Rhizobacteria Bacillus spp. mitigate osmotic stress and improve seed germination in mustard by regulating osmolyte and plant hormone signaling
Abstract
Drought, a widespread abiotic stressor, exerts a profound impact on agriculture, impeding germination and plant growth, and reducing crop yields. In the present investigation, the osmotolerant rhizobacteria Bacillus casamancensis strain MKS-6 and Bacillus sp. strain MRD-17 were assessed for their effects on molecular processes involved in mustard germination under osmotic stress conditions. Enhancement in germination was evidenced by improved germination percentages, plumule and radicle lengths, and seedling vigor upon rhizobacterial inoculation under no stress and osmotic stress conditions. Under osmotic stress, rhizobacteria stimulated the production of gibberellins and reserve hydrolytic enzymes (lipases, isocitrate lyase, and malate synthase), bolstering germination. Furthermore, these rhizobacteria influenced the plant hormones such as gibberellins and abscisic acid (ABA), as well as signalling pathways, thereby promoting germination under osmotic stress. Reduced proline and glycine betaine accumulation, and down-regulation of transcription factors BjDREB1_2 and BjDREB2 (linked to ABA-independent signalling) in rhizobacteria-inoculated seedlings indicated that bacterial treatment mitigated water deficit stress during germination, independently of these pathways. Hence, the advantageous attributes exhibited by these rhizobacterial strains can be effectively harnessed to alleviate drought-induced stress in mustard crops, potentially through the development of targeted bio-formulations.
1 INTRODUCTION
Agriculture is one of the foremost vulnerable sectors to global climate change and is strongly influenced by climate and weather. Drought is one of the most negatively impacting climate extremes; even temporary water deficits can cause massive yield and economic losses in agriculture production (Kim et al., 2019). Globally, at least 70% of the irrigated area is periodically exposed to drought and over half of the rainfed lands have sub-suitable productivity; it is likely to increase under increasing climate change, leading to more water shortage (Zhang & Cai, 2013). Mustard is one of the key oilseed crops in the world. It requires less irrigation and is usually grown under rainfed conditions in India. Water deficit stress at critical stages of growth adversely affects a variety of vital physiological and biochemical processes, which lead to severe impacts on mustard yield (Bandeppa et al., 2019). Under drought stress, yield reductions ranging between 17–94% have been reported in mustard. Reduction in the plant height and yield, and adverse effects on physiological attributes (relative water content, membrane stability index and chlorophyll content) were reported as a result of water deficit stress on fields (Paul et al., 2022).
Germination is a vital process and, as the first stage in plant development, it can be considered as determinant for plant productivity (Parihar et al., 2015). Germination processes, like enzymatic hydrolysis of lipids, proteins and carbohydrates and metabolites transportation, are dependent on water availability. Due to climate change, modification of rainfall patterns and frequent periods of extreme weather are expected, which may result in increased plant evapotranspiration and lower soil moisture (Zhang & Cai, 2013). Given these ongoing conditions, germination of crops will likely be largely affected by water deficit stress at the time of sowing, leading to poor seedling development or stand establishment and eventually to reduced crop production (Saux et al., 2020). Mustard seed germination is also severely affected by water deficit stress; decreased germination percentage and seedling fresh weight were reported under PEG 6000-induced osmotic stress (Bandeppa et al., 2015; Nivetha et al., 2021).
Phytohormones, especially abscisic acid and gibberellic acid (GA), play a key role in regulating the process of germination. Seed imbibition results in the activation of GA biosynthesis, GA diffuses to aleurone layer and initiates the synthesis of enzymes involved in food reserve hydrolysis. Glyoxylate cycle enzymes, mainly isocitrate lyase and malate synthase, play a crucial role in lipid degradation and are essential in oil seed germination (Ali & Elozeiri, 2017). ABA has been reported to inhibit water uptake and is inhibitory for germination. During germination under favourable conditions, GAs represses the effect of ABA levels on germination (Ali & Elozeiri, 2017 and Liu et al., 2019). The level of ABA in plants increases under stress conditions, which regulates the expression of drought stress-responsive genes and the level of osmolytes like proline and glycine betaine (Bandeppa et al., 2019).
Plant growth-promoting rhizobacteria (PGPR) are soil bacteria living in the rhizosphere and roots of plants. They have direct and indirect involvement in plant growth and development. Germination and enhanced seedling growth through seed priming with PGPR is well established and reported by many authors (Manjunatha et al., 2017; Paul et al., 2017 and Nivetha et al., 2021). Inoculation with rhizobacteria Pseudomonas putida, Azospirillum lipoferum, Bacillus subtilis, Paenibacillus polymyxa, Streptomyces spp., Mesorhizobium ciceri spp, and Rhizophagus irregularis has been reported to impart drought stress tolerance in plants (Paul et al., 2017). Improved root elongation, biochemical and physiological processes, nutrient uptake, phytohormone regulation and antioxidant production were reported as the mechanisms for imparting drought tolerance to plants (Chieb and Gachomo, 2023; Zhao et al., 2022; Zhu et al., 2023). Bacillus spp., one of the most abundant PGPR in soil and rhizosphere, has been reported to improve seed germination, growth, and plant biomass in mustard (Bandeppa et al., 2019; and Paul et al., 2017). Inoculation with osmotolerant rhizobacteria Bacillus sp. MRD-17, Bacillus cereus NAD-7 in mustard and Shewanella putrefaciens strain MCL-1, Cronobacter dublinensis strain MKS-1in pearl millet, increased seedling vigour, fresh weight of seedlings, plumule and radicle length under osmotic stress conditions (Bandeppa et al., 2015; and Manjunatha et al., 2017).
In an earlier study, Nivetha et al. (2021) investigated the effect of different population densities of rhizobacteria Bacillus sp. strain MRD-17 and Bacillus casamancensis strain MKS-6 on seed germination and plant growth. They observed that rhizobacterial population densities ranging from 105 to 107 cfu/ mL stimulated germination and seedling fresh weight under control and water deficit stress conditions. There are limited studies related to the mechanism of seed germination enhancement by rhizobacteria and its effect on hydrolytic enzymes and osmolytes under water deficit stress during germination. Regulation of phytohormone levels, expression of germination-related genes and stress-responsive genes by PGPR during germination under osmotic stress also have rarely been demonstrated. Keeping the above-given facts in view, in the present investigation, we made an attempt to elucidate the mechanism behind the effect of Bacillus sp. strain MRD-17 and Bacillus casamancensis strain MKS-6 on seed germination under control as well as water deficit stress. We also assessed the influence of osmotic stress on the metabolic processes during germination and changes in the expression of stress-responsive and germination-related genes under water deficit stress.
