A QTL on chromosome 17 identified by Genome-Wide Association Mapping controls postharvest cold tolerance of Cucurbita pepo L.
Abstract
The worldwide cultivated Cucurbita pepo L. is one of the most diverse species in the plant kingdom. In this study, chilling tolerance over a wide range of cultivars was characterized to discover the allelic variants to improving the postharvest quality of the immature fruit during cold storage. For this purpose, fruits from 126 accessions of worldwide origin have been evaluated for weight loss and chilling injury after 3, 7 and 14 days of cold storage, classifying them into tolerant, partially tolerant, and sensitive accessions. To verify this classification, antioxidant capacity and lipid peroxidation (MDA) of contrasting accessions (tolerant vs. sensitive) were assessed. The antioxidant capacity significantly decreased during cold storage in the sensitive accessions, while it was maintained in tolerant accessions. Additionally, the sensitive accessions presented a higher accumulation of MDA during this period. Finally, a GWAS analysis using GBS data available in CuGenDBv2, combined with weight loss percentage data, led to the identification of a candidate QTL located on chromosome 17 that regulates postharvest cold tolerance in zucchini. The region contains four SNPs whose alternative alleles were significantly associated with lower weight loss percentage and chilling injury indices during cold storage. Two SNPs are located in the 3’ UTR region of the gene CpERS1, a gene involved in ethylene perception. The other two SNPs generate missense mutations in the coding region of a Pectin methyl esterase inhibitor gene (CpPMI). The role of this QTL and these variants in chilling tolerance is discussed.
1 INTRODUCTION
Among the Cucurbitaceae family, zucchini (Cucurbita pepo L.) is a non-climacteric fruit, harvested immature, and represents one of the most consumed commodities worldwide. Cold storage is one of the preferred methods for conserving and transporting fruits after harvesting. However, due to its subtropical origin, preservation at low temperatures can trigger a syndrome known as chilling injury (CI), characterized by pitting on the fruit's exocarp, which results in loss of cellular integrity due to membrane damage. In addition, this syndrome is accompanied by significant dehydration, leading to fruit deterioration (Carvajal et al., 2011; Megías et al., 2016). These factors contribute significantly to a decline in fruit quality and, consequently, to increased food loss and waste. Chilling injury affects not only zucchini but also other crops such as tomato, cucumber, and melon, which are sensitive to cold storage, thereby limiting their postharvest life (Zhang et al., 2021).
In previous research, a wide range of approaches have been followed in zucchini fruit to enhance defense against cold stress. These include physical treatments such as preconditioning (Carvajal et al., 2015b), individual shrink-wrapping (Megías et al., 2015), UV-B irradiation (Tossi et al., 2024), and application of polysaccharide-based edible coatings (Castro-Cegrí et al., 2023b). Additionally, exogenous application of the ethylene inhibitor 1-MCP (Megías et al., 2016), polyamines (Palma et al., 2015), gamma-aminobutyric acid (Palma et al., 2019), nitric oxide (Jiménez-Muñoz et al., 2021) or ABA (Carvajal et al., 2017) have shown a positive effect on fruit shelf life in this species. Although the fruits of other species, such as tomato or melon, are consumed at a mature stage, CI remains a common consequence of cold storage during postharvest, regardless of the degree of maturation. Common methodologies to alleviate CI in crops like cucumber or pepper include the application of methyl jasmonate or nitric oxide (Ma et al., 2020; Liu et al., 2016), or of melatonin in tomato, pepper or cucumber (Aghdam et al., 2019; Kong et al., 2020; Liu et al., 2022), among others. These treatments have successfully alleviated symptoms of cold stress, providing insight into the metabolic pathways contributing to enhanced cold tolerance.
The appearance of CI during cold storage is correlated with increased membrane lipid peroxidation and ROS accumulation, along with elevated respiration rate and ethylene production (Carvajal et al., 2015b; Megías et al., 2016; Palma et al., 2015). Conversely, metabolites such as soluble sugars (Castro-Cegrí et al., 2023a; Palma et al., 2014b), antioxidative enzymes (Carvajal et al., 2011), phenolic compounds (Castro-Cegrí et al., 2023c), polyamines, proline, ɣ-amino butyric acid (GABA) (Palma et al., 2014a), and abscisic acid (ABA) (Carvajal et al., 2017) have been related to chilling tolerance. Additionally, fruits exhibiting lighter CI symptoms under different treatments also develop better antioxidant capacity via enzymatic (Carvajal et al., 2015b) or non-enzymatic antioxidant systems (Castro-Cegrí et al., 2023c). In addition, transcriptomic analysis in ABA-treated fruit has revealed the complex transcriptional regulation underlying zucchini postharvest cold tolerance. This regulation is mediated by several specific pathways related to phytohormones, Ca2+ and MAPK signaling components, and several transcription factors, as well as cell wall degrading enzymes (Benítez et al., 2022). While this research has provided valuable insights into the genetic mechanisms underlying the tolerance to cold stress, further investigation is needed to gain a better understanding of the genes responsible for cold tolerance.
