Disturbed nutrient accumulation and cell wall metabolism in panicles are responsible for rice straighthead disease
Abstract
Rice straighthead disease substantially reduces crop yield, posing a significant threat to global food security. Dimethylarsinic acid (DMA) is the causal agent of straighthead disease and is highly toxic to the reproductive tissue of rice. However, the precise physiological mechanism underlying DMA toxicity remains unknown. In this study, six rice varieties with varying susceptibility to straighthead were utilized to investigate the growth performance and element distribution in rice panicles under DMA stress through pot experiments, as well as to explore the physiological response to DMA using transcriptomic methods. The findings demonstrate significant variations in both DMA accumulation and straighthead sensitivity among cultivars. The susceptible varieties exhibited higher DMA accumulation indices and displayed typical symptoms of straighthead disease, including erect panicles, deformed rachides and husks, and reduced seed setting rate and grain yield when compared to the resistant varieties. Moreover, DMA addition promoted mineral nutrients to accumulate in rachides and husks but less in grains. DMA showed preferential accumulation in rice grains with a distribution pattern similar to that of Copper (Cu) and zinc (Zn) within the panicle. Transcriptome analyses underscored the substantial impact of DMA on gene expression related to mineral metabolism. Notably, DMA addition significantly up-regulated the expression of pectin methylesterase, pectin lyase, polygalacturonase, and exogalacturonase genes in Nanjingxiangzhan, while these genes were down-regulated or weakly expressed in Ruanhuayou 1179. The alteration of pectin metabolic pathways induced by DMA may lead to abnormality of cell wall assembly and modification, thereby resulting in deformed rice panicles.
1 INTRODUCTION
Straighthead disease, a physiological disorder in rice (Oryza sativa L.), is characterized by the persistence of upright mature panicles with unfilled grains and distorted husks (Tang et al., 2020; Liu et al., 2023a). This disease poses a significant threat to global rice production and food security due to its potential to cause substantial reduction or complete failure in grain yield, particularly in highly susceptible varieties (Yan et al., 2005). Straighthead disease is prevalent in rice-growing regions worldwide, including the United States, Portugal, Japan, Australia, Southeast Asia, and South America (Agrama and Yan, 2010; Rahman et al., 2012). In China, straighthead disease, also known as “green straighthead disease” or “dry green straighthead disease”, easily occurs in rice fields converted from drylands (Liu et al., 2020). This disease is widely distributed in China, especially in the middle and lower reaches of the Yangtze River (Chen et al., 2019; Tang et al., 2020).
Arsenic (As) is a toxic metalloid that has the capacity to accumulate significantly in rice plants. The most abundant As forms found in rice are arsenate (As(V)), arsenite (As(III)), monomethylarsonic acid (MMA), and dimethylarsinic acid (DMA) (Mitra et al., 2017). The uptake, transport, and metabolism of various As forms in rice have been extensively documented in detailed reports (Kalita et al., 2018; Zhao and Wang, 2020; Liu and Li, 2022). Rice lacks the ability to methylate As and methylated As in grains primarily originate from root uptake from the external environment (Jia et al., 2012; Lomax et al., 2012). In rice plants, OsLsi1 and OsPTR7 play crucial roles in mediating root uptake and upward transport of DMA, respectively (Li et al., 2009; Tang et al., 2017). DMA exhibits high mobility within rice plants due to its inability to be immobilized by non-protein thiols (NPTs) (Carey et al., 2010; Mishra et al., 2017). Previous studies have demonstrated that DMA primarily exhibits toxicity towards the reproductive organs in rice and leads to distorted glumes and abnormal floral structures, which may be attributed to the high root-to-shoot transport efficiency of DMA and the induction of strong oxidative stress (Zheng et al., 2013; Tang et al., 2016; Mishra et al., 2017). Indeed, the excessive accumulation of DMA in rice plants can induce straighthead disease, resulting in sterility of spikelets and substantial yield reductions (Limmer et al., 2018; Tang et al., 2020). Tang et al. (2020) found that DMA treatment induced significant alterations in the expression of genes associated with cell wall modifications, nutrient transport and oxidative stress responses and pointed out that cell wall metabolism may represent a susceptible target for DMA toxicity. As previously mentioned, despite extensive evidence on the high mobility and toxicity of DMA in rice, the underlying molecular mechanism responsible for DMA-induced straighthead disease remains elusive.
Elements with similar chemical properties may share common proteins, chelating agents, binding mechanisms, as well as metabolic and transport pathways (Baxter and Dilkes, 2012). For instance, cadmium (Cd) uptake in rice roots is primarily facilitated by manganese (Mn) transporter OsNRAMP5, while OsHMA2 plays a crucial role in transporting zinc (Zn) and Cd from roots to shoots (Sasaki et al., 2012; Satoh-Nagasawa et al., 2012). The accumulation of arsenic in rice is closely associated with silicate and phosphate transport systems (Baxter and Dilkes, 2012). Although numerous studies have explored the interactions between total or inorganic As and mineral elements, limited information exists regarding DMA (Williams et al., 2009; Feng et al., 2017; Yao et al., 2020). Given its high mobility and shared transporters in rice plants, DMA might influence the uptake of mineral nutrients such as nitrogen (N), silicon (Si), and zinc (Zn) through competition among transporters or regulation of transporter gene expression (Dunn et al., 2006; Murphy et al., 2020; Gao et al., 2023).