2 MATERIALS AND METHODS
2.1 Plant and rhizobacterial cultures
Drought-susceptible mustard (Brassica juncea) cultivar Pusa Karishma LES-39, procured from the Division of Genetics, IARI, New Delhi, was used in the present study. Rhizobacterial cultures Bacillus sp. strain MRD-17 and B. casamancensis strain MKS-6, used in the present study were obtained from the Division of Microbiology, IARI, New Delhi (Bandeppa et al., 2018; Rathi et al., 2018). These cultures were screened earlier for osmotic stress tolerance and both cultures were reported to be osmotolerant.
2.2 Sample preparation
Mustard seeds were surface-sterilized with 0.1% HgCl2 solution, followed by several washing with sterile water to remove the traces of HgCl2. Seeds were dipped in 106cfu/ mL of B. casamancensis strain MKS-6 and 107cfu/ml of Bacillus sp. strain MRD-17 for one hour, concentrations that had the most stimulatory effect on mustard seed germination according to a previous study (Nivetha et al., 2021). Then, these seeds were kept for germination in Petri plates containing 0.8% agar as control and 0.8% agar supplemented with 20% PEG 6000 as osmotic stress treatment. Plates were incubated at 25 ± 2 °C for the required period (24–96 h). After germination, seedlings were collected and tested for various physiological and biochemical parameters.
2.2.1 Germination measurements
The following parameters were determined as a measure of germination (Noorhosseini et al., 2017).
Mean speed of Germination (MS) = ∑(nt)/ ∑n, where n = number of normal germinated seeds at time t.
Mean daily germination (MDG) = GP/T, where GP = germination percentage and T = number of days after the start of the test.
Mean germination time (MGT) = ∑dn / ∑n, where n = number of seeds germinated until day d.
Radicle and plumule length in cm were recorded at 72 h after incubation.
Seedling vigour index = GP × SL, where GP = germination percentage and SL = Seedling length.
2.2.2 Enzymes related to germination
The titrimetric method of Malik et al. (2000) was used for the analysis of lipase enzyme activity. One gram of seedlings were homogenized in 10 mL acetone, centrifuged at 3000 rpm, at 4°C for 20 min. The pellet was collected, dissolved in double distilled water, vortexed, centrifuged at 7500 rpm for 20 min at 4 °C and supernatant was collected as crude enzyme. To one mL of crude enzyme, 5 mL olive oil emulsion (180 mL distilled water, 0.4 g sodium benzoate, 20 mL olive oil and 1 g gum arabic) and 5 mL 0.1 M Tris buffer (pH 8.0) was added. This mixture was incubated at 35°C for 10 min, 10 mL of 1:1 solution of acetone and methanol was added and titrated against 0.025 N NaOH using 1% phenolphthalein as indicator. The volume of NaOH used in the titration was used to calculate lipase activity from the standard curve. Standard curve was prepared using lipase enzyme. One unit of lipase was defined as the amount of enzyme required to liberate 1 μmol of free fatty acid from olive oil per min under the standard assay conditions.
2.2.3 Analysis of osmolytes
Osmolytes, proline and glycine betaine contents were determined from 72-h-old seedlings. Bates et al. (1973) method was used for determining the proline content. For that, one g seedling from various treatments was ground in 5 mL of 3% 5-sulfosalicylic acid, centrifuged at 10,000 rpm at 4°C for 30 min and supernatant was collected. One ml of the supernatant was mixed with 2 mL of ninhydrin reagent, boiled for 1 hr in a boiling water bath, cooled and 2 mL of toluene was added for colour extraction. Absorbance was measured at 520 nm using a spectrophotometer and proline content in the samples was quantified based on the proline standard curve. Glycine betaine was analyzed based on the method given by Grieve & Grattan (1983). One g seedling samples were ground in 5 mL of chilled 0.2 M phosphate buffer (pH 7.4), centrifuged at 10,000 rpm at 4°C for 30 min. The pellet was collected and 2 mL of 0.5 M perchloric acid was added, incubated for 10 min in the cold and 3 M KOH was added until pH of the samples raised to 4.5. The suspension was again centrifuged at 10,000 rpm for 15 min and supernatant was collected. Out of this, 2 mL supernatant was incubated overnight with 0.2 mL potassium tri-iodide solution at 4°C. After incubation, the mixture was centrifuged at 10,000 rpm for 10 min, 1 mL of supernatant was transferred to a test tube and 20 drops of concentrated H2SO4 was added. The mixture was incubated at 0°C for 2 h and the precipitate formed was collected by centrifugation at 10,000 rpm for 10 min. These was dissolved in 10 mL dichloroethane, incubated for 2 h and the absorbance was measured at 365 nm using a spectrophotometer. Glycine betaine content was quantified from the standard curve.
2.2.4 Phytohormones
Total gibberellins and abscisic acid content were analysed from 72-h-old seedlings of various treatments. Gibberellins was determined using the method of Bhalla et al. (2010). Seedling (2 g) were homogenized with 10 mL sodium phosphate buffer (50 mM, pH 7.5) containing 0.02% sodium diethyl dithio-carbonate extract and incubated overnight at 150 rpm, 4°C. After incubation, the suspension was centrifuged at 10,000 × g at 4°C for 10 min, supernatant was collected and volume was made upto 10 mL with sodium phosphate buffer (50 mM, pH 7.5). In a separating funnel, the supernatant was partitioned with 5 mL diethyl ether, aqueous phase was collected and pH was adjusted to 2.5 using 1 N HCl. This was partitioned twice with 5 mL petroleum ether; the aqueous phase was recollected at each step. The extract was repartitioned twice with diethyl ether and aqueous phase was collected. The extract was again partitioned twice with 2.5 mL ethyl acetate and the organic phase was collected from each step and pooled. This was partitioned twice with 5 mL 0.2 M K2HPO4; the aqueous phase was collected and pH was adjusted to 2.5 using concentrated H3PO4. The extract was again partitioned twice with 5 mL ethyl acetate, the organic phase from each step was collected and dried by filtering through anhydrous sodium sulphate. The dried extract was dissolved in 2 mL methanol, filtered through 0.45 μm Millipore syringe filter and injected into HPLC for the estimation of total gibberellins. Variable length detector with 65% methanol:35% HPLC water as mobile phase was used for the analysis. Abscisic acid was determined using HPLC using the method given by Ali et al. (2011). Samples were homogenized thrice with 10 mL of the extraction mixture (80 mL acetone, 1 mL glacial acetic acid and 100 mg of 2, 6 di-tart-butyl 4-methyl phenol in a total volume of 100 mL) for 30 min each and the extract was collected, pooled, and filtered through Whatman No.1 filter paper; acetone was removed by rotary flask evaporator and the remaining lipid soluble material was dissolved in 5 mL acetic acid. The extract was filtered through 0.45 μm Millipore syringe filter and injected into HPLC for abscisic acid estimation. Mobile phase used was 1% acetic acid in 95% methanol and the detector used for the analysis was Variable wavelength detector (265 nm).