Previous studies have demonstrated that different cultivars display diverse responses to fruit cold preservation (Megías et al., 2016, 2017), highlighting the cultivar-dependent nature of this trait. The USDA germplasm collection of C. pepo contains a wide range of genetic diversity, as demonstrated by genotyping-by-sequencing and other genomic analyses (Hernandez et al., 2023; Yu et al., 2023). The current research aimed to assess postharvest quality under cold storage in 126 zucchini accessions conserved in germplasm banks, searching for genetic factors involved in cold tolerance. Genome-Wide Association Studies (GWAS) were conducted to identify the genomic regions associated with this trait using the GBS data available at CuGenDBv2.
2 MATERIALS AND METHODS
2.1 Plant material
A total of 126 accessions of C. pepo were evaluated for postharvest quality under cold storage. These accessions were provided by the Germplasm Bank of the United States Department of Agriculture (National Plant Germplasm System, NPGS; https://npgsweb.ars-grin.gov/gringlobal/search) and propagated and preserved in the germplasm bank of the University of Almería (BSUAL). Geographically, these accessions are distributed worldwide, coming from different countries of origin (Table S1; Figure S1). Additionally, 10 pre-commercial and inbred lines of C. pepo were also included. The accessions were cultivated during the autumn season of 2022 under standard greenhouse conditions in Almería, Spain.
Twelve plants were cultivated per accession, and fruits from each plant were harvested daily and stored in permanent darkness at 4°C with 85–90% relative humidity. Fruits were harvested at the commercial stage (weighing between 250 and 350 g) and without deformations.
2.2 Postharvest quality evaluation
Wi is the initial weight of the fruit (T0), and Wf is the final weight of the fruit (T7 or T14).
Chilling Injury (CI) in fruits from each accession was assessed using a subjective scale of visual symptoms described by Martínez-Téllez et al. (2002). The fruits were classified as follows: 0, no damage; 1, 10% or less of the fruit surface affected; 2, 10–20% of the fruit surface affected; and 3, more than 20% of the fruit surface affected. CI index for each accession was determined by calculating the arithmetic mean of the CI scores for each analyzed fruit.
2.3 Biochemical analyses
For the biochemical analyses, three biological replicates were obtained from the exocarp of three uniform fruits each. Samples were collected from freshly harvested fruit (T0) and after 3 (T3) and 14 (T14) days of cold storage. The exocarp was completely removed, frozen in liquid nitrogen, lyophilized and powdered, and stored at room temperature in permanent darkness.
2.3.1 Lipid peroxidation
Lipid peroxidation determined as malondialdehyde (MDA) content was measured by TBARS procedure described by Heath and Packer (1968), with some modifications. 20 mg of lyophilized exocarp material were homogenized in 1.5 mL of 20% trichloroacetic acid (TCA) and 0.2 mL of 4% butylated hydroxytoluene (BHT) for extraction. The homogenate was centrifuged at 10,000 × g for 15 minutes and 4°C. 0.75 mL of 0.5% 2-thiobarbituric acid (TBA) was added to 0.25 mL of supernatant. The mixture was heated at 95°C in a water bath for 30 minutes, immediately cooled on ice to stop the reaction, and then centrifuged at 4°C and 10,000 × g for 10 minutes. The absorbance of the supernatant was measured at 532 and 600 nm. The TBA-reactive compounds were calculated by subtracting the non-specific absorption at 600 nm from the absorption at 532 nm. Data were calculated according to a calibration curve to obtain μg of MDA per Kg of dry weight. Results were expressed as the % of increment of T3 and T14 against T0.
