Metabolomic insights into the multiple stress responses of metabolites in major oilseed crops
Abstract
The multidimensional significance of metabolomics has gained increasing attention in oilseeds research and development. Sesame, peanut, soybean, sunflower, rapeseed, and perilla are the most important oilseed crops consumed as vegetable oils worldwide. However, multiple biotic and abiotic stressors affect metabolites essential for plant growth, development, and ecological adaptation, resulting in reduced productivity and quality. Stressors can result in dynamic changes in oilseed crops' overall performance, leading to changes in primary (ex: saccharides, lipids, organic acids, amino acids, vitamins, phytohormones, and nucleotides) and secondary (ex: flavonoids, alkaloids, phenolic acids, terpenoids, coumarins, and lignans) major metabolite classes. Those metabolites indicate plant physiological conditions and adaptation strategies to diverse biotic and abiotic stressors. Advancements in targeted and untargeted detection and quantification approaches and technologies aided metabolomics and crop improvement. This review seeks to clarify the metabolomics advancements, significant contributions of metabolites, and specific metabolites that accumulate in reaction to various stressors in oilseed crops. Considering the response of metabolites to multiple stress effects, we compiled comprehensive and combined metabolic biosynthesis pathways for six major classes. Understanding these essential metabolites and pathways can inform molecular breeding strategies to develop resilient oilseed cultivars. Hence, this review highlights metabolomics advancements and metabolites' potential roles in major oilseed crops' biotic and abiotic stress responses.
1 BACKGROUND
A wide range of environmental stresses, including high temperatures, cold, light, drought, waterlogging, and nutrient deficiencies, affect plant performance (Manna & Sinha, 2023). As with other crops, oilseed crops are seriously facing multiple stress effects, reducing the quality and quantity of the product. Soybean, sesame, peanut, perilla, rapeseed, and sunflower are the top oilseeds produced and consumed worldwide (Kefale et al., 2023; Viana et al., 2022; Peng et al., 2022), with soybean, rapeseed, and sunflower being the top 2nd, 3rd, and 4th vegetable oil sources globally, following palm fruit (FAO, 2023). However, climatic variability negatively impacts food supply systems by affecting crop production and productivity (Lakhanpaul et al., 2012). Several studies have shown that climate change exacerbates the frequency and severity of extreme weather events such as drought, cold, heat, and salinity, negatively impacting plants simultaneously or in succession (Patel et al., 2022). While soybean, peanut, sesame, perilla, and sunflower plants are frequently affected by drought, salinity, alkalinity, and heat stresses (Manna & Sinha, 2023; Patel et al., 2022; Xue et al., 2018), rapeseed is more influenced by salinity, cold, and waterlogging stress (Xin et al., 2019). Additionally, biotic factors such as diseases, insect pests, and weeds significantly affect the output, quality, and other components of oilseed crops (Silva et al., 2021; Chowdhury et al., 2017; Prats et al., 2006).
In plants, metabolites are involved in multidimensional physiological, ecological, and biochemical activities for normal growth, development, and reproduction. Plant-origin metabolites are also used as sources of nutraceutical, pharmacological, and cosmetic products that are helpful for human health. In crop improvement, metabolites can be used as identification markers to develop special-function (pigmentation, nutritional, cosmetic, medicinal, aroma, etc.) and stress (biotic and abiotic)-tolerant varieties. Therefore, scientific advances place metabolomics, studying an organism's metabolome, at the forefront of current research and development as a tool for improving crops (Ncube et al., 2022). All living forms on earth have a metabolome, a qualitative and quantitative compilation of all the small and low-molecular-weight compounds that serve as functional outputs of cellular reactions necessary for cell growth, repair, and proper biological functioning (Mishra et al., 2022). These compounds reflect the phenotypic parameters of organisms and their variations, which are tools for breeders to obtain elite genotypes for traits of interest. These changes either trigger crop survival or accelerate senescence and death. The metabolic changes in a crop in response to stressors are essential for designing crops resistant to specific or multiple stresses.
Oilseed crops contain relatively high levels of primary and secondary metabolites. Primary metabolites are essential biomolecules (lipids, amino acids, saccharides, vitamins, phytohormones, organic acids, and nucleotides), considered building blocks, playing a significant role in the cellular functions of plants, such as growth, development, and reproduction. In contrast, secondary metabolites are products of primary metabolism and serve as a defense and signaling response, resulting in a greater contribution to adaptive traits and ecological fitness (Ku et al., 2020); they include phenolic acids, flavonoids, lignans, coumarins, alkaloids, terpenoids, quinones, glucosinolates, and resveratrol (Hou et al., 2022; Li et al., 2022; Shen et al., 2021). The biosynthesis of plant secondary metabolites (PSMs) involves several precursors from primary metabolites, including acetyl-CoA, fatty acids, and amino acids. These precursor molecules are either start points or end products of the shikimate and tricarboxylic acid cycles. Although it is estimated that there are ~1 million metabolites in the plant metabolome, nearly 200,000 secondary metabolites have been identified, less than the total number of plant species (391,000) on earth (Divekar et al., 2022). These include the most important secondary metabolites known for their stress response and adaptation roles, such as terpenoids (>25,000), alkaloids (>21,000), and phenolics (~10000) (Razzaq et al., 2019).
In this review, we investigated specialized metabolites and their role in the plant response to biotic and abiotic stresses to maintain plant health. Although significant achievements have been made on a single crop or a combination of two crops against various stresses, comprehensive and comparative literature studies are limited. This review aims to define the specific metabolites and significant metabolic pathways in response to distinct stressors and improve the understanding of how these crops respond to biotic and abiotic stressors. Therefore, metabolites identified from the literature can serve as markers of plant physiological status and adaption methods. This review focuses on the stress response of metabolites in major oilseed crops.
2 ECONOMIC IMPORTANCE, DISTRIBUTION, AND GEOGRAPHIC SUITABILITY OF MAJOR OILSEED CROPS
Sesame, soybean, sunflower, peanut, perilla, and rapeseed are all key oilseed crops with great economic significance worldwide. Sesame (Sesamum indicum L.) seeds are primarily used for oil, food, cosmetics, and pharmaceuticals (Tripathy et al., 2019). Sesame oil is high in polyunsaturated fatty acids, tocopherols, polyphenols, and antioxidant components (lignans). Although it is originated in sub-Saharan Africa (Ethiopia), India and China have grown sesame for over 3,500 years (Teklu et al., 2021). Today, it is grown in tropical and subtropical locations worldwide, with significant producers including India, China, Sudan, Myanmar, and Ethiopia (Kefale & Wang, 2022). Sesame is heat and drought-tolerant and grows well in arid and hot conditions. It needs a long growing season of 100 to 150 days and prefers well-drained sandy loam soils.
Soybean (Glycine max) is produced for oil and protein. The annual production of soybeans reached over 348.8 million tonnes worldwide (Figure 1) (FAOSTAT, 2024). and its oil makes up 30% of all edible oil produced (Zhao et al., 2020) and is used for food, biofuel, and industrial purposes. The phytochemical composition of soybean shows that seeds, tissues, leaves, and different parts of the crop are sources of flavonoids (mainly isoflavones), proteins (amino acids), alkaloids, terpenoids, and phenolic compounds like saponins. Today, soybeans are grown worldwide, with the US, Brazil, Argentina, and China being the top producers (Ku et al., 2022). Although it may grow in various soil types, it favors loamy, well-drained soils. Soybean needs a long growing season (100 to 150 days) and a modest amount of water.
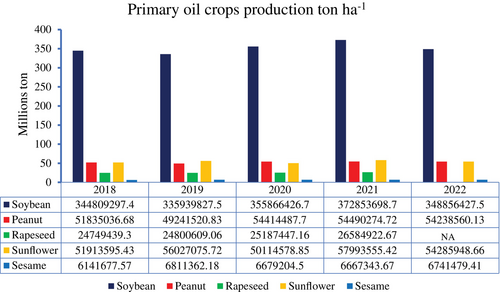
Data source: FAOSTAT, 2024. The “NA” in the table for rape seed means no data in 2022.
Peanuts (Arachis hypogaea) are used in various culinary products, such as peanut butter, chocolates, and snacks, and peanut oil is used in cooking (Toomer, 2018). Although originating from Brazil, peanuts are currently farmed all over the world, with China, India, the United States, Myanmar, and Nigeria being the main producers (El Idrissi et al., 2024). Peanut seeds and oils are rich in various phytochemical components like phytohormones, amino acids, vitamins, stilbenes (resveratrols), carotenoids, and fatty acids. The ideal growing conditions for peanuts are subtropical climates (warm, humid weather with evenly distributed rainfall) and well-drained sandy loam soils. It needs a lengthy growing season of 120 to 150 days.
Perilla (Perilla frutescens) seeds contain an edible oil with nutritional and medicinal benefits (Kaur et al., 2024). Perilla is most known for its leaves, which are used in Asian cuisine, and its seeds, which are used as a spice. Perilla oil contains omega-3 fatty acids, tocopherols, and phytosterols, and it is rich in phenolic and antioxidant compounds, making it significantly crucial in cosmetics and pharmaceuticals (Kim et al., 2012). It is native to East Asia, notably China, but is currently grown across Asia, including Japan, Korea, and Vietnam. Perilla enjoys well-drained, loamy soils and requires moderate watering (Shang et al., 2023). It thrives in fertile soils and humid, warm areas with temperatures ranging from 20 – 30°C.
