Accumulation of reactive carbonyl species in roots as the primary cause of salt stress-induced growth retardation of Arabidopsis thaliana
Abstract
Salt stress on plants induces an increase in reactive oxygen species (ROS), which then leads to the formation of reactive carbonyl species (RCS) such as acrolein and 4-hydroxy-(E)-2-nonenal (HNE), potent cytotoxins generated from lipid peroxides. We recently showed that salt-stress treatment of Arabidopsis thaliana plants increased RCS levels, and exogenously added RCS-scavenging chemicals alleviated the stress symptoms, indicating that RCS were responsible for the tissue damage in salt-stressed plants. To obtain deeper insights into the role of RCS in stressed plants, we here analyzed changes in the levels of various RCS in the roots and shoots of A. thaliana. NaCl (90 mM) addition to the culture medium as a salt-stress treatment caused growth inhibition and leaf chlorosis. Carbonyl analysis using HPLC revealed that the stress treatment induced a 2-fold increase in the root levels of RCS, including acrolein, HNE and 4-hydroxy-(E)-2-hexenal (HHE). In the shoots, basal levels and stress-induced increases of the RCS were lower than in roots. In the transgenic A. thaliana plants that overexpress the RCS-scavenging enzyme 2-alkenal reductase (AER) cDNA under the β-estradiol (β-ED)-responsive promoter, salt stress induced less damage than in the wild-type under β-ED supplementation. The AER overexpression suppressed the stress-induced increases in HNE, acrolein, HHE and (E)-2-hexenal in roots and in HNE in leaves, but not the ROS increase. These results suggest that the RCS increase in roots was the primary cause of salt-induced damages. Enhancing RCS-scavenging abilities, such as by AER overexpression, could be a new strategy to confer salt-stress tolerance to plants.
1 INTRODUCTION
Among abiotic stressors to plants, soil salinity is one of the most detrimental, as it limits plant survival and hampers crop production (Parihar et al., 2015). Under saline conditions, high osmolarity and a high concentration of Na+ ions disturb the metabolism and thus inhibit plant growth (Munns & Tester, 2008). Salinity causes ionic imbalance in cells, which leads to K+ ion deficiency and, as a consequence, an increase in the levels of reactive oxygen species (ROS) via two distinct mechanisms. In the first, K+ deficiency decreases the CO2 fixation capacity, and the resulting over-reduced state of the chloroplast enhances ROS generation in chloroplasts and peroxisomes (Asada, 2006). In the second, K+ ion deficiency leads to activation of the plasma membrane-bound nicotinamide adenine dinucleotide phosphate (NADPH) oxidase to produce O2− (Cakmak, 2005).
ROS are thought to cause damage to cells by oxidizing biomolecules. Cys residues in proteins are oxidized to sulfenyl by ROS. At least 11 proteins that undergo Cys sulfenylation have been identified in Arabidopsis thaliana plants that were fed with H2O2 from the culture medium (Akter et al., 2017). Other amino acid residues are also oxidized by ROS to carbonyl groups (Møller et al., 2007). In salt-stressed A. thaliana leaves, we have detected an increase in the carbonylation level in at least 28 proteins (Mano et al., 2014).
In addition to ROS, reactive carbonyl species (RCS), which are endogenous electrophilic molecules secondarily generated from ROS, also cause cell damage. RCS is a collective term for a group of aldehydes and ketones that are produced via the decomposition of lipid peroxides and have α,β-unsaturated bonds (Figure 1A; Mano, 2012; Biswas & Mano, 2021). These compounds are also known as oxylipin reactive electrophile species (Farmer & Mueller, 2013). In plants, over a dozen types of RCS with different carbon chain lengths and degrees of oxygenation, such as acrolein and 4-hydroxy-(E)-2-nonenal (HNE), have been detected, and their levels have been shown to increase under stress conditions (Mano et al., 2010; Yin et al., 2010; Yamauchi et al., 2012; Sultana, 2022). Because of the electrophilicity of its β-carbon, an RCS molecule can covalently modify proteins at the Cys-, Lys- and His residues (Esterbauer et al., 1991; Matamoros et al., 2018) and the lipoate moiety in certain enzymes (Taylor et al., 2004), thereby mediating oxidative damage to proteins (Biswas & Mano, 2021; Mano et al., 2009; Mano et al., 2019; Winger et al., 2007). Matamoros et al. (2018) scrutinized the structure of carbonylated amino acids in legume nodule proteins and found that the carbonylation due to RCS such as 4-hydroxy-(E)-2-hexenal (HHE), crotonaldehyde, HNE, 4-oxo-nonenal and acrolein predominated over the carbonylation due to ROS. Exogenous addition of RCS to plants caused injury in A. thaliana (Alméras et al., 2003; Matsui et al., 2012) and tobacco (Mano et al., 2005) leaves and inhibited the germination of lettuce seeds (Reynolds, 1977) and root growth of tobacco seedlings (Yin et al., 2010). Thus RCS, downstream products of ROS, have the potential to exacerbate oxidative stress symptoms in plants.