2.2.5 Analysis of the expression of germination-related and drought-responsive genes
Priming effect of inoculation on the expression of germination-related and drought stress-responsive genes were determined from 72-h-old seedlings. Samples were frozen in liquid nitrogen and stored at −80°C for RNA isolation. Total RNA was isolated using RNeasy® Plant Mini Kit (QIAGEN), according to the manufacturer's instructions. cDNA was synthesized by using Prime Script™ 1st strand cDNA Synthesis Kit (TaKaRa) and cDNA was stored at −20°C. Gene specific primers for qRT-PCR were designed manually based on the sequence information available in databases (NCBI) and the primer characteristics were analysed using Oligoanalyzer (http://eu.idtdna.com/calc/analyzer). Semi-quantitative RT-PCR was performed using StepOne real-time PCR machine (Applied biosystems) using cDNA, 5 μL of 2x KAPA master mix, 0.2 μL each of forward and reverse primer and 0.2 μL of 50x ROX high. Conditions for qRT-PCR were as follows: Holding at 95°C for 3 min followed by 40 cycles of denaturation at 95°C for 10 sec and annealing/extension at 60°C for 20 sec. Expression data were normalized using BjACTIN as endogenous control gene. Relative fold change was calculated using the 2−ΔΔCt method (Livak & Schmittgen, 2001). List of primers used is given in Table S1.
2.3 Statistical analysis
Data were analysed by using WASP 2.0 statistical package from ICARGOA and Analysis of variance (ANOVA) was used to calculate the least significant difference (LSD) at a probability level of 0.05%. Tukey's honestly significance difference (HSD) test at p < 0.05 was used to differentiate the single means from ANOVA. Principle component analysis (PCA) of mixed data was done in ‘R' using the package FactoMineR. Pearson correlation coefficient analysis of the mixed data was done with RStudio 1.3.1056.
3 RESULTS
3.1 Effect of inoculation and water deficit stress on the seed germination
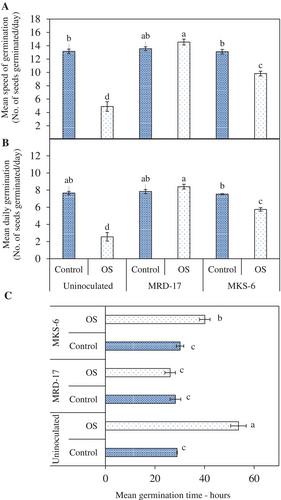
The effect of inoculation with rhizobacteria Bacillus sp. strain MRD-17 and B. casamancensis strain MKS-6 on seed germination was examined under both no stress and osmotic stress conditions (Figure 1). Osmotic stress led to a reduction in germination (Figure 1B); however, inoculation with the rhizobacteria significantly enhanced seed germination. The average speed of germination was found to be similar in all the treatments under no stress conditions. Under osmotic stress conditions, a 63% reduction in the mean speed of germination was observed, but the treatments with rhizobacteria showed a significant enhancement (Figure 2A). Notably, the highest mean germination speed was observed in Bacillus sp. strain MRD-17-treated seeds under osmotic stress conditions. Additionally, seeds treated with rhizobacteria showed a similar mean daily germination under no stress conditions (Figure 2B). However, osmotic stress had a significant impact on the mean daily germination, leading to a 67% reduction. Nonetheless, treatments with rhizobacteria, especially Bacillus sp. strain MRD-17, showed a 230% increase and significantly enhanced the mean daily germination under osmotic stress conditions (Figure 2B). Mean germination time (Figure 2C) under no stress conditions was observed to be similar for all treatments, ranging between 28.3–30.1 hours. Osmotic stress significantly affected mean germination time, as uninoculated seeds took 53.7 hours to germinate under such conditions. However, Bacillus sp. strain MRD-17 treatment significantly reduced the time taken for germination (26.1 hours) under osmotic stress conditions, followed by B. casamancensis strain MKS-6 (40.17 hours).
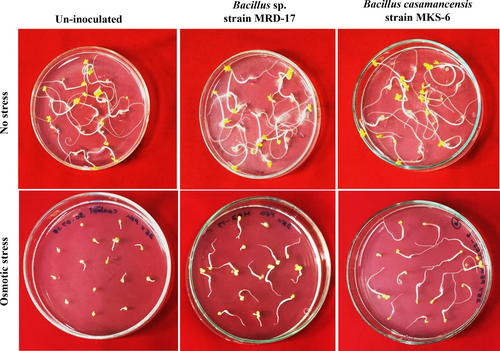
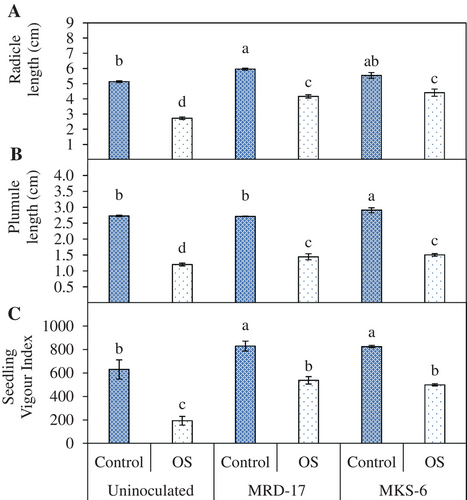
Inoculation with both the rhizobacteria led to significant improvement in the seedling length at 72 h of germination. The highest radicle length was observed in Bacillus sp. strain MRD-17-inoculated seedlings, followed by B. casamancensis strain MKS-6 under no stress condition (Figure 3A). Moreover, plumule length of B. casamancensis strain MKS-6 inoculated treatments also showed 1.2-fold enhancement compared with the no stress control, while there was no effect on Bacillus sp. strain MRD-17-inoculated seedlings (Figure 3B). Osmotic stress not only delayed germination but also inhibited the growth of germinated seedlings; however, inoculation with both rhizobacteria improved these parameters. Under osmotic stress conditions, 1.5 to 1.6-fold and 1.2 to 1.25-fold increase in the radicle and plumule length, respectively, were observed in the seedlings inoculated with rhizobacteria. The highest seedling vigour was observed due to inoculation with both rhizobacteria under no stress condition (Figure 3C). Additionally, osmotic stress significantly reduced the seedling vigour by 69%; however, seedling vigour was significantly improved upon inoculation with rhizobacteria by 1.58 to 1.79-fold under osmotic stress conditions.