The sunflower (Helianthus annuus L.) oil is the best source of polyunsaturated fatty acids (PUFAs) and vitamin E, mainly α-tocopherol (Guo et al., 2017). Sunflower oil has significantly contributed to the 61% rise in the world's production of edible vegetable oils over the past ten years. Due to the sunflower seed's high sulphuric amino acid content, it can be dehulled and utilized as cooking oil and eaten as a roasted or salted snack (Guo et al., 2017). Although they were first cultivated in the central United States of America, sunflowers are today planted worldwide, with Argentina and the United States being the leading producers (Dai et al., 2022). Loamy soils with good drainage are ideal for sunflower growth and need a modest amount of water. Sunflower favors extended growing seasons (120–150 days) and warm areas with temperatures between 20 and 30°C.
Rapeseed (Brassica napus) or oilseed rape produces an oil that is utilized in industrial applications, biodiesel, and cooking (Yin et al., 2019). Rapeseed is rich in fatty acids, phenolic acids, anthocyanins, and glucosinolates, which are helpful for cosmetic and pharmacological applications. Its consumption as a cooking oil was previously hampered due to the high erucic acid and glucosinolate content in the seed and its products (Hu et al., 2017), but the development of canola (low erucic and glucosinolates rape) lifted this restriction. Although first cultivated in Europe and Asia, rapeseed is currently farmed worldwide, with China, Canada, India, Germany, France, and Russia being the main producers (Viana et al., 2022). Rapeseed needs more rainfall and thrives in loamy, well-drained soils. It prefers more excellent areas with temperatures between 10 – 20°C and a long growing season of 100 to 150 days.
Due to these oilseed crops' adaptability to various ecological settings and diverse phytochemical compositions, they are widely grown worldwide to satisfy the rising demand for edible oils and other derived goods. Perillas and sunflowers have relatively similar ecological requirements, while sesame, soybean, and peanut can grow in tropical and subtropical areas with warm climatic conditions. Rapeseed also needs cooler weather conditions and higher rainfall. According to FAOSTAT (20204), among the six crops, soybean, sunflower, and peanut have been leading in total production, respectively, in the past five years (Figure 1).
3 ADVANCES IN METABOLOMICS RESEARCH IN OIL CROPS
Metabolomics combines multiomics and phenomics, representing the gene products and final phenotypic expression, respectively (Figure 2A). There has been ongoing and dynamic research progress in studying metabolites in various oil crops. Various high-throughput screening and metabolite profiling technologies have been recently introduced, improving the understanding of phytochemical composition and its potential health effects. In addition, studies have investigated the factors that influence metabolite composition, including genetic factors, environmental conditions, agricultural practices, and postharvest handling. It is crucial to understand how metabolite profiles change in response to abiotic and biotic stimuli with the help of metabolomics. Understanding the changes in metabolite profiles and underlying mechanisms can aid in developing crop varieties with increased metabolite content and resilience to multiple stresses (Ahmad et al., 2021).
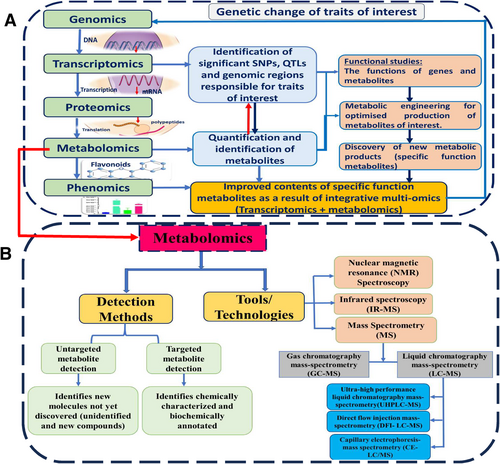
Various detection methods are used in metabolomics. Current molecular biology protocols highlight targeted and untargeted metabolomics as the two standard analysis methods (Roberts et al., 2012; Vinayavekhin & Saghatelian, 2010). Targeted metabolomics is a mass spectrometry-based analytical technique that detects defined groups of chemically and biochemically described metabolites. It allows for the confirmation and precise quantification of a selected subset of metabolites (such as those found in a specific metabolic pathway). This approach provides methodological optimization and enhanced resolution of biological processes of recognized importance and is particularly helpful for investigating known processes and reactions (Crandall et al., 2020). It focuses on quantifying discrete groupings of metabolites and offers the possibility of absolute quantification. Untargeted metabolomics analysis measures molecules not yet discovered (Vinayavekhin & Saghatelian, 2010). The primary aim of untargeted or discovery-based metabolomics is to globally detect and quantitatively assess small molecules within a sample. This approach facilitates hypothesis generation by enabling the comprehensive analysis of all metabolites present in the sample. Untargeted analysis serves as a discovery tool to identify novel chemicals and previously uncharacterized entities, enriching our understanding of various conditions, sample types, cells, tissues, organs, and organisms. For untargeted metabolite analysis, an integrated platform consisting of a reversed-phase ultrahigh-performance liquid chromatography (RP/UPLC) system coupled to tandem mass spectrometry (MS/MS) in positive ion mode electrospray ionization (ESI), (RP)/UPLC–MS/MS in negative ion mode ESI, and hydrophobic interaction liquid chromatography (HILC)/UPLC–MS/MS in negative ion mode ESI was developed for metabolomic profiling (Evans et al., 2009; Klevorn et al., 2019).
Various metabolomic approaches or tools have been successfully used to identify, quantify, and evaluate the metabolite profiles of oil seed crops (Table 1), including nuclear magnetic resonance (NMR) spectroscopy, infrared spectrometry, and mass spectrometry (Figure 2B). The mass spectrometry methods are also further grouped into gas mass spectrometry (GC–MS) (Peluffo et al., 2010; Koek et al., 2011) and liquid chromatography-mass spectrometry (LC–MS) (Qu et al., 2020; Chen et al., 2007). Stable isotope labeling, metabolome imaging (spatial metabolomics), single-cell analysis, cheminformatics, computational mass spectrometry (Tsugawa et al., 2021; Misra, 2016), capillary electrophoresis (CE-MS) (Fernie et al., 2011), and direct flow injection mass spectrometry (DFI-MS) (Razzaq et al., 2019) are among the other approaches used in metabolomics research to detect, identify, and quantify as many small molecules as possible.
| Crops | Trait considered | Plant parts studied | Number | Methods & tools used | Source |
|---|---|---|---|---|---|
|
Different tissues | leaves, seeds, flowers, and carpels | 776 | Targeted (UHPLC–MS/MS) | Dossou et al. (2021) |
| Seed coat color | Seeds | 671 | Targeted (UHPLC–MS/MS) | Dossou et al. (2022) | |
| Seed coat color | Seeds | 557 | LC–MS/MS | Wang et al. (2018) | |
| Lipid metabolism | Seeds | 243 | LC–MS/MS & GC–MS/MS | Lee et al. (2020) | |
| Drought stress tolerance | Not mentioned | 345 | UHPLC–MS & GC–MS/MS | You et al. (2019) | |
| Salinity stress response | Roots, leaves, and shoots | 282 | UHPLC–MS & GC–MS/MS | Zhang et al. (2019) | |
|
Geographic origin of seeds | Seeds | 365 | Untargeted (UHPLC–MS/MS) | Klevorn et al. (2019) |
| Salt stress | Root & shoot | 391 | GC–MS/MS | Cui et al. (2018) | |
| Seed coat color | Seed coat/testa | 160 | GC–MS/MS | Patel et al. (2022) | |
| Cold stress | Leaves | 563 | LC–MS/MS | Wang et al. (2021) | |
| Oil content | Seed | 547 | UHPLC–MS/MS | Liu et al. (2020) | |
| Roasting | Seeds | 738 | UHPLC–MS/MS | Zhang et al. (2023) | |
|
Alkali/salt stress | Seeds | 404 | UHPLC–MS/MS | Lu et al. (2021) |
| Lipid metabolism | Seeds | 687 | UPLC-MS/MS | Chernova et al. (2021) | |
| Disease resistance | Leaves | 169 | UPLC-MS/MS | Peng et al. (2022) | |
| Disease resistance | Flower head | 63 | GC–MS/MS | Peluffo et al. (2010) | |
|
Low-nitrogen stress response | Root & leaf | 108 | GC–MS/MS | Li et al. (2018) |
| Salt stress | Leaf (leaves) | 98 | GC–MS/MS & LC − FT/MS | Lu et al. (2013) | |
| Nutrient deficiency | Roots | 531 | Targeted (HPLC-MS/MS) | Mo et al. (2019) | |
| Salt stress(alkali) | Leaves | 68 | GC–MS/MS | Zhang et al. (2016) | |
| Nutritional/taste quality | Seeds and sprouts | 452 | UPLC & GC–MS/MS | Gu et al. (2017) | |
| Nutritional and fungal stress | Seeds and sprouts | 700 | UPLC-MS/MS | Haruya et al. (2021) | |
|
Cold stress | Leaves | 559 | UPLC-MS/MS | Jian et al. (2020) |
| Different tissues | Stems, leaf, roots, flower, & seeds | 152 | UPLC-MS/MS | Yang et al. (2019) | |
| Nitrogen deficiency | Leaves and roots | 574 | UPLC-HESI-MS/MS | Shen et al. (2022) | |
| Seed coat color | Seeds | 248 | UPLC-HESI-MS/MS | Qu et al. (2020) | |
| Seed development | Seeds | 443 | GC–MS | Tan et al. (2015) | |
| Seed coat | Silique wall | 641 | GC–MS | Tan et al. (2015) | |
| Light effect | Siliques of rapeseed | 249 | UPLC-HESI-MS/MS | Kamal et al. (2022) | |
|
Antioxidant properties | Leaf | 26 | UPLC-ESI-MS/MS | Hee et al. (2017) |
| Geographic origin of seeds | Seeds | 63 | GC–MS/MS | Kim et al. (2020) | |
| Leaf color | Leaf | 71 | UPLC-TQ-MS/MS | Zheng et al. (2020) | |
| Time of harvesting | Leaf | 118 | LC–MS/MS & GC–MS/MS | Chen et al. (2022) |
Although NMR, DFI-MS, and IR-spectroscopy tools have high throughput and minimum sample size and are preferred for better molecular fingerprinting, the combined tools GC–MS, LC–MS, and CE-MS have the ability to quantify and identify diverse chemical compounds/metabolites (Koek et al., 2011). A highly effective platform of integrated, nontargeted ultrahigh-performance liquid chromatography-electrospray ionization–tandem mass spectrometry (UHPLC-ESI-MS/MS) for the identification and relative quantification of small molecules in biological systems was developed (Evans et al., 2009). This analytical technique involves chemical analysis, process identification and relative quantification, data reduction, and quality assurance components to address the difficulties associated with metabolomics analysis, including identifying and eliminating experimental artifacts. The development of high-resolution mass spectrometry imaging has provided unparalleled information and insight into the spatial distribution of metabolites, enabling a better understanding of physiological processes in biological samples (Hansen & Jin Lee, 2017). The reliability, precision, speed, selectivity, and accuracy of the selected analytical tool are prerequisites for correct biological data interpretation and can affect the selection of a metabolomics approach (Razzaq et al., 2019; Koek et al., 2011).
