SlPHL1 positively modulates acid phosphatase in response to phosphate starvation by directly activating the genes SlPAP10b and SlPAP15 in tomato
Abstract
Increased acid phosphatase (APase) activity is a prominent feature of tomato (Solanum lycopersicum) responses to inorganic phosphate (Pi) restriction. SlPHL1, a phosphate starvation response (PHR) transcription factor, has been identified as a positive regulator of low Pi (LP)-induced APase activity in tomato. However, the molecular mechanism underlying this regulation remains to be elucidated. Here, SlPHL1 was found to positively regulate the LP-induced expression of five potential purple acid phosphatase (PAP) genes, namely SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b. Furthermore, we provide evidence that SlPHL1 can stimulate transcription of these five genes by binding directly to the PHR1 binding sequence (P1BS) located on their promoters. The P1BS mutation notably weakened SlPHL1 binding to the promoters of SlPAP7, SlPAP12, and SlPAP17b but almost completely abolished SlPHL1 binding to the promoters of SlPAP10b and SlPAP15. As a result, the transcriptional activation of SlPHL1 on SlPAP10b and SlPAP15 was substantially diminished. In addition, not only did transient overexpression of either SlPAP10b or SlPAP15 in tobacco leaves increase APase activity, but overexpression of SlPAP15 in Arabidopsis and tomato also increased APase activity and promoted plant growth. Subsequently, two SPX proteins, SlSPX1 and SlSPX4, were shown to physically interact with SlPHL1. Moreover, SlSPX1 inhibited the transcriptional activation of SlPHL1 on SlPAP10b and SlPAP15 and negatively regulated the activity of APase. Taken together, these results demonstrate that SlPHL1-mediated LP signaling promotes APase activity by activating the transcription of SlPAP10b and SlPAP15, which may provide valuable insights into the mechanisms of tomato response to Pi-limited stress.
1 INTRODUCTION
Phosphorus (P) is an essential mineral nutrient that is not only a component of many biological components like membranes and nucleic acids but it is also involved in the transfer and storage of energy in both photosynthesis and respiration (Zhang et al., 2014; Wang et al., 2018). The primary type of P that plants can directly take is soluble inorganic phosphate (Pi, H2PO4− and HPO42−) (Lambers, 2022). However, Pi is easily fixed and precipitated in the soil as insoluble complexes, making poor availability of Pi a key concern constraining plant growth and agricultural production (Zhang et al., 2014).
When soluble Pi is deficient in the soil, plants initiate a series of Pi starvation responses (PSRs) that include increased acid phosphatase (APase) activity, thereby maintaining growth via enhanced P utilization efficiency (PUE) (Wang et al., 2018; Wang and Liu, 2018). APases are a family of hydrolases widespread in living organisms and are responsible for the release of Pi by the hydrolysis of monoesters of orthophosphate in organophosphate (Po) under acidic conditions (Tian and Liao, 2015). A variety of plants, including Arabidopsis (Arabidopsis thaliana), rice (Oryza sativa), and tomato (Solanum lycopersicum), have been found to exhibit an increase in APase activity in response to low Pi (LP) stress (Lu et al., 2016; Sun et al., 2016; Wang and Liu, 2018; Zhang et al., 2021). Purple acid phosphatases (PAPs) are a unique group of APases that have been extensively studied for their role in the release of Pi from phosphomonoesters in soil and senescent tissues, thus contributing to the absorption and distribution of Pi in plants (Tian and Liao, 2015; Wang and Liu, 2018; Bhadouria and Giri, 2022). PAPs hydrolyze a variety of phosphomonoester substrates, including phytate-P, adenosine triphosphate (ATP), adenosine diphosphate (ADP), and phosphoenolpyruvate (PEP), and appear pink or purple in aqueous solution (Srivastava et al., 2020; Bhadouria and Giri, 2022). The active binuclear metal center of these enzymes consists of Fe(III)-Me(II) (Me stands for iron, zinc, or manganese), and there are seven invariant metal-binding amino acid residues (DXG/GDXXY/GNH(D/E)/VXXH/GHXH, bold letters) in the five conserved domains of the PAPs, which are necessary for their activity (Bhadouria and Giri, 2022). Notably, specific Po, such as 5-bromo-4-chloro-3-indolyl-phosphate (BCIP), para-nitrophenyl-phosphate (pNPP), and β-naphthyl acid phosphate (β-NAP), are widely used to detect APase activity (Wang et al., 2014a; Wang and Liu, 2017). PAPs exist in plants as a multigene family. For instance, 29 AtPAPs, 26 OsPAPs, and 25 SlPAPs have been annotated in Arabidopsis, rice, and tomato, respectively (Li et al., 2002; Zhang et al., 2011; Srivastava et al., 2020). Based on protein size, they are usually classified into high molecular weight (HMW) PAPs and low molecular weight (LMW) PAPs. HMW PAPs can function as monomers, dimers, and tetramers, producing a variety of APase isomers (Schenk et al., 2013; Bhadouria and Giri, 2022; O'Gallagher et al., 2022).