3.2 Rhizobacteria and osmotic stress on enzymes related to germination
Germinating oil seeds usually have two types of lipases: acid lipase active during the initial stages of germination followed by alkaline lipases, whose activity increases during 3–5 days of germination (Muto and Beevers, 1974). Under no stress conditions, the rhizobacteria inoculated seeds showed less lipase activity at 48 h of germination compared with control, indicating that the germination process was faster with rhizobacteria, which led to a faster reduction in lipase activity (Figure 4A). Lipase activity at 72 h was observed to be higher in seedlings inoculated with rhizobacteria compared with the control and there was a continuous increase in the lipase enzyme activity over the period. Under osmotic stress conditions, seedlings treated with the rhizobacteria showed higher lipase activity compared with uninoculated stressed seedlings at 72–96 h of germination. The lipase activity was reduced after 72 h in rhizobacteria-treated seedlings, while its activity increased in uninoculated stressed seedlings.
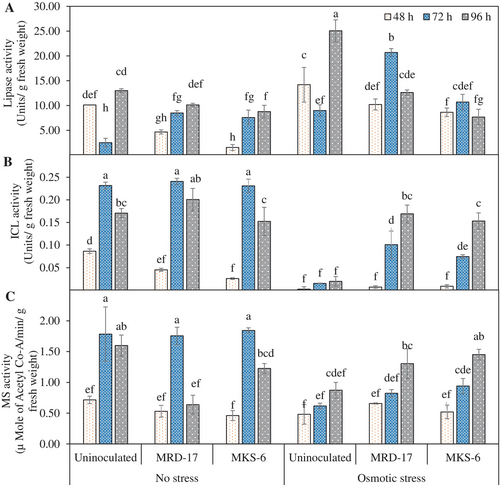
Activities of isocitrate lyase (ICL) and malate synthase (MS) increased between 48 h-72 h of germination and then decreased at 96 h under no stress condition (Figure 4B,C). ICL and MS activities of seedlings treated with both rhizobacteria were similar to uninoculated control 72 h after germination. Under osmotic stress conditions, isocitrate lyase activity was lowest in the uninoculated control at all time points. Inoculation with the rhizobacteria significantly improved ICL activity by 5–7 folds and 8 folds during 72 and 96 h of germination, respectively. There was no significant difference in the ICL activity of different treatments at 48 h of germination under osmotic stress conditions (Figure 4B). Isocitrate lyase activity was lowest in the uninoculated control during 48–96 h of germination. Seedlings inoculated with the inhibitory populations of rhizobacteria showed 1.7 to 3 and 4 to 5-fold increase in ICL activity at 72 h and 96 h, respectively. Under osmotic stress conditions, MS activity was significantly increased in the seedlings treated with the rhizobacteria, especially at 96 h, which was 1.5 to 1.6-fold higher than the uninoculated control (Figure 4C).
3.3 Rhizobacteria on osmolyte accumulation
Seedlings inoculated with Bacillus sp. strain MRD-17 showed higher proline content than uninoculated control and B. casamancensis strain MKS-6 treatments under no stress conditions (Table 1). Seedlings treated with B. casamancensis strain MKS-6 recorded lower proline content (1.3-fold) than uninoculated control treatments. Proline and glycine betaine content of seedlings under osmotic stress were significantly higher than under no stress. Under osmotic stress conditions, uninoculated seedlings showed significantly higher proline content than treatments with both rhizobacteria. Seedlings inoculated with Bacillus sp. strain MRD-17 showed significantly higher proline content than B. casamancensis strain MKS-6. Uninoculated seedlings showed higher glycine betaine content that inoculated ones. Glycine betaine content of Bacillus sp. strain MRD-17 was significantly higher than B. casamancensis strain MKS-6.
| Osmolyte accumulation | Phytohormone | ||||
|---|---|---|---|---|---|
| Proline | Glycine betaine | ABA | GA | ||
| Treatments | (m Moles/ g fresh weight) | (μ Moles/ g fresh weight) | (ng/ g fresh weight) | (ng/ g fresh weight) | |
| No stress | Uninoculated | 0.37 ± 0.0012e | 76.27 ± 0.52d | 1.43 ± 0.04d | 0.66 ± 0.13d |
| MRD-17 | 0.4 ± 0.0053d | 56.27 ± 4.76e | 1.48 ± 0.16d | 0.65 ± 0.24d | |
| MKS-6 | 0.29 ± 0.0022f | 72.55 ± 3.16d | 1.49 ± 0.02d | 0.52 ± 0.09d | |
| Osmotic stress | Uninoculated | 1.27 ± 0.0031a | 175.07 ± 6.27a | 7.46 ± 0.67b | 1.56 ± 0.05c |
| MRD-17 | 1.25 ± 0.0009b | 155.47 ± 3.61b | 8.59 ± 0.33a | 1.91 ± 0.06b | |
| MKS-6 | 1.23 ± 0.003c | 139.73 ± 7.82c | 5.7 ± 0.2c | 3.96 ± 0.61a | |
- Note: Values are means of three replications.
- Data followed by different letters are significantly different (P < 0.05).
3.4 Rhizobacteria on phytohormones
ABA content of the seedlings treated with rhizobacteria was at par with the uninoculated control under no stress condition (Table 1). Seedlings exposed to osmotic stress conditions recorded higher ABA content than under no stress conditions. The ABA content of seedlings treated with B. casamancensis strain MKS-6 was significantly lower and Bacillus sp. strain MRD-17 was significantly higher than uninoculated stressed seedlings. The GA content of the seedlings treated with rhizobacteria was at par with uninoculated control, under no stress condition. Uninoculated stressed seedlings showed significantly lower GA content compared with inoculated ones under osmotic stress conditions. Seedlings inoculated with B. casamancensis strain MKS-6 had 60% higher GA and Bacillus sp. strain MRD-17 had 18% higher GA content than uninoculated seedlings.