The literature reveals that diverse analytical tools have been used to identify, quantify, and evaluate metabolites in different oil crops under certain conditions (Table 1). The collected data show how various studied plant parts, analytical tools, and crop types influence the diversity of metabolites in a biological sample. Multiple techniques and tools are often used in combination to gain a comprehensive understanding of the metabolome. The nature of the metabolites of interest, the nature of the sample, and the study objectives determine the choice of analytical method. Advances in technology and methodology continue to refine and expand metabolomics approaches. Due to its higher chromatographic resolving power, the UHPLC approach detects approximately 100% more compounds than the HPLC method, and the complete list of compounds detected by the UHPLC method permits baseline separation of compounds that were previously unresolved by conventional HPLC (Evans et al., 2009). Therefore, UHPLC–MS/MS, commonly used in metabolomics analysis of oil crops, could improve metabolite detection, identification, and quantification. Recent publications have shown the increased demand for and application of widely targeted ultrahigh-performance liquid chromatography-mass spectrometry (UHPLC–MS/MS) (Cao et al., 2023). Moreover, UHPLC/GC–MS/MS-based identification and quantification methods boosted metabolomics.
4 METABOLITES IN OILSEED CROPS IMPROVEMENT
Metabolomics is more practical than other omics technologies because changes in the composition and concentration of metabolites promptly reflect differences in plants, plant parts, tissues, and other traits (Li et al., 2023). This enables breeders to develop new varieties resistant to biotic and abiotic stresses and varieties with unique traits (high nutritional, quality, flavor, aroma, color, pharmacological, and cosmetic advantages) (Figure 3). We focused on the contributions of metabolites to abiotic and biotic stress effects in major oilseed crops. Various pathways could be modulated to synthesize specific or multiple stress-induced metabolites, including the pentose phosphate, shikimate, phenylpropanoid, and MEP/MVA pathways. Based on various research outputs, we developed a descriptive figure that shows an overview of the classification and contribution of primary and secondary metabolites in major oil crops (Figure 3). The details of the metabolite contributions reported in metabolomic studies of oilseed crops are presented in the following subsections.
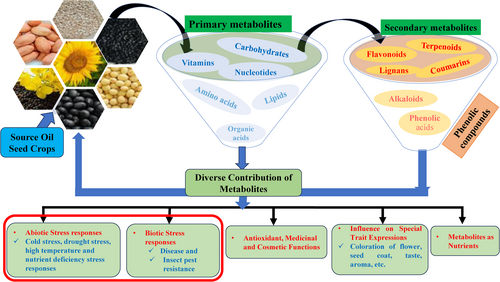
4.1 Metabolites Involved in the Biotic Stress Response
Naturally synthesized plant metabolites contribute to disease and insect pest resistance (Patel et al., 2022). Plant secondary metabolites (PSMs) function as defense mechanisms and signals, protecting plants from herbivorous organisms. When exposed to biotic factors, plants are forced to synthesize protective or defensive compounds specific to the host. For example, injury caused by herbivores triggers intricate processes that eventually result in the synthesis and accumulation of PSMs (Divekar et al., 2022). Numerous PSMs, such as phytoalexins, are produced by plants for defense against fungal infections and can partially improve plant health (Haruya et al., 2021). Additionally, microorganisms that live in a plant's endosphere but do not cause disease symptoms have been shown to create host (plant) metabolites such as taxol, vinblastine, vincristine, hypericin, and camptothecin by influencing various responses (enhanced availability of nutrients and tolerance to multiple stresses) and modulating the host plant's metabolism (Mishra et al., 2022).
4.1.1 Metabolites involved in disease resistance
Head/stem rot, anthracnose, soybean mosaic virus, charcoal rot, sunflower rust, verticillium wilt, soybean rust, and Rhizoctonia stem rot are the most devastating diseases reported in major oilseed crops (Table 2). Metabolites, including saccharides, organic acids (OAs), amino acids (AAs), phenolic acids, coumarins, flavonoids, phytohormones, and alkaloids have been reported to act against various biotic stressor agents in major oilseed crops (Table 2). For instance, in response to Sclerotinia sclerotiorum (Lib) disease, the levels of two saccharides (trehalose and ononitol) and four organic acids (glycerate, citrate, isocitrate, and succinate) reportedly increase in the resistant genotype (RHA801) of sunflower (Peluffo et al., 2010). Wagner et al. (2012) reported that some metabolites, especially amino acids, were positively associated with clubroot disease symptoms in some genotypes of rapeseed. The accumulation of glucose and fructose was negatively correlated with club-root disease symptoms in some genotypes. Consequently, Syed et al. (2015) reported that the compounds extracted from sesame leaves, stems, and roots exhibited inhibitory effects, suggesting that various secondary metabolites contributed to the antifungal response of the tissue extracts. The resistant genotype (Nirmala) of sesame showed greater accumulation of salicylic acid (SA), jasmonic acid (JA), and ethylene (ET) than did the susceptible genotype (VRI-1) against the fungal disease charcoal rot (Chowdhury et al., 2017). In their study, the JA, SA, and ET biosynthesis pathways and associated genes were activated in response to charcoal rot infection, resulting in the upregulation of downstream metabolites. Due to the nature of phytohormone crosstalk, the induction of a single plant hormone catalyzes other compounds for cooperative functions. Moreover, the stems of resistant soybean genotypes showed increased accumulation of phenolic acids (benzoic, ferulic, and caffeic acids), flavonoids (daidzein and malonyl), and coumarins (7-methoxy coumarin, scopoletin, xanthyletin, and osthole) in response to soybean diseases (Silva et al., 2021; Al-Wakeel et al., 2013).