2.3.2 Antioxidant Capacity assessment: FRAP
The Ferric Reducing Antioxidant Power (FRAP) assay was conducted according to Benzie and Strain (1996), with some modifications. 10 mg of lyophilized exocarp material was extracted in 4 mL of 80% acetone. The extracts were shaken for 2 hours at 4°C and then centrifuged at 5,000 × g for 15 minutes at 4°C. 0.1 mL of supernatant was allowed to react with 0.9 mL of FRAP solution [0.3 M acetate buffer pH 3.6, 0.01 M TPTZ (2,4,6-tripyridyl-s-triazine) in 0.04 M HCl, and 0.02 M FeCl3 6H2O (10:1:1, v/v/v)] for 30 minutes in darkness at 37°C. Subsequently, readings of the colored product were taken at 595 nm. Data were calculated using a standard FeSO4 curve and expressed in mg kg−1 DW.
2.4 Genome-Wide Association (GWAS) mapping approach
The genome sequence and the GBS SNPs of 828 C. pepo cultivars are available at the Cucurbit Genomics Database v2 (CuGenDBv2) (Yu et al., 2023). A diversity panel of 126 accessions of C. pepo was used for GWAS. The collection was phenotyped for postharvest quality, having robust phenotyping data in 99 accessions. These 99 accessions were subjected to GWAS analysis by using a total of 47,544 biallelic SNPs.
The analysis was performed with TASSEL software (Bradbury et al., 2007), using the linear mixed model (MLM), which considers both population structure (PCA) and relatedness (kinship matrix), and the generalized linear model (GLM), which only considers the population structure in the association analysis. Genome-wide significance thresholds of GWAS were determined using the Bonferroni correction at p-value = 0.05 (False discovery rate FDR 5%) and p-value = 0.01 (FDR 1%) for significant and extremely significant associations, respectively, as described in Li et al. (2012). Linkage disequilibrium (LD) was calculated as r2 values between pairwise SNPs using TASSEL with a significant level of 0.1 and represented using R studio software.
Principal component analysis (PCA) and Quantile-quantile (Q-Q) plots, where distributions of P-values expected the null hypothesis distribution, were performed by using TASSEL software (V 5.2.92) (Bradbury et al., 2007).
2.5 Transcriptomic analysis
RNA-seq of samples from different plant organs of the inbred line MU-CU-16, generated by the research group BIO293 at the University of Almería, were used for expression analysis of selected genes: CpERS1, CpPMEI7, CpDI19, CpMYB. Transcriptomic profiling was assessed in dry, soaked, and germinated seeds, roots from seedlings 17–21 days after germination, meristems, leaves of 10 mm, corolla of male and female flowers with a length of 30 mm, ovaries, and commercial zucchini fruit. For each sample, three biological replicates have been taken from at least three plants per replicate. For RNA isolation, the Omega Biotek EZNA® plant RNA kit (R6827-01) was used following the manufacturer's protocol. The RNA was then eluted in nuclease-free water and immediately prepared for sequencing. Samples were sequenced by BGI Genomics using the DNBseq platform, generating 150 bp paired-end reads and 6 Gb of raw data per sample. Fragments per kilobase of transcript per million mapped reads (FPKM) values were obtained for the analysis of results using the BALLGOWN package in R (Frazee et al., 2015). Log2(FPKM+1) data were used to create heatmaps with TBtools.
3 RESULTS
3.1 Analysis of postharvest cold tolerance-related traits
By using a diversity panel with 126 accessions of C. pepo from the USDA-GRIN germplasm bank (Table S1), the cold tolerance of immature commercial zucchini fruit was assessed. The accessions within the panel were selected based on the morphotype; all of them were elongated fruits of Zucchini, Cocozelle, and Vegetable Marrow types. Apart from these 126 accessions, 10 additional lines, including pre-commercial lines and hybrids, were included for evaluation and phenotypic analysis. Since external chilling injury (CI) and weight loss (WL) are the two most important symptoms of cold damage (Carvajal et al., 2011; Megías et al., 2014), %WL was measured at 7 and 14 days and CI Index at 3, 7 and 14 days of cold storage at 4°C (Table S2). The evaluation was performed on most of the accessions, excluding those with predominantly male flowers, which did not produce enough fruits for postharvest analysis.