3.5 Expression analysis of genes related to enzymes involved in reserve hydrolysis
The expression of BjICL and BjMLS, which are involved in the biosynthesis of isocitrate lyase and malate synthase, key enzymes of glyoxylate pathway, was analysed to elucidate the effect of rhizobacterial inoculation and water deficit stress condition on the events related to germination. Under no stress conditions, inoculation with the rhizobacterial strain MRD-17 alone or combined with MKS-6 led to an increase in the BjICL and BjMLS gene expression (Figure 5A,B). Under no stress conditions, BjICL expression increased by 18.5% in seedlings inoculated with Bacillus sp. strain MRD-17. The osmotic-stressed seedlings showed 207% reduction in BjICL expression. Inoculation with Bacillus sp. strain MRD-17 and B. casamancensis strain MKS-6 led to 42.5% and 44.8% increase in BjICL expression, respectively, under osmotic stress conditions. BjMS expression increased by 45.6% and 37.5% under no stress conditions with Bacillus sp. strain MRD-17 and B. casamancensis strain MKS-6, respectively. Under osmotic stress conditions, an increase of 53.2% and 30.6% in the expression of BjMS gene was observed with Bacillus sp. strain MRD-17 and B. casamancensis strain MKS-6, respectively.
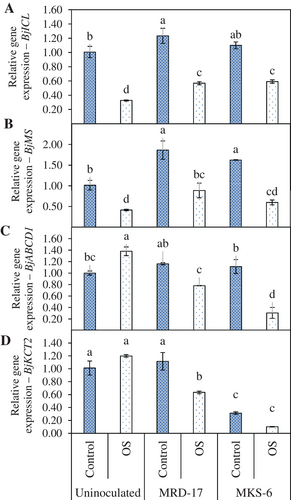
The BjABCD1 (ATP BINDING CASSETTE SUBFAMILY D MEMBER 1) gene provides instructions to produce ALDP (ADRENOLEUKODYSTROPHY PROTEIN), located in the peroxisome membranes, involved in the transport of fatty acids for breakdown (Zolman et al., 2001). The expression of BjABCD1 in uninoculated and inoculated seedlings was at par under no stress conditions (Figure 5C). Under osmotic stress, the uninoculated seedlings showed significantly higher expression of BjABCD1 compared with the inoculated seedlings.
BjKCT2 is involved in the long-chain fatty-acid β-oxidation prior to gluconeogenesis during germination and subsequent seedling growth. Under no stress conditions, the expression of BjKCT2 in Bacillus sp. strain MRD-17-inoculated seedlings was at par with the uninoculated seedlings, while it was lower by 223% in seedlings inoculated with B. casamancensis strain MKS-6 (Figure 5D). Inoculation with rhizobacteria led to a decreased expression of BjKCT2 compared with the uninoculated seedlings under osmotic stress.
3.6 Expression analysis of the genes related to osmolyte accumulation under osmotic stress conditions
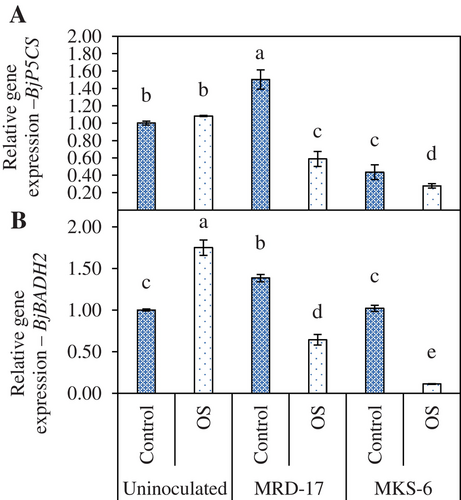
BjP5CS and BjBADH2 are genes playing a key role in the proline and glycine betaine biosynthesis; osmolytes accumulating in response to environmental stresses. Inoculation with rhizobacteria led to reduced BjP5CS expression under osmotic stress conditions (Figure 6A). Seedlings inoculated with Bacillus sp. strain MRD-17 showed 84% lower BjP5CS expression, while it decreased by 289% in the B. casamancensis strain MKS-6-treated seedlings compared to uninoculated seedlings. Osmotic stress led to the upregulation of BjBADH2 expression in uninoculated seedlings, but rhizobacteria-inoculated seedlings showed significant reduction in the expression compared with the uninoculated control (Figure 6B).
3.7 Priming effect of rhizobacterial inoculation on the genes related to phytohormone synthesis and regulation under osmotic stress condition
BjNCED3 encodes a 9-cis-epoxy carotenoid dehydrogenase, a key enzyme in the biosynthesis of abscisic acid. BjNCED3 was up-regulated in the seedlings inoculated with B. casamancensis strain MKS-6 compared to Bacillus sp. strain MRD-17 and uninoculated control under no stress conditions (Figure 7A). Under osmotic stress conditions, inoculation with B. casamancensis strain MKS-6 led to up-regulation of BjNCED3 expression compared to Bacillus sp. strain MRD-17 and uninoculated control. GA 20-oxidase (GA 20-ox) is an enzyme that catalyses the late step in the formation of active GA and is also involved in the regulation of GA biosynthesis. GA20Ox1 was up-regulated in the seedlings treated with rhizobacteria B. casamancensis strain MKS-6 compared with the uninoculated seedlings under no stress condition (Figure 7B). It was observed that, under osmotic stress conditions, the expression of BjGA20Ox1 gene was higher in the uninoculated seedlings than in no stress condition. Inoculation with rhizobacteria B. casamancensis strain MKS-6 led to decreased expression of BjGA20Ox1 under osmotic stress conditions; it was also similar to control without stress.
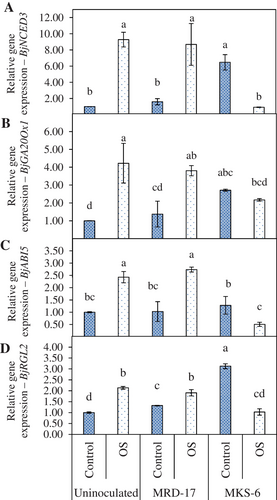
Protein abscisic acid- insensitive 5 (ABI5) participates in the ABA-regulated gene expression. Under no stress condition, inoculation with B. casamancensis strain MKS-6 led to increased expression of BjABI5 compared with Bacillus sp. strain MRD-17 and uninoculated control (Figure 7C). However, the expression levels were statistically similar with control. Under osmotic stress conditions, upregulation of BjABI5 gene expression was observed in uninoculated seedlings compared with no stress conditions. There was downregulation in the expression of BjABI5 gene in B. casamancensis strain MKS-6 inoculated seedlings compared with Bacillus sp. strain MRD-17 inoculated seedlings and uninoculated treatment. The expression of RGL2, which is a probable transcriptional regulator that acts as a repressor of the gibberellin (GA) signalling pathway, was up-regulated in rhizobacteria-inoculated seedlings compared to control (Figure 7D). Under osmotic stress conditions, inoculation with B. casamancensis strain MKS-6 led to the down-regulation of BjRGL2.
