Insights into Bacillus zanthoxyli HS1-mediated systemic tolerance: multifunctional implications for enhanced plant tolerance to abiotic stresses
Abstract
Abiotic stresses significantly impact agricultural productivity and food security. Innovative strategies, including the use of plant-derived compounds and plant growth-promoting rhizobacteria (PGPR), are necessary to enhance plant resilience. This study delved into how Bacillus zanthoxyli HS1 (BzaHS1) and BzaHS1-derived volatile organic compounds (VOC) conferred systemic tolerance against salt and heat stresses in cabbage and cucumber plants. Direct application of a BzaHS1 strain or exposure of BzaHS1-derived VOC to cabbage and cucumber plants promoted seedling growth under stressed conditions. This induced systemic tolerance was associated with increased mRNA expression and enzymatic activities of superoxide dismutase (EC 1.15.1.1), catalase (EC 1.11.1.6), or ascorbate peroxidase (EC 1.11.1.1), leading to a reduction in oxidative stress in cabbage and cucumber plants. Plants co-cultured with BzaHS1 and exposed to BzaHS1-derived VOC triggered the accumulation of callose and minimized stomatal opening in response to high salt and temperature stresses, respectively. In contrast, exogenous treatment of azelaic acid, a well-characterized plant defense primer, had no significant impact on the seedling growth of cabbage and cucumber plants grown under abiotic stress conditions. Taken together, BzaHS1 and its VOC show potential for enhancing plant tolerance responses to salt and heat stresses through modulation of osmotic stress-regulatory networks.
1 INTRODUCTION
Environmental stresses, such as high salinity, drought, flooding, noxious temperatures (heat and cold), and UV radiation, are the most disastrous threats to sustainable agriculture worldwide. Due to tremendous climate change, the adverse effects of environmental stresses on plant production are worsening gradually (Mohammadi et al., 2023). Additionally, climate change that plants have never experienced yet can influence the disease susceptibility of plants. Therefore, it is likely that environmental stresses lead to unpredictable transitions in the agroecosystem (Velásquez et al., 2018; Yang et al., 2023). Among them, soil salinity and high temperature are detrimental aspects of agriculture and farming systems that limit crop production worldwide (Cohen & Leach, 2020; Machado & Serralheiro, 2017). When plants reside in soils with higher salinity or temperature than crops can tolerate, plant growth and development are retarded, and crop productivity is significantly reduced. These inhibitions result from an assortment of physiological and biochemical changes in plants, including less water uptake, excess water loss from the leaf, reduced nutrient assimilation by roots, and expanded oxidative stress (Zhang et al., 2022; Zhu, 2016).
Physical and chemical management methods for controlling reasonable salt content in soils may need to be more environmentally friendly or offer long-lasting effects (Shaygan & Baumgartl, 2022). Genetic approaches to breed tolerant plants require a lengthy process, and biotech crops may not be applicable owing to social issues in many countries (He et al., 2018; Kotula et al., 2020). As an alternative way to manage abiotic stresses, plant growth-promoting rhizobacteria (PGPR) and their associated secondary metabolites are being highlighted as biostimulants because of their capabilities to confer tolerance on plants against abiotic stresses (Dimkpa et al., 2009; Yang et al., 2009). For instance, inoculating tomato (Solanum lycopersicum) plants with a PD1.5 strain (Arthrobacter sp.) and RC5.5 strain (Pseudomonas sp.) more effectively alleviated a disadvantage caused by salt stress than chemical fertilizer treatments. Moreover, this inoculation strategy effectively mitigated the morphological imbalances induced by chemical fertilizers (Cordero et al., 2018). Some PGPR strains, especially Bacillus spp., emit bacterial volatile organic compounds (VOC) that play important roles in plant-microbe interactions and positively affect plant growth and tolerance response to abiotic stresses, such as drought, salinity, and mineral deficiency (Costa Almeida et al., 2023; Fincheira et al., 2021; Liu & Zhang, 2015). Tolerance of Arabidopsis (Arabidopsis thaliana) plants against drought stress increased when plants were treated with 2,3-butanediol, a volatile compound produced by a P. chlororaphis O6 strain. This positive effect resulted from enhanced stomatal closure and reduced water loss during drought conditions (Cho et al., 2008). Nonetheless, since induced systemic tolerance (IST) by PGPR and their derived compounds was often restricted depending on plant species, growth/developmental stages, and environmental conditions, continuous identification of beneficial PGPR and PGPR-derived compounds should be necessary.
The exogenous application of plant-derived compounds to plants can also enhance plant tolerance against abiotic stresses. Salicylic acid (SA) applied exogenously on both tomato leaves and roots mitigated the harmful effects of salt stress on plant growth (Souri & Tohidloo, 2019). Exogenous proline treatment promoted the accumulation of osmotic adjustments and the activation of antioxidant enzymes in false wheatgrass (Leymus chinensis) plants, ameliorating the harmful effects of abiotic stresses (Sun & Hong, 2010). In addition, unsaturated fatty acids were engaged in the tolerance response of plants against abiotic stresses (Fan et al., 2017; He & Ding, 2020; Mata-Pérez et al., 2016). Azelaic acid (AzA), identified as a primer for systemic resistance during plant immunity, is a lipid-derived dicarboxylic acid produced by plants (Cecchini et al., 2019; Jung et al., 2009). Additionally, AZELAIC ACID INDUCED 1 (AZI1), whose expression was positively regulated by AzA, is involved in the freezing tolerance response in Arabidopsis (Xu et al., 2011), indicating that AzA can have multiple roles in plant responses to biotic and abiotic stresses. Thus, revealing the interactions between AzA and beneficial plant-associated microbes may present a promising avenue for agricultural innovation.
A few years ago, our research group isolated a Bacillus zanthoxyli HS1 (hereafter BzaHS1) strain from an agricultural field that promoted plant growth at the juvenile stage under high salinity conditions and inhibited disease development (Usmonov et al., 2021). This study implies the potential of BzaHS1 as a biostimulant capable of enhancing disease resistance and stress tolerance in plants. However, how the BzaHS1 conferred IST on vegetable plants has yet to be fully elucidated. In this study, we investigated the effects of the BzaHS1 and BzaHS1-derived VOC on the tolerance response of cabbage (Brassica rapa) and cucumber (Cucumis sativus) plants grown under stressed conditions. Soil drenching with BzaHS1 and exposing plants, especially roots, to the BzaHS1-derived VOC effectively triggered plant tolerance response to high salt stress. BzaHS1-treated cabbage and cucumber plants also exhibited thermotolerance. Given the previously characterized role of AzA in plant stress responses, we explored whether there would be any synergistic effects when combining AzA with BzaHS1 or BzaHS1-derived VOCs. Unexpectedly, no synergistic or additional effect exists between AzA and BzaHS1 or BzaHS1-VOC on tolerance response. Furthermore, we also examined several physiological and biochemical changes seen in AzA-, BzaHS1- and BzaHS1-derived VOC-treated plants compared with those in mock-treated plants. These findings propose that the BzaHS1 strain and its associated VOC could serve as valuable tools for enhancing the resilience of cabbage and cucumber plants in response to high salt and temperatures, offering promising approaches for sustainable agricultural practices in the face of changing environmental conditions.
