level with exogenous melatonin increased total tissue melatonin levels. Additionally, this combination acted as a bio-stimulant, accelerating the biosynthesis of betalains and phenolic acids. This contributes to the mitigation of ROS-dependent stress and the enrichment of osmolytes for a well-balanced water status, avoiding physiological dryness in plants. In this study, higher NaCl concentrations (150 and 250 mM NaCl) combined with melatonin application further increased endogenous melatonin levels, which were cultivar-specific in beets. This can regulate the biosynthesis of betalains and phenolic acids in response to salt stress, as evidenced by the dose-dependent increase or decrease in their contents.
Similar to anthocyanins, betalains can also be used as free radical scavengers to eliminate ROS, either directly or indirectly, and can maintain homeostasis as osmotic substances that regulate osmotic pressure in cells to help plants adapt to harsh external environments (Li et al., 2019). Sepúlveda-Jiménez et al., (2004) reported a seven-fold increase in betacyanin content in wounded beet leaves compared to untreated beet leaves. Photoprotection and an efficient ROS scavenging potential have been demonstrated in recent studies on betalains using the example of the succulents D. australe (Jain et al., 2015) and Portulacca oleraceae (Sdouga et al., 2019) as well as the halophyte Salicornia fruticosa under salt stress (Duarte et al., 2013). Those authors confirmed a decrease in chlorophyll content and a four-fold increase in betalain concentrations in S. fruticosa and a prompt increase in betalain concentrations in P. oleraceae (Duarte et al., 2013; Sdouga et al., 2019). Increasing doses of UV-B radiation have been shown to cause a dose-dependent increase in total betalain content in sugar beet leaves compared to control plants (Rahimzadeh Karvansara and Razavi, 2019). Under our experimental conditions, exogenous melatonin application led to a dose-dependent and cultivation-specific increase in betanin, neobetanin and isobetanin contents, especially in red beets. It may be hypothesised that melatonin administration in beet cultures under salinity conditions increases betalain content in a cultivation- or betalain-specific manner, contributing to osmotic adjustments and a ROS scavenging system. Our results are consistent with the findings of these previous studies, which together show that betalains play a strong and crucial role in improving beet salt tolerance.
Recent studies have shown that exogenous melatonin administration also plays a positive role in the accumulation of secondary metabolites such as alkaloids, phenolic acids, flavonoids/anthocyanins and lignans in plants under various environmental stresses (Yin et al., 2022). This is related to increasing the antioxidant capacity of plants and protecting them from the adverse effects of various types of abiotic stresses (Yin et al., 2022; Colak et al., 2022; Esmaeili et al., 2023; Kurt-Celebi et al., 2023). In addition, numerous studies have shown that phenolic acids e.g. hydroxycinnamic acid derivatives (HCAs) accumulate in barley (Ma et al., 2019; Wang et al., 2020; Yin et al., 2022) and Brassica cultivars (Linić et al., 2019) grown under salt stress and in wheat under ionising radiation (Colak et al., 2021; Kurt-Celebi et al., 2023). In the present study, HCAs like FeA, SiA, m- and o-CoA, accumulated in significant amounts in the investigated beet cultures, mainly in the conjugated forms (SEC and SGC) and especially in the ester form (SEC), followed by CWB and SGC. These results are consistent with recently published data for wheat varieties (Colak et al., 2021; Kurt-Celebi et al., 2023). Yin et al., (2022) reported that Ca2+ plays an important role in intercellular signal transduction in barley seedlings in response to salt stress and phenolic acid biosynthesis promoted by melatonin. In the current study, melatonin application in conjunction with salt stress significantly enhanced the salt stress-induced decrease in Ca2+ influx and increased the level of conjugated HCAs. Consistent with the results of Yin et al., (2022) and Kurt-Celebi et al., (2023), phenolic acids in the four forms can be increased by enhancing the activity and relative expression of key enzymes in the phenylpropanoid metabolism in beets exposed to melatonin application plus salt stress treatment. It may be noted that exogenous melatonin further enhances the ROS scavenging activity in beet seedlings under salt stress, interacting with a series of inter-related antioxidant enzyme activities (by SOD, CAT, POX, GR, etc.) and the content of non-enzymatic antioxidants (e.g., phenolic acids, betalains and GSH) as efficient H2O2 scavengers. It may also be suggested that leaves enriched with exogenous melatonin or supported to some extent by this, maintain a balance between the generation and destruction of ROS e.g., H2O2, O2− and MDA. Notably, salt stress-induced activities of POX, CAT, SOD and GR in the tested beets, and particularly in the white beets, decreased when salt stress was combined with melatonin. Under our experimental conditions, the activities of the antioxidant enzymes in the red beets were significantly lower than in the white beets. From that perspective, a non-enzymatic antioxidant compound pool including betalains, phenolic acids and GSH, enhanced by salt stress or salt stress + melatonin treatment can contribute an additional ROS scavenging system in red beets compared to white beets. In agreement with this suggestion, previous studies by Wang et al., (2017) and Zhang et al., (2021) confirmed the presence of salt stress-induced increases in the activities of the antioxidant enzymes ASA-GSH and non-enzymatic antioxidants like betaine, proline and total phenolics/flavonoids/anthocyanins in white sugar beets. Zhang et al., (2021) also confirmed that exogenous melatonin application further enhanced antioxidant enzyme activities and the antioxidant compounds' contents. However, in the present beet cultivars, we observed regular cultivar-specific and dose-dependent decreases in the activities of POX, CAT, SOD and GR, while the activities of MDHAR and DHAR were enhanced by salt stress alone and in combination with melatonin application, and also to some extent in GSH content.