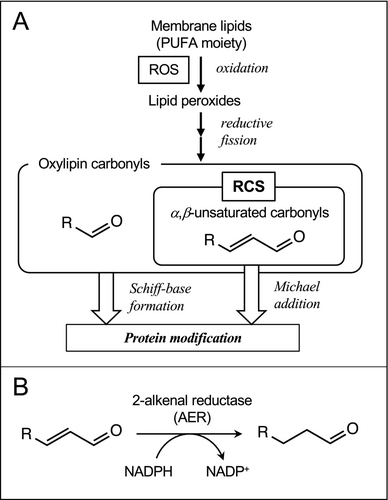
Indeed, endogenously generated RCS are involved in plant damage under environmental stress, as described below. An A. thaliana NADPH-dependent reductase 2-alkenal reductase (AER, EC 1.3.1.74, encoded by the gene At5g16970) specifically catalyzes the reduction of the α,β-unsaturated bond in RCS molecules (Figure 1B; Mano et al., 2002). Transgenic tobacco (Nicotiana tabacum) plants that overexpress AER (AER-OE plants) suffered less severe damage in leaves under strong light (Mano et al., 2005, 2010) and in roots under Al3+ stress (Yin et al., 2010). The levels of RCS, such as acrolein and (E)-2-pentenal, were increased by these stress treatments prior to the appearance of damages in wild type plants, while their increases were significantly lower or eliminated in AER-OE plants. Notably, the overexpression of AER did not affect the ROS level in stressed tissues (Yin et al., 2010), indicating that RCS were a more direct cause of the damage than ROS.
The involvement of RCS in salinity-induced damage has also been suggested. Papdi et al. (2008) constructed transgenic A. thaliana plants that harbor the AER cDNA under the control of the XVE promoter, which drives the overexpression of AER in response to exogenously added β-estradiol (β-ED). These plants [controlled cDNA overexpression system (COS)-AER plants] showed tolerance to salt stress in the presence of β-ED, while they suffered germination delay and growth inhibition in its absence, as did the wild type plants. This provided conditional evidence that RCS caused the stress symptoms, but it was not clear which specific RCS were responsible. We recently investigated the relationship between the RCS composition and plant damage in salt-stressed A. thaliana (Sultana et al., 2022). Our results showed that NaCl treatment (100 mM) of seedlings increased the levels of ROS, protein carbonylation and the RCS acrolein, crotonaldehyde, (E)-2-pentenal and HNE and delayed the growth. We further showed that addition of carnosine, an RCS-scavenging dipeptide occurring in mammalian muscles (Aldini et al., 2005), suppressed the increases in these RCS and protein carbonylation levels and mitigated the growth inhibition under salt stress. It should be noted that carnosine did not suppress the ROS increase (Sultana et al., 2022). This demonstrated that RCS, rather than ROS, were the major cause of protein carbonylation and, hence, of plant damage.