Herein, we conducted pot experiments to evaluate the growth performance, As accumulation, and mineral ion composition of rice plants treated with DMA. Transcriptome analysis was employed to investigate the differential expression patterns of genes associated with cell wall metabolism between rice cultivars under DMA stress. The aim is to validate two hypotheses: (1) DMA induces alterations in the distribution pattern of mineral ions in rice panicles, leading to physiological disorders related to imbalanced nutrition; and (2) DMA alters the expression of key genes involved in the cell wall metabolic pathway. The findings from this investigation contribute significantly towards comprehending the molecular mechanism of deformed panicles in diseased rice and developing effective strategies for mitigating straighthead disease.
2 MATERIALS AND METHODS
2.1 Experimental materials
The six rice varieties used in experiments were Ruanhuayou 1179 (RHY), Nanjingxiangzhan (NJXZ), Meixiangzhan No.2 (MXZ), Huahang 11 (HH), Xiangyaxiangzhan (XYXZ), and Shennong 9903 (SN). All the varieties are indica rice widely cultivated in South China, with the exception of SN being japonica. Based on our preliminary experiment, RHY exhibited susceptibility to straighthead disease, while NJXZ showed resistance. The soils were obtained from a typical paddy field located in the Zengcheng district of Guangzhou city at coordinates 23.3° N and 113.7° E and then dried, crushed, and sieved. The soil contained organic matter 17.1 ± 0.4 g kg−1, alkali-hydrolyzable N 53.9 ± 5.4 mg kg−1, available P 26.5 ± 0.9 mg kg−1 and available K 50.6 ± 5.2 mg kg−1, with the pH value of 5.80 ± 0.01. The content of total As in soil was 6.7 ± 0.0 mg kg−1.
2.2 Pot experiment
Rice seeds were disinfected with 30% H2O2 solution for 15 minutes, followed by rinsing with tap water and overnight soaking. Subsequently, the seeds were evenly distributed in seedling trays filled with clean quartz sand and supplied with 1/2 Kimura B nutrient solution (pH = 5.5) for seedling development. Seedlings with three leaves were transplanted into individual PVC pots (16 cm in diameter and 17.5 cm in height) containing 2.5 kg of soil, with one rice seedling per pot. Prior to transplanting, basic fertilizers (N: 0.15 g kg−1, P2O5: 0.075 g kg−1, K2O: 0.15 g kg−1) were uniformly incorporated into the soil. The pot trials were conducted in a greenhouse in 2022, with natural light and temperature. Two levels of DMA (0 and 0.5 mg As kg−1) in each variety were arranged for each rice variety. Each treatment was replicated four times for mature plant analysis. Three additional replicates in the treatments of RHY and NJXZ were employed to perform transcriptome analysis. DMA solution was injected into the rhizosphere soils at the tillering stage. The soil was waterlogged until one week prior to the rice harvest.
2.3 Plant sample analysis
The unopened young panicles of RHY and NJXZ were collected at the booting stage and immediately frozen in liquid nitrogen for subsequent transcriptome analysis. Before the harvest, rice plant height and chlorophyll content (SPAD) were measured. Once the rice plants reached maturity, their above-ground portions were collected and subjected to oven drying at 60°C until a consistent weight was achieved. The straw and grain biomass were recorded after threshing, and then the rachis in panicles were obtained. The filled and unfilled grains were separated by water immersion to assess the seed setting rate (SSR) for each individual plant. Subsequently, the grains and husks were separated using a husker (TP-JLG-2018), ground with a mill (JXFSTPRP-CL), and then sieved with a sieve size of 0.15 mm and stored at 4°C until further analysis.
The rachis, husk, and grain samples were digested using concentrated HNO3/H2O2, as described by Liu et al. (2023b). In brief, 0.25 g of the sample was subjected to digestion, resulting in a colorless and transparent solution. Subsequently, total As, boron (B), molybdenum (Mo), copper (Cu), and iron (Fe) in the solution were determined using inductively coupled plasma mass spectrometry (ICP-MS, Agilent 7700x), while Zn, Mn, and magnesium (Mg) were quantified using an atomic absorption spectrophotometer (AAS, Hitachi ZA3000). Calcium (Ca) and sulfur (S) in the solution were analyzed via an inductively coupled plasma optical emission spectrometer (ICP-OES, Varian 710-ES). Potassium (K) was determined using a flame photometer (INESA FP6400A), and phosphorus (P) was measured utilizing the molybdenum antimony colorimetric method (Sjösten and Blomqvist, 1997). Total N level was detected following the Kjeldahl procedure (Lou et al., 2007). Rice standard material (CRM NIST 1568b) was included during sample analysis for quality control purposes regarding both digestion procedure and analytical process.
The cellulose, pectin, and lignin contents in panicles (divided into rachides and husks) were quantified using the kits purchased from Suzhou Comin Biotechnology Co. Ltd. According to the manufacturer's instructions, the furfural derivative formed from cellulose after acid hydrolysis and dehydration has a characteristic absorbance at 620 nm. The pectin product, after acid hydrolysis, is condensed with carbazole to form a purple compound with a characteristic absorption at 530 nm. The absorbance of the product at 280 nm after acetylation of lignin is positively correlated with lignin content.