2 MATERIALS AND METHODS
2.1 Plants and bacterium
Cabbage (Brassica rapa spp. pekinensis cv. Ryeong-gwang) and cucumber (Cucumis sativus cv. Jo-eun-baeg-log-da-da-gi) seeds were sterilized with 70% ethanol (30 s) and 20% NaOCl (5 min), then rinsed with sterile distilled water. We sowed these sterilized seeds on Murashige and Skoog (MS) medium (pH = 5.8) (Duchefa), which contained 0.9% phytoagar (w/v) and 2% sucrose (w/v). We kept them at 4°C for 2 days (for cabbage) or 3 days (for cucumber) until transplanting to soils (Bioplug®, Nongwoo Bio). These seedlings were cultured in an environmentally-controlled growth chamber during experiments (12 h night at 25°C/12 h day at 28°C).
A Bacillus zanthoxyli HS1 strain, previously isolated from the rhizosphere soils of field-grown cucumbers in Gunsan, Korea (Usmonov et al., 2021), was freshly cultured in tryptic soy broth media (30 g L−1, Difco) at 30°C overnight. The resulting bacterial culture was diluted to approximately 1.3 × 107 CFU mL−1 (OD600 = 0.5). We then poured 10 mL of freshly prepared bacterial suspension on a magenta box containing 50 g of Bioplug® soils before transplanting.
2.2 Analysis of seedling growth in vitro culture
On the day preceding the plant experiments, 5, 15, and 25 μL of freshly prepared bacterial cultures (approximately 1.3 × 107 cfu mL−1) were dropped and cultured on one side of divided Petri dishes (I-plate; Fisher Scientific) filled with tryptic soy agar. Meanwhile, 4 sterilized cabbage and cucumber seeds were placed on the other side of the I-plates containing MS solid medium. As a mock, sterile distilled water was applied dropwise on the side. To mitigate CO2 influence, 5 mL of 0.3 N Ba(OH)2 was applied in a small round Petri dish (∅ = 6 mm) (Lee et al., 2012). One day before the plant experiments, 5, 15, and 25 μL of freshly prepared bacterial cultures (approximately 1.3 × 107 cfu ml−1) were dropped and cultured on another small Petri dish containing tryptic soy agar. Subsequently, both Petri dishes were positioned inside a larger square plate filled with MS, and we planted 5 sterilized cabbage and cucumber seeds on the square plate. After one week of cultivation, the total fresh weights of the plants were recorded. As a control, sterile distilled water was applied dropwise on the tryptic soybean agar media.
2.3 Treatments of various stimuli and environmental stresses
Four 2-day-old cabbage and 3-day-old cucumber seedlings were transplanted to soils treated with AzA and BzaHS1 and/or exposed to BzaHS1-derived VOC, as described in Figure 1A. Seven days after transplanting (DAT), we drenched 10 mL of 100 mM (for cabbage) or 150 mM (for cucumber) NaCl solution into the pots every 2 days to induce high salt stress. For heat stress, 7-day-old plants were exposed to high temperatures of 36 ± 2°C for 2 days and then moved to normal growth conditions (12 h night at 25°C/12 h day at 28°C). We evaluated tolerance responses against high salt or high temperature one week later (Figure 1B).
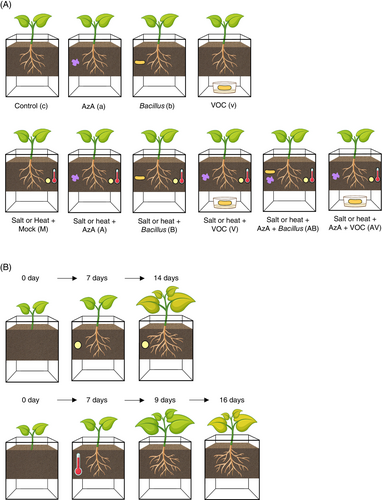
2.4 Measurement of growth parameters
Plants were further harvested after being exposed to stress for 7 days, and their shoot and root weights (mg), heights (cm), and leaves areas were measured. The total leaf chlorophyll and chlorophyll a (chla) and chlorophyll b (chlb) contents were determined at 7 days after salt and high-temperature treatments, as previously described (Arnon, 1949). Briefly, we collected leaf discs (0.2 g) from 4 plants, placed them in a falcon tube containing 3 mL of 80% acetone, and kept the tube at −10°C overnight. The absorbances of chla and chlb were read at 663 nm and 645 nm wavelengths by UV–visible spectrophotometer (EPOCH2TSC, BioTek). The concentrations of total chlorophyll, chla, and chlb were calculated according to the following equations (Arnon 1949): chla = (0.0127𝐷663–0.00269𝐷645), chlb = (0.0229𝐷645–0.0468𝐷663), and total chlorophyll = chla + chlb (𝐷663, OD = 663 nm; 𝐷645, OD = 645 nm).
The stomatal aperture of 9-day-old seedlings exposed to high temperature for 2 days was measured microscopically after placing epidermis peels on glass slices (Kostaki et al., 2020). The ratio of width to height of stomatal apertures was determined using Image J. Statistical analysis was conducted using a one-way ANOVA-Tukey (n = 12, p < 0.05) or a two-tailed Student's t-test (n = 12, *p < 0.05).
2.5 Real-time quantitative reverse transcription PCR analysis
Total RNA extraction was performed using the RNeasy Plant Mini kit (Qiagen) according to the manufacturer's instructions. For DNase I (Roche) treatment: 3 μL of 10 X buffer, 2 μL of DNase I (200 U mL−1), and 25 μL of total RNAs were gently mixed, and then the reactants were incubated at 37°C for 30 min. After adding 270 μL of DEPC-treated distilled water and PCI (phenol:chloroform:isoamyl alcohol = 25:24:1) to the reactant, the mixtures were centrifuged (16,000 x g) at 4°C for 15 min, and 200 μL of supernatants were taken from them for ethanol precipitation. The resulting precipitates were dissolved in 50 μL of DEPC-treated RNase-free water. To synthesize cDNA, 10.5 μL of total RNA (~1 μg) from each sample was mixed with 10 μM of oligo dT and 2 μL of 10 mM dNTP, and the mixture was incubated at 65°C for 5 min. Next, we added 5 X first strand buffer, 2 μL of 0.1 mM DTT, and 0.5 μL reverse transcriptase (Invitrogen) to the previous mixture and then incubated the reactant at 42°C and 70°C for 1 h and 15 min, respectively. The synthesized cDNAs were diluted 10 times and stored at −20°C. RT-qPCR was performed by mixing cDNA, gene-specific primers, and SYBR Green PCR master mix (Q-Master Mix (2X), GENET BIO) in a reaction volume of 20 μL. The instrument program was set for initial denaturation at 95°C for 3 min, then proceeded to 30 cycles of 30 sec each at 95°C, 50°C, and 72°C for cabbage, and 40 cycles at 95°C, 60°C, and 72°C for 30 sec each for cucumber. The relative transcript levels of genes involved in detoxification and antioxidant response were analyzed using gene-specific primers (Table S1). Three biological replicates with 3 technical repeats were used for the mRNA analyses. Relative mRNA expression values were normalized against BrACTIN (accession no. XM_009147610.3) and CsACTIN (accession no. XM_011659465.1) genes for cabbage and cucumber, respectively, and calculated by using the 2−∆∆Ct method (Livak & Schmittgen, 2001).