In a salt-stressed plant, the primarily affected site is the root. It has been reported that the responses of ROS metabolizing enzymes to salt stress treatments in roots are different from those in leaves (Cavalcanti et al., 2007). Li et al. (2022) recently showed that high activities of catalase and peroxidases in the root were correlated with the salt tolerance of heterografted chrysanthemums. Conversely, Lodeyro et al. (2016) reported that the genetic suppression of chloroplast ROS levels prevented leaf damage but did not prevent growth arrest in salt-stressed plants. Because ROS levels are reflected in RCS generation, it is expected that RCS also have different effects on the leaves and roots under salt stress. Exogenously added HNE inhibited the root growth in tobacco (Yin et al., 2010) and A. thaliana (Yin et al., 2017), and (E)-2-hexenal caused programmed cell death in wheat roots (Liang et al., 2023). Biswas and Mano (2015) showed that the overexpression of AER in tobacco suppressed the salt stress-induced injury in root epidermal cells, suggesting the involvement of RCS in the initiation or development of stress symptoms. It is therefore likely that the changes in the endogenous RCS levels will affect root growth. Thus far, however, there have been no analyses of the effects of RCS in roots; such investigations have been conducted only for leaves (Mano et al., 2010; Yamauchi et al., 2012) and whole seedlings (Sultana et al., 2022; Yin et al., 2010).
In this study, we investigated the role of RCS in the salt stress symptoms in roots and shoots. We employed A. thaliana because it is a salinity-sensitive species (Kazachkova et al., 2018) and has been utilized as an experimental model for investigating the mechanisms of stress sensitivity (Yalcinkaya et al., 2019). We utilized COS-AER plants to examine the effects of induced overexpression of AER and analyzed the stress-induced changes in the RCS levels both in roots and shoots. Analysis of the carbonyl composition revealed the importance of RCS in roots for the suppression of plant growth.
2 MATERIALS AND METHODS
2.1 Plant material and stress conditions
Two independent homozygous lines of Arabidopsis thaliana, COS-AER #3 and COS-AER #13, were selected for their hygromycin resistance from the T1 seeds of the N180 line (Papdi et al., 2008). The A. thaliana ecotype Columbia-0 (Col-0) was employed as a control wild-type (WT) line. Seeds were surface-sterilized with 5% sodium hypochlorite containing 0.1% Tween 20 for 20 min with intermittent shaking, then washed with deionized sterilized water for 5 min. Washing was repeated six times. Then the seeds were placed on 1% agar plates containing half-strength Murashige and Skoog medium supplemented with 1.5% sucrose, pH 5.7–5.9 (1/2 MS agar), with or without 90 mM NaCl and with or without 4 μM β-ED. The plates were kept at 4°C for stratification for 2 days and then transferred to a 25°C room with a photoperiod of 16-h light/8-h dark. Light intensity was 125 μmol m−2 s−1.
2.2 Determination of photosystem II (PSII) activity
The relative PSII activity in vivo, represented as the maximal photochemical yield of chlorophyll fluorescence (Fv/Fm value) as formulated by Genty et al. (1989), was determined with a MINIPAM chlorophyll fluorometer (Walz). The plants were incubated in darkness for 5 min before measurement.
2.3 Determination of AER (EC 1.3.1.74) activity
Plants (approx. 0.1 g) subjected to each treatment were collected, washed and within 3 min frozen in liquid nitrogen and ground into powder with a pre-chilled mortar and pestle. The powder was then homogenized with extraction medium (50 mM KH2PO4, 1 mM EDTA and 1X protease inhibitor cocktail (Sigma-Aldrich)). The plant extract was centrifuged at 1,000 × g for 5 min at 4°C and the resulting supernatant was collected for the enzyme assay. AER activity was determined by measuring the oxidation rate of 0.1 mM NADPH at 340 nm in 1 mL of reaction mixture containing 50 mM MES-NaOH, pH 6.0, and 0.1 mM HNE (Mano et al., 2002). Protein concentrations were determined by using Pierce 660 nm Protein Assay Reagent (Thermo Scientific) with bovine serum albumin as a standard.
2.4 Determination of H2O2
In situ staining of H2O2 with 3,3′-diaminobenzidine (DAB) was done according to Daudi and O'Brien (2012), as follows. Whole seedlings were vacuum-infiltrated with an aqueous DAB solution (1 mg/mL) containing 10 mM Na2HPO4, pH 3.0. The seedlings were kept in the solution in darkness for 4 h with shaking at 90 rpm. To bleach unreacted DAB, seedlings were incubated with the combination of ethanol: acetic acid:glycerol at 3:1:1 at 90°C for 10 min.