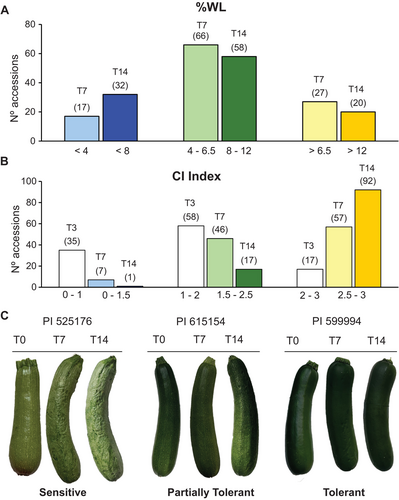
Significant variability in %WL was observed among harvested fruits in the studied population, leading to their classification as tolerant, partially tolerant, and sensitive based on their %WL after 14 days of cold storage. Specifically, 32 accessions exhibited relatively low %WL, ranging from 0 to 8% after 14 days, and were considered tolerant. The distribution indicates that approximately 50% of the lines evaluated were partially tolerant, with 58 cultivars showing a %WL between 8 and 12% after 14 days. Conversely, 20 cultivars exhibited higher values, ranging from 12 to 16.5, and were classified as sensitive (Figure 1A; Table S2). The CI index was also determined for harvested fruits after 3, 7, and 14 days of storage at 4°C. As expected, the CI index increased across all the accessions over the period (Figure 1B; Table S2). After 3 days at 4°C, 58 accessions exhibited low CI index (1–2), while 17 accessions showed severe exocarp damage (2–3). Just 4 days later (T7), the number of tolerant accessions was reduced to 7 (0–1.5), while the number of sensitive accessions increased to 57 (2.5–3). By the end of the experiment (T14), only 1 accession presented a CI index below 1.5, and 92 accessions had CI Index values between 2.5 and 3, demonstrating the high sensitivity of zucchini fruit in the appearance of pitting in the exocarp. Figure 1C shows fruits from PI 525176 accession classified as sensitive, PI 615154 as partially tolerant, and PI 599994 classified as tolerant stored for 0, 7, and 14 days at 4°C, noticing the differences in chilling injury. To assess the correlation between the two parameters, %WL and CI Index, Pearson correlation analysis was conducted. The analysis revealed correlations of r = 0.57 and r = 0.53 between %WL and the CI Index after 7 and 14 days of cold storage, respectively (p < 0.01), indicating a moderate correlation.
To validate the tolerant and sensitive phenotypes observed in relation to differences in %WL at T14 and considering that antioxidant defense is crucial to avoid decay, antioxidant capacity and lipid peroxidation were measured in both groups. Varieties that showed %WL <8 were tolerant to cold stress (in blue), and varieties with %WL over 12 at T14 showed high sensitivity to cold (in yellow) (Figure 2; Table S2). Considering the high variability of the population, the biochemical parameters were measured in contrasting accessions in freshly harvested fruit (T0) and after 3 (T3) and 14 days (T14) of cold storage (Table S3). The % of variation for each time point in every accession was compared to its initial value (T0). These data provide valuable insights into the effect of cold storage on the antioxidant capacity and lipid peroxidation of each genotype (Figure 2). Figure 2A shows that most of the accessions classified as tolerant exhibit minimal changes in antioxidant capacity over the storage period (T14), while sensitive accessions show predominantly lower values, indicating a decline in antioxidant capacity. Figure 2A further emphasizes this observation by comparing the % of increment in sensitive and tolerant groups. Considering the % of increment average, the tolerant group maintains antioxidant capacity levels similar to T0, 3 for T3 and 0.3% for T14, whereas the sensitive group shows a substantial decrease in antioxidant capacity, with an average % of decrements of −15.6% and − 13.6% for T3 and T14, respectively. Significant differences were found between tolerant and sensitive groups after 3 days of cold storage, whereas no significant differences were found after 14 days due to the high variability found in the sensitive group. This substantial reduction in antioxidant capacity underscores the vulnerability of sensitive accessions to oxidative stress induced by cold storage conditions.
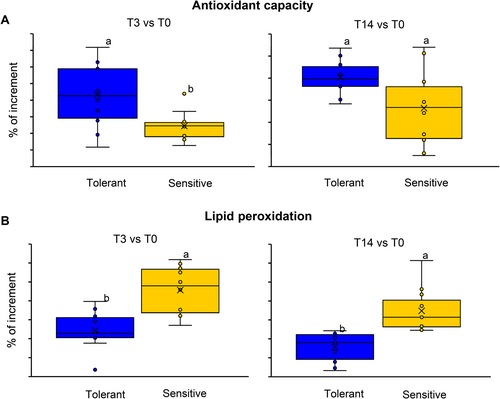
As antioxidant capacity, lipid peroxidation is a physiological marker of fruit decay. The % of MDA increment compared to T0 shows a notable variation for all evaluated accessions (Figure 2B). However, this increase is more pronounced in sensitive accessions than in tolerant ones (Figure 2B). On average, after 3 days of cold storage, the sensitive group exhibited an increase in lipid peroxidation of 51.4%, while the MDA content was incremented by only 8.8% in the tolerant accessions (Figure 2B). After 14 days of cold storage, MDA increased by 77.9% in the tolerant accessions, while it increased by 173.8% in sensitive ones. Data show that sensitive accessions are more susceptible to lipid damage during cold storage, further highlighting their increased sensitivity to cold stress-induced deterioration.