2.6 Determination of SOD, CAT, and APX activities
Frozen leaf tissues (0.2 g) collected from 14-day-old cabbage and 16-day-old cucumber plants were homogenized using a mortar and pestle, along with 2 mL of ice-cold phosphate buffer (pH 7.8, 50 mM) containing 1 mM EDTA. After centrifugation (14,000 x g) for 15 minutes at 4°C, the supernatant was used for assessing the activities of superoxide dismutase (SOD), catalase (CAT), and ascorbate peroxidase (APX) (Qui et al., 2008).
SOD activity (EC 1.15.1.1) was assessed by evaluating its ability to impede the photoreduction of nitro blue tetrazolium (NBT), according to Chen & Zhang (2016), with minor modification. The reaction mixture contained 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 130 mM methionine, 0.75 mM NBT, 0.02 mM riboflavin, and 0.1 mL plant extract. Reaction mixtures kept under dark and light for 15 min each without crude extract were used as control I and II, respectively. The absorbance of the reaction mixture was recorded at OD = 560 nm, and SOD activity was quantified based on the enzyme amount necessary to induce a 50% inhabitation in the NBT photoreduction rate and reported as unit (U) mg−1 protein.
To measure CAT activity (EC 1.11.1.6), plant crude extract (0.1 mL) was added to the reaction mixture (100 mM phosphate buffer [pH 7.8], 0.1 μM EDTA, and 0.1% hydrogen peroxide [H2O2]) (Chen & Zhang, 2016). The changes of hydrogen peroxide were monitored immediately, followed by every 15 sec for 3 minutes at OD = 240 nm and calculated as U per mg of protein using the following formula: CAT activity (U mg−1 protein) = ΔA240 x (V/Vt)/(0.1 x t)/Cp (ΔA240, the change of absorbance at 240 nm during every 15 sec; V, the total volume of crude enzyme solution; Vt, the volume of crude enzyme used in the testing tube; t, the reaction time (min); Cp: the crude protein concentration (mg mL−1).
The activity of APX (EC 1.11.1.1) was assessed following the method of Nakano & Asada (1981). Hydrogen peroxide (0.1 mM) was added to the reaction mixture, which comprised 50 mM phosphate buffer (pH 7.8), 0.1 mM EDTA, 0.5 mM ascorbate, and 0.1 mL plant crude extract. The oxidation of ascorbate was monitored at OD = 290 nm for 3 minutes. Enzyme activity was determined using the molar extinction coefficient for ascorbate (2.8 mM−1 cm−1).
2.7 Analysis of stress-related metabolites
The first leaves of 14-day-old cabbage and cotyledons of 16-day-old cucumber plants were harvested to evaluate the callose deposition responding to high salt stress. We destained detached leaves using an alcoholic lactophenol (phenol:glycerol:lactic acid:water:ethanol = 1:1:1:1:8) overnight and stained them with 0.01% aniline blue (w/v) for 4 h (Nguyen et al., 2010). Deposited callose in leaves was observed under epifluorescence microscopy with excitation/emission wavelengths of OD = 377 nm/447 nm (Olympus BX60, Olympus) and quantified using digital photographs and analyzed with Image J software (Mafurah et al., 2015). Different letters indicate statistical differences (n = 5, p < 0.05, one-way ANOVA-Tukey).
The experimental procedure previously described by Bates et al. (1973) was followed with some modifications to measure the proline concentration in plants. Leaf tissue (0.1 g) was homogenized with a mortar and pestle in 10 mL of 3% sulfosalicylic acid (Sigma-Aldrich). After centrifugation (10,000 x g) for 10 min, 2 mL of the supernatant was transferred to a 45 mL Falcon tube (SPL). The same volume of an acid-ninhydrin solution (1.25 g ninhydrin [Sigma-Aldrich] in 30 mL glacial acetic acid and 20 mL of 6 M orthophosphoric acid [Sigma-Aldrich]) was added to the supernatant. Then, the mixture was heated at 100°C in a water bath for 1 h. The mixture was cooled on ice and agitated vigorously for 20 ~ 30 sec in a shaker incubator at 250 rpm after adding 4 mL of toluene. The samples were then kept in the dark at room temperature for 30 min, and 240 μL of aqueous toluene-containing phase was aspirated for spectrophotometric absorbance measurement at OD = 520 nm using a spectrophotometer (EPOCH2TSC, BioTek).
Malondialdehyde (MDA) was determined by a typical procedure with some modification (Heath & Pacher, 1969). Cabbage and cucumber leaves (0.2 g) were homogenized in 1.5 mL trichloroacetic acid (TCA) (5%, w/v) and centrifuged (10,000 x g) at 4°C for 10 min. The reaction mixture (0.5 mL of supernatant and 1 mL of 5% thiobarbituric acid (TBA) diluted in 20% TCA) was kept at 95°C for 25 min, followed by cooling on ice and centrifugation (10,000 x g) at 4°C. Absorbance was read with a spectrophotometer at OD = 532 nm and OD = 600 nm. MDA was calculated using an extinction coefficient of 155 mM cm−l.
Deposited hydrogen peroxide in leaves was detected by a 3,3-diaminobenzidine (DAB) staining method, as described previously (Huang et al., 2019). Leaf samples were cut and placed in 1 mg mL−1 DAB-HCl (pH 3.8, Sigma-Aldrich) for 6 h. The stained leaf discs were transferred to a destaining solution (ethanol:acetic acid:glycerine = 3:1:1) and boiled for 10 min. This process revealed the presence of stable reddish-brown DAB polymerizing sediments.
Hydrogen peroxide contents in the leaf tissues were quantified using spectrophotometry following its reaction with potassium iodide (KI) as described previously (Alexieva et al., 2001). The reaction mixture consisted of 0.5 mL of 0.1% TCA leaf extract supernatant, 0.5 mL of 100 mM potassium phosphate buffer, and 2 mL of a 1 M KI reagent. The reaction proceeded for 1 hour in darkness, after which the absorbance was measured at 390 nm. The concentration of hydrogen peroxide was determined by referencing a standard curve prepared with known concentrations of hydrogen peroxide.
2.8 Statistical analysis
Statistical analysis was conducted using ANOVA, and post-hoc comparisons were carried out using Tukey tests, using Minitab version 21.3.1. Significant differences were identified at a significance level of probability value ≤0.05. Specific replication numbers for the reported data are provided in each figure legend.