H2O2 concentrations were also determined spectrophotometrically according to Kamara and Pflugmacher (2008). In brief, seedlings (approx. 100 mg) were harvested and immediately frozen and ground to powder under liquid nitrogen. The powder was then suspended in 100 mM potassium phosphate (0.5 mL), pH 6.8, and centrifuged at 13,000 × g for 10 min, and the resulting supernatant was subsequently centrifugated at 19,100 × g for 5 min. The supernatant (160 μL) was then mixed with 480 μL titanium sulphate solution (0.1% in 20% H2SO4) and incubated for 1 min, and then a V-750 spectrophotometer (JASCO) was used to measure the absorbance at 410 nm. The H2O2 concentration was determined using an absorption coefficient of 0.28 mM−1 cm−1.
2.5 Determination of carbonyls by using HPLC
Seedlings (100 mg) were collected from the culture plate, put in acetonitrile (2.5 mL) containing 10 μM 2-ethylhexenal and 0.005% butylated hydroxytoluene and incubated at 60°C for 30 min. Then, the extract was collected and derivatized with DNPH (0.5 mM), and the carbonyl-DNP derivatives were determined by reverse-phase HPLC as previously reported (Mano & Biswas, 2018).
3 RESULTS
3.1 Induction of the AER activity in COS-AER plants by β-ED
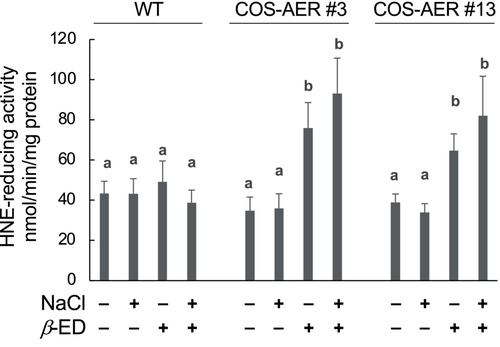
The overexpression of AER in COS-AER plants is driven by β-ED (Papdi et al., 2008). To evaluate the extent of overexpression, we determined the NADPH-dependent HNE-reducing activity in the COS-AER plants in the presence and in the absence of β-ED (Figure 2). Seedlings of the WT and the COS-AER lines #3 and #13 were grown on 1/2 MS agar for 6 days, transferred to the medium with or without β-ED, and then cultured for 3 d. Both WT and COS-AER plants showed a basal level of HNE-reducing activity. Because HNE contains both an aldehyde moiety and the α,β-unsaturated bond, the activity value determined here represents the total activities of AER and other aldehyde-reducing enzymes such as aldo-keto reductases (Simpson et al., 2009). Both in the WT and COS-AER lines, the level of HNE-reducing activity was not significantly changed by salt stress treatment (90 mM NaCl) in the absence of β-ED. This shows that RCS-scavenging enzymes, including AER, were not significantly induced by the stress treatment. Application of β-ED to WT plants, both salt-stressed and non-stressed, did not affect the HNE-reducing activity, indicating that β-ED does not induce the genes of RCS-scavenging enzymes. When β-ED was added to the culture medium used to grow COS-AER seedlings, the HNE-reducing activity increased to 2.5 times compared to when β-ED was not added, and the extent of increase was not significantly different between salt-stressed and non-stressed plants. Thus, under this experimental condition, β-ED exerted a specific action on the plants to induce the XVE promoter-driven transgene (i.e., the recombinant AER gene).
3.2 Salt stress-induced damages were mitigated by the overexpression of AER
We observed the growth of plants under the salt stress condition for 6 days (Figure 3). As compared with the plants without salt stress treatment, the stressed plants of both the WT and COS-AER lines showed smaller root elongation (Figure 3B) and suppression of growth, represented as the seedling dry weight (DW) (Figure 3C). Leaves were also damaged; the PSII activity, as determined by the Fv/Fm value of chlorophyll fluorescence, was lower (Figure 3D) and some leaves exhibited chlorosis (Figure 3A).