Gradually, the functions of numerous PAPs in plants are becoming apparent. Overexpression of AtPAP10, which encodes a Pi starvation-induced APase primarily associated with the root surface, stimulates plant growth (Wang et al., 2011; Wang et al., 2014a; Sun et al., 2016). Overexpression of AtPAP17 increases APase activity, Pi content, and plant biomass (Farhadi et al., 2020; Jamali Langeroudi et al., 2023). OsPAP10c-overexpressed rice grows faster and produces more grain (Lu et al., 2016; Deng et al., 2020). Overexpression of OsPAP26 in rice has the potential to increase the conversion of ATP into Pi relative to the wild type (WT) (Gao et al., 2017). SAP1 and SAP2, two PAP isozymes isolated from Pi-starved tomato cell cultures, were demonstrated to scavenge Pi from extracellular Pi-esters (Bozzo et al., 2002). SlPAP1, the first PAP to be cloned in tomato, is projected to be a 50.7 kDa secreted protein (Suen et al., 2015).
Transcriptional induction of numerous PAPs occurs in response to Pi deficiency. For instance, the expression of 11 AtPAPs in Arabidopsis, 10 OsPAPs in rice, and at least 17 SlPAPs in tomato is enhanced following Pi starvation treatment (Haran et al., 2000; Zhang et al., 2011; Srivastava et al., 2020). The mechanism underlying LP's ability to promote the expression of these PAP genes seems to be more intriguing. A group of TFs referred to as PHOSPHATE STARVATION RESPONSE (PHR), which possess both MYB and coiled-coil (CC) domains, notably AtPHR1, its homolog AtPHR1-Like2 (AtPHL2), and OsPHR2, have been extensively studied and are thought to be crucial to the regulation of plant PSRs (Zhou et al., 2008; Bustos et al., 2010; Sun et al., 2016). The majority of PAP gene promoters exhibit the presence of the PHR1 binding site (P1BS, 5’-GNATATNC-3′), which is commonly seen in the promoters of Pi starvation-induced (PSI) genes. However, there is limited evidence available on the direct interaction between PHRs and these specific sites (Bustos et al., 2010; Zhang et al., 2011; Sun et al., 2016; Gao et al., 2017; Srivastava et al., 2020; Zhang et al., 2021). Notable instances include AtPAP10 and OsPAP21b, which are modulated by AtPHR1 and OsPHR2 via direct binding to the P1BS motifs located on their promoters, respectively (Sun et al., 2016; Mehra et al., 2017). Moreover, high-throughput data show that a substantial number of PAP genes are probable targets of PHRs in Arabidopsis (Bustos et al., 2010; Sun et al., 2016; Barragán-Rosillo et al., 2021). In addition, there is growing evidence indicating that SPX (Syg1/Pho81/Xpr1) domain-containing proteins adversely affect PSR via controlling PHR translocation from the cytoplasm to the nucleus or preventing PHR from activating the downstream PSI genes transcriptionally (Lv et al., 2014; Puga et al., 2014; Wang et al., 2014b; Zhong et al., 2018; Osorio et al., 2019). Under Pi-replete conditions, inositol pyrophosphates (PP-InsPs) 1,5(PP)2-InsP4 (InsP8) accumulates and triggers the formation of an SPX-InsP8-PHR complex, thereby inhibiting PHRs from binding to target PSI gene promoters, whereas in the absence of Pi, InsP8 levels decrease and the complex dissociates, releasing PHRs and inducing PSI gene expression (Wild et al., 2016; Dong et al., 2019; Osorio et al., 2019; Ried et al., 2021). Although prior studies have established the SPX-PHR module's significance in regulating PSI gene expression, anthocyanin biosynthesis, and arbuscular mycorrhizal symbiosis in response to LP stress (He et al., 2021; Shi et al., 2021; Das and Gutjahr, 2022; Liao et al., 2022; Wang et al., 2023), its involvement in Pi deprivation-induced APase activity has not been thoroughly investigated.