3 RESULTS
3.1 B. zanthoxyli HS1 strain and its-derived VOC enhance salinity tolerance in cabbage and cucumber plants
Induced disease resistance and IST stimulated by the BzaHS1 strain occurred in several vegetables against high salinity stress and bacterial pathogen infection (Usmonov et al., 2021), which proposed that the BzaHS1 strain is a potential biostimulant to control (a)biotic stresses. As aforementioned, Bacillus spp. could often produce VOC that may act as external signals among intra-, inter-species, and even inter-kingdoms (Costa Almeida et al., 2023; Poulaki & Tjamos, 2023). To see if the BzaHS1-derived VOC promotes plant growth, we cultured different amounts (5, 15, and 25 μL) of bacterial cultures (1.3 × 107 CFU mL−1) on tryptic soy agar in small Petri dishes. Seedlings of cabbage and cucumber plants exposed to the BzaHS1-derived VOC were heavier than those of non-treated plants (Figure S1A). We observed that the 15 μL concentration most significantly impacted the seedling growth of cabbage and cucumber. Based on these results, we used this bacterial concentration for subsequent experiments. To minimize the impact of the excess amount of CO2 released from the bacterial strain on seedling growth, 5 mL of Ba(OH)2 was placed in another small Petri dish in the closed system. Even though seedling growth was decreased under Ba(OH)2-treated conditions compared with non-treated conditions, the strain BzaHS1 continued to enhance plant growth even when CO2 was captured with Ba(OH)2 (Figure S1B). This suggests that CO2 is not the sole factor responsible for the observed growth promotion and indicates that BzaHS1 could employ its VOC in interacting with plants.
To test if these results were reproducible in real soil system and if the increased tolerance stimulated by the BzaHS1 strain was due to VOC released from the strain, we designed a stacked magenta box system to allow plants to smell VOC without any physical contact with the BzaHS1 strain (Figure 1A). In addition, this system allowed us to check the synergistic or additive effect of the BzaHS1 or its VOC and AzA on plant tolerance response. Under non-stressed conditions, treatments of azelaic acid, BzaHS1, and BzaHS1-derived VOCs did not significantly increase the fresh weight of cabbage and cucumber seedlings (Figure 2A,B). The fresh weights of both shoot and root in 14-day-old cabbage and 16-day-old cucumber seedlings treated with the BzaHS1 strain showed no significant difference compared to the control group (Figure S2A). While there was no notable increase in shoot growth rate, applying BzaHS1-derived VOC demonstrated a capacity to enhance root development (Figure S2A). Compared to control plants, those exposed to the VOC exhibited substantial enhancements in root growth, with increases of 77.34% in cabbage and 41.35% in cucumber, respectively (Figure S2A). Under non-stress conditions, neither co-incubation with BzaHS1 nor exposure to its derived VOC significantly affected the leaf area (Figure 2C,D). While direct treatment with BzaHS1 did not induce notable changes in shoot and root length in cabbage and cucumber plants compared to control plants, exposure to its VOC led to a reduction in root length (Figure S2B). Even though chlorophyll b level in cabbage plants was significantly higher in those treated with BzaHS1 compared to the control plants, total chlorophyll and chlorophyll a did not show a significant increase in plants treated with BzaHS1 and VOC compared to other plants (Figures 2E,F and S2C). These results indicate that supplements of BzaHS1 and its VOC do not promote plant growth when plants grow under favorable conditions.
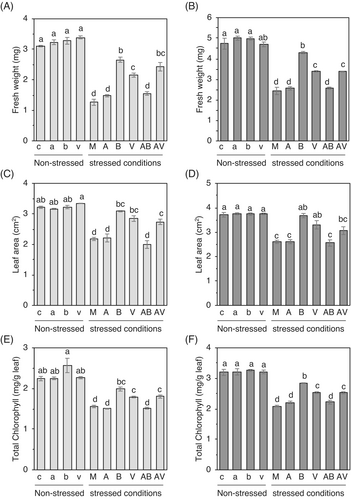
We grew cabbage and cucumber plants under different conditions and evaluated several growth parameters to examine the biological activity of the BzaHS1 and the BzaHS1-derived VOC for inducing IST against high salt stress. Compared to control plants, salt stress significantly decreased the growth rate by 59.3% and 48.59% in cabbage and cucumber (M), respectively (Figure 2A,B). Furthermore, other growth parameters, including leaf area, shoot and root weight, and shoot and root height, were observed to significantly decrease by varying degrees (Figures 2 and S2). The application of BzaHS1 (B) and its VOC (V) effectively promoted shoot and root growth in both cabbage and cucumber plants under high salt stress conditions, as compared to plants treated with salt alone (M) (Figure 2A,B). Fresh weights of shoot and root significantly increased nearly 2-fold in 14-day-old cabbage and 16-day-old cucumber seedlings nurtured with the BzaHS1 strain (B) than mock-treated plants (M) (Figure S2A). Even though the promotion rate was lower than that by direct treatment of the BzaHS1 strain, the BzaHS1-derived VOC could also facilitate seedling growth under high salt stress conditions (Figures 2A,B and S2A,B). Compared to the mock treatment, plants exposed to the BzaHS1 strain and its VOC exhibited improved seedling growth by 111.11% and 71.03% in cabbage and 76.91% and 39.38% in cucumber, respectively (Figure 2A,B). In addition, leaf areas of plants co-incubated with the BzaHS1 or exposed to the BzaHS1-derived VOC were larger than those in mock-treated plants (Figure 2C,D). Direct treatment of the BzaHS1 strain in soils also triggered the higher growth of shoots and roots on cabbage and cucumber plants than in mock treatment (Figure S2B). However, these shoot and root length increments were not observed in plants exposed to the BzaHS1-derived VOC (Figure S2B). Chlorophyll a and total chlorophyll were considerably higher in plants treated with the BzaHS1 and its VOC compared to the mock. In contrast, chlorophyll b only increased in the BzaHS1-treated plants compared to other salinized plants (Figures 2E,F and S2C).
To further analyze the IST in cabbage and cucumber against high salt stress, we measured the extent of callose deposition, a histochemical marker for tolerance response (Wang et al., 2022). Plants grown with the BzaHS1 strain deposited a high level of callose compared to those grown under different conditions employed in this study (Figure S3). Levels of deposited callose in VOC-exposed plants were also statistically different from those in mock-treated plants. Under non-stressed conditions, none of the treatments changed callose deposition (Figure S3), suggesting a nuanced response dependent on environmental cues.