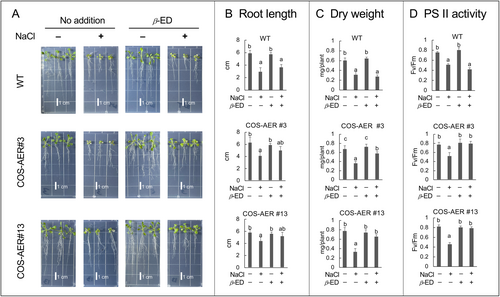
The addition of β-ED to the COS-AER#3 and #13 plants significantly restored their growth under salt stress, while it did not have any effects on the growth of the WT as judged by DW (Figure 3C). The elongation of the primary root in COS-AER#3 and #13 plants under salt stress also tended to recover in response to β-ED, while root elongation was not affected by β-ED in the WT (Figure 3B). The addition of β-ED to the COS-AER lines suppressed the decrease in the Fv/Fm value due to salt stress, while it did not reduce the loss of PSII activity in the WT plants (Figure 3D). Thus, the induced overexpression of AER in the COS-AER lines improved the ability of the plants to tolerate salt stress.
3.3 Changes in the carbonyl contents in roots due to salt stress treatment
To elucidate the carbonyl species that are relevant to salt-induced damage, we compared the changes in the carbonyl profiles in the roots between damaged plants (WT with β-ED) and protected plants (COS-AER#13 with β-ED). Six-day-old plants grown on 1/2 MS plates were transferred to 90 mM NaCl medium with 4 μM β-ED and cultured for 3 days, allowing the induction of transgenic AER cDNA (Figure 2) that should bring about salt tolerance in the subsequent culture period (Figure 3). Then the plants were separated into the shoot (including the hypocotyl) and the root parts for carbonyl analysis with HPLC, as described in the Materials and Methods. COS-AER#13 line was taken as a representative of COS-AER lines.
Figure 4 shows the carbonyls detected in roots. We could identify 6 types of RCS and 4 non-RCS carbonyls in both the WT and COS-AER roots. The RCS levels in the non-stressed WT roots were as follows: crotonaldehyde, 42.4 nmol (g FW)−1; (E)-2-hexenal, 31.5 nmol (g FW)−1; HNE, 27.7 nmol (g FW)−1; (E)-2-pentenal, 14.7 nmol (g FW)−1; acrolein, 11.5 nmol (g FW)−1; and HHE, 8.4 nmol (g FW)−1. The non-RCS carbonyl levels were the following: acetone, 557.4 nmol (g FW)−1; n-butyraldehyde, 9.0 nmol (g FW)−1; (Z)-3-hexenal, 4.5 nmol (g FW)−1; and propionaldehyde, 2.5 nmol (g FW)−1. In COS-AER roots, the levels of RCS and non-RCS carbonyls were not significantly different from those in the WT. Thus, the basal carbonyl composition in the roots was almost identical between the WT and COS-AER#13 plants in the presence of β-ED.
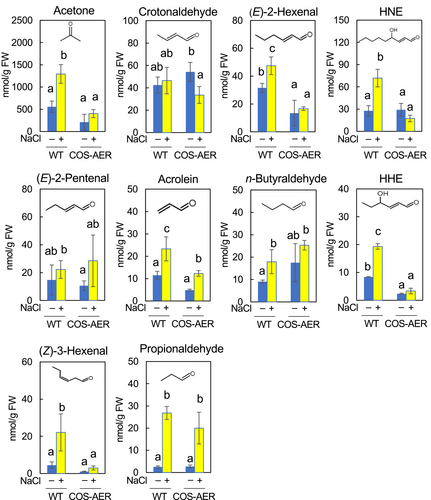
When the WT plants were treated with 90 mM NaCl, the levels of RCS in the roots increased to the following extents: HNE by 2.6-fold, HHE by 2.3-fold, acrolein by 2.0-fold, and (E)-2-hexenal by 1.5-fold. Non-RCS carbonyls also increased as follows: propionaldehyde by 11.0-fold, acetone by 2.5-fold, and (Z)-3-hexenal by 4.0-fold. In COS-AER#13 roots, under the salt stress condition, the fold increase of carbonyls was lower than that observed in the WT. HNE, HHE, (E)-2-hexenal and (Z)-3-hexenal contents increased significantly in the WT, while they showed no increases in COS-AER#13. Acrolein was increased by 2.0-fold in the WT, whereas it was increased by 1.5-fold in COS-AER#13. The acetone content increased by 2.5-fold in the WT under stress, whereas in COS-AER#13 it did not increase significantly. As described above (3.1), under the present experimental conditions, the β-ED treatment increased the AER activity only; no other types of RCS-scavenging reductases were induced. The salt tolerance and the suppression of the RCS increase observed in the β-ED-treated COS-AER plants were, therefore, attributable solely to the activity of the overexpressed AER protein.