3.2 Identification of QTL and allelic variants associated with %WL
GWAS is a valuable tool in discerning the genetic basis of variation for agronomic traits. As a result, this methodology allows the identification of single nucleotide variants (SNPs) associated with the trait under study. GWAS analysis was performed with %WL after 7 and 14 days of cold storage data and by using the variation approach after genotyping-by-sequencing (GBS) reported in the USDA for the C. pepo collection. Firstly, accessions with insufficient production of fruits, and therefore, no optimal data, were filtered out. Secondly, accessions showing high variability phenotype of the %WL in their fruits were also eliminated from the analysis. A panel of 99 PI accessions was finally selected (Table S1) for %WL evaluation. Biallelic positions were included in the analysis, removing SNPs with more than 80% missing data and minor allele frequency (MAF <0.05). A total of 47,544 GBS-SNPs with a density of SNPs per chromosome ranged between 161.9 and 225.7 SNPs per Mb, with an average of one SNP for each 5.1 Kb.
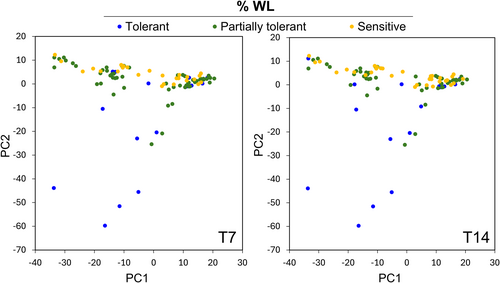
Principal component analysis (PCA) was used to determine population structure. PCA analysis was conducted to show the relationship between the genotypic data (99 accessions) and the parameters studied in the population (%WL after 7 and 14 days of cold storage). The same %WL intervals for tolerant, partially tolerant, or sensitive established in Figure 1A were used to represent PCA graphs. This analysis is performed to ensure any possible association between SNPs and the trait under study is not attributable to population structure. Figure 3 shows PCA plots with the superimposition over the first two PCs of the %WL at T7 and T14 and genotyping data across the 99 accessions used for GWAS analysis. The aleatory distribution of the phenotype indicated that there is no population structure and that the panel is suitable for the analysis.
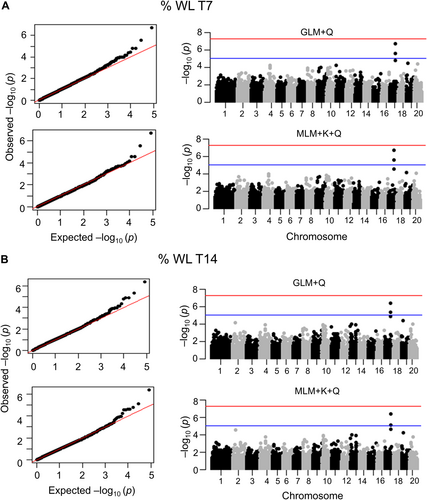
The selected panel, coupled with GLM + Q and MLM + Q + K statistical models, allowed the identification of a region in chromosome 17 significantly associated with %WL at T7 and T14. In the region, four SNPs at positions 6075118, 6075157, 6076443, and 6076445 overcome the threshold in the multidimensional-scaling (MDS) method (Figure 4) and principal components (PC) (Figure S2). QQ-plots for each analysis are also shown. The analysis of this putative QTL by Linkage disequilibrium decay analysis (p < 0.01), a region of 20,278 bp upstream and downstream from the detected GWAS signal, is expected to contain casual mutations and causal genes controlling the phenotype (Figure S3A). The genomic region identified contains four annotated genes. Among these, the Dehydration-induced 19-like protein (Cp4.1LG17g07350) and the L10-interacting MYB domain-containing protein (Cp4.1LG17g07390) genes are included in the previously described region but do not contain SNPs in their genomic sequences. Table 1 shows the variant location of those significant SNPs, with two affecting the 3’ UTR of the ethylene receptor CpERS1, and the other two altering the coding region of a pectin methyl esterase CpPMEI7. As expected for the close distance between each other, LD revealed that the 4 significant SNPs are cosegregating (Figure S3B). Transcriptomic profiling of different tissues of C. pepo revealed that CpERS1, CpPMEI7, CpDI19 and CpMYB are expressed in commercial fruits, likely having an effect on the postharvest quality. CpDI19 and CpMYB genes have a higher expression than CpERS1 and CpPMEI7 in fruits (Figure S4).