| Biotic stresses | Metabolites | Major class | Crop | Crop part | Source |
|---|---|---|---|---|---|
|
|||||
Head/stem rot (Sclerotinia sclerotiorum) |
Trehalose, ononitol | Saccharides | Sunflower | Florets | Peluffo et al. (2010) |
| Glycerate, citrate, isocitrate, succinate | Organic acids | Sunflower | Florets | ||
| Benzoic acids, chlorogenic acid, caffeic acids and ferulic acids | Phenolic acids | Soybean | Silva et al., (2021); Al-Wakeel et al. (2013) | ||
| Scopolin, ayapin, and scopoletin | Coumarins | Sunflower | Leaves | Prats et al. (2006) | |
Anthracnose (Colletotrichum truncatum) |
Ginsenoside, taracxasterone, cedrelone, alisol A, aterobin A, picrasin B, kauroneic acid, torskolin, burceine D, abietic acid, marocarpal B, guaiol, curzerene, picropcropin, isopulegol, geraylacetate, cantharidin | Terpenoids | Soybean | Zhu et al. (2022) | |
| JA, auxin | Phytohormones | soybean | |||
| Soybean mosaic virus | Isoflavone |
Flavonoids |
Soybean |
leaves |
Zhang et al. (2020) |
Charcoal rot (Macrophomina phaseolina) |
JA, SA, Ethylene | Phytohormones | Sesame | Roots | Chowdhury et al. (2017) |
| Scopolin, ayapin, and scopoletin | Coumarins | Sunflower | Leaves | AL-Wakeel et al. (2013) | |
| Salicylic acid (JA) | Phytohormone | sunflower | Leaves | ||
| Sunflower rust (Puccinia helianthin) | Scopolin, ayapin, and scopoletin | Coumarins | Sunflower | Leaves | Prats et al. (2007) |
Verticillium wilt (Verticillium dahlia) |
Proline, serine, threonine | Ammino acid | Sunflower | Leaves | Peng et al. (2022) |
| Phenethylamine | Alkaloid | Sunflower | Leaves | ||
| Soybean rust (Phakopsora pachyrhizi) | Daidzein, malonyl | Isoflavonoids | Soybean | Silva et al. (2021) | |
| 7-methoxycoumarin, scopoletin, xanthyletin, osthole | Coumarins | Soybean | |||
Rhizoctonia stem rot (Rhizoctonia solani) |
Daidzein, 2′-hydroxydaidzein, 2′-hydroxyformononetin, maakian | Isoflavonoids | Soybean | Stem | Aliferis et al. (2014) |
| SA, JA, ABA, GA | Phytohormones | Soybean | Stem | ||
|
|||||
Cutworm (Spodoptera litura) |
4′,7-dihydroxyflavone, daidzein, and formononetin | Flavonoids | Soybean | Leaves | Murakami et al. (2014) |
Sobean aphids (Aphis glycines) |
JA and ABA | Phytohormones | Soybean | Chapman et al. (2018) | |
Diamondback moth Plutella xylotella |
Ferulic acid, Sinapoyl malate, feruloyl | Phenolic acid | rapeseed | Leaves | Widarto et al. (2006) |
| Gluconapin | Glucosinolate | ||||
| Threonine and alanine | Amino acids | ||||
| Sucrose and glucose | Saccharides | ||||
| Sunflower moth (Homeosoma electellum) | Niveusin B, argophyllone B, and 15-hydroxy-3-dehydrodesoxyfruticin | Sesquiterpenes | Sunflower | Leaves | Prasifka et al. (2015) |
Bollworm (Helicoverpa armigera) and Aphid (Aphis craccivora) |
Chlorogenic acid, ferulic acid, caffeic acid, and vanillic acid, cinnamic acid | Phenolic acids | Peanut | Leaves | War et al. (2016) |
| Quercetin, catechin, and genistin | Flavonoids | ||||
| Umbelliferone | Coumarin | ||||
| Flea beetle (Psylliodes chrysocephala) | Neoglucobrassicin and glucobrassicin. | Indole glucosinolates | Rapeseed | Stems and roots | Koritsas et al. (1991) |
Caterpillars (Anticarsia gemmatalis) |
Quercetin 3-O-rutinoside, quercetin-3,7-O- di-glucoside, quercetin-3-O-rhamnosylglycoside-7-O-glucoside, quercetin-3-O-rhamnopyranosyl-glu- copyranoside-rhamnopyranoside, genistein-7-O-diglucoside-dimalonylated, genistein-7-O-6-O-malonylglucoside, daidzein-7-O-glucoside-malonate | Flavonoids | Soybean | leaves | Gomez et al. (2018) |
| C. Parasitic Weeds | |||||
| Broomrape/Beggarweed (Orobanche cumana) | scopoletin | Coumarins | Sunflower | Root | Rial et al. (2021) |
- Where: SA: salicylic acid, JA: jasmonic acid, ABA: abscisic acid, GA: gibberellic acid.
In a combined transcriptomic and metabolic analysis study, the resistant genotype of soybean (ZC-2) showed a resistance response against the fungal disease anthracnose through jasmonic acid, terpenoid, and auxin hormone accumulation compared with the susceptible genotype (Zhu et al., 2022). Their study indicated that the molecular mechanism underlying the resistance of the soybean genotype ZC-2 to anthracnose (C. truncatum) could involve the activation of signal transduction components (JA, AUX, Ca+, and MAPK), transcription factors (bHLH and WRKY genes), and pathogenesis-related and resistance genes (PR and R-genes (RPS6, RGA2, ULP2B, PR14, and CH1)). At the same time, they confirmed that the exogenous application of methyl jasmonate (JA) and auxin (indole 3-buthyric acid (I3BA) enhanced the resistance of soybean pods to anthracnose. Sixteen differential terpenoid metabolites were upregulated in response to anthracnose disease in soybean (Table 2). Interestingly, during pathogen infection, plants undergo oxidative stress and signal transduction due to the expression of PR genes and the biosynthesis of their metabolites. Then, the activation of primary and secondary metabolites as a defense response to specific or multiple stress effects initiates the synthesis of phytoalexins such as coumestrol, coumarins, coumarates, glyceollin, maackiain, and resveratrol due to phytoalexin inducers (jasmonate) (Aliferis et al., 2014).
Peng et al. (2022) identified and reported a total of 169 secondary metabolites associated with virulent Verticillium dahlia (V33) and hypo-virulent Gibellulopsis nigrescens (Vn-1) pathogen responses in sunflower. They also detected 48 differentially abundant metabolites (DMs) responsible for resistance against two bacterial species of sunflower diseases. In their study, a significant and greater accumulation of 17 metabolites in response to Vn-1 inoculation than to V33 inoculation was reported (Peng et al., 2022). Metabolites, including pyruvate, 2-phenyl butyric acid, and phenethylamine, revealed considerable potential for sunflower disease resistance in Gibellulopsis nigrescens. Furthermore, the coumarin scopolin significantly contributed to the response against sunflower head rot (Sclerotinia sclerotiorum) (Prats et al., 2006).
Additionally, flavonoid compounds, particularly anthocyanins and rutins, could be crucial in boosting root cell resistance to oxidative damage and infections caused by soil pathogens (Shen et al., 2022). A notable study by Zhang et al. (2020) demonstrated that CRISPR/Cas9-mediated multiplex gene editing and metabolomic engineering enhanced isoflavone content in soybean leaves in response to mosaic virus infection. Specifically, the targeted knockout of genes GmF3H1, GmF3H2, and GmFNSII elevated the synthesis of genistein, an isoflavone. The findings indicate that increased isoflavone levels in soybean plants contribute to enhanced resistance against mosaic virus disease. This suggests that it is possible to develop crop varieties resistant to disease with the help of an untouched alternative approach to metabolome engineering coupled with CRISPR/Cas9. Therefore, a metabolomics study could effectively identify specific metabolites and pathways involved in defense against diseases by designing new biochemical pathways to address plant disease issues, making crops responsive and resistant to oil crop pathogens.
4.1.2 Metabolites involved in insect pest resistance
Aphids, leafhoppers, pod borers, stem borers, caterpillars, pod bugs, termites, leaf miners, and white grubs are the most common and devastating insect pests of major oilseed crops (Murugesan & Balaji, 2023). Inherently, all plants are equipped with protection mechanisms against insect pest attacks through biochemical or morphological adaptations (Tanda, 2022; War et al., 2019). Morphological defenses include cuticular waxes, thorns (spines), and trichomes. In contrast, biochemical defenses usually involve the dynamic synthesis of secondary metabolites that are potentially harmful to the organisms that attack the plant.
Pesticides have been used to reduce agricultural loss due to insect pest damage. However, pesticide options have negatively impacted environmental and crop quality, harmed human health, and interfered with the natural ecosystem balance. Thus, it is imperative to enhance nonchemical methods for mitigating insect damage that are safe, cost-effective, and long-lasting through the development and use of pest-resistant genotypes (Arora & Sandhu, 2017). This could be achieved by identifying responsible metabolites, biosynthesis pathways, and genes underlying plant defence.