3.4 Changes in the carbonyl contents in shoots due to salt stress treatment
In shoots, we could detect 5 types of RCS and 1 non-RCS carbonyl, i.e. propionaldehyde (Figure 5). Their levels in untreated WT leaves were as follows: crotonaldehyde, 28.3 nmol (g FW)−1; HNE, 10.8 nmol (g FW)−1; (E)-2-hexenal, 3.5 nmol (g FW)−1; acrolein, 2.0 nmol (g FW)−1; HHE, 1.1 nmol (g FW)−1; and propionaldehyde 2.8 nmol (g FW)−1. The crotonaldehyde content in leaves was 1.8-fold lower than that in the roots. Similarly, the contents of HNE, (E)-2-hexenal, acrolein and HHE in leaves were lower than those in roots by 1.5-fold, 8.0-fold, 4.7-fold and 6.6-fold, respectively. Thus, the shoots of A. thaliana contained fewer carbonyls at detectable levels than did the roots, and the basal levels of these carbonyls in leaves were lower than those in the roots. In the COS-AER #13 plants, the basal level of crotonaldehyde was 1.3-fold higher than that in the WT. For other carbonyls, the basal levels were not significantly different between the COS-AER #13 and WT plants.
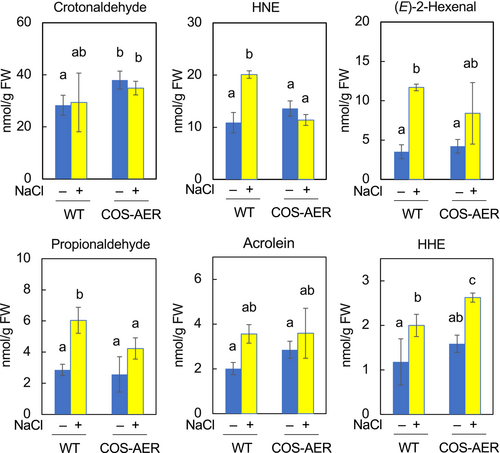
In salt-stressed WT shoots, the contents of HNE, (E)-2-hexenal and HHE were higher than in the non-stressed shoots by 2.0-fold, 3.3-fold and 1.6-fold, respectively. The propionaldehyde level was also higher by 2.1-fold. It should be noted that the levels of these RCS in shoots were lower than their basal levels in roots, even under the stress condition. For example, the HNE level in stressed shoots was ca. 22 nmol (g FW)−1, while that in non-stressed roots was ca. 28 nmol (g FW)−1. For acrolein, these values were 3.8 nmol (g FW)−1 in stressed shoots and 11.5 nmol (g FW)−1 in non-stressed roots. The crotonaldehyde content in shoots was not significantly changed by the salt stress treatment.
In the shoots of COS-AER #13 plants, the level of HNE was not higher with salt stress than without it, while salt stress increased HNE by 2.0-fold in the WT shoots. Propionaldehyde was increased by 0.6-fold in the COS-AER #13 versus 1.1-fold in the WT shoots. The increases in HNE and propionaldehyde in shoots were correlated with the level of damage in leaves.