Tomato is an important horticultural crop that is widely cultivated worldwide, and increasing APase activity is a common feature of its PSR (Bozzo et al., 2002; Suen et al., 2015; Srivastava et al., 2020; Zhang et al., 2021). Recently, the PHR and SPX proteins of tomato were identified (Zhang et al., 2021; Liao et al., 2022; Singh et al., 2023). SlPHL1, an AtPHR1 homolog identified from tomato, plays a key role in the regulation of tomato PSR, including APase activity, root hair development, Pi uptake, and anthocyanin biosynthesis (Zhang et al., 2021; Wu et al., 2023). Nonetheless, it remains unclear how SlPHL1 modulates LP-induced APase activity. In the present study, we discover that SlPHL1 can stimulate the transcription of SlPAP10b and SlPAP15 by binding directly to the P1BS motifs on their promoters. Furthermore, SlPHL1 is capable of interacting with both SlSPX1 and SlSPX4, and the transcriptional activation of SlPHL1 to SlPAP10b and SlPAP15 is negatively regulated by SlSPX1. Thus, our study reveals a signal pathway of LP induction of APase activity in tomato, which not only aids in the comprehension of how tomato responds to LP stress but also serves as a reference for breeding plant germplasm with improved PUE.
2 MATERIALS AND METHODS
2.1 Plant materials and growth conditions
For tomato-related experiments, Solanum lycopersicum L. cv. Micro-Tom (MT) was used for all physiological experiments. Overexpression plants SlPHL1-OE and SlPAP15-OE have MT as the genetic background. Tomato seedlings were cultured, and Pi-starvation was treated as described previously (Zhang et al., 2019; Zhang et al., 2021; Wu et al., 2023). In general, the seeds were surface-sterilized with bleach and then transferred to agar-solidified Murashige and Skoog (MS, Caisson Labs) media for germination. The seedlings that had fully developed cotyledons were hydroponically cultured with either 1/4 Hoagland's solution containing 250 μM H2PO4−, designated as high Pi (HP), or 1/4 Hoagland's solution containing 2.5 μM H2PO4−, designated as low Pi (LP). The discrepancy in the K+ concentration between them was offset by K2SO4. The germination and culture were conducted in a chamber with a photoperiod of 16 h light and 8 h dark at 20–23°C. The light intensity is approximately 80 μmol m−2 s−1.
For Arabidopsis-related experiments, the Columbia-0 (Col-0) ecotype was used as the WT, which was the background of the allogenic overexpression plant SlPAP15-OX. Following surface sterilization with bleach, Arabidopsis seeds were deposited onto a solid medium comprising 1/2MS salt (Caisson Labs), 3% sucrose, 0.05% MES, and 0.8% agar (pH 5.8). After stratification in the dark at 4°C for 3 days, the seeds germinated in a growth chamber under identical conditions to those used in tomato culture. Seven days after germination, seedlings were transferred to Pi-sufficient (HP), Pi-deficient (LP), or LP medium containing varying concentrations of ATP. HP was identical to the germination medium containing 1.25 mM KH2PO4. In contrast to HP, the concentration of KH2PO4 in LP was reduced to 10 μM, while the difference in concentration of K+ was compensated by K2SO4.