2.4 Transcriptome analysis
Transcriptome analyses of young panicle samples (2 varieties, 2 DMA levels, 3 replicates) were performed at Biomarker Technologies. Detailed protocols for RNA extraction, library construction, and sequencing can be found in Text S1. Differentially expressed genes (DEGs) were identified using a false discovery rate (FDR) < 0.01 and a fold change ≥2. All DEGs were annotated via the GO database (http://www.geneontology.org/) and the KEGG database (http://www.genome.jp/kegg/), respectively. Based on the annotation results, GO classification and KEGG enrichment were performed. GO term and KEGG pathway with corrected P-value <0.05 were considered significantly enriched by DEGs.
2.5 Quantitative real-time PCR (qRT-PCR) analysis
We selected a total of 18 DEGs from RHY and NJXZ for qRT-PCR analysis to validate the RNA-seq results. Total RNA was extracted from young panicles using the RNA Prep Pure Plant Kit (Tiangen), and purified RNA was reversely transcribed into first-strand cDNA using the TransScript First-Strand cDNA Synthesis kit (AiDLAB Biotech) according to the manufacturer's instructions. Subsequently, quantitative real-time PCR (qRT-PCR) was performed using the SYBR Green QPCR Mix (DF Biotech). OsUbiquitin and 18S rRNA were used as the normalization reference (Yu et al., 2012; Tang et al., 2020). All samples were tested in triplicate. The 2−ΔΔCt method was used to calculate the relative expression levels of the candidate genes. The primer sequences used for qRT-PCR analysis are provided in Table S1. Figure S1 shows the relative expression levels of the selected DEGs validated by qRT-PCR.
2.6 Statistical analysis
All the data were expressed as mean ± standard deviation (SD). Statistical analysis was performed using SPSS Statistics version 24.0, and GraphPad Prism version 9.0 was used for data visualization. A two-way analysis of variance (ANOVA) was conducted to analyze rice plant phenotypes, levels of As, mineral elements and cell wall components in panicles. Duncan's test was applied for post hoc comparisons, with significance set at P < 0.05. The significant differences between the control and the DMA treatments were assessed by Student's t-test at the probability of P < 0.05.
3 RESULTS
3.1 Growth performance and As accumulation in rice
The effect of DMA on the growth performance of rice plants varied with rice cultivars (Figure 1). Compared to the control, the panicles of RHY, HH, and SN became erect and shorter under DMA treatment, while the other cultivars almost remained unchanged (Figure 1A). DMA addition caused curved rachides and distorted husks in RHY but had no obvious effects on NJXZ (Figure 1B). Seed setting rate and grain yield are usually used to assess the severity of rice straighthead disease. In contrast to the control, the seed setting rate of RHY and HH decreased by 25.3 and 9.6%, and the yield decreased by 33.6 and 33.9%, respectively, under DMA treatment (Figure 1C and D). Additionally, DMA addition significantly reduced straw biomass and plant height of RHY and HH (P < 0.05), but increased chlorophyll levels (SPAD) in flag leaves at the maturity stage (Figure 1E-G). Conversely, these traits of NJXZ, MXZ (except for chlorophyll), and XYXZ under DMA treatment did not show significant differences compared to those of the control group, indicating strong resistance against straighthead disease.
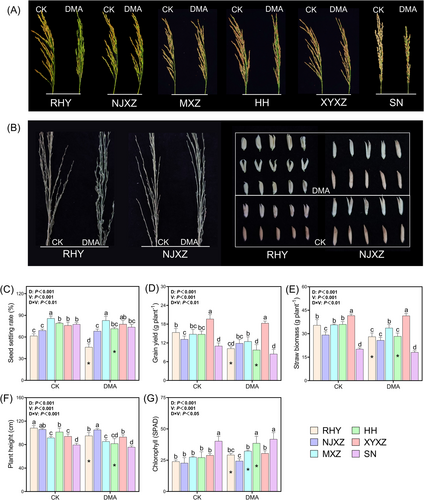
As depicted in Figure 2, the total As and As species in rice panicles displayed significant differences based on DMA level and rice variety. DMA treatment resulted in a significant increase in total As, MMA, and DMA contents in rice panicles (P < 0.05). Additionally, there was an observed elevation of iAs content in the rachides or husks of certain varieties (RHY, NJXZ, HH, and SN) due to DMA stress. Notably, DMA primarily accumulated within the grains, while iAs and MMA were predominantly located in the rachides and husks (Figure 2E-G), suggesting a high mobility of DMA within plants. The accumulation index (AI) was used to evaluate the ability of rice to accumulate DMA. Specifically, the AI of RHY, HH, and SN were 26.3, 22.1, and 18.1 for husks and 28.6, 26.2, and 22.9 for grains, respectively (Figure 2H). Conversely, the As accumulation capacity was at a low level in NJXZ, MXZ, and XYXZ, with the AI of 15.3, 13.2, and 17.6 for grains, respectively. These findings suggest that rice varieties susceptible to straighthead disease often exhibit higher ability to accumulate DMA. Pearson correlation analysis showed that there was a significant negative correlation between seed setting rate and DMA content in grain (r = − 0.895*) and in rachis (r = − 0.945**) (Table S2).