On the other hand, the supplement of AzA, a primer and long-distance mobile signal for systemic acquired resistance in plants (Cecchini et al., 2019; Jung et al., 2009), could not promote growth under non-stressed conditions and protect cabbage and cucumber seedlings from high salt stress (Figures 2, S2, and S3). Instead, AzA treatment adversely affected the BzaHS1's effect on tolerance response (please compare (B) with (AB) in Figure 2) because AzA has a direct antimicrobial efficacy to this strain (Figure S4). When AzA was introduced to the soils exposed to VOC (AV), the addition did not further increase or decrease tolerance beyond the effects observed with VOC treatment alone (V) (Figures 2, S2, and S3), indicating that AzA may not be effective in tolerance of cabbage and cucumber plants against high salt stress. These results suggest that soil treatment of the BzaHS1 strain and exposure of seedlings to the BzaHS1-derived VOC can diminish the harmful effects of high salt on the seedling growth of cabbage and cucumber in this experimental system. However, AzA does not effectively induce IST on cabbage and cucumber seedlings against high salt stress. Notably, the beneficial effects of the bacteria and VOC are not observed under normal conditions but are initiated when plants are stressed. Note that the multiplication of the BzaHS1 strain remained unaffected by the salt concentration introduced into our soil (Figure S5A).
3.2 B. zanthoxyli HS1 strain confers thermotolerance on cabbage and cucumber plants
Considering the rising global temperature, unexpected fluctuations threaten maintaining a sustainable agricultural ecosystem (Velásquez et al., 2018; Yang et al., 2023). To test if the BzaHS1 could induce thermotolerance in plants, we incubated 7-day-old seedlings at 36 ± 2°C for 2 days and transferred heat-treated plants to normal growth conditions (12 h night at 25°C/12 h day at 28°C). Exposure to high temperatures (M) led to a significant reduction in all measured growth parameters of the seedlings, compared with those of plants grown under normal conditions (c, a, b, and v) (Figures 3 and S6). Contrary to high salt stress conditions, treating soils with the BzaHS1 only led to increased growth in both shoots and roots, in weight and length, for cabbage and cucumber plants under heat stress (Figures 3A,B and S6A,B). A significant difference was also observed in the leaf area in cabbage and cucumber plants co-cultivated with the BzaHS1 (Figure 3C,D). Notably, compared with mock-treated plants, cabbage plants treated with the BzaHS1 showed increased levels of leaf chlorophyll a and total chlorophyll. In contrast, the BzaHS1-treated cucumber plants exhibited higher total chlorophyll contents than mock-treated plants under heat stress conditions (Figures 3E,F and S6C).
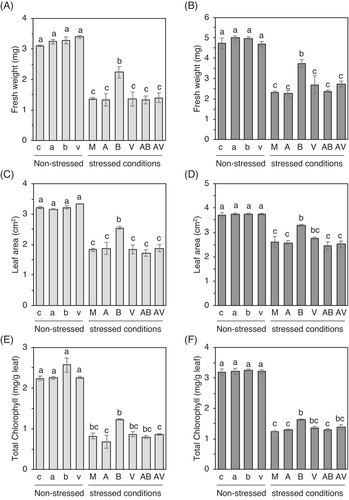
High temperature causes increased leaf temperature but also severe water loss from the leaves of plants; thus, plants need to modulate stomatal closure with sophisticated mechanisms (Sadok et al., 2021). To disclose the relationship between BzaHS1-induced thermotolerance and stomata-mediated tolerance, we directly measured the ratio of width to height of stomatal aperture in the leaves of 9-day-old cabbage and cucumber plants pre-exposed to high temperature for 2 days. The measurement of the stomatal aperture revealed that, compared to unstressed controls, both cabbage and cucumber actively close the stomata aperture to avoid dehydration under high temperature conditions. Moreover, BzaHS1 treatment in soils further triggered stomatal closure, amplifying this natural response and aiding in the mitigation of water loss in leaf tissues following heat stress (Figure S7). Note that the statistical difference between the mock-treated and the BzaHS1-treated plants was detected by a Student's t-test (p < 0.05), not an ANOVA-Tukey test (p < 0.05). Other treatments did not elicit significant changes in the stomata aperture (Figure S7). These results demonstrated that soil treatment of the BzaHS1, which grew well at 30 and 37°C (Figure S5B), allows plants to hold water effectively against high temperatures via a stomata-mediated tolerance response.
3.3 Bacillus and its VOC regulate the transcription and enzyme activity of antioxidant proteins in cabbage and cucumber plants under stresses
In general, environmental stresses cause the generation of excess amounts of reactive oxygen species (ROS) in plants. ROS leads to oxidative stress that damages plant cells in many ways, even though ROS is a key player in tolerance response (Gechev & Hille, 2005; Triantaphylidès et al., 2008; You & Chan, 2015). Therefore, to scavenge and detoxify ROS for maintaining cellular homeostasis, plants have developed highly efficient enzymatic antioxidant defense systems that collaborate to regulate uncontrolled oxidation cascades, thus safeguarding plant cells against oxidative damage (Gill & Tuteja, 2010). To test if exogenous treatments of the BzaHS1 strain or its VOC influenced antioxidant responses, we checked mRNA levels and enzymatic activities of superoxide dismutase, catalase, and ascorbate peroxidase in leaves of cabbage and cucumber plants growing with different combinations before and after stress treatment. In cabbage, AzA, BzaHS1, and its VOC did not significantly alter mRNA levels and enzyme activities compared to the control plants grown under normal growth conditions (Figures 4 and S8). In contrast, salt stress induced varied responses in the mRNA levels of BrSOD and BrCAT, with differing magnitudes of increase observed (Figure 4A,B; please compare non-stressed plants to stressed plants). In the case of BrAPX, plants did not show a comparable increase by high salt treatment, compared with non-stressed plants (Figure S8A). At the same time, heat stress led to a significant elevation of BrAPX mRNA levels (Figure S8B). Under high salt stress, mRNA levels of BrSOD and BrCAT significantly increased by 1.73 and 1.58-fold, respectively, in cabbage plants co-cultivated with the BzaHS1 strain (B) (Figure 4A,B). The sensing of VOC (V) by cabbage plants also increased the relative expression of BrSOD and BrCAT compared to that of mock-treated plants (M) (Figure 4A,B). In the same line with transcriptional regulation, cabbage plants treated with the BzaHS1 and its VOC showed a significant increase in SOD and CAT activities under salt stress (Figure 4C,D). On the other hand, the exogenous application of AzA (A) did not induce transcription and activate the enzymatic activity of these antioxidant enzymes in cabbage plants (Figure 4).
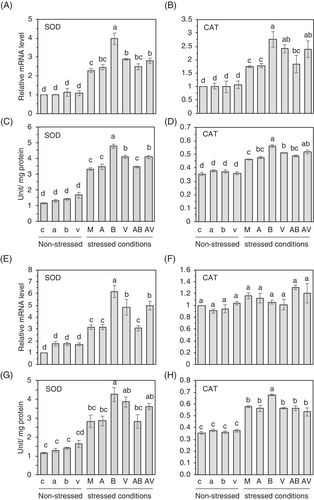
In the case of response to heat stress, treatment of the BzaHS1 strain and its VOC increased the expression of BrSOD mRNA in cabbage plants after heat stress treatment (Figure 4E). None of the treatments could regulate the mRNA expression of BrCAT and BrAPX in plants that were under high temperature for 2 days (Figures 4F and S8B). In addition, treatment of the BzaHS1, but not the BzaHS1-derived VOC, was influential in elevating SOD and CAT activities in the leaves of cabbage plants pre-exposed to high temperature (Figure 4G,H).