In most cases, plants produce sulfur-containing compounds (terpenoids and flavonoids), nitrogen-containing compounds [alkaloids, nonprotein amino acids, glucosides, and indoles (I3AA and indole alkaloids)] to resist insect pest attack (War et al., 2019). Phenolic compounds from various plant sources, mainly flavonoids, lignin, tannins, coumarins, and terpenes (geraniol, humulene, lycopene, and cafestol), are very important phytoprotectants of insect pests (Khuram et al., 2023). Some phytochemicals do not directly destroy insect pests but are building blocks for synthesizing other secondary metabolites. For example, instead of instantly killing insects, flavonols and anthocyanins are precursors for synthesizing tannins, which are poisonous and deterring insects (Khuram et al., 2023). The contents of sesquiterpene lactones (niveusin B, argophyllone B, and 15-hydroxy-3-dehydrodesoxyfruticin) were greater in sunflower genotypes resistant to sunflower moth (Homeosoma electellum) (Prasifka et al., 2015). Glucosinolates in mustard family (Cruciferae) crops, mainly Brassica napus, (Brassica oleraceae, Brassica juncea, B.rapa, and B. nigra, play a significant role in the response to various insect pests. Studies have indicated that the products of glucosinolate hydrolysis (isothiocyanates) could protect the plant directly through an effect on insect biology or behavior or indirectly through the attraction of the pest's predators in Brassica spp. (Tanda, 2022). The insect-resistant genotypes of peanuts showed increased levels of total phenols, malondialdehyde, proteins, and hydrogen peroxide as well as increased activity of defensive enzymes (superoxide dismutase, peroxidase, phenylalanine ammonia-lyase, polyphenol oxidase, ascorbate peroxidase, and catalase) against Helicoverpa armigera larvae and Aphis craccivora (War et al., 2013). In line with this, differential accumulation of phenolic acids (chlorogenic acid, ferulic acid, caffeic acid, and vanillic acid), coumarin (umbelliferone), and flavonoids (quercetin, catechin, and genistin) was observed against chewing (Helicoverpa armigera) and sucking (Aphis craccivora) insects in peanut (War et al., 2016). Similarly, Helicoverpa larvae demonstrated noticeable variations in their weights and rates of survival when fed plants that were previously or simultaneously treated with SA and JA and untreated controls (War & Sharma, 2014).
The different forms of herbivory on rapeseed caused changes in both primary and secondary metabolites. Elevated glucosinolate (gluconapin), phenolic acid (ferulic acid, feruloyl, and sinapoyl malate), amino acid (alanine and threonine), and saccharide (glucose and sucrose) contents were detected in the leaves of herbivorous insect pest (Plutella xylotella)-infested rape seeds (Widarto et al., 2006). Although the genetic resistance of soybeans to a variety of herbivores has been linked to isoflavonoid phytoalexins, in some cases, reduced levels of metabolites could have a positive effect on reducing insect pest populations. The contents of flavonoids, mainly quercetin, caempferol, genistein, and daidzein, and their conjugates were elevated in soybean plants resistant to caterpillars (Anticarsia gemmatalis) (Gomez et al., 2018). In this regard, higher contents of isoflavonoids (genistein and genistin) were detected in soybean genotypes infested with soybean aphids (Scott et al., 2022). However, their results showed that the most resistant soybean variety had, on average, reduced levels of certain free amino acids (Met, Tyr, and His) compared to the most susceptible variety, indicating that within the tested varieties, nutrient quality may be more critical in stimulating aphid feeding than isoflavonoids, which are detrimental to aphid feeding or growth (Scott et al., 2022). Therefore, plants can respond to or induce the accumulation of defensive compounds when infested or attacked by insect pests.
4.1.3 Metabolites involved in weed infestation resistance
Weeds are a significant problem in oil crop production, productivity, and quality concerns (Stefanic et al., 2022). Limited research has investigated the metabolites oilseed crops produce in response to weed invasion. Most of these studies have focused on weed interference's effects on yield and herbicides' application to suppress weeds in oilseed crop farming. Some studies have shown that phenolic compounds such as benzoxazinoids, terpenes, glucosinolates, sorgoleone, alkaloids, and momilactones are some of the most important allelochemicals/allelochemical groups found in major field crops (Jabran, 2017). Plants communicate and influence the growth of other plants and microbes through the secretion of allelochemicals from their roots, leaves, and flowers (Jabran, 2017). They release exudates containing diverse metabolites into the rhizosphere via root exudates, engaging actively with soil organisms (Tsuno et al., 2018). These root exudates include antibiotic phytoalexins that protect plants from soil-borne fungi, bacteria, and herbivores. Likewise, studies have shown that some crop species' leaves, flowers, and stems can produce various compounds incorporated into the soil during rain splash effects or as fallen parts (Hussain et al., 2017). For example, sesame leachate was reported to affect the biophysical and biochemical parameters of invasive weed nutsedge (Cyperus rotundus L.); as a result, sprouting, growth, multiplication, and photosynthetic pigments were suppressed (Hussain et al., 2017). Nutsedge are perennial invasive weeds that can outcompete native plants for resources in the tropics and subtropics (Kumar & Varshney, 2008). Leachate, a liquid that seeps through porous surfaces of plants and soil, contains dissolved or suspended chemical compounds that negatively (allelopathic) impact the plants, especially when it comes to invasive species control and agricultural activities (Hussain et al., 2017). These compounds are primarily secondary metabolites produced by plants, including flavonoids, quinones, coumarins, phenolic acids, steroids, and alkaloids (Babu et al., 2023). The allelopathic effect of sesame leachate, which can prevent the growth of neighboring plants, including crops and beneficial flora, impacts the invasive weed nutsedge (Babu et al., 2023). The allelopathic effect of leachate may decrease the disruption of local ecosystems and biodiversity losses. Furthermore, adding specific leachate compounds can change the pH of the soil, which can impact microbial activity and nutrient availability (Kumar & Varshney, 2008). Leachate chemicals might potentially eutrophicate and contaminate groundwater, endangering aquatic ecosystems and drinking water quality if it includes dangerous chemicals (Kumar & Varshney, 2008). Therefore, understanding the effects of sesame leachate on the weeds and environment (mainly soil chemicals and water) could be very significant in taking necessary solutions and positive effects (use as a biopesticide).
Sunflower (Helianthus annuus L.) exhibits significant allelopathic properties, with its phytotoxic potential evidenced in laboratory, greenhouse, and field trials across several parameters, including concentration, test species, genotype, and growth stages (Ravlić et al., 2022). Applications of secondary metabolites such as phenolic acids, terpenoids, and coumarins extracted from sunflowers have been reported to manage various weed species due to the allelopathic effects of their extracts (Jabran, 2017). For example, sunflower water extracts inhibited the germination and development of lettuce seedlings (Ravlić et al., 2022). Due to the crop suppressive effect, a reduced total number of companion crops, weeds, and biomass was reported in sunflowers (Alsaadawi et al., 2011). They also found increased contents of phenolic acids (ferulic acids, caffeic acid, and syringic acids) and terpenoids (terpinol) from suppressive genotypes, suggesting that the reduction might be linked to phenolic acids and terpenoids in sunflower genotypes. Further, among sunflower weeds, broomrape (Orobanche cumana) is the most devastating and outcompeting parasitic weed (Rial et al., 2021). Coumarin (scopoletin) levels were increased in response to O. cumana in resistant sunflower genotypes compared with susceptible ones (Rial et al., 2021). Coumarins have a 5,6-benz-2-pyrone backbone, can be variously hydroxylated, alkoxylated, alkylated, or acylated, and can be used to discourage food intake and disrupt insect pest or weed development (Arora & Sandhu, 2017). Pretreatment with brassinosteroids (BRs) improved sunflower resistance to O. cumana infection by improving plant growth and biomass and photosynthesis, activating the antioxidant defense system, reducing oxidative stress and cellular damage, and modulating the expression of BR synthesis and signaling genes (Zhang et al., 2023). Sunflowers' allelopathic action makes them a great tool in integrated weed management and sustainable agriculture. Understanding and utilizing these natural suppressive features allows farmers and researchers to increase crop yield while reducing dependency on synthetic pesticides, supporting a more ecologically friendly agricultural system.
Moreover, the details about the particular metabolites that the plant uses to react to weed infestation have been limited. Accordingly, no study has directly addressed the specific metabolites linked to oilseed crop responses to weed infestation. Investigations or particular studies on the metabolomic response of economically important oilseeds to weed infestation are required to elucidate weed control mechanisms in oilseed crops because metabolomics could be more important for crop improvement, especially for the development of genotypes resistant to weeds. Overall, applying metabolic engineering and biotechnological techniques provides a substitute strategy for generating bioactive, commercially advantageous, and noteworthy compounds present in plants that would help mitigate the issue of restricted availability (Khuram et al., 2023). This would help develop insect pest, weed, and disease-tolerant/resistant genetic resources that are eco-friendly, cost-effective, organic, market-oriented, healthy, and high-quality products.
4.2 Metabolites Involved in the Abiotic Stress Response
Sesame, soybean, peanut, and perilla are grown in high-temperature areas and are frequently influenced by high temperatures and drought (Patel et al., 2022; Wei et al., 2015) but are mainly stressed by salinity, waterlogging, nutrient deficiencies, and other extreme conditions. Rapeseed is grown in cold to moderate temperature ranges and is significantly influenced by cold (chilling and freezing) stress (Jian et al., 2020). Sunflower can grow in broader ecological regions, including temperate, subtropical, and tropical areas (Andrea et al., 2021) and is also hampered by drought, salinity, waterlogging, nutrient deficiency, and other extreme conditions. When these stresses impact crops, they produce reactive oxygen species (ROS), Ca+, NO, and phosphatidic acid (PA) as a signaling response to induce the genes responsible for the synthesis, accumulation, or degradation of many metabolic components in cells (Lakhanpaul et al., 2012) (Figure 4). Stress-induced primary and secondary metabolites synthesized upon stress can most likely act as oxidative damage reducers, osmolytes, and osmoprotectants for osmotic adjustment and reduce the effects of single or multiple stresses (Cui et al., 2018). When ROS and other signaling compounds are produced, they directly activate kinase proteins (MAPKs) to direct the synthesis of particular metabolites for specific/multiple stresses, which are then translocated throughout the plant body or to a particular site where the plant part or cell is damaged for further adaptive strategies. Understanding the function of downstream proteins involved in the up- or downregulation of ROS-scavenging enzymes is crucial for manipulating the ROS-mediated MAPK pathway to increase plant stress tolerance (Manna & Sinha, 2023).