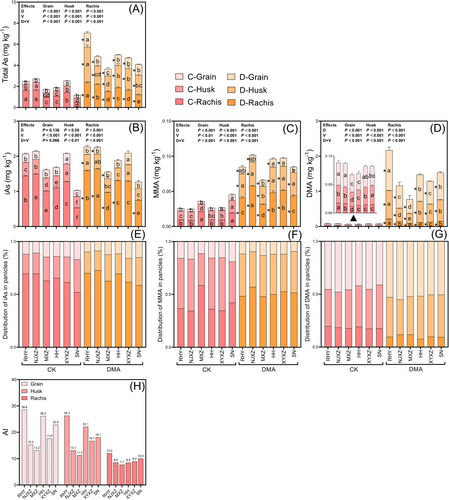
3.2 Mineral elements in rice panicles
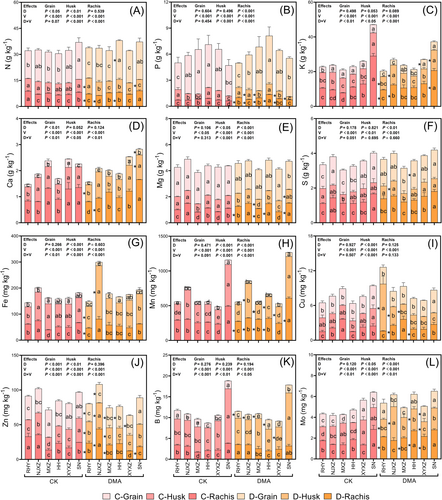
The addition of DMA significantly altered the distribution of elements in rice panicles, with notable variations observed among cultivars and organs (Figure 3). Among macroelements, N, P, Mg, and S were predominantly enriched in grains, while K and Ca exhibited a higher distribution in rachides and husks. A consistent trend was observed where several elements in rachides and husks increased due to DMA addition, whereas the corresponding elements in grains decreased. For example, under DMA treatment, N and Ca in the husks of RHY, P, K, Ca and S in the rachides/husks of NJXZ, and N and Mg in the rachides of HH, were significantly higher than those in the control (P < 0.05). Microelements such as Fe, Mn, B, and Mo primarily accumulated in rachides and husks, while Cu and Zn demonstrated relatively even distribution across all three organs. Similarly, DMA addition also inhibited the transport of microelements into the grains, resulting in their accumulation within rachides or husks, particularly for Cu and Zn. These findings indicate that DMA could potentially impede the transport of mineral elements into the grain.
3.3 Element accumulation patterns associated with abnormal phenotype
Principal component analysis (PCA) was used to analyze the element groups associated with abnormal phenotypes in rice under DMA treatment (Figure 4). As depicted in Figure 4A, the first two principal components explained 67.43% of the total variance. Grain, husk, and rachis samples exhibited distinct separation, indicating variations in ion composition among different rice organs. N, P, Mg, S, Zn, Cu and DMA showed high enrichment levels in grains. B and Fe were primarily in the husks, and K and Ca in the rachides, while Mn and Mo were concentrated in both rachides and husks. The relationship of agronomic traits and elements in grains, husks and rachides are shown in Figure 4B-D, respectively. In grains, DMA was distinctly separated from other factors and negatively correlated with PC2, whereas seed setting rate and some nutrients such as P, K, Ca, Mg, Fe, and Zn were positively correlated with PC2 (Figure 4B). Husk elements, including K, Ca, Fe, Mn, Zn, B, Mo, and DMA, were loaded on the positive axis of PC1 and showed aggregation, while yield and straw biomass were loaded on the negative axis of PC1. Husk Cu and SSR were loaded on the negative and positive axes of PC2, respectively (Figure 4C). In rachides, DMA and N, K, S, Mn, Mo and chlorophyll loading lay on the positive axis of PC1, and yield, straw biomass, and plant height fall on the negative axis of PC1. Meanwhile, rachis Zn and SSR were loaded on the negative and positive axes of PC2, respectively (Figure 4D). These results indicated that seed setting rate under DMA treatment was positively associated with P, K, Ca, and Fe content in the grains but negatively associated with husk Cu content and rachis Zn content. Furthermore, grain yield was negatively associated with various mineral elements presented within husks or rachides. The above implies a strong association between changes in these minerals and abnormal phenotypes observed in diseased rice plants.
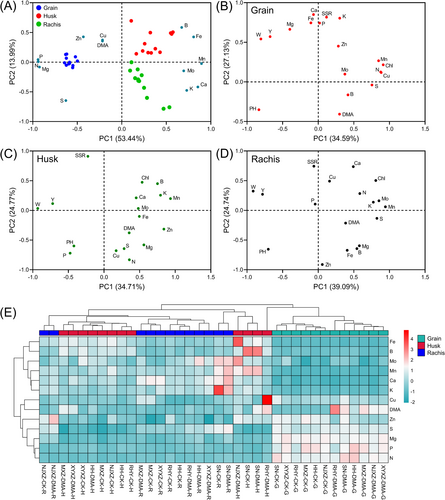
Hierarchical cluster analysis (HCA) was used to analyze the distribution pattern of mineral elements in rice panicles further. The 13 elements were divided into two main clusters. Cluster 1 mainly consisted of N, P, Mg, S, Cu, Zn, and DMA, which exhibited enrichment primarily in the grains (Figure 4E). On the other hand, K, Ca, Fe, Mn B, and Mo formed cluster 2 and showed higher concentrations in rachides and husks. Notably, the distribution pattern of DMA in panicles displayed a high similarity to that of Cu. Additionally, all the grain samples were classified into the same cluster, and there was a clear separation between grain samples in the control and DMA treatment, indicating an alteration in elemental composition within the grains.