In cucumber plants, the applications of the BzaHS1 strain and its VOC resulted in increased expression of CsSOD and CsCAT under salt stress conditions (Figure 5A,B). However, treatment of the BzaHS1 strain and its VOC did not trigger the transcription of CsAPX in cucumber leaves under high salt conditions (Figure S8A). Notably, the treatment with AzA positively regulated CsSOD mRNA expression, even though co-treatment of AzA with the BzaHS1 strain or its VOC did not display an additive/synergistic effect (Figure 5A). BzaHS1- and its VOC-treated plants exhibited increased SOD and CAT activity in comparison to the other treatments under salt stress conditions (Figure 5C,D). In concordance with the observed elevation in relative mRNA levels, exposure to VOC induced the highest increase in SOD activity (Figure 5C). However, the BzaHS1 strain and its VOC did not affect the transcription and enzymatic activity of APX in cucumber leaves (Figure S9).
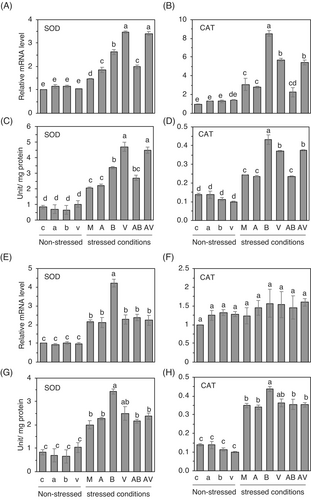
After heat treatment, the BzaHS1 treatment also increased CsSOD mRNA expression in cucumber plants (Figure 5E). mRNA levels of CsAPX and CsCAT after heat stress were not altered by any treatments examined in this study in cucumber (Figures 5F and S9). Moreover, following heat treatment, only the BzaHS1 treatment exhibited a significant increase in SOD and CAT activity, while neither Bacillus treatment nor any other interventions demonstrated discernible effects on APX activity (Figures 5G,H and S9). AzA treatment did not have any synergic impact on enzyme activities when it was applied in soil alone (A) or with the BzaHS1-derived VOC (AV) (Figures 4 and 5). Instead, AzA suppressed the BzaHS1-induced enzyme activity when AzA was applied to the soils with the BzaHS1 (AB) in cabbage and cucumber under both salt and heat stress (Figures 4 and 5). Collectively, these results show that plants cultured with the BzaHS1 or exposed to the BzaHS1-derived VOC may activate transcription and enzymatic activities of antioxidant enzymes, particularly SOD and CAT, to detoxify superoxide generated by oxidative stress, thereby establishing salt tolerance and thermotolerance.
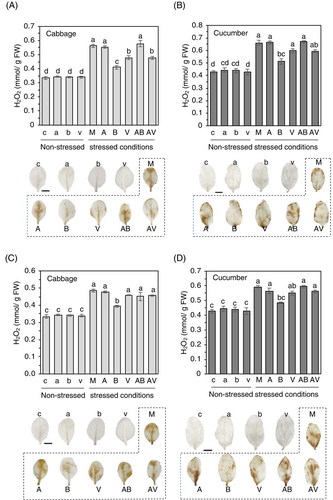
3.4 Hydrogen peroxide levels significantly decrease in stressed cabbage and cucumber leaves treated with Bacillus and its VOC
Considering the increased enzymatic activities of SOD and CAT in cabbage and cucumber plants, we presumed that levels of hydrogen peroxide would be reduced in plants treated with the BzaHS1 strain or its VOC. Exogenous treatments of AzA, BzaHS1, and its VOC could not affect hydrogen peroxide levels in non-stressed cabbage and cucumber plants (Figure 6). However, exposure to salt and heat stresses led to a notable increase in hydrogen peroxide levels (Figure 6). This suggests that high salt and high temperature triggered oxidative stress in the plants, possibly impacting their physiological responses and stress tolerance. Expectedly, treatment of the BzaHS1 and the BzaHS1-derived VOC decreased the level of hydrogen peroxide, compared with mock treatment, in both cabbage and cucumber leaves grown under high salt conditions (Figure 6A,B). Contrary to the salinity stress response, exposure to the BzaHS1-derived VOC alone did not exert any discernible effect on hydrogen peroxide production under heat stress conditions (Figure 6C,D). Treatment with AzA did not exhibit any mitigating effect on hydrogen peroxide production under either salinity or heat stress conditions. Intriguingly, AzA treatment interrupted the alleviating effect of the BzaHS1 (Figure 6), supported by an inhibition effect of AzA on the growth of the Bacillus strain (Figure S4). Notably, plants exposed to the BzaHS1 strain exhibited a significant decrease in hydrogen peroxide levels, indicating a unique influence of the BzaHS1 strain in mitigating oxidative stress during heat exposure (Figure 6C,D).
3.5 Induced tolerance by B. zanthoxyli HS1 and its VOC does not tightly accompany proline and MDA accumulation
Plants grown with the BzaHS1 strain or exposed to the BzaHS1-derived VOC exhibited reduced sensitivity and more robust antioxidant responses than mock-treated plants to high salt and temperature stresses (Figures 2-6). To see whether or not treatments with the BzaHS1 and its VOC could regulate the level of osmoprotectants in plants, we measured endogenous proline levels, one of the representative metabolites (Szabados & Savouré, 2010; Yoshiba et al., 1997), in leaves of cabbage and cucumber grown under various conditions, as aforementioned (Figure 1). The presence of any of the treatments tested in this study did not induce significant changes in the levels of proline and MDA in cabbage and cucumber plants grown under non-stressed conditions (Figure 7). On the other hand, cabbage and cucumber plants exposed to high salt and temperature (M) exhibited elevated levels of proline and MDA compared with non-stressed plants (Figure 7). Cabbage and cucumber plants grown in soils treated with AzA (A) under salt stress conditions showed slightly decreased levels of proline, as compared to those in mock-treated plants (M) (Figure 7A,B). Exogenous treatment of the BzaHS1 (B) or its VOC (V) caused the dramatic decline of proline levels in cabbage and cucumber leaves grown under high salt conditions compared to that in mock-treated plants (Figure 7A,B). Under heat stress conditions, applying the BzaHS1 was the only one significantly decreasing proline levels in cabbage plants (Figure 7C). In contrast, no such proline reduction was detected in cucumber plants following the treatment (Figure 7D). These observations suggest that IST by the BzaHS1 against high salt and high temperature might be independent of the proline or plants treated with the BzaHS1, and its VOC might be less sensitive to environmental stresses.