2.2 Plant transformation, transient expression, and virus-induced gene silencing (VIGS)
For overexpression of SlPHL1 in MT, vector construction and plant transformation were performed as described previously (Zhang et al., 2021). The full-length coding sequence (CDS) of SlPAP10b and SlPAP15 was PCR amplified from complementary DNA (cDNA) of MT leaves using DNA polymerase KOD-Plus-Neo (TOYOBO) and cloned into binary vector pRI101-AN downstream of cauliflower mosaic virus (CaMV) 35S promoter using the restriction enzymes SalI and KpnI to generate constructs 35S:SlPAP10b and 35S:SlPAP15, respectively. To obtain the transgenic plants, 35S:SlPAP15 was introduced into Agrobacterium tumefaciens (Agrobacterium) strain GV3101, and Arabidopsis transformation was carried out using the floral-dip method (Clough and Bent, 1998), while MT transformation was carried out using the cotyledon infection method (Shikata and Ezura, 2016; Zhang et al., 2021). The selection of stable transgenic lines was carried out using MS medium supplemented with kanamycin or G418, followed by subsequent PCR analysis. The primers utilized for vector construction and PCR confirmation can be found in Table S1. For transient expression in tobacco leaves, 35S:SlPAP10b and 35S:SlPAP15 were transformed into Agrobacterium GV3101 (pSoup). Bacteria were harvested overnight and resuspended in an Infiltration Buffer (10 mM MES, 10 mM MgCl2, 150 μM acetosyringone, pH 5.6) to a cell density of OD600 = 0.5, the bacteria cells were infiltrated into fully expanded leaves of Nicotiana benthamiana (N. benthaniana) using a 1-mL needleless syringe. The penetration region was designated with a pen. The leaves were taken 2 days later to determine APase activity.
Tobacco rattle virus (TRV)-based VIGS, first established by Liu et al. (2002), is an effective approach for studying gene function in plants. The fragment used to silence SlPHL1 was ‘213’, which could efficiently down-regulate the expression of SlPHL1 (Wu et al., 2023). Specific primers were generated for silencing SlSPX1 based on the target DNA fragment recommended by the online software SGN VIGS Tool (http://vigs.solgenomics.net/), and PCR amplification of this fragment was done using MT leaf cDNA as a template. The resultant PCR product was cloned into the vector pTRV2 using the restriction enzymes EcoRI and XhoI to create the pTRV2-SlSPX1 construct. The primers are listed in Table S2. The plasmid transformation into GV3101 and subsequent invasion into MT cotyledon were carried out as previously described (Zhang and Liu, 2014; Wu et al., 2023). In brief, the seedlings were then cultivated under HP conditions for one week after being infiltrated into cotyledons with either pTRV2 or pTRV2-SlSPX1. Subsequently, each type of infiltrated seedlings was subdivided into two groups: one was moved to the LP solution for Pi starvation treatment, while the other was left in the HP solution to continue growing as a control. The initial true leaves of the seedlings were utilized for APase activity measurement and gene expression assay following a period of two weeks.
2.3 In-gel APase profiling and APase activity analysis
Plant tissues were ground to a fine powder in liquid nitrogen and homogenized in an ice-cold Plant Cell Lysis Buffer for Western & IP (Beyotime). The sample was placed on ice for 1 h, reversed 3–4 times during the reaction, and then centrifuged at 13,000 g at 4°C for 20 min. The supernatant was transferred to a new tube for immediate use or temporarily stored at −20°C. The protein content was quantified using a Detergent Compatible Bradford Protein Assay Kit (Beyotime). In-gel APase profiling and APase activity assay were carried out according to the previous methods (Liu et al., 2016; Lu et al., 2016; Gao et al., 2017; Wang and Liu, 2017; Deng et al., 2020). Briefly, 10 μg of proteins were separated on a 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE) gel at 4°C. After gel washing, gel equilibration and gel staining with β-NAP-containing detection buffer, the isozyme profile of APases was visualized as red-brown bands on the gel and photographed with a digital camera. To quantify internal APase activities, a solution was prepared by combining 600 μL of reaction buffer (10 mM pNPP, 50 mM sodium acetate, pH 5.5) with 10 μg of the extracted proteins. The reactions were initiated at 25°C for 30 min before being terminated with 1.2 mL of 1 M NaOH. Absorbance at 410 nm was determined using a spectrophotometer (UV1800, MAPADA). APase activity was expressed as OD410 per minute per milligram of protein (OD410 min−1 mg−1 protein). To stain root-associated APase activity, an agar solution (0.5%, w/v) containing 0.01% (w/v) BCIP was evenly overlaid on the roots. Following 12 h of color development, the roots were captured using a camera.