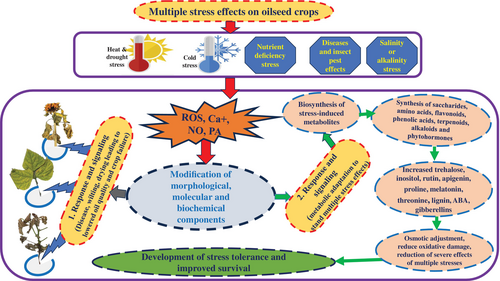
A summary of various metabolites induced in response to multiple abiotic stresses in major oilseed crops is presented in Table 3. The levels and diversity of most primary metabolites and secondary metabolites increased in response to abiotic stressors. The following subsections also present the details of each abiotic stress and metabolic insight.
| Traits/stress conditions | Increased/upregulated metabolites | Major class | Crops studied | Plant part | References |
|---|---|---|---|---|---|
| Abiotic stresses response | |||||
| Drought stress | Proline, isoleucine, leucine, valine, tryptophan, tyrosine, asparagine, phenylalanine | Amino acids | Sesame, Peanut |
You et al. (2019) Patel et al. (2022) |
|
| Chlorogenic acid, caffeic acid, p-coumaric acid, gallic acid, and ellagic acid | Phenolic acids | Sesame | Seed | Kermani et al. (2019) | |
| Rutin, apigenin | Flavonoid | Sesame | Seed | ||
| Glycine, betaine | Sunflower | Root | Manivannan et al. (2007) | ||
| Proline | Amino acid | Sunflower | Root | ||
| Glucose, fructose, sucrose, myo-inositol | Saccharides | Rapeseed | shoot | Viana et al. (2022) Viana et al. (2022) |
|
| Malic acid | Organic acid | Rapeseed | Shoot and root | ||
| Waterlogging | Glucose, sucrose, myo-inositol | Saccharides | Rapeseed | Root | |
| Valine, proline | Amino acid | Rapeseed | Root | ||
| Citric acid | Organic acid | Rapeseed | root | ||
| Heat stress | Glutamic acid and serine | Amino acids | Peanut | Patel et al. (2022) | |
| Isoleucine, alanine, glycine | Amino acids | Rapeseed | Shoot | Viana et al. (2022) | |
| Serine | Amino acids | Rapeseed | root | ||
| Cold stress | Lignin and lipids | Lipids & Lignin | Peanut | Wang et al. (2021) | |
| Malic acid | Organic acid | Rapeseed | Jian et al. (2020) | ||
| Quercetin 4′-O-glucoside (spiraeoside), quercetin 3-O-glucoside (isotrifoliin), selgin 5-O-hexoside, hesperetin C-hexosyl-O- hexosyl-O-hexoside | Flavonoids | Rapeseed | |||
Salt stress/ Alkali stress |
Increased contents of Saccharolipids, glycerolipids | Lipids | Sunflower | Lu et al. (2021) | |
| Proline, L-aspartate, and L-glutamate | Amino acids | Sunflower | Roots | ||
| 2-Isopropylmalic acid, caproic acid, citraconic acid, citramalic acid, eicosapentaenoic acid, mesaconic acid, trans-vaccenic acid, and traumatic acid | Lipids | Sunflower | Leaves | ||
| Increased contents of D-lactose, myo-inositol, D-mannose, raffinose, sorbose, and trehalose | Saccharides | Sunflower | Leaves | ||
| Increased contents of myo-inositol, D-galactarate, erythritol, fructose 1-phosphate, galactinol, glyceric acid, isomaltose, and sucrose | Saccharides | Sunflower | Roots | ||
| Increased contents of ABA, GA1, and ACC | Phytohormones | Sunflower | Leaves | ||
| Increased contents of TY, Iso- pentenyladenosine (iPR), and cis-OPDA | Phytohormones | Sunflower | roots | ||
| Proline, glutamic acid, aspartic acid, l-allothreonine, isoleucine, serine, alanine, 5-methoxytryptamine, and fluorene | Amino acids | Wild Soybean | Roots | Li et al. (2017) | |
| Arachidic acid, oleic acid, cis-gondoic acid | Lipids | Wild Soybean | |||
| Fumaric acid, l-malic acid, citric acid, malonic acid, gluconic acid | Organic acids | Wild Soybean | |||
| Salicylic acid | Phytohormone | Wild Soybean | |||
| Low nitrogen stress | Increased contents of L-threonine, alanine, glycine, ethanolamine (aliphatic amino acid), and sphingosine | Amino acids | Wild Soybean | Leaves | Liu et al. (2019) |
| Increased contents of xylose, sucrose, maltose, maltotriose, and melezitose | Saccharides | Cultivated Soybean |
Leaves | ||
| Increased contents of itaconic, methylmalonic, maleic, gluconic and fumaric, malic, citramalic, succinic, and succinic acids | Organic acids | Wild Soybean | Leaves | ||
| Prunin | Flavonoid | Wild Soybean | Leaves | ||
| Salicylic acid | Phytohormone | Wild Soybean | Leaves | ||
| Ferulic acid | Phenolic acids | Wild Soybean | Leaves | ||
| Phosphorus deficiency (-P) | α-D-glucose and Sucrose | Saccharides | Soybean | Root | Yang et al. (2020) |
| Vanillic acid | Phenolic acid | Soybean | Root | ||
| Glucuronic acid | Organic acid | Soybean | Root | ||
| Abscisic acid, gibberellin | Phytohormones | Soybean | Root | ||
| Quercetin | Flavonoid | Soybean | Root | ||
| Kaempferol, quercetin, apigenin, and liquiritigenin, rotenone, daidzein, daidzein 7-O-glucoside, ampelopsin, kaempferitrin, isohemoheloin, rotenone, vitexin, isohoilfolin, cosmosin, isovitexin, velutin O-glucuronic acid, tricin-Ohexoside, 5-methoxyflavanone, biorobin, daidzin, medicarpin, protocatechuic acid & protocatechuic acid O-hexoside | Flavonoids | Soybean | Roots | Luo et al. (2020) | |
| Glucose, inositol, gluconic acid | Saccharides | Soybean | roots | ||
| Citrulline, tryptophan, leucine, theanine, isoleucine, asparagine, and methionine | Amino acids | Soybean | roots | ||
4.2.1 Cold stress response
Low temperatures can negatively affect plant development and growth and decrease agricultural yield (Xin et al., 2019). Plants have evolved various defense and response mechanisms to confront low-temperature stress. In China, cold stress damage has become the greatest obstacle to early-sowing efforts to ease spring-sowing drought in peanuts (Zhang et al., 2019). Wang et al. (2021) identified 563 metabolites related to peanut cold stress. They also confirmed the presence of key regulatory and metabolic pathways contributing to cold stress, resulting in a profound accumulation of metabolites. Amino acids, carbohydrates, terpenoids, alkaloids, flavonoids, nucleotides, and lipids were predominantly observed in response to cold. However, the expression of two metabolite components, lignin and lipids, was upregulated and significantly affected the stress response to cold in peanut crops (Wang et al., 2021).
Although rapeseed plants also respond to vernalization and have the potential to tolerate cold stress, a greater risk of freezing injury during the cold winter and spring months was recently observed in early-maturing cultivars with limited cold resistance (Xin et al., 2019). In the spring and winter ecotypes of rapeseed, 559 metabolites were identified and categorized into 30 classes (Jian et al., 2020). Organic acids (74, 13.12%), amino acids and their derivatives (55, 9.8%), and nucleotides and their derivatives (52, 9.3%) were the top three classes. In addition, 41 differentially expressed metabolites (DEMs) in the spring ecotypes of rapeseed were responsible for the cold response. In their study, seven genes encoding enzymes responsible for lignin biosynthesis [PHE ammonia lyase1 (PAL1), cinnamate-4-hydroxylase (C4H), O-methyltransferase1 (OMT1), cinnamyl alcohol dehydrogenase5 (CAD5), ferulic acid 5-hydroxylase1 (FAH1), caffeoyl-CoA 3-O-methyltransferase (CCOAMT), and hydroxycinnamoyl-CoA shikimate/quinate hydroxycinnamoyl transferase (HCT)] were primarily upregulated in both winter and spring rapeseed ecotypes. However, lipids and amino acids were the most prominent differentially accumulated metabolites in the winter and spring Brassica napus ecotypes. These metabolites could be essential for rapeseed breeding and improvement programs to develop cold-resistant varieties. Therefore, the metabolic pathway of these compounds needs to be addressed in intensive research to develop cold-resistant oilseed crops via metabolic engineering.