| SNP | Ref | Alt | Gene ID | Location | Annotation | Protein |
|---|---|---|---|---|---|---|
| S17_6075118 | T | C | Cp4.1LG17g07380 | 3’ UTR | Ethylene receptor | CpERS1 |
| S17_6075157 | T | C | Cp4.1LG17g07380 | 3’ UTR | Ethylene receptor | CpERS1 |
| S17_6076443 | C | T | Cp4.1LG17g07190 | Exon | Pectinesterase | CpPMEI7 |
| S17_6076445 | T | G | Cp4.1LG17g07190 | Exon | Pectinesterase | CpPMEI7 |
3.3 Allele association for CI index and %WL
CI index and %WL data were used to study the allele effect of the four significant identified SNPs in chromosome 17. Firstly, Figure 5A shows the classification of the different accessions according to the postharvest behavior as tolerant, partially tolerant, or sensitive based on the %WL at 7 days of cold storage, indicating the genotypes for each group. The percentage of accessions harboring both reference alleles (in yellow), heterozygous (in green), or homozygous alternative (in blue) for the four SNPs identified by GWAS was assessed, showing that the haplotype of about 50% of the tolerant accessions is homozygous for the alternative allele for each SNP identified. However, the haplotype of the region for accessions classified as sensitive showed that none of them harbor the alternative allele in homozygosity. Similar results were obtained for 14 days of cold storage (Figure S5A; Table S2).
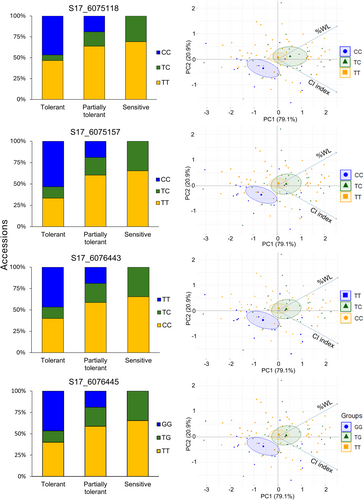
A multivariate data analysis was performed in Figure 5B showing the combination of CI index and %WL data at 7 days of cold storage and the association with each allele. The separation of the postharvest phenotype was mainly due to the %WL (79.1%). Thus, the accessions that produced cold-tolerant fruit were located in the downer left quadrant of the graph, clustering the accessions harboring the homozygous alternative allele for each significant SNP identified by GWAS (in blue). Heterozygous and homozygous for the reference allele plants were clustered in the central zone, indicating a major variation of the trait in these two groups, being significantly separated from the homozygous alternative. At 14 days of cold storage, the influence of WL was lower, 77.3%, due to the state of the fruit under a long term of storage (Figure S5B). Nevertheless, the homozygous alternative allele was also significantly clustered and separated from the heterozygous and homozygous reference alleles and showed a significant number of tolerant accessions influenced by the genotype.
4 DISCUSSION
4.1 Phenotypic characterization of cultivars for chilling tolerance
The phenotypic characterization of 126 accessions distributed worldwide has shown that the parameters weight loss, development of CI, antioxidant capacity and lipid peroxidation are implicated in postharvest tolerance. Although these parameters may or may not be correlated with each other, all of them are responsible for the tolerance to postharvest cold storage, suggesting that there are different physiological pathways implicated in the acquisition of tolerance (Carvajal et al., 2015b; Megías et al., 2017; Valenzuela et al., 2017). Figure 1 illustrates the high variability observed in the population for %WL, displaying a normal distribution with about 50% of the accessions classified as partially tolerant (8–12% %WL). Notably, 32 accessions exhibited a %WL of less than 8 after 14 days of exposition to chilling temperatures, making them potential candidates for being chilling tolerant genotypes. In contrast, most accessions (92) were more susceptible to pitting, showing a CI index of over 2.5. In a previous screening performed in fruit of 25 accessions of C. pepo, 20 showed higher %WL values and only 1 accession presented a CI under 2.5 at T14. These results point to a high variability concerning chilling tolerance parameters in C. pepo, and the same has been observed in other commercial hybrids analyzed (Carvajal et al., 2011; Megías et al., 2016), as well as in other cucurbit species such as cucumber (Hakim et al., 1999).