2.4 Gene expression analysis
RNA extraction from the tomato leaves and subsequent real-time quantitative reverse transcription PCR (RT-qPCR) were performed as described previously (Liu et al., 2015; Zhang et al., 2021; Wu et al., 2023). RNA extraction was performed using the RNA-easy Isolation Reagent (Vazyme). The analysis of RNA quality and concentration was conducted using a NanoDrop Spectrophotometer (Thermo Fisher Scientific). The cDNA was generated with the EasyScript One-Step gDNA Removal and cDNA Synthesis kit (TransGen). The PCR reactions were conducted with the CFX96 Real-Time PCR Detection System (Bio-Rad) and the TransStart Tip Green qPCR SuperMix (TransGen). The SlACT7 gene was employed as an internal control to normalize gene expression. The primers for qPCR analysis are documented in Table S3. All PCR reactions were carried out in at least three biological replicates and four technical replicates. Relative expression (fold change) was quantified utilizing the 2−ΔΔCt method (Livak and Schmittgen, 2001).
2.5 Yeast one-hybrid (Y1H) and modified yeast one-hybrid (mY1H)
To generate a variety of pro::LacZ constructs, various promoter fragments were PCR amplified from tomato genomic DNA (gDNA) using the DNA polymerase KOD-Plus-Neo (TOYOBO) and introduced into the vector pLacZi-2μ. C terminus of SlPHL1 (cSlPHL1) that contains MYB and CC domains was cloned into the vector pJG4-5 to produce the GAD-cSlPHL1 construct, while the N-terminus of SlSPX1 (nSlSXP1) that contains four SPX domains was introduced into the vector pGADT7 to generate AD-nSlSPX1 construct. The primers utilized for construction are shown in Table S4. For Y1H, the constructs of each pro::LacZ and GAD-cSlPHL1 were transformed into Saccharomyces cerevisiae (S. cerevisiae) strain EGY48 through the Matchmaker GAL4-based Two-Hybrid System (Clontech) in pairs, and the interaction between cSlPHL1 and promoter fragments was subsequently analyzed using Y1H according to the previous method (Lin et al., 2007; Liu et al., 2022; Wu et al., 2023). Briefly, after transformed yeast cells were grown on drop-out synthetic dropout (SD) media lacking tryptophan (Trp) and Uracil (Ura) (SD/−Trp/-Ura, Coolaber) agar media for 4 days at 30°C, the positive clones for each transformation were randomly selected and cultured in SD/−Trp/-Ura broth overnight at 30°C, and then the cells were collected by centrifugation and spread on SC media (SD without carbon) devoid of Trp and Ura but containing 5-bromo-4-chloro-3-indolyl-β-D-galactopyranoside (SC/−Trp/-Ura/+X-gal) for blue color development. For mY1H, three constructs, including each pro::LacZ, GAD-cSlPHL1, and AD-nSlSPX1, were co-transformed into Saccharomyces cerevisiae strain EGY48 using the same technique as Y1H. The positive clones were screened on synthetic dropout (SD) media lacking Trp, Ura, and leucine (Leu) (SD/−Trp/-Ura/−Leu, Coolaber) agar medium at 30°C and the blue color development was carried out on SC media devoid of Trp, Ura, and Leu but containing X-gal (SC/−Trp/-Ura/−Leu/+X-gal) after about 3 days of culture at 30°C. At least four randomly selected clones were employed for analysis with similar results.
2.6 Site-directed mutagenesis
To mutate all P1BS motifs in each promoter fragment of proSlPAP7(I), proSlPAP10b(I), proSlPAP12(I), proSlPAP15(I) and proSlPAP17b(I), specific primers were designed using Vazyme online software (https://crm.vazyme.com/cetool/singlepoint.html). The sequences are shown in Table S5. Mutagenesis at certain sites in the pro::LacZ constructs were created using the Mut Express II Fast Mutagenesis Kit (Vazyme) in accordance with the manufacturer's instructions. Sequencing verified the success of the alterations.
2.7 Electrophoretic mobility shift assay (EMSA)
Expression of the fusion protein MBP-cSlPHL1 in Escherichia coli (E. coli) and its purification in vitro have been described by Zhang et al. (2021). The probe preparation and labeling procedures were performed according to the manufacturer's protocol of the EMSA Probe Biotin Labeling Kit (Beyotime). The oligonucleotide sequences are detailed in Table S6. EMSA was conducted according to the manufacturer's instructions for the LightShift Chemiluminescent EMSA Kit (Beyotime). The ChemiDoc MP Chemiluminescence Imaging System (Bio-Rad) was employed for luminous signal detection and photography.