3.4 Transcriptome analysis of young panicles
3.4.1 Overview of transcriptome data
A total of 90.32 Gb of clean data was obtained from 12 young panicle samples. The average clean data was ≥6.04 Gb in each sample and the average percentage of bases reaching the Q30 criteria in each clean dataset was ≥92.5%, and 92.34–93.51% of clean reads in each sample could be mapped to the reference genome (Table S3). PCA analysis based on the transcriptome results shows that the samples had a clear segregation between cultivars (Figure S2A), and that DMA stress markedly changed the gene expression in panicles of NJXZ. The total number of genes expressed in RHY and NJXZ were 24366 and 24811, respectively (Figure S2B and C). Using the screen standards of FDR <0.01 and |log2(fold change) | ≥ 2, a total of 145 and 387 differentially expressed genes (DEGs) were identified in RHY and NJXZ, respectively (Figure S2D). In RHY, there were 79 up-regulated and 66 down-regulated genes, accounting for 54.5 and 45.5% of the total DEGs, respectively. In NJXZ, 307 up-regulated and 80 down-regulated genes were identified, and the up-regulated genes accounted for nearly 80% of the DEGs. The Venn diagram displays that there were 13 mutual DEGs in both cultivars (Figure S2E). There was a strong linear relation between the transcriptome sequencing data and the qRT-PCR (R2 = 0.8862**) (Figure S2F), indicating the high reliability of the transcriptome sequencing.
3.4.2 GO and KEGG enrichment analyses of DEGs
Under DMA treatment, 74 and 253 DEGs in RHY and NJXZ were annotated by GO analysis and classified as biological process, cellular component and molecular function (Figure S3). The significantly enriched GO terms of each ontology are shown in Figure 5A and B, and Table S4. Regarding biological processes, 13 and 62 terms were significantly annotated in RHY and NJXZ, respectively (P < 0.05). “Exocytosis”, “lipid transport” and “carbohydrate metabolic process” were significantly annotated in both varieties, and the specific annotations in RHY included “chorismate metabolic process”, “mitochondrial calcium ion homeostasis”, “anther wall tapetum development”, “transposition”, “malate transport” and so on. In NJXZ, “pectin catabolic process”, “glutamine biosynthetic process”, “plant-type cell wall organization”, “regulation of plant organ formation”, “oxidation–reduction process” and others were specially annotated. The two cultivars shared the notable annotations “exocyst” and “apoplast” in regard to cellular component, and “cell wall”, “extracellular region”, and “external encapsulating structure” were specific to NJXZ. In molecular function, “oxidoreductase activity”, “heme binding” and “iron ion binding” were significantly annotated in both RHY and NJXZ, while “pectate lyase activity” and “pectinesterase inhibitor activity” were specifically annotated in NJXZ.
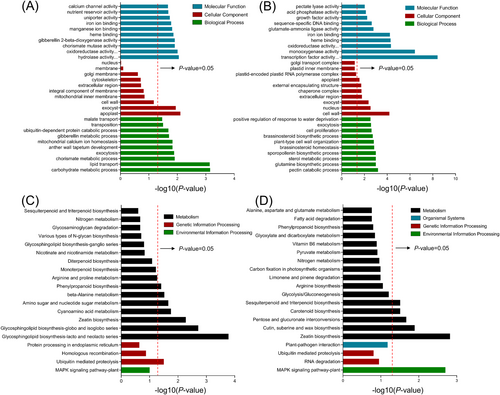
KEGG enrichment analysis was performed on DEGs in RHY and NJXZ to identify the metabolic pathways associated with DMA stress. Figure 5C and D show the top 20 enriched KEGG pathways in RHY and NJXZ, respectively. Specifically, zeatin biosynthesis (ko00908) was the common enriched pathway for both species (P < 0.05). The specific enrichment pathways were glycosphingolipid biosynthesis (ko00601 and ko00603), cyanoamino acid metabolism (ko00460), amino sugar and nucleotide sugar metabolism (ko00520), beta-alanine metabolism (ko00410), ubiquitin-mediated proteolysis (ko04120), and phenylpropanoid biosynthesis (ko00940) in RHY (P < 0.05). DEGs of NJXZ were mainly enriched in MAPK signaling pathway-plant (ko04016), cutin, suberine and wax biosynthesis (ko00073), pentose and glucuronate interconversions (ko00040), carotenoid biosynthesis (ko00906), and sesquiterpenoid and triterpenoid biosynthesis (ko00909).