3.8 Priming effect of inoculation on drought stress-responsive genes
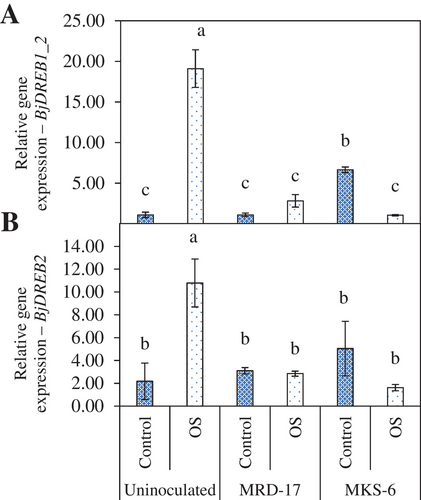
DREBs (Dehydration Responsive Element Binding) are important transcription factors involved in the regulation of stress-responsive genes. The expression of BjDREB1_2 and BjDREB2 were analysed (Figure 8). Inoculation with B. casamancensis strain MKS-6 led to the upregulation of BjDREB1_2 under no stress conditions. There was a considerable upregulation in the expression of BjDREB1_2 and BjDREB2 genes in uninoculated seedlings under osmotic stress compared with no stress conditions. Under osmotic stress conditions, both BjDREB1_2 and BjDREB2 were down-regulated, but not significantly, in the seedlings inoculated with rhizobacteria.
3.9 PCA and correlation analysis
Correlation analysis of the data indicated that there was a strong positive correlation of seedling vigour, root length, shoot length with activities of isocitrate lyase and malate synthase (at 72 hr); and a negative correlation with lipase, proline, glycine betaine and ABA content (Figure S1A). There was a strong positive correlation between the expression of genes such as BjICL and BjMLS; between BjABCD1, BjKCT2, BjP5CS and BjBADH2 genes; and between BjNCED3, GA20Ox1 and BjAB15 genes. The expression of BjRGL2 was positively correlated with the expression of BjNCED3; and BjDREB1_2 with BjDREB2 (Figure S1B).
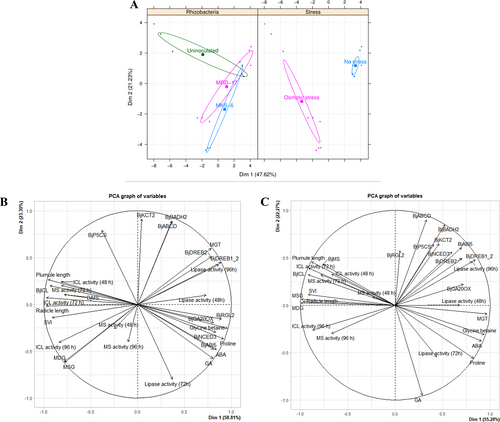
Osmotic stress considerably affected the activity and expression of various parameters as indicated by the distinct clusters formed for no stress and osmotic stress in PCA factor map (Figure 9A). Inoculation with Bacillus sp. strain MRD-17 and B. casamancensis strain MKS-6 also differentially regulated the activity and expression of the plant parameters compared with the uninoculated plants. Moreover, the two rhizobacterial strains formed distinct clusters, which were close but not overlapping, indicating that their effect on certain plant parameters was not similar. The variable map of MRD-17 (Figure 9B) showed that expressions of BjP5CS, BjBADH2, BjABCD1, BjKCT2, DREB2 and DREB1_2 were positively correlated and clustered together. These variables were negatively correlated with the cluster containing BjRGL2, BjGA20OX1, BjNCED3, BjABI5, ABA, GA, glycine betaine and proline. The clustering was observed to be different from the variable map of MKS-6 (Figure 9, 10). The variables glycine betaine, ABA, proline and GA were clustered together, which were negatively correlated with the cluster with BjRGL2, BjABCD1, BjKCT2, BjP5CS, BjBADH2, BjNCED3, BjABI5, BjDREB2, BjDREB1_2 and BjGA20OX1. These results clearly showed that MRD-17 and MKS-6 regulated the phytohormones differently during germination under water deficit stress.
4 DISCUSSION
4.1 Rhizobacteria improved germination under osmotic stress conditions
In the present investigation, an attempt was made to elucidate the influence of inoculation with rhizobacteria on seed germination of mustard under osmotic stress and the mechanism behind it. Osmotic stress was observed to have an adverse effect on mean germination time, speed of germination, seedling fresh weight and seeding length (Figures 2 and 3). Rhizobacterial inoculation had a beneficial effect on these parameters with reduced mean germination time and increased speed of germination, along with enhancement in the fresh weight and seedling length, under both no stress and osmotic stress conditions. Similar results were reported in our previous study (Nivetha et al., 2021), where inoculation with rhizobacterial population densities, ranging from 105 to 107 cfu/ mL, stimulated seedling fresh weight in vitro, and shoot and root fresh weight at 30 days after sowing (DAS) in a pot experiment, under both no stress and water deficit stress conditions. Inoculation with rhizobacteria generally enhances seed germination, fresh weight and vigour of seedlings under osmotic stress; this has been reported in many crops such as mustard, pearl millet and cluster bean (Bandeppa et al., 2015; Manjunatha et al., 2017; Rathi et al., 2018).
4.2 Rhizobacteria inoculation enhanced reserve hydrolysis under osmotic stress condition
In oilseeds, lipids cannot be used directly as a source of energy and need to be converted first to soluble sugars, which are then used for seedling growth (Wu et al., 2020). Massive conversion of triacylglycerol to sugar after germination occurs via the oil mobilization pathway, involving β- oxidation and glyoxylate cycle. β-oxidation of fatty acids derived from triacylglycerol produces acetyl-CoA, which ultimately is converted to sucrose through the glyoxylate cycle and gluconeogenesis. The sucrose produced is transported throughout the seedling, where it supports growth and development (Eastmond et al., 2000). Lipase catalyses the release of fatty acids from triacylglycerol stored within oil bodies. Lipase II is the first enzyme to be activated and is involved in the hydrolysis of fat at the stage of imbibition (Vijayakumar & Gowda, 2013). Post germination, the lipase II enzyme activity slowly decreases and the activity of lipase I increases. Similar observations were recorded in the present study, where a dip in the total lipase activity was observed at 24 h, followed by an increase in its activity (Figure 4). The increase in lipase activity was reported in the seedlings inoculated with rhizobacteria under no stress as well as osmotic stress conditions. Higher expression of these genes upon inoculation with rhizobacteria under no stress condition compared to control indicated that there was an enhanced transport of fatty acids for hydrolysis to peroxisomes, but also a higher β-oxidation of long chain fatty- acid with rhizobacteria inoculation. Under osmotic stress condition, down-regulation of BjABCD1, BjKCL2 and up-regulation of BjICL and BjMLS genes were observed with rhizobacterial inoculation. Reduced lipase enzyme activity at 96 h compared to control supported the results obtained in gene expression analysis studies under osmotic stress condition. This indicated that the rhizobacteria-inoculated seeds were in the initial stages of germination, with up-regulated BjICL and BjMLS for further hydrolysis and growth. In contrast, uninoculated seeds were still in the germination process with up-regulated BjABCD and BjKCL2 for fatty acid transport and hydrolysis. These observations were also supported by increased mean speed and time of germination in the inoculated seeds under both no stress and osmotic stress conditions.