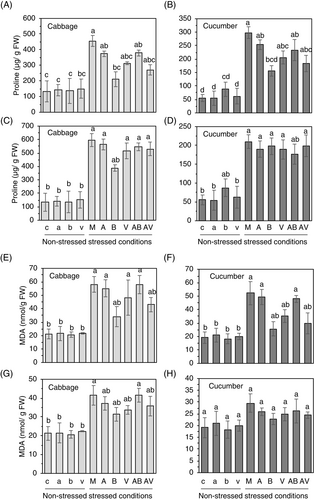
MDA is used as a marker to determine whether or not plants were damaged by oxidative stresses (Jbir-Koubaa et al., 2015; Morales & Munne-Bosch, 2019). Under high salt stress conditions, MDA levels in both cabbage and cucumber leaves decreased after treatment with BzaHS1 and its VOC compared to those in mock-treated plants (Figure 7E,F). In the case of heat stress, only the BzaHS1 and its VOC treatments resulted in slightly decreased MDA accumulation compared to the mock treatment in cabbage, but not in cucumber plants (Figure 7G,H). Taken together, these results demonstrate that the exogenous treatment of the BzaHS1 strain alleviates oxidative stresses in plants caused by high salt and high temperature.
4 DISCUSSION
Numerous previous studies have well-documented the roles of PGPR, including Bacillus spp., Pseudomonas spp., and Rhizobium spp., in regulating abiotic stress tolerance and induced resistance through both direct and indirect interaction between plants and PGPR (Costa Almeida et al. 2023; Dimkpa et al., 2009; Fincheira et al., 2021; Liu & Zhang, 2015; Pieterse et al., 2014; Yang et al., 2009). Our preceding study indicated that the exogenous application of the BzaHS1 strain could initiate IST to protect plants from high salinity stress (Usmonov et al., 2021). Herein, we have characterized the mechanisms stringently regulated by the interaction between plants and BzaHS1 and its VOC. These mechanisms include (1) the increased transcription and enzymatic activity of antioxidant enzymes, (2) the increase in chlorophyll levels, (3) the accumulation of callose in the leaves as a defense against high salt stress, and (4) the reduction of water evaporation facilitated by a decrease in stomata aperture width, which is a response to high temperature stress. Consequently, this interaction protects cabbage and cucumber plants (at least until the 4-leaf stage) from high salt and temperature stresses. The interactions between plants and the BzaHS1 or its VOC can cause significant changes in plant tolerance response to stress. However, there are usually no substantial alterations in the absence of stress, except for roots treated with the BzaHS1-derived VOC. Similarly, the B. subtilis HAS31 strain did not trigger potato plant growth or stimulate stress response under well-watered conditions; however, supplementing this strain rendered potato plants tolerant to drought stress (Batool et al., 2020). On the other hand, the BzaHS1-derived VOC promotes seedling growth in an in vitro culture system, although plants were not exposed to obvious stresses. We deduce that the nutrient availability of MS media differs from that in soils, which results in different biological effects on seedling growth. This observation underscores how plants selectively modulate their physiology and regulate interactions with microbes through stress-induced signaling pathways, enabling tailored responses for adaptation and survival under environmental stress.
Pouring bacterial culture into the soil was the most efficient way to initiate IST in cabbage and cucumber plants. The growth-promoting activity was observed through significant improvements in shoot and root biomass and leaf area in cabbage and cucumber seedlings. The biological importance of Bacillus spp. within agroecosystems is robustly underpinned by these findings (Poulaki & Tjamos, 2023). Notably, the direct application of the BzaHS1 strain was more efficient at inducing IST than exposure to its VOC, suggesting that the BzaHS1 strain influences plant growth in multiple ways. In addition to volatile compounds, this strain may be able to secrete non-volatile compounds that are potent activators of IST in plants. From a different point of view, the BzaHS1 itself and its derived compounds might be able to restructure the rhizosphere microbiota community in cabbage and cucumber plants, as described in previous case studies (Afridi et al., 2022; Escudero-Martinez et al., 2022; Kong & Liu, 2022). While further research is warranted, either reshaping rhizosphere microbiota or diminishing dysbiosis by high salt and temperature might be another viable mechanism by which the BzaHS1 activates IST in vegetables. Therefore, the BzaHS1 shows promise as a multifaceted agent that enhances plant growth under high salinity and temperature environments and serves as a natural modulator of the rhizosphere microbiome.
Chlorophyll plays a crucial role in plant photosynthesis by helping plants capture energy from light. Abiotic stresses, such as salinity and heat, commonly lessen the chlorophyll levels within plants, negatively affecting plant photosynthesis efficiency (Chauhan et al., 2023). Applying PGPR has significantly boosted chlorophyll levels in crops exposed to abiotic stresses (Enebe & Babalola, 2018; Khanna et al., 2019; Nadeem et al., 2007). In this study, exposure to the BzaHS1 and its VOC led to increased chlorophyll contents of cabbage and cucumber leaves grown under stress conditions, indicating their role in mitigating the adverse impacts of salt and heat stress on photosynthesis.
PGPR, as mutualists of plants, actively regulate the expression and activity of the antioxidant enzymes in plants, leading to improved tolerance to abiotic stresses (Batool et al., 2020; Bharti et al., 2016; Khan et al., 2020; Liu et al., 2020). Exogenous treatments of the BzaHS1 and its VOC upregulated the expression of the SOD and CAT genes under salt stress conditions and transcription of the SOD gene following high-temperature treatment in cabbage and cucumber seedlings. These mRNA analyses suggest that plants co-cultured with the BzaHS1 and exposed to its VOC employ SOD and CAT-dependent pathways to detoxify superoxide radicals under stressed conditions. Additionally, significantly higher levels of antioxidant enzymatic activities were detected in the salt- or high-temperature-stressed cabbage and cucumber plants treated with BzaHS1 and its VOC compared with those in mock-treated plants. This demonstrates that plants could utilize an antioxidant defense system to alleviate the oxidative damage caused to plants by high salt and temperature stress. Increased mRNA and enzyme activity of SOD, an initial antioxidant enzyme, was notably observed in cabbage and cucumber plants after the BzaHS1 or its VOC treatment, followed by a significant rise in CAT activity to scavenge hydrogen peroxide. Consequently, the level of hydrogen peroxide drastically decreased in the leaves of the BzaHS1- and/or its VOC-treated cabbage and cucumber plants grown under stress conditions. While an upregulation in BrCAT and CsCAT genes' expression was not detected, their enzymatic activities exhibited a remarkable increase of 17.33% and 25.57% in cabbage and cucumber plants exposed to the BzaHS1 strain under heat stress, respectively. The modulation of CAT activity is influenced by various factors, including the availability of its substrate, hydrogen peroxide. Increasing substrate availability can elevate enzyme activity without a corresponding change in mRNA expression.