2.8 Transient transcription expression assay (TTEA)
Various WT or mutant promoter fragments were PCR amplified from MT leaf gDNA or other existing constructs and then cloned into the vector pGreenII 0800-LUC to generate a series of reporters pro:LUC, while the full-length CDS of SlPHL1 and SlSPX1 were separately cloned into the vector pGreenII 62-SK to generate the effectors 35S:SlPHL1 and 35S:SlSPX1. The primers for the constructions are shown in Table S7. The resultant constructs were transformed into GV3101 (pSoup). After overnight culture, the bacteria were resuspended in the Infiltration Buffer to a cell density of OD600 = 0.5. The cells harboring different constructs were mixed and co-infiltrated to the fully expanded leaves of N. benthamiana using a 1-mL needleless syringe. Two days later, the leaves were sprayed uniformly with D-luciferin (Sangon). After 5 min of exposure in the dark, luminescent images were captured with a cooled charge-coupled imaging device of the Tanon 5200 Automatic Chemiluminescence Image Analysis System (Tanon) according to the manufacturer's instructions. The images shown in the figures are representative of at least three leaves from a single experiment. Relative LUC activity was quantified using ImageJ software (https://imagej.net).
2.9 Yeast two-hybrid (Y2H)
The full length of CDS, N terminus, and C terminus of SlSPX1, SlSPX2, SlSPX3, SlSPX4, and SlSPX5 were individually fused in frame to the AD domain in the pGADT7 vector to generate AD-SlSPX1, AD-SlSPX2, AD-SlSPX3, AD-SlSPX4, AD-SlSPX5, AD-nSlSPX1, AD-nSlSPX2, AD-nSlSPX3, AD-nSlSPX4, AD-nSlSPX5, AD-cSlSPX1, AD-cSlSPX2, AD-cSlSPX3, AD-cSlSPX4, and AD-cSlSPX5. Meanwhile, cSlPHL1 was cloned into vector pGBKT7 in fusion to the BD domain to generate BD-cSlPHL1. The primers for the constructions are listed in the Table S8. The resulting constructs were transformed in pairs (BD-cSlPHL1 coupled with each AD construct) into Saccharomyces cerevisiae strain AH109. The presence of the transgenic clones was screened on SD media devoid of Trp and Leu (SD/−Trp/−Leu, Clontech). To test the possible interactions, each selected clone was suspended in 100 μL sterile water, and the values of OD600 were adjusted to about 1.0, then they were diluted with sterile water 101,102,103 and 104 times, and finally, 5 μL of bacterial droplet for each concentration was placed on the surface of SD media lacking Trp, Leu, and histidine (His) (SD/−Trp/−Leu/−His, Clontech), SD media lacking Trp, Leu, His, and adenine (Ade) (SD/−Trp/−Leu/−His/−Ade, Clontech), and SD/−Trp/−Leu. After 4 days of incubation at 30°C, the yeast growth was photographed with a digital camera.
2.10 Pull-down assay
In addition to obtaining the recombinant protein MBP-cSlPHL1 as described previously (Zhang et al., 2021), we created GST-nSlSPX1 and GST-nSlSPX4 constructs by subcloning the N-terminal CDS of SlSPX1 and SlSPX4 from AD-nSlSPX1 and AD-nSlSPX4, respectively, into the vector pGEX-4T-1 in frame of Glutathione S-Transferase (GST). Empty vector, GST-nSlSPX1 and GST-nSlSPX4 were transformed into E. coli BL21 (DE3) independently. The proteins were purified using glutathione agarose resin (Transgen) after they were expressed in a soluble state. The pull-down and Western Blot procedures were performed following the previous methods (Qi et al., 2014; Zhang et al., 2018). Primary antibodies anti-MBP and anti-GST, as well as the secondary antibody goat anti-mouse, were ordered from Transgen. Following immunoblotting analyses, bright signals were detected and photographed using the ChemiDoc MP Chemiluminescence Imaging System (Bio-Rad).