3.5 AER overexpression did not affect the ROS increase due to salt stress treatment
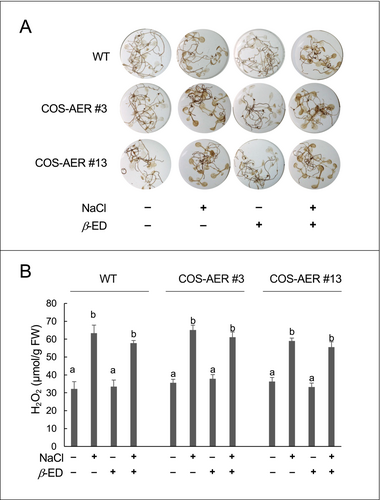
The above results suggest that the overexpressed AER contributed to salt tolerance in COS-AER plants by detoxifying RCS. Although the AER protein does not exhibit ROS-scavenging activity (Mano et al., 2002), there was a possibility that the β-ED treatment or the overexpression of AER might suppress the ROS levels in those plants via an unexpected mechanism. To investigate this possibility, we analyzed the ROS level in the seedlings by DAB staining and also determined the H2O2 content by spectrophotometry (Figure 6). Both in the WT and COS-AER plants, the ROS levels determined by these two methods were increased by the salt stress treatment. The induced overexpression of AER did not affect the extent of ROS increases due to the salt stress treatment. Thus, the observed suppression of the RCS increases in the β-ED-fed COS-AER plants was solely attributable to the enzymatic scavenging of RCS.
4 DISCUSSION
The roots of a plant are the first parts exposed to high salt concentrations. Therefore, although there have been few investigations of oxidative damages to the roots, it is very likely that the metabolism of ROS in the roots is critical to the salt tolerance of plants. Indeed, the capacity of the roots to counteract oxidative stress contributes to the salt tolerance of the whole plant (Li et al., 2022). On the other hand, we have recently demonstrated that RCS, downstream products of ROS, directly contribute to the salt stress-induced damages in A. thaliana seedlings (Sultana et al., 2022). Accordingly, in this study, we separately analyzed the changes in the RCS compositions due to salt stress in the roots and shoots, and examined the effect of RCS scavenging in the roots on the stress tolerance of the whole seedlings. We found that the basal levels of RCS in the roots were higher than those in shoots; on the FW base, the contents of HNE, acrolein, HHE and (E)-2-hexenal were 3- to 10-fold higher in the roots than in the shoots (Figures 4 and 5). The salt stress treatment caused increases in the levels of (E)-2-hexenal, HNE, acrolein, and HHE in both the roots and shoots. Notably, the shoot RCS levels of the plants under salt stress were lower than the basal RCS levels in the roots. The overexpression of AER successfully alleviated the stress symptoms. It was found that the overexpressed AER efficiently suppressed in the roots the 4 types of RCS mentioned above. On the other hand, in the shoots, only the HNE increase was suppressed; the increases in the (E)-2-hexenal, acrolein and HHE levels were not. These results indicate that the RCS production in the roots was critical for the salt-induced damages to the plant.
The 4 RCS types that were suppressed by the overexpressed AER have different levels of cytotoxicity. Reynolds (1977) comprehensively analyzed the toxicity of aliphatic carbonyls on the germination of lettuce seeds. Among the RCS tested, acrolein showed the most potent toxicity, with an IC50 of 0.043 mM, while crotonaldehyde had an IC50 0.344 mM, an 8-fold difference. If we apply these values to carbonyl's toxicity on the roots, we can estimate that acrolein made a 4-fold greater contribution to the root cell damage compared to crotonaldehyde because the level of crotonaldehyde in stressed WT roots was 2-fold higher than that of acrolein (Figure 4). Another highly toxic RCS is HNE. An analysis of the toxic effect of RCS against CO2 fixation in isolated chloroplasts showed that acrolein showed the greatest inactivation of chloroplast CO2 fixation, followed in order by HNE, (E)-2-hexenal and HHE (Mano et al., 2009). Also, acrolein and HNE induced programmed cell death (PCD) in tobacco cells at a concentration as low as 0.2 mM (Biswas & Mano, 2015). Both in roots and shoots, salt stress induced a greater increase in HNE levels than in acrolein levels (Figures 4 and 5). Thus, the effective toxicity of HNE would be comparable to that of acrolein in roots.