As observed for WL and CI, values for antioxidant capacity and lipid peroxidation (MDA) also oscillate among tolerant and sensitive cultivars. Increased production of reactive oxygen species (ROS) can accelerate zucchini fruit softening and CI when exposed to cold (Carvajal et al., 2011; Valenzuela et al., 2017), whereas the accumulation of antioxidant compounds can enhance the antioxidant defense mechanisms (Castro-Cegrí et al., 2023c). In fact, most of the tolerant accessions that had lower WL showed the highest values of antioxidant capacity (T0 vs. T3), and some of them presented lower values at the beginning (T0 vs. T3) followed by an increment to the end of the experiment (T0 vs. T14), as observed for PI_169465. Data suggest that the mechanisms to generate antioxidant activity are genotype-dependent. Likewise, MDA content, an indicator of stress, is lower in tolerant cultivars that show reduced WL. MDA, as a product of lipid peroxidation, leads to changes in membrane fluidity and stability and correlates with CI as described in peach (Song et al., 2022), cucumber (Chen & Yang, 2013) or zucchini (Carvajal et al., 2011; Megías et al., 2015). Our data clearly indicate that tolerant accessions presented a lower lipid peroxidation in the exocarp, thereby reducing cellular damage. Overall, data suggest that zucchini cold tolerance may be acquired through various mechanisms, which are cultivar-dependent.
4.2 A QTL on chromosome 17 controls postharvest quality in the C. pepo population
Cold tolerance is a complex trait that involves numerous genetic factors. The molecular mechanisms underlying cold stress tolerance during plant development have been previously studied in various species, including maize (Yi et al., 2020), wheat (Zhao et al., 2020), rice (Thapa et al., 2020), cucumber (Li et al., 2022) or pea (Beji et al., 2020). However, the genetic basis of postharvest cold tolerance remains poorly understood. This trait is of maximum interest for breeding C. pepo, whose fruit is harvested at an immature stage, being then highly susceptible to chilling injury. Although numerous studies have elucidated the physiological and biochemical changes occurring when the fruit is stored at low temperatures (Carvajal et al., 2015b; Megías et al., 2015, 2014; Palma et al., 2014a), no genomic studies have been conducted to date to detect genomic regions and allelic variants linked to this trait. GWAS approach has been useful in identifying QTL and genes associated with extended postharvest shelf life in other crops, such as cucumber (Nandi et al., 2024), tomato (Shah et al., 2024), blackberry (Chizk et al., 2023), lettuce (Sthapit Kandel et al., 2020) or Vitis vinifera L. (García-Abadillo et al., 2024).
The GWAS analysis for %WL at T7 and T14 led to the identification of a QTL on chromosome 17 controlling postharvest cold tolerance in the population. Within the QTL region, LD analysis indicates that four genes were included. Among the genes, Dehydration-induced 19-like protein (Cp4.1LG17g07350) and L10-interacting MYB domain-containing protein (Cp4.1LG17g07390) did not contain genomic variants; however, they are strongly expressed in the fruit (Figure S4) and hence they could play a role in the postharvest cold tolerance of zucchini fruit. Supporting the relevance of Cp4.1LG17g07350, the Dehydration-induced 19-like protein gene is involved in pepper cold stress (Kong et al., 2019). Interestingly, the region also contains 4 significant SNPs, two affecting the 3’ UTR extreme of CpERS1, a gene involved in the ethylene perception pathway, and the other two fall into the exonic region of one CpPMI gene, involved in cell wall remodeling. The four alternative SNPs markers identified were significantly associated with tolerant varieties whose fruits showed less WL during cold storage and less CI (Figure 5). Therefore, this analysis has provided a valuable source of genetic variants and candidate genes that can be incorporated into breeding programs across the globe.