2.11 Luciferase complementation imaging (LCI)
The vector construction and luciferase imaging were carried out according to Chen et al. (2008). The sequence of cSlPHL1 was cloned into pCambia1301-nLuc and pCambia1301-cLuc, resulting in the generation of cSlPHL1-nLuc and cLuc-cSlPHL1, respectively. N terminus of SlSPX1 and SlSPX4 were introduced into both vectors to generate four constructs: nSlSPX1-nLuc, cLuc-nSlSPX1, nSlSPX4-nLuc, and cLuc-nSlSPX4. The primers for the constructions are listed in Table S9. All constructs and the empty vector were turned into GV3101. Bacteria that had been cultivated overnight were collected and resuspended in the Infiltration Buffer to an OD600 = 0.5 cell density. Equal amounts of the bacteria cells harboring the different constructs were mixed and then co-infiltrated into the leaves of N. benthamiana using a 1-mL needleless syringe. After 2 days of growth, the leaves were sprayed evenly with D-luciferin (Sangon), and the luminescent images were captured with the Tanon 5200 Automatic Chemiluminescence Image Analysis System (Tanon).
2.12 Statistical analysis
GraphPad Prism 8.3.0 was utilized to process the data, and a paired Student's t-test was employed to ascertain the statistical significance of differences between data sets.
2.13 Accession number
Sequence data in this study can be found in the Sol Genomics Network (http://solgenomics.net/) under accession numbers: SlACT7 (Solyc03g078400), SlPHL1 (Solyc09g072830), SlSPX1 (Solyc08g060920), SlSPX2 (Solyc12g009480), SlSPX3 (Solyc01g090890), SlSPX4 (Solyc02g067160), SlSPX5 (Solyc02g088210), SlPAP1 (Solyc05g012260), SlPAP4 (Solyc04g008260), SlPAP7 (Solyc04g008250), SlPAP9a (Solyc04g005450), SlPAP10a (Solyc01g110050), SlPAP10b (Solyc01g110060), SlPAP12 (Solyc04g080920), SlPAP15 (Solyc09g091910), SlPAP17b (Solyc03g098010), SlPAP18a (Solyc07g064500), SlPAP18b (Solyc10g006300), SlPAP20 (Solyc09g009610), SlPAP23b (Solyc04g051650), SlPAP26a (Solyc12g009800), SlPAP26b (Solyc07g007670), SlPAP27c (Solyc07g008570).
3 RESULTS
3.1 Effect of SlPHL1 on the expression of SlPAP genes
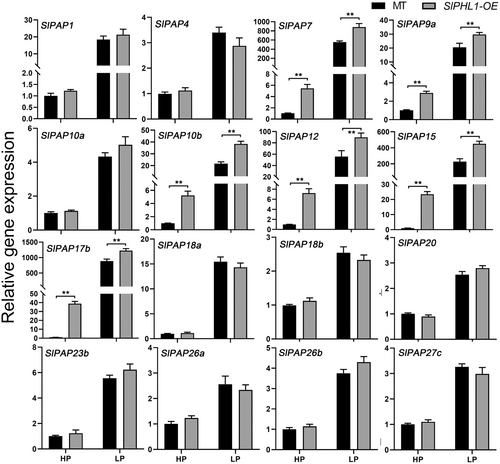
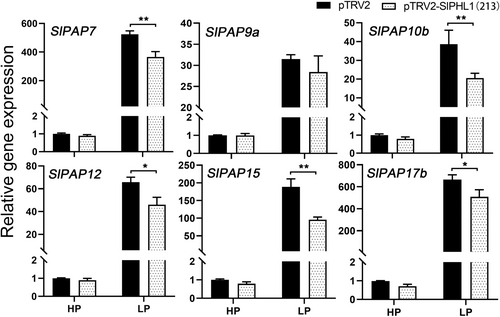
Overexpression of SlPHL1 was found to boost the activity of root-associated APase in a prior study (Zhang et al., 2021). Furthermore, we discovered that SlPHL1-OE leaves had marginally higher intracellular APase activity than MT leaves under HP conditions, but considerably higher than MT leaves under LP conditions (Figure S1). Given that PAPs comprise the most extensive class of plant APases (Jamali Langeroudi et al., 2023), it was postulated that SlPHL1 might facilitate the transcription of SlPAP genes, thereby positively modulating APase activity. To that purpose, the first true leaves of SlPHL1-OE and MT seedlings treated with LP for one week were taken for RNA extraction and RT-qPCR analysis of SlPAP gene expression. Of the genes examined, it was found that 16 SlPAP genes exhibited a significant upregulation in response to Pi deficiency (Figure 1). Notably, the expression levels of SlPAP7, SlPAP9a, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b were substantially higher in SlPHL1-OE than in MT under both HP and LP conditions (Figure 1), indicating that SlPHL1 is a positive regulator of these six genes. We considered whether attenuating the expression of SlPHL1 would reduce their transcription. Since the reliable SlPHL1 knockout mutant has not been developed, VIGS technology was utilized to suppress SlPHL1 expression. The expression of SlPHL1 fell dramatically two weeks following VIGS with the fragment ‘213’, as described by Wu et al. (2023), and the transcript level of SlPAPs was evaluated at this time. As shown in Figure 2, there was no discernible difference in the expression levels of the six SlPAP genes between pTRV2 and pTRV2-213 under HP conditions. However, under LP conditions, pTRV2 exhibited significantly higher levels of expression for SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b (excluding SlPAP9a) (Figure 2). These findings imply that SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b may be SlPHL1 regulatory targets, increasing APase activity and adapting to the LP environment.