Isocitrate lyase and malate synthase are key enzymes of the glyoxylate cycle (Eastmond & Graham, 2001). Isocitrate lyase regulates the formation of succinate and glyoxylate from isocitrate, and malate synthase produces malate from isocitrate during the glyoxylate cycle for the conversion of lipid reserves to carbohydrates needed for seed germination and growth (Eastmond et al., 2000). In an earlier study, the activity of these enzymes gradually increased after imbibition and decreased after a few days post-germination (Wu et al., 2020). In the present investigation, a similar gradual increase in the activity of these enzymes was observed, up to 96 h (Figure 4). The expression of genes BjICL and BjMLS, which are related to isocitrate lyase and malate synthase, was up-regulated due to rhizobacterial inoculation under both stress and no stress conditions (Figure 5). Strong positive correlation of seedling vigour, plumule and radicle length with ICL and MS activity at 72 h indicated the important role of these enzymes in germination (Figure 9A).
4.3 Rhizobacteria modified hormonal-induced processes for enhanced germination under osmotic stress
Our results showed that there was a modulation of plant hormones in the mustard seedlings upon inoculation with rhizobacteria under osmotic stress condition (Table 1). Generally, seed imbibition leads to the activation of gibberellic acid biosynthesis and the reduction of abscisic acid levels. GA represses the inhibitory effect of ABA, stimulates the genes encoding enzymes responsible for the hydrolysis of storage sugars, lipids, and proteins and thus initiates the synthesis of hydrolytic enzymes involved in the food reserve hydrolysis (Leubner-Metzger et al., 1995; Nonogaki et al., 2000; Ali & Elozeiri, 2017). The presence of adequate GA levels stimulated the synthesis and activity of hydrolytic enzymes, resulting in the release of nutrients required for embryo growth (Vieira et al., 2002). Gibberellin biosynthesis was studied by analysing the gene BjGA20Ox1 from the Gibberellin 20-oxidase gene family. GA 20-oxidase (GA 20-ox) catalyses a late step in the formation of active GA and is also involved in the regulation of GA biosynthesis (Ait-Ali et al., 1999). BjGA20Ox1 was up-regulated in the seedlings inoculated with rhizobacteria under no stress condition (Figure 7). This may have led to the initial germination enhancement, probably because of the increased and faster release of nutrients due to the enhanced activity of hydrolytic enzymes, as was observed in the present investigation. Similar improvement in the GA levels of plants inoculated with plant growth-promoting rhizobacteria was observed in many plants (Park et al., 2017; Khan et al., 2020). Under osmotic stress, uninoculated plants showed higher expression of BjGA20Ox1 compared to rhizobacterial treatments. However, giberellins content was observed to be increased in the rhizobacteria-inoculated plants under osmotic stress condition, leading to the observed enhancement in seed germination compared to uninoculated treatment. Higher giberellins content under osmotic stress was observed with MRD-17-inoculated seedlings. Five paralogs of GA20Ox genes have been reported in A. thaliana, a close relative of mustard (Plackett et al., 2012). These genes might have contributed to the synthesis of GA in the rhizobacteria-treated plants, resulting in the higher giberellins content observed in these treatments. In the present study, however, the expression of only one gene (BjGA20Ox1) involved in the biosynthesis of GA was analysed. Moreover, rhizobacterial strains used in this study B. casamancensis strain MKS-6 and Bacillus sp. strain MRD-17, were observed to produce gibberellins during plant-rhizobacterial interaction and might have contributed to the increased endogenous GA pool (Bandeppa et al., 2018; Rathi et al., 2018).
ABA is reported to have an inhibitory effect on seed germination and seedling growth. ABA prevents the cell wall loosening of embryo, thus inhibiting water uptake and reducing the potential of embryo growth (Koornneef & Karssen, 1994). A rapid decrease of endogenous ABA content during the phase two of germination is one of the many key factors that influence the successful completion of germination (Weitbrecht et al., 2011). Increased ABA and reactive oxygen species levels were observed during drought stress, which suppressed germination (Lie et al., 2019). Considerable increase in the ABA levels under osmotic stress condition compared with no stress condition led to delayed germination and inhibited seedling growth in the uninoculated treatment, as was also indicated by the strong negative correlation between ABA level and seedling vigour, radical and plumule length (Table 1; Figure 9A). In the present investigation, seedlings inoculated with rhizobacteria showed similar ABA levels than uninoculated control under no stress condition. The expression of NCED3 gene encoding 9-cis-epoxy carotenoid dehydrogenase, a key enzyme in the biosynthesis of abscisic acid, was up-regulated due to inoculation with rhizobacteria under no stress condition (Figure 7). In case of osmotic stress condition, BjNCED3 expression was down-regulated upon inoculation with B. casamancensis strain MKS-6, while it was up-regulated upon Bacillus sp. strain MRD-17 inoculation. This was supported by the increased levels of ABA with Bacillus sp. strain MRD-17 inoculation and reduced levels with B. casamancensis strain MKS-6 inoculation under osmotic stress compared with uninoculated seedlings. These observations indicated that ABA levels were differentially regulated by Bacillus sp. strain MRD-17 and B. casamancensis strain MKS-6.
Abscisic acid-Insensitive 5 (ABI5) participates in the ABA-regulated gene expression during seed development and subsequent vegetative stage. Ral Guanine Nucleotide Dissociation Stimulator Like 2 (RGL2) is a probable transcriptional regulator that acts as a repressor of the GA signalling pathway (Vishal and Kumar, 2018). Under osmotic condition, the higher expression of BjABI5 and BjRGL2 probably had a germinative suppressive effect on the seedling growth of uninoculated seedlings (Figure 7). The reduced expression of these two genes, observed in B. casamancensis strain MKS-6 and Bacillus sp. strain MRD-17 inoculated seedlings, respectively, under osmotic stress may have led to the observed germination enhancement.