Among the several factors previously identified to be involved in zucchini postharvest cold tolerance, the four SNPs affect genes associated with important processes: ethylene pathway and cell wall remodeling. Several studies have described the negative role of ethylene in zucchini fruit cold tolerance (Megías et al., 2014, 2016). In fact, treatments with the ethylene inhibitor 1-methylcyclopropene (1-MCP) reduced CI under cold storage in different varieties of C. pepo, along with a downregulation of the ethylene biosynthesis genes CpACO1 and CpACS1, and also the perception and signaling genes CpETR1, CpCTR1 and CpEIN3.1 (Megías et al., 2015, 2016). Additionally, the ethylene-insensitive mutant etr2b develops fruits with enhanced postharvest cold tolerance compared to wild-type fruits (García et al., 2020). Thus, cold tolerance in zucchini is associated with an inhibited ethylene response and reduced basal ethylene production, and the opposite happens in the cold-damaged fruit of more sensitive varieties (Megías et al., 2017). In concordance, our results point to a relevant role of the Ethylene response sensor 1 (CpERS1) gene in the population studied, modulating ethylene perception in response to cold, thereby influencing the postharvest behavior of the accessions. In other plant species, ethylene mediates the mechanisms of response to cold stress, including cold storage of fruits (Pareek et al., 2014; Pons et al., 2014). Abscisic acid (ABA) in zucchini has the opposite effect to ethylene. In fact, the transcriptomic analysis of ABA-treated fruits showed a reduction of ethylene pathway genes during cold storage. As ABA has a protective role, low ethylene levels in ABA-treated fruit could be associated with a delayed appearance of CI (Benítez et al., 2022; Carvajal et al., 2017). Transcriptomic in ABA-treated fruit also unmasked the role of other phytohormones during cold storage, suggesting a negative role for auxin and brassinosteroids and a positive role for jasmonates on postharvest quality (Benítez et al., 2022). The positive role of jasmonates in postharvest cold tolerance was also supported by the metabolomic analysis of ABA-treated fruits containing a higher accumulation of methyl jasmonate during cold storage (Castro-Cegrí et al., 2024). This treatment also revealed the induction of the cytokinin t-zeatin and riboflavin biosynthesis during the first day of cold storage (Castro-Cegrí et al., 2023a). Our findings suggest that postharvest cold tolerance is influenced by a complex crosstalk between phytohormones in which ethylene plays a pivotal role.
Two of the variants associated with WL in chromosome 17 result in aminoacidic substitutions of a Pectin methyl esterase inhibitor (CpPMI) protein. Pectin acts as the matrix of the microfibrils of cellulose crosslinked by hemicelluloses for the cell wall (Vicente et al., 2007), playing roles in cell adhesion and wall hydration, thereby influencing wall porosity in fruits (Carvajal et al., 2015a; Xue et al., 2020). Thus, pectin metabolism is related to CI and cell wall degradation (Carvajal et al., 2015a). One of the major enzymes influencing pectin solubilization is pectin methylesterase (PME), which converts methyl-esterified pectin into de-esterified pectin, the substrate of polygalacturonase (PG). In C. pepo, the enzymatic activity of both PME and PG increased during the cold storage period, being significantly reduced by the preconditioning treatment that alleviates CI symptoms (Carvajal et al., 2015a). Besides, pectin methylesterases in strawberry fruit are crucial for determining fruit firmness and softening (Xue et al., 2020). The two missense variants found on the CpPMEI of chromosome 17 could be responsible for the reduction of PME activity, explaining the lower WL and enhanced postharvest cold tolerance in the accessions possessing these variants.
5 CONCLUSION
The characterization of postharvest cold tolerance using the variability of C. pepo conserved in germplasm bank has led to the identification of a QTL on chromosome 17, along with the obtention of four genetic variants associated with the trait. These results advance our understanding of the genetic regulation underlying zucchini fruit cold tolerance but are also highly important for zucchini postharvest breeding programs, using these genetic variants as molecular markers to develop new varieties with enhanced cold tolerance.
AUTHOR CONTRIBUTIONS
M.J., C.M. and AG: design and coordination of the research; A.G: conducting most of the experiments and data analysis; A.C., A.L., M.S., F.P: collaborated in the experimental process and data analysis; M.J., C.M., and A.G: writing and revision. D.G. revised the manuscript. All authors contributed to the article and approved the submitted version.
ACKNOWLEDGEMENTS
This work was supported by grant PID2020-118080RB-C21 at UAL and PID2020-118080RB-C22 at UGR, funded by ‘Ministerio de Ciencia, Innovación y Universidades’ together with EU FEDER funds. AG received a Margarita Salas postdoctoral fellowship (RR_A_2022_05) of ‘Ministerio de Universidades’ funded by NextGenerationEU program.
FUNDING INFORMATION
This work was supported by grant PID2020-118080RB-C21 at UAL and PID2020-118080RB-C22 at UGR, funded by ‘Ministerio de Ciencia, Innovación y Universidades’ together with EU FEDER funds.
Open Research
DATA AVAILABILITY STATEMENT
All relevant data can be found within the manuscript and its supporting materials. The GBS data of C. pepo used in this study is available at CuGenDBv2 database (http://cucurbitgenomics.org/v2/).
The transcriptomic data were deposited in NCBI-SRA database (https://www.ncbi.nlm.nih.gov/sra/). Project number: PRJNA1019120.