3.4.3 The expression profiles of DEGs linked to mineral elements
Based on KEGG annotation, a total of 44 DEGs related to element ion metabolism were obtained from the two varieties, mainly involving N, P, K, Ca, Fe, Cu, Zn, B and As (Table S5). Specifically, several up-regulated genes encoding enzymes involved in nitrogenous material transport and amino acid synthesis were observed in NJXZ. The DEGs linked to phosphorus metabolism were mainly involved in substrate phosphorylation and dephosphorylation, as well as inorganic phosphate transport. A variety of potassium transporter and calmodulin genes were found to be differentially expressed in NJXZ. There were many differential genes involved in Fe metabolism, mainly encoding Fe transporters and cytochrome P450. Furthermore, the DEGs related to Cu (laccase and Cu transporter), Zn (Zn finger protein and Zn transporter), B (B transporter), and As (As/Si transporter) were specifically present in NJXZ. Overall, DMA accumulation altered the expression patterns of genes related to ion metabolism, and a range of up-regulated genes suggested that NJXZ showed a positive physiological response toward DMA stress.
3.5 Cell wall composition and gene expression of the key metabolic pathways
In Figure 6A, the addition of DMA significantly reduced cellulose content in RHY panicles (P < 0.05), while increasing pectin levels in rachis and husk by 26.1 and 74.0%, respectively, compared to the control. There was no significant change in cellulose content in NJXZ, but the pectin level was increased by DMA addition as well. Moreover, lignin contents in panicles of both rice varieties treated with DMA were slightly lower than those of the control.
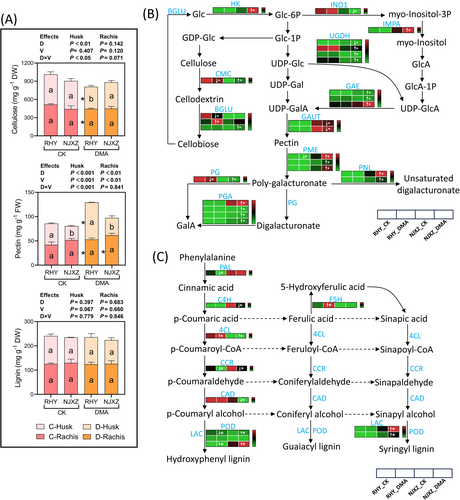
A total of 26 genes from cellulose and pectin metabolic pathways were found to be differentially expressed in RHY or NJXZ (Figure 6B). Significant differences in the expression of genes encoding beta-glucosidase (BGLU) and endoglucanase (CMC) were observed in cellulose. Regarding pectin metabolism, the genes significantly regulated by DMA stress mainly encode UDP-glucose-6-dehydrogenase (UGDH, mixed-regulated), UDP-glucuronate-4-epimerase (GAE, up) and alpha-1,4-galacturonosyltransferase (GAUT, down), pectin methylesterase (PME, up), polygalacturonase (PG, up), pectate lyase (PNL, up), and galacturan-1,4-alpha-galacturonidase (PGA, up). In RHY, with the exception of the significantly downregulated expression of PME and PG genes by DMA addition, other genes exhibited relatively low or no expression at all. A total of 11 DEGs were annotated in the phenylpropanoid synthesis pathway, mainly encoding enzymes such as phenylalanine ammonia-lyase (PAL), trans-cinnamate 4-monooxygenase (C4H), 4-coumarate-CoA ligase (4CL), cinnamyl-alcohol dehydrogenase (CAD), peroxidase (POD), laccase (LAC), ferulate-5-hydroxylase (F5H), and cinnamoyl-CoA reductase (CCR) (Figure 6C). The down-regulation of genes encoding key enzymes (CAD and CCR) induced by DMA addition might be responsible for the slightly decreased lignin content in panicles.
4 DISCUSSION
4.1 Rice cultivars with high DMA accumulation are susceptible to straighthead disease
In the present study, RHY treated with DMA showed typical symptoms of straighthead disease, such as erect panicles, deformed rachides and husks, reduced seed setting rate and grain yield (Figure 1), which appears to be related to the high DMA concentration in RHY panicles (Figure 2 and Table S2). The results of Zheng et al. (2013) and Tang et al. (2020) also showed that excessive accumulation of DMA can cause toxicity to the reproductive organs in rice and lead to straighthead disease. In contrast to inorganic As and MMA, DMA exhibits a preference for accumulating in rice panicles due to its resistance to chelation with NPTs and subsequent vacuolar isolation (Mishra et al., 2017), which explains the high DMA concentration in rice panicles in this study. Moreover, DMA addition apparently reduced straw biomass and plant height in RHY and HH, indicating that DMA also had a negative effect on the vegetative growth of rice (Figure 1), aligning with the findings of Yan et al. (2005), which may be attributed to the higher oxidative stress in plants caused by DMA addition (Tang et al., 2016).