4.4 Rhizobacteria inoculation reduced the accumulation of osmolytes and stress-responsive genes in seedlings under osmotic stress
There is an increased accumulation of osmolytes in the plants exposed to osmotic stress. Proline and glycine betaine are known to play an important role in the osmotic adjustment under drought stress. The increased osmolytes content in the uninoculated plants exposed to water-deficit stress as compared with no stress plants indicated an increase in the stress levels under these treatments (Figure 6; Table 1). Likewise, there was a considerable increase in the accumulation of osmolytes in barley and Axonopus compressus under drought stress (Tabassum et al., 2018; Nawaz & Wang, 2020). Inoculation with rhizobacteria did not have any effect on the osmolyte accumulation under no stress condition. However, under osmotic stress condition, inoculation led to reduced accumulation of proline and glycine betaine in the seedlings. This indicated that there was a lowering of the stress level in the inoculated seedlings, leading to the reduced accumulation of the osmolytes. Similar decreases in the osmolyte content have been reported in PGPR-inoculated paddy and chickpea under osmotic stress (Jha & Subramanian, 2014; Khan et al., 2020). Moreover, various authors suggested that higher accumulation of osmolytes was not correlated to tolerance to abiotic stresses but rather to the stressed condition, as potassium ions were more involved in maintaining osmotic balance (Wang et al., 2003; Rabie & Almadini, 2005; Shukla et al., 2012). Expression of genes BjP5CS and BjBADH2, playing a key role in the proline and glycine betaine biosynthesis, also showed similar pattern of downregulation in the rhizobacteria-inoculated seedlings under osmotic stress condition, while there was upregulation of these genes in the uninoculated plants exposed to osmotic stress. Thus, the expression levels of BjP5CS and BjBADH2 supported the reduced accumulation of osmolytes in the rhizobacteria-inoculated stressed plants and enhanced accumulation in the uninoculated stressed plants.
DREBs are important transcription factors involved in the regulation of the expression of many stress-responsive genes via ABA-independent signaling pathways and play a crucial role in improving the stress tolerance of plants (Lata & Prasad, 2011). Expression of BjDREB1_2 and BjDREB2, transcription factors involved in the regulation of an ABA-independent signaling pathway, was downregulated in the rhizobacteria-inoculated seedlings under osmotic stress condition, indicating that the ABA-independent pathway was possibly not involved in the rhizobacteria-mediated alleviation of osmotic stress (Figure 8). Moreover, the expression of these transcription factors was variable under no stress condition.
The improved germination under drought stress due to seed priming was attributed to processes such as early mobilization of seed reserves, weakening of endosperm and elongation of embryo cells. Saha et al. (2022) reported that these processes led to an enhancement in pre-metabolic processes. From our study, it is clear that rhizobacteria inoculation facilitates the mobilization of seed reserves by regulating the phytohormones and enzymes. Additionally, it controlled the accumulation of osmolytes, enabling the seeds to endure osmotic stress. These demonstrate the ability of rhizobacteria to alleviate stress during the germination process.
5 CONCLUSION
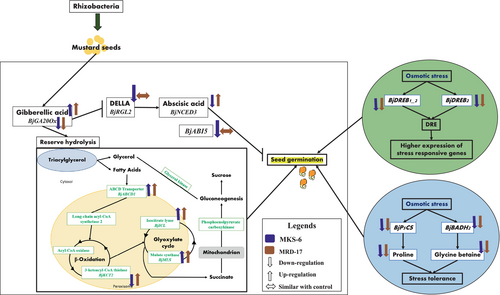
In conclusion, in the present study, the overall mechanism for the effect of inoculation with rhizobacteria under no stress and osmotic stress was deciphered, as described in Figure 11. Enhancement of GA levels upon inoculation with rhizobacteria may have led to the observed increase in the activity of hydrolytic enzymes under no stress and osmotic stress condition. Eventually, these led to the observed improvement in seed germination upon rhizobacterial inoculation. Rhizobacteria presumably regulated the production of GA for initiating the reserve hydrolysis process and sugar production, which may have led to the reduced sensitivity of germination to ABA levels in inoculated seedlings under osmotic stress condition. Moreover, BjABI5 and RGL2 genes, involved in the signalling pathway of ABA and GA, also had a role to play in the germination process by suppressing the effect of ABA and enhancing the effect of GA. At germination stage, seeds inoculated with rhizobacteria showed increased activity of hydrolytic enzymes (lipase, ICL and MS), presumably due to the regulation of GA- and ABA-related genes' expression by the inoculated rhizobacteria. Increased giberellin levels and reduced ABA levels may have played a key role in the germination enhancement by B. casamancensis strain MKS-6 inoculation. B. casamancensis strain MKS-6-inoculated seedlings also showed less accumulation of osmolytes, which indicated that B. casamancensis strain MKS-6 increased the seeds' tolerance to osmotic stress and enhanced the germination by regulating plant hormones and enhancing the activity of hydrolytic enzymes involved in germination. Increased giberellin levels with increased ABA levels were observed in seedlings inoculated with Bacillus sp. strain MRD-17. Increased accumulation of osmolytes was also observed in Bacillus sp. strain MRD-17 inoculated seedlings compared with B. casamancensis strain MKS-6. It showed the possibile activation of the ABA-dependent regulation of osmotic stress tolerance by Bacillus sp. strain MRD-17 inoculation. Reduction of DREB expression showed the reduced effect of osmotic stress in rhizobacteria-inoculated seeds. This clearly indicated that Bacillus sp. strain MRD-17 and B. casamancensis strain MKS-6 enhanced the germination process by differentially regulating the plant hormones and osmolytes under osmotic stress condition.
AUTHOR CONTRIBUTIONS
Nagarajan Nivetha: Conceptualization, Methodology, Validation, Formal analysis, Investigation, Writing - original draft. Arambam Devi Asha: Methodology, Investigation. Gopinathan Kumar Krishna: Methodology, Investigation. Viswanathan Chinnusamy: Conceptualization, Methodology, Supervision. Sangeeta Paul: Conceptualization, Methodology, Supervision, Formal analysis, Writing - original draft, Review & editing.
ACKNOWLEDGEMENTS
This study was supported under the scheme “Innovation in Science and Pursuit for Inspired Research” (INSPIRE) by Department of Science and Technology, Govt. of India (DST INSPIRE Fellowship/2016/ IF160668). The authors are also thankful for the facilities provided by the Division of Microbiology, Post Graduate School and National Phytotron Facility, ICAR-IARI, New Delhi-110012.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