Based on the above findings, it may be suggested that the exogenous melatonin-mediated enhancement of ROS scavenging in salt stress-exposed red and white sugars beets promotes a set of inter-linked antioxidant enzyme activations and antioxidant compounds content production in a cultivar-specific or salt stress dose-dependent manner. During exogenous melatonin application, all these activations/productions of the antioxidants in the beets described above can further be enhanced by endogenous melatonin biosynthesis in the same manner. This may probably result in a slight further enhancement of the existing ASA-GSH enzyme activities and non-enzymatic antioxidant pool, thus making exogenous melatonin application more effective in terms of ROS scavenging. All these findings indicate that exogenous melatonin application may regulate efficient ROS scavenging capacity in the beets, not only by using salt stress and salt stress + melatonin-mediated enhanced antioxidant enzyme activities, but also by contributing to the enzyme activations through the support of the existing non-enzymatic antioxidant pool.
5 CONCLUSIONS
In general, the growth of sugar beet seedlings was significantly inhibited by salt stress treatment in a dose-dependent and cultivation-specific manner in red and white sugar beets. This study investigated the alleviation of salt stress by exogenous melatonin application in beet seedlings in terms of biomass, ions like Na+, K+, Ca2+, antioxidant enzymes and compounds (GSH, betanins, neobetanins and isobetanins, and phenolic acids). The results suggest that exogenous melatonin administration and melatonin-enhanced salt tolerance are due to three factors: osmotic adjustment, ionic balance and the attenuation of salt-induced oxidative stress by betalains and phenolic acids. The results of the current study suggest that exogenous melatonin administration has great potential to improve salt tolerance in crops and that further studies are needed to confirm its performance in the future.
AUTHOR CONTRIBUTIONS
F.A.A. and N. C. developed the concept. The methodology was developed by F.A.A., N.C., H.T., and A.K.-C. Validation was performed by F.A.A., A.K.-C., N.C., A.S., A.M., G.D., J.D., and T.E. The formal analysis was conducted by F.A.A., N.C., H.T., A.S., A.M., G.D., J.D., and T.E. The investigation was led by F.A.A. and N.C., while resources were provided by F.A.A., N.C., H.T., A.S., G.D., and T.E. Data curation was handled by F.A.A., N.C., H.T., A.S., G.D., and T.E. The original draft preparation was completed by F.A.A., N.C., H.T., A.S., G.D., and T.E., and visualization was carried out by F.A.A., N.C., and A.S. T.E., A.S., F.A.A. and N.C. reviewed and edited the manuscript. Supervision was overseen by F.A.A., with project administration managed by the Scientific and Technological Research Council of Turkey (TUBITAK).
ACKNOWLEDGEMENTS
This study was funded by the Scientific and Technological Research Council of Turkey (TUBITAK) ARDEB 1001 Grant No 119Z085. This work is partly a part of the program Horticulture No. P4-0013-0481, funded by the Slovenian Research Agency (ARRS).
FUNDING INFORMATION
This project was funded by the Scientific and Technological Research Council of Turkey (TUBITAK) ARDEB 1001 (Grant No 119Z085) to the corresponding author.
CONFLICT OF INTEREST STATEMENT
On behalf of all authors, the corresponding author states that there is no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as all new created data is already contained within this article.