4.2.2 Drought and High-Temperature Stress Response
Drought is among the most important stressors that reduce the nutritional value and productivity of the world's most important oil crops (El Sabagh et al., 2019). As water is an essential resource for a plant's metabolic activity, water deficit or drought can impact plant growth, development, and overall physiological activity due to inefficient physiological and biochemical processes. Plants may experience unbalanced osmotic adjustment and dysfunctional photosynthesis due to extended drought stress, resulting in variations in the synthesis of primary metabolites (Razzaq et al., 2019). This harms crop survival and can lead to total failure or loss of the whole plant (Song et al., 2022). One of the critical aspects of future climates could be variability and the possibility of occasional high temperatures (Djanaguiraman et al., 2011) that could result in drought and its consequences. High temperature results in increased thylakoid membrane damage, decreased chlorophyll content, decreased photosynthesis, and decreased stomatal conductance, resulting in reduced photoassimilates and reduced economic yield and quality of crop produce (Santos et al., 2022; Djanaguiraman et al., 2011). Reduced levels of biochemical compounds, including chlorophyll a, b, total chlorophyll, and carotenoids, were observed to a greater extent due to drought's destructive effect on sunflower crops (Manivannan et al., 2007). When these essential compounds are reduced, the net effects of photosynthesis and dry matter partitioning are reduced, thus affecting the seed yield and oil quality of oilseed crops (Hussain et al., 2018).
Studies have revealed that melatonin is the most vital metabolite for mitigating abiotic stresses (heat, cold, drought, and salt) in plants (Tiwari et al., 2020). The exogenous administration of melatonin enhanced the drought and salt stress tolerance of soybean plants (Wei et al., 2015). Furthermore, melatonin was found in a much greater quantity in sesame than in other crops (0.04 to 298.62 ng g−1) (Wang et al., 2022), which might enhance the ability to confer drought stress. The authors have identified the gene (SiWRKY67) responsible for the melatonin spike from the GWAS of sesame.
Moreover, Sicher (2015) revealed a decrease in inorganic acid levels and increased rates of photorespiration due to increasing temperature. Their results showed that citrate, fumarate, malonate, malate, and succinate compounds decreased by 40–80% in soybean leaflets grown in higher temperature chambers (36/28°C). This could be due to the impairment of the metabolic pathway and the alteration of photorespiratory flux. Higher levels of metabolites such as 4-hydroxyproline, D-saccharic acid, cyclic GMP, guanine, and adenine were detected in drought-tolerant sesame plants than in drought-susceptible sesame plants under all circumstances (You et al., 2019). In this study, the drought-tolerant sesame genotype also exhibited increased accumulation of benzoic acid, lactobionic acid, putrescine, and several amino acids (such as asparagine, arginine, tyrosine, and lysine) under drought stress conditions.
A comparative hormonal and metabolic profiling analysis of sunflower (Helianthus annus L.) inbred lines revealed that water deficit stress is regulated by various water deficit stress sensitivities (Andrea et al., 2021). Although sunflower can tolerate stress conditions to a greater extent than other oilseed crops, it is susceptible to drought stress from the flowering to grain-filling stages in arid and semiarid environments due to inadequate soil water availability and high temperatures (Hussain et al., 2018). When short- and long-term droughts occur during crop grain/seed-filling stages, they can negatively impact grain yield and crop quality (El Sabagh et al., 2019). This means that the reproductive stages of oil crops could be critical for regulating stress, resulting in metabolite changes and reduced oil quality.
Studies have shown that proline, valine, threonine, tryptophan, and phenylalanine are significantly associated with the cumulative effect of heat and drought stress in peanuts (Patel et al., 2022). In their study, individual stresses such as drought increased the content of asparagine and isoleucine, whereas heat stress enhanced serine and glutamic acid accumulation. Under drought stress conditions, all sunflower varieties exhibited increased proline and free amino acid contents and increased γ-glutamyl kinase activity in the leaves, roots, and stems (Manivannan et al., 2007). The reduced chlorophyll content could be attributed to chlorophyll damage, which led to increased activity of γ-glutamyl kinase and associated compounds in response to drought. These compounds could significantly contribute to osmotic adjustment, resulting in drought stress resistance in sunflower crops. Drought-tolerant cultivars of Brassica napus revealed increased enzymatic activities (catalase, superoxide peroxide, and dismutase), and increased contents of ascorbic acid (AsA), glutathione, proline, total soluble sugars (TSSs), and total soluble proteins (TSPs) (Mehak et al., 2021). Therefore, these findings suggest that metabolites contribute to the drought stress response and are areas of interest for further metabolome engineering of oil crops.
4.2.3 Salt Stress Tolerance
The effects of salinity on crop growth and development lead to decreased production. Naturally, salty soils are characterized by more prevalent ions, which include K+, Cu2+, Zn2+, Na+, Ca2+, Mg2+, Cl−, NO−3, Na2HCO3 NaHCO3, HCO3−, and SO42− (Lu et al., 2021). Elevated soil salinity leads to metabolic syndromes, osmotic imbalances, growth retardation, ion toxicity, ion uptake dysfunction, and other abnormal physiological activities (R. Guo et al., 2015). The responses and recovery of plants from salt stress may be explained by changes in plant physiological parameters associated with the regulation of metabolites and proteins involved in the process (Cui et al., 2018).
In response to salt stress, 98 metabolites were identified from the leaves of soybean genotypes (Lu et al., 2013). Similarly, 119 metabolites (59 in shoots and 60 in roots; 27 in common) were found at significantly different levels in response to salt stress in peanuts (Cui et al., 2018). Based on their results, amino acids, polyols, and organic acids were the most common metabolites, accounting for 29.67, 28.57, and 21.98%, respectively. Concurrently, Zhang et al. (2019) observed the critical metabolic pathways involved in the adaptive salt stress response of two distinct sesame genotypes via transcriptome and metabolome analyses. They identified 152 metabolites involved in the response to salt from two of the studied sesame genotypes. In another study, the levels of most metabolic components, such as organic acids, free amino acids, and sugar groups, in the shoots and roots decreased under salt stress in rapeseed crops (Viana et al., 2022).
Furthermore, lipidomic analysis of sunflower seeds revealed that alkali stress increased the accumulation of glycerophospholipids and saccharolipids while reducing glycerolipid synthesis (Lu et al., 2021). This result implies that alkali stress may modify the lipid components of sunflower seeds grown in alkaline agricultural areas, resulting in an altered quality of sunflower oil. The sunflower plants had increased contents of several fatty acids, carbohydrates, amino acids, and organic acids in the roots under alkali stress conditions. Four distinct categories of metabolites, including amino acids, organic acids, fatty acids, and carbohydrates, were involved in alkali tolerance. In the roots, alkali stress increased the contents of L-aspartate, proline, and L-glutamate, while the L-arginine and L-glutamine contents decreased. Alkali stress reduced the concentrations of proline, aspartic acid, L-glutamate, L-glutamine, L-serine, and ornithine in leaves while stimulating the accumulation of eight fatty acids and reducing the concentrations of five fatty acids. However, almost all the organic and fatty acids in the roots significantly increased due to alkali stress.
4.2.4 Nutrient Deficiency Stress Response
A lack of an essential nutrient results in nutrient shortages, which can cause stunted growth and physiological problems in plants. Significant metabolic changes, including fructose, fumaric acid, alanine, ferulic acid, serine, proline, glycine, myoinositol, 4-aminobutyric acid, malonic acid, and isoleucine accumulation, were detected in the leaves of soybean plants during growth in low-nitrogen (N-deficient) environments (Li et al., 2018). Forty-eight metabolites in the N-deficient soybean leaves were markedly different from those in the control group. Concurrently, recent research has indicated that N deficiency (nitrogen) significantly hampers rapeseed shoot growth and root architectural changes (Shen et al., 2022). A total of 572 metabolites were identified in the leaves and roots under varying nitrogen conditions. Specifically, 175 metabolites in leaves and 166 metabolites in roots exhibited differential accumulation in response to high versus low nitrogen levels. Similarly, Mo et al. (2019) observed the accumulation of metabolites under P-nutrient deficiency in soybean. They also observed that P shortage in soybean significantly decreased the levels of approximately 43 phosphate-containing metabolites, including 23 lipids and glycerophospholipids, 10 nucleotide-related metabolites, 3 carbohydrates, 2 choline, 1 vitamin, and 4 other metabolites, in the roots. This information provides a potential method for genetically enhancing soybean low-Pi tolerance and insights into the intricate regulatory mechanism of Pi deficiency tolerance in soybean plants. Higher concentrations of flavonoids, phenylamides, and phenolic acids were detected in the stylo roots of the soybeans. These compounds can help plants absorb phosphorus (P) while interacting with helpful microbes in the rhizosphere to support the uptake and utilization of P under P stress conditions (Luo et al., 2020).