As a representative osmoprotectant and antioxidant defense molecule, proline helps maintain osmotic balance and reduce ROS levels by functioning as a hydroxyl-free radical scavenger. Some strains of Azotobacter spp., for instance, enhance plant response to salt stress by improving Na+ exclusion and K+ uptake and increasing proline and chlorophyll content (Rojas-Tapias et al., 2012). Soybean plants exposed to the VOC produced by P. simiae AU strain and P. pseudoalcaligenes displayed intensified tolerance to salt and drought stress, evidenced by higher proline levels and upregulation of proteins related to photosynthesis and ion homeostasis (Vaishnav et al., 2015; Yasmin et al., 2021). However, there have been reports of PGPR treatments reducing proline levels (Hmaeid et al., 2019; Luo et al., 2022), suggesting that PGPR-treated plants might be less sensitive to environmental stress or that PGPR might trigger IST in a proline-independent manner. These previous findings strongly support our observation that the BzaHS1- or its VOC-treated cabbage and cucumber plants had lower proline levels than mock-treated plants under high salt conditions or after heat treatment. Excessive ROS disrupts cell membranes, particularly due to lipid peroxidation (Farmer & Mueller, 2013). MDA is a primary byproduct of cellular membrane perturbation. In this study, cabbage and cucumber seedlings treated with BzaHS1 and its VOC contained lower MDA levels than mock-treated plants under stress conditions. These findings align with earlier observations that link reduced lipid peroxidation with improved stress tolerance mechanisms (Kotchoni et al., 2006; Luo et al., 2022). Given the lower proline and MDA levels in plants treated with the BzaHS1- or its VOC under stress conditions, we hypothesized that plants mitigate oxidative stress through mechanisms dependent on antioxidant enzymes and independent of proline, as mentioned above.
Callose, a polysaccharide that contributes to reinforcing the plant cell wall, is significantly deposited in plant tissues as a response to a range of biotic and abiotic stresses (Lia et al., 2023; Luna et al., 2011; Oʼlexy et al., 2018). Although it may indirectly serve as a signal compound for tolerance/resistance responses, its role in enhancing cell wall integrity is crucial for plant defense mechanisms (German et al., 2023; Wang et al., 2022). Additionally, PGPR induced intense callose deposition at infection courts compared with non-treated plants (Rodríguez et al., 2020; Tyagi et al., 2020). Our findings indicate that treating with BzaHS1 and its VOC positively regulates callose deposition, which protects plant cells from the dehydration and shrinkage associated with high salt stress.
High temperature can directly increase the transpiration rate to facilitate evaporation cooling, a process largely dependent on stomatal regulation (Sadok et al., 2021). However, stomatal response to heat stress varies among plant species and is influenced by the severity of temperature increase and the vapor pressure difference in microenvironments (Kostaki et al., 2020; Mott & Peak, 2010; Weston & Bauerle, 2007). PGPR can also control water export from the leaf by modulating stomatal conductance (Zheng et al., 2018). Moreover, B. velezensis SQR9 induced an oxidative burst in cucumber and Arabidopsis under stress-free conditions, potentially affecting ROS content and stomatal aperture. This oxidative burst mechanism may also influence stomatal responses under stress conditions (Zhang et al., 2021). In this study, we measured the stomata aperture in leaves of cabbage and cucumber plants exposed to a high temperature (36 ± 2°C) under dark conditions for 2 days. Typically, under such conditions, plants might open their stomata aperture to keep lower leaf temperature by releasing water vapor. Contrary to this, we observed reduced stomata aperture in the leaves of cabbage and cucumber plants grown with the BzaHS1 strain exposed to a high temperature for 2 days. This observation aligns with findings from studies on B. subtilis and B. amyloliquefaciens strains, which have shown that PGPR can induce stomatal closure under stress conditions through modulation of ROS pathways (Kumar et al., 2012; Wu et al., 2018). The present study demonstrated that the BzaHS1 in the soils, probably rhizosphere, contributes to inhibiting stomatal opening to reduce excessive water loss from leaves of cabbage and cucumber plants exposed to high temperature for 2 days. This adaptation may occur even at the cost of reduced evaporation cooling. The enhanced stomatal closure observed in the presence of BzaHS1 despite the reduced H2O2 levels suggests that the bacteria might be activating additional signaling pathways that promote stomatal closure. For instance, this could involve the modulation of hormonal signaling pathways, which are known to play crucial roles in stomatal regulation. The bacteria might induce a more complex defense response that includes, but is not limited to, ROS signaling. Thus, while H2O2 is a known signal for stomatal closure, BzaHS1 might help the plant achieve a more balanced oxidative state while still promoting stomatal closure through other signaling mechanisms, ensuring the plant's defense and water conservation under high-temperature stress. In addition, the potential role of abscisic acid (ABA) in the observed stomatal closure induced by BzaHS1 under high-temperature stress conditions should be considered (Jiang & Zhang, 2002). Studies have shown that certain PGPRs, such as B. licheniformis, can synthesize ABA, which can enhance plant tolerance to drought conditions by lowering water loss (Salomon et al., 2013). An alternative explanation is that cabbage and cucumber can tolerate the temperature (36 ± 2°C), and therefore, they do not need evaporation cooling for temperature regulation.
An initial aim in treating AzA in plants was to examine the synergistic or additive effect of the BzaHS1 and AzA on tolerance response in cabbage and cucumber plants. However, the addition of AzA to soils containing the BzaHS1 strain appeared to inhibit the proliferation of the BzaHS1 strain, likely due to an antibacterial property of AzA, especially Gram-positive bacteria (Charnock et al., 2004; Figure S4). Indeed, simultaneous application of AzA with the BzaHS1 interrupted the BzaHS1-induced IST. Although AzA is reported to enhance plant immunity against pathogens, as previously mentioned (Cecchini et al., 2019; Jung et al., 2009), our findings indicate that AzA did not significantly increase plant growth or defense under normal or stressed conditions in this study. This suggests that while AzA and BzaHS1 enhance plant immunity and tolerance, their effects might be context-dependent and influenced by specific environmental conditions or plant species. This observation underscores the complexity of interactions between biostimulant and microbial communities, suggesting that not all beneficial effects on plants are additive.
Taken together, we confirmed that the BzaHS1 and its VOC induce systemic tolerance in cabbage and cucumber seedlings in response to high salt and temperature stresses. Further studies are necessary to identify the specific BzaHS1-derived VOC that are responsible for enhancing plant tolerance and to decipher their mode of action. By exploring the interactions between exogenous compounds and plants and identifying crucial molecular mediators, we can develop innovative approaches toward achieving sustainable agriculture in the face of escalating environmental challenges.
AUTHOR CONTRIBUTIONS
A.B. and H.W.J. designed the research. A.B. performed the research, and A.B. and H.W.J. analyzed and interpreted the data. A.B. and H.W.J. wrote the paper.
ACKNOWLEDGEMENTS
This work was supported by the National Research Foundation of Korea (NRF) grants funded by the Korea government (2020R1A6A1A03047729) and an Agenda Research Program, the Rural Development Administration, Korea (RS-2020-RD009081).
FUNDING INFORMATION
A Basic Science Research Program through the National Research Foundation of Korea (NRF), the Ministry of Education, Korea, Grant Number: 2020R1A6A1A03047729.
An Agenda Research Program, the Rural Development Administration, Korea, Grant Number: RS-2020-RD009081.
Open Research
DATA AVAILABILITY STATEMENT
The data supporting the findings of this study are available from the corresponding author, Ho Won Jung, upon request.