Significant variation in susceptibility to straighthead disease was observed among cultivars, with RHY exhibiting the typical disorder, while NJXZ, MXZ, and XYXZ showed resistance (Figure 1). A previous study reported that Zhe733 from China was immune to straighthead and had no symptoms or yield loss, while Cocodrie, Mars, Kaybonnet and Bengal were susceptible genotypes (Yan et al., 2005), indicating that plants have evolved mechanisms that reduce DMA toxicity, possibly via regulation of DMA uptake and transport (Pinson et al., 2022). The susceptibility to straighthead disease has also been found to be potentially associated with the accumulation of DMA in rice, whereby the severity of the disease increases proportionally with higher concentrations of DMA in the husk (Tang et al., 2020; Gao et al., 2023), while grain As contents of the resistant varieties was generally lower (Hua et al., 2013). Similarly, the susceptible cultivar (RHY) in the present study accumulated more DMA in panicles than other cultivars (Figure 2). Methylated As in grains is known to originate mainly from As methylation mediated by soil microorganisms (Lomax et al., 2012), and the uptake and upward transport of DMA in rice depend on the silicate transporter (OsLsi1) and the nitrate/peptide transporter (OsPTR7), respectively (Li et al., 2009; Tang et al., 2017). However, the expression levels of these key transporters tend to differ among rice varieties (Ma et al., 2007), which could explain the differences observed in DMA accumulation among cultivars in the current work.
4.2 DMA affects the distribution and metabolism of mineral ions in rice panicles
Abnormal development of rice panicles is a typical symptom of straighthead disease. Elemental analysis is helpful in revealing the distribution of minerals under DMA stress and determining the ion clusters closely associated with the aberrant panicle phenotype. We observed that DMA stress promoted the accumulation of several mineral elements (P, Ca, Mg, Fe, Mn, Cu, Zn, and Mo) in the rachides and/or husks, while reduced grain yield and seed setting rate (Figures 3 and 4). Some studies have also reported that organic As application increases the contents of many minerals in rice flag leaves and panicles (Yan et al., 2008), but decreases the elements in rice grains (Zheng et al., 2021). The changes in water potential caused by DMA destroyed the phloem transport function, which may explain the decrease in nutrient accumulation in grains (Zheng et al., 2013). In addition, the accumulation of some microelements in rachides and husks may be attributed to the increase in pectin content within panicles under DMA addition (Figure 6A), since the carboxyl group of pectin polymer is the main binding site of positive metal ions in plants (Meychik et al., 2014; Szatanik-Kloc et al., 2017). We also found that DMA has a similar distribution pattern with Cu and Zn in panicles (Figure 4), which is consistent with the results of Zheng et al. (2021). DMA is distributed throughout the rice grain, while inorganic As typically accumulates to the outer edges, localized in the ventral ovular vascular trace (OVT) of grains (Zheng et al., 2013). The OVT is the only pathway for many nutrients to enter the grain and also represents a major barrier for As species to enter the endosperm (Krishnan and Dayanandan, 2003; Lombi et al., 2009). As(III)-thiol complexes are identified within the OVT and rice bran. These complexes limit the mobility of iAs and prevent further transport into the grain, whereas DMA can effectively enter the endosperm through the OVT as it cannot effectively complex with NPT (Carey et al., 2010; Wu et al., 2019). Therefore, DMA may impede nutrient translocation into the grain by competing for transport proteins in the OVT or decelerating certain metabolic reactions within the grain to reduce the substrate requirements, which needs to be further demonstrated.
Element distribution in rice plants under stress conditions is essentially regulated by gene network (Xiao et al., 2021). Therefore, transcriptome analyses were performed to better understand the possible genes involved in the translocation and metabolism of elements in the rice plants (Table S5). OsPTR7 (OsNPF8.1), a nitrate/peptide transporter, has been shown to be involved in long-distance transport and unloading of DMA in grains (Tang et al., 2017), suggesting that DMA accumulation may overlap with the transport pathways of N compounds, which may partially explain the differential expression of transporter genes for N-containing compounds in this study. Genes related to phosphorus metabolism are mainly contributing to the transport of inorganic phosphorus as well as substrate phosphorylation and dephosphorylation processes, highly indicating possible changes in energy metabolism in rice plants under DMA stress (Ahsan et al., 2009; Bechtaoui et al., 2021). In addition, phosphorylation and dephosphorylation of proteins play a switching role in the molecular transmission process of plants that sense external stress signals (Schweighofer et al., 2004). Up-regulation of multiple K+ transporter genes in NJXZ may help alleviate DMA-induced oxidative damage, maintain cell wall metabolism stability, and promote normal floral organ development (Tang et al., 2020; Liu et al., 2021; Li et al., 2022). DMA can up-regulate a number of DEGs associated with Fe metabolism, such as Fe transporter and cytochrome P450 (CYPs). Notably, CYPs are directly involved in the secondary metabolism of plants and facilitate the detoxification of external compounds or those produced as a byproduct of metabolism in response to stress (Pandian et al., 2020; Zhao et al., 2023). Similarly, genes related to Cu and Zn transport were up-regulated by DMA (Table S5), which may explain the similarity in the distribution of Cu, Zn and DMA in this work and the previous study (Zheng et al., 2021). Additionally, it has been demonstrated that the application of Zn can drastically reduce the accumulation of DMA in rice roots (Ma et al., 2020). The findings suggest a complex association between DMA stress and Cu or Zn accumulation in rice plants, potentially indicating their involvement in the same transport pathway.