5 THE BIOSYNTHESIS AND DEGRADATION PATHWAYS OF METABOLITES AS INFLUENCED BY STRESS IN OILSEED CROPS
Multiple stresses frequently impact oilseed crops; as a result, their economic yield, as well as their seed quality, are reduced. Both biotic and abiotic stresses affect the crop life cycle simultaneously or sequentially. Plants produce various morphological and biochemical responses to avoid multiple stress effects. In this case, the degradation and biosynthesis of lipids, amino acids, organic acids, saccharides, phenolic acids, flavonoids, coumarins, lignins, alkaloids, and terpenoids influence the stress responses of oilseed crops. The critical metabolic pathways that connect plant glucose, fat, and protein metabolism are the tricarboxylic acid cycle (TCA cycle) and glycolysis (Yadav et al., 2021). However, under certain conditions, plants can reduce the synthesis of primary metabolites and turn them into secondary metabolites to synthesize and accumulate secondary metabolites with specific functions. The most significant class of secondary metabolites in a plant's body are phenolic compounds, which have crucial functions in synthesizing and regulating various phytochemicals vital for stress responses (Jian et al., 2020). In addition to the stress response, the development and advancement of metabolic pathway engineering technology have created the possibility of altering genes in the metabolic pathway of fatty acid biosynthesis and producing desired fatty acids with C8-C24 chain lengths within a particular oil crop (Rahman & Jiménez, 2016).
The key route of phenolic and other secondary compounds (terpenoids and alkaloids) arises from the shikimate or mevalonate pathway and is then extended to various precursors for different phenolic compounds (Figure 5). Phenylalanine and tyrosine are the two main precursors that enter the phenylpropanoid pathway to produce phenolic acids and other polyphenols with the help of many enzymes involved in multiple stress effects (Ku et al., 2022). Then, P-coumaroyl-CoA is converted to naringenin chalcone to synthesize many flavonoid compounds. Chalcone synthase (CHS) or chalcone reductase (CHR) enzymes produce naringenin chalcone and liquiritigenin from coumaroyl-CoA (Ku et al., 2022). Chalcone isomerase was the first flavonoid pathway enzyme discovered to be the sole substrate for reactions to subsequent flavonoid classes, catalyzing the stereospecific cyclization of chalcones to (2S)-flavanones, flavones, and isoflavonoids, which are particularly enriched in most oilseed crops. Additionally, alkaloids can be synthesized from three precursors: aspartic acid-lysine, tyrosine, or tryptophan. The most important compound, melatonin, can be synthesized from tryptophan, tryptamine, and serotonin. In the same procedure, various important alkaloids are produced in greater quantities under normal conditions and when crops are stressed. As shown in Figure 5, terpenoids and phytohormones can be synthesized from the main pathways of mevalonic acid (MVA) (the precursor is acetyl-CoA) or methylerythritol phosphate (MEP) (the precursor is 3PGA), which commonly produces isopentenyl diphosphate (IPP) (Huang et al., 2022). Then, IPP is involved in the synthesis of terpenoids and phytohormones.
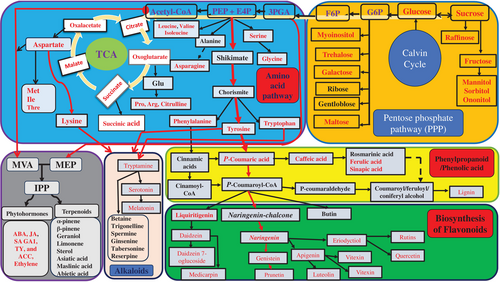
Understanding the key routes or pathways for synthesizing the main compounds is essential for reconstructing and producing new metabolites with specific functions. We identified the seven most important pathways responsible for multiple stress responses: the pentose phosphate pathway (PPP), TCA, shikimate, MVA/MEP, phenylpropanoid, tryptamine, and flavonoid pathways. For example, as indicated in Figure 5, the PPP is responsible for the synthesis of saccharides, TCA for organic acids, shikimate for aromatic amino acids and downstream phenolic compounds, MVA/MEP for terpenoids and phytohormones, phenylpropanoids for phenolic acids and polyphenols, and the flavonoid pathway for various flavonoid class metabolites. Additionally, tryptamine is the most crucial precursor for synthesizing many alkaloid compounds. Based on this, we developed a comprehensive general model pathway comprising these major pathways to develop stress-tolerant crop varieties via re-engineering the genes responsible for the biosynthesis of each pathway (Figure 5).
6 CURRENT METABOLOMICS STUDY LIMITATIONS AND FUTURE PROSPECTS
Metabolomics is the latest and most robust approach for studying the small molecule metabolites present in a biological sample (Yang et al., 2019). Despite technological advances, none of today's analytical platforms can ultimately measure the entire metabolome (Danzi et al., 2023). There are limitations and challenges associated with metabolomics. Some of the critical limitations of the current methods in metabolomics studies include metabolite coverage, quantification, identification, data analysis, standardization, reproducibility, sample handling and storage, sample size, biological variation of the sample, metabolite interaction, interpretation, and validation (Minno et al., 2022; Vuckovic, 2018). Many metabolomics methods may not detect or identify all metabolites in a sample, resulting in incomplete metabolome coverage (Peters et al., 2018). Some metabolites may be present at deficient concentrations or have unique chemical properties that make them difficult to detect. The ability to identify and quantify a broad range of metabolites in a biological sample is known as “coverage of a metabolite,” and several technical, biological, and experimental concerns determine this ability. The analytical technique, sample preparation, instrumentation, sensitivity, metabolite class, database and reference metabolite, metabolite stability and biological variability, concentration of the compound, and diversity affect the coverage of metabolites in a sample (Yang et al., 2019; Vuckovic, 2018).
Researchers often combine analytical methods and optimize workflows to address the abovementioned factors to improve metabolite coverage. In addition, the coverage of metabolites in metabolomics studies is being expanded by ongoing advances in analytical technologies and database resources. High-throughput chemical annotation is a substantial bottleneck in processing metabolic information, leaving metabolomic profiles sparser and more confounding than transcriptomics (Zhao & Rhee, 2023). The complexity of processing and managing metabolomic data is another challenge in metabolomics (Peters et al., 2018). Researchers in metabolomics continue to work to address these limitations by developing methods, standardizing data, and improving reference databases. Additionally, there is limited research on metabolites responsible for weed infestation and management in major oilseed crops. Therefore, we suggest developing technologies for whole metabolome detection and quantification, as well as precise, updated, and standardized comprehensive metabolome databases, tools, and software for integrated omics. Meanwhile, future research works shall focus on investigating metabolites responsible for acidic stress response, identifying stress signaling pathways, and identifying regulatory genes and genomic regions for accelerated breeding of stress-tolerant genotypes. Investigating specific metabolites responsible for specific or multiple weed species infestation in major oilseed crops is recommended. Application of genetic engineering, including gene editing for identified metabolites and significant pathways, shall be the focus of future oil crop breeding. Finally, integrated omics approaches such as genomics, transcriptomics, proteomics, and metabolomics would be more critical for biotechnological approaches to oil crop improvement to facilitate physiological, biochemical, and molecular understanding of the complex phenomena stresses.
7 CONCLUSION
Metabolome profiling studies have become an area of research interest in various oilseed crops. A dozen metabolites have been identified in the growth, development, reproduction, and regulation of many traits involved in oilseed crops' physiological and adaptation processes. The roles of these genes in multiple stress responses, such as drought, cold, disease, nutrient deficiency, salinity, high-temperature stress, disease, weed, and insect pest resistance responses, have been investigated. Metabolites with extreme quantities of one species and species-specific compounds could potentially be used as metabolic markers and in developing new genotypes. Although bioactive compounds have potential functions in nutraceutical, pharmacological, and cosmetic discoveries and the expression of unique traits, this review focused on multiple stress responses. Lipids, lignin, amino acids, and other organic acid metabolites are upregulated under cold stress. Some metabolites, including proline, jasmonic acid, salicylic acid, threonine, trehalose, scopoletin, ferulic acid, caffeic acid, and melatonin, are essential for chemical defense against multiple stress conditions. Several metabolites generally contribute to oil crops' biotic and abiotic stress resistance/tolerance. We also identified seven significant pathways responsible for multiple stress responses and developed a comprehensive pathway model. Therefore, this review could provide helpful insight into metabolomics studies, prevailing functions (applications) of metabolites, and other relevant scientific information to the scientific world of oilseed crop improvement and other economically important crops.
AUTHORS' CONTRIBUTION STATEMENT
H.K. wrote the original draft, investigated it, did critical reviewing and editing, and revised it; L.W., J.Y., and C.O.O. contributed to reviewing, editing, project administration, and supervision. Y.Z., S.W., M.B, and A.A.A., contributed to reviewing and editing the manuscript. All authors read and approved the final version of the manuscript.
ACKNOWLEDGMENTS
This project was funded by the Agricultural Science and Technology Innovation Program of the Chinese Academy of Agricultural Sciences (CAAS-ASTIP-2024-OCRI), the China Agriculture Research System (CARS-14), the Hubei International Science and Technology Cooperation Project (2022EHB034, 2024EHA055), the Science and Technology Innovation Project of Hubei province (2024-620-000-001-031), the Fundamental Research Funds for Central Non-profit Scientific Institution (1610172023003), and the National Center for Crops Germplasm Resources (NCCGR-2023-016).
AVAILABILITY OF DATA AND MATERIAL
Not applicable.