We also found a high acetone content in roots; its basal level was ca. 560 nmol (g FW)−1, and its level under stress conditions reached 1300 nmol (g FW)−1, which was 50-fold greater than the acrolein level. However, based on the very weak toxicity of acetone as compared with acrolein (1700-fold greater IC50; Reynolds, 1977), the contribution of acetone to the root damage would be relatively small.
The overexpression of AER also prevented damage in leaves. Interestingly, HNE was the only RCS with a suppressed increase by AER overexpression. This suggests that HNE contributed greatly to the injury of leaf cells. Ji et al. (2023) recently found that HNE modifies the D1 subunit of PSII and inhibits the dimerization of repaired PSII monomers, a crucial process for the maintenance of PSII activity under light. Alternatively, the alleviation of photooxidative damage may have been an indirect effect of the root protection. When roots are damaged, the water supply from roots to leaves will be hampered, leading to stomata closure and enhancing photoinhibition. Root protection by the overexpressed AER via scavenging of RCS might help maintain the water supply to leaves.
The overexpression of AER effectively suppressed the stress-induced increases of a broad range of carbonyls, including non-RCS carbonyls such as propionaldehyde, acetone, and (Z)-3-hexenal (Figure 4). We also have observed that the constitutively overexpressed AER in tobacco suppressed increases in a broad range of RCS and non-RCS carbonyls in Al3+-stressed seedlings (Yin et al., 2010) and in abscisic acid-stimulated leaf epidermis (Islam et al., 2016). It is interesting that the overexpressed AER showed such a “universal” carbonyl-suppression effect in spite of its strict substrate specificity for RCS; that is, AER catalyzes the reduction of the α,β-unsaturated bond of RCS molecules but does not react with the carbonyl moiety (Mano et al., 2002, 2005). A possible explanation for our observation that AER suppressed an unexpectedly broad range of carbonyls is as follows. When membrane lipids are oxidized by ROS, long-chain RCS(s) may be generated first, and then these decompose to generate various shorter-chain RCS and non-RCS carbonyls such as acetone and propionaldehyde. Because AER prefers long-chain hydrophobic RCS such as 9-oxo-octadecadienoic acid (Mano et al., 2005), it might scavenge this long-chain “precursor” RCS and eventually suppress the formation of various types of carbonyls. Non-RCS carbonyls such as acetone were not directly scavenged by AER but could be suppressed as secondary effects. To verify this hypothesis and to elucidate the process of the generation of various carbonyls in stressed cells, we are currently pursuing the identity of this putative long-chain precursor RCS.
In summary, in this study, we provided answers to several open questions. (1) The salt tolerance of the β-ED-fed COS-AER plants, previously reported, can now be attributed to the RCS scavenging activity of the overexpressed AER protein. (2) We previously reported that RCS rather than ROS are the main cause of salt stress-induced growth inhibition, but the primary site of RCS action was unknown. This study revealed that RCS were increased to a greater extent in the roots than in the shoots. (3) When the roots were protected by AER from salt stress injury, the increases of acrolein, HNE, and HHE were suppressed while the ROS increase was unaffected. Specifically, these RCS were mainly responsible for the root injury. These results indicate that the increase in the RCS levels in the roots is a critical cause of salt stress-induced damage to plants. Considering that oxidative damage is a common stress injury mechanism in many plant species and that research into A. thaliana is currently being translated to crop plants through the use of the latest genomic tools (Krukowski et al., 2020), we can expect that enhancing the RCS scavenging ability in roots through AER overexpression would be a promising molecular breeding strategy for the development of salt-tolerant crops.
AUTHOR CONTRIBUTIONS
M.S.S. and J.M. conceived and designed the experiments. M.S.S. mainly conducted the experiments. M.S.S., C.S. and M.S.B. analyzed the HPLC data. L.S. selected the homozygous COS-AER lines.
ACKNOWLEDGEMENTS
This work was supported by KAKENHI grants from the Japan Society for the Promotion of Science (17H3700, 20H03278 and 21F21383).
Open Research
DATA AVAILABILITY STATEMENT
All data supporting the findings of this study are available within the paper published online.