4.3 Pectin metabolism disorder may be the main cause of rice panicle deformity
Although DMA has been shown to induce straighthead disease, the mechanism of toxicity is still unclear, and there is insufficient evidence to explain panicle deformity in diseased rice. Down-regulation of the cellulose synthase active subunit and PME genes caused by DMA stress may affect cellulose synthesis and methylesterification of pectin polymers in the cell wall, resulting in abnormal development of plant organs (Ma et al., 2020; Tang et al., 2020). Pectin is an important polysaccharide of primary cell walls and is mainly involved in cell wall plasticity and cellular adhesion, playing an important role in plant growth and development as well as stress resistance (Tang et al., 2020; Du et al., 2022). Heavily methylated homogalacturonan (HG) is a dominant form of pectin polymers, is selectively demethylesterified by PME activity to produce low methylated HG, and this process is simultaneously regulated by PME inhibitor (PMEI) proteins (Peaucelle et al., 2008; Xiao et al., 2014; Nguyen et al., 2017). This study shows that many genes of the pectin metabolic pathway in RHY and NJXZ have opposite expression patterns. The increase in pectin content may be attributed to the severe oxidative stress caused by the high DMA content in panicles (Figure 6A), since studies have shown that hydrogen peroxide promotes pectin accumulation (Xiong et al., 2015; Tang et al., 2016). Furthermore, pectin-synthesis genes (GAUT) were significantly down-regulated, and downstream modified genes (PME, PNL, PG, PGA) were significantly up-regulated in NJXZ under DMA stress, while the expression of these genes was low expressed or down-regulated (PME and PG) in RHY (Figure 6B). Overexpression of PMEI in Arabidopsis significantly inhibits PME activity and results in increased cell adhesion and abnormal developmental phenotypes, including stem distortion, organ (flower and stem) fusion, flower organ absence or inhibited growth (Peaucelle et al., 2008; Müller et al., 2013). Transgenic rice plants with overexpressing OsPMEI28 show increased levels of pectin methylesterification, dwarf phenotypes and reduced culm diameter (Nguyen et al., 2017). These typical symptoms caused by the inhibition of PME activity were very similar to the phenotypes of rice under DMA stress, as observed in previous studies (Marin et al., 1992; Zheng et al., 2013). In addition, hypomethylated HGs are substrates for pectin-degrading enzymes such as polygalacturonases (PG) and pectin/pectate lyases (PL/PNL) to form oligogalacturonic acid, thereby inducing cell wall loosening and ensuring cell expansion or growth (Hocq et al., 2017). The overexpression of the PG gene in rice induced by abiotic stress can reduce pectin content and cell adhesion and promote cell expansion (Liu et al., 2014). It is evident that PME and PG can function as the downstream components of the regulatory networks that control cell expansion as well as floral phyllotaxis and organogenesis (Xiao et al., 2014). And, under DMA addition, changes in lignin and cellulose content may be related to the disturbed pectin metabolism because altering pectin causes changes in other wall components and remodelling of the whole cell wall, shifting the plant's ability to grow, develop and respond to stimuli (Xiao et al., 2017; Du et al., 2022). In summary, a hypothetical model based on the pectin metabolic pathway is proposed (Figure 7), with the aim to explain panicle deformity in diseased rice and phenotypic differences between the resistant and the susceptible cultivars.
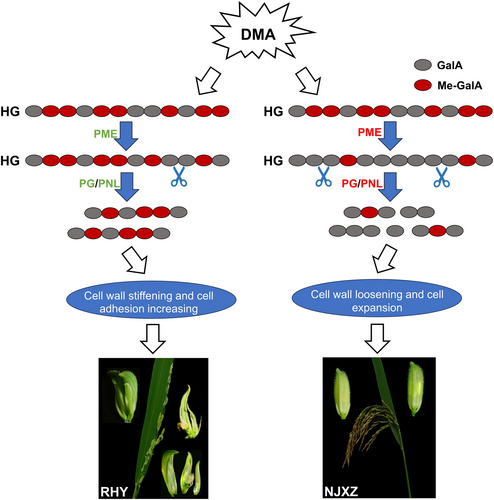
5 CONCLUSION
Our results suggest that there were significant varietal differences in the incidence of straighthead disease. The susceptible cultivars accumulated higher levels of DMA in the panicles than the resistant ones, accompanied by typical straighthead symptoms such as erect and deformed panicles, reduced yield and seed setting rate. DMA was more readily unloaded into the grain and altered the distribution and metabolism of mineral ions in panicles. Notable changes were observed in the pectin metabolic pathway in rice with straighthead disorder. Differential expression of pectin modification genes may contribute to deformed panicles in diseased rice and the phenotypic differences between the resistant and the susceptible varieties. In the future, the application of soil ameliorants containing Cu and/or Zn may be utilized to alleviate rice straighthead disease. Gene editing is expected to provide a deeper understanding of molecular mechanisms underlying straighthead disease through silencing or overexpressing key genes associated with pectin metabolism, such as PME or PG.
AUTHOR CONTRIBUTIONS
Qinghui Liu: Investigation, chemical analysis, data curation, validation, and writing-original draft. Zhijun Zhang: Investigation assistance and chemical analysis. Cuihua Bai: Analysis method exploration and supervision. Yi Li, Xueying Yin and Wanting Lin: Investigation assistance and chemical analysis. Lixian Yao: Funding acquisition, methodology, writing review and editing.
ACKNOWLEDGEMENTS
This research was supported primarily by Science and Technology Planning Project of Guangdong Province (NO.2021B1212040008).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