3.2 SlPHL1 binds directly to the promoters of the genes SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b and promotes their transcription
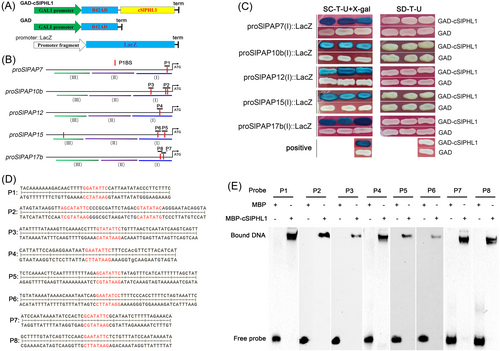
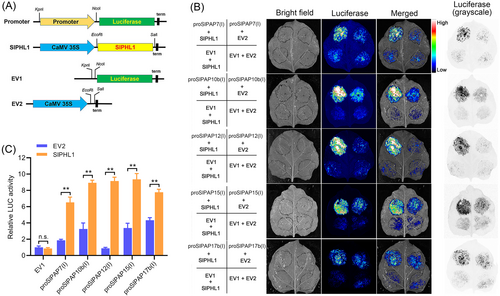
To see if SlPHL1 directly regulates the expression of these five genes, we first used Y1H to examine the interaction between SlPHL1 and their promoters. The promoter of SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b were each divided into three segments (called I, II, and III) and cloned into the pLacZi-2μ vector, yielding a series of promoter::LacZ constructs; concurrently, the plasmids of GAD-cSlPHL1 and GAD were prepared (Figure 3A, B). Remarkably, the Y1H analysis revealed that the yeast cells co-transformed with GAD-cSlPHL1 and each of the I-fragments exhibited a blue coloration in the presence of X-gal (Figure 3C). Conversely, no such effect was observed with the remaining fragments (Figure S2). Based on these results, it looks like SlPHL1 binds to the I-fragments. Given that the promoters of SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b contain 1, 3, 1, 3, and 2 P1BS motifs, respectively, with most of them being situated in the I-fragments (Figure 3B), our next hypothesis was that SlPHL1 has binding affinity towards these I-fragments through the P1BS motif, as previous studies have demonstrated its interaction with this particular element (Zhang et al., 2021). For this purpose, eight probes were constructed depending on DNA sequences that contained P1BS motifs and were designated sequentially as P1 through P8 (Figure 3D). EMSA results show that the eight biotin-labeled probes were all retained by MBP-cSlPHL1 but not by MBP alone during electrophoresis (Figure 3E), suggesting that the DNA sequences harboring P1BS motifs situated on the promoters of SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP7b serve as specific binding sites for SlPHL1. Subsequently, an additional inquiry emerged: what would happen to the transcriptional activity of the promoters if SlPHL1 bound to these DNA fragments? To do this, each of the five I-fragments was cloned in front of the luciferase gene of pGreenII 0800-LUC (EV1), and the full-length CDS of SlPHL1 was cloned downstream of CaMV 35S promoter on pGreenII 62-SK (EV2) (Figure 4A). After that, TTEA was done on the leaves of N. benthamiana. EV2 and SlPHL1 had similar effects on EV1 as measured by imaging and relative quantification of LUC activity (Figure 4B, C). While the presence of each I-fragment remarkably elevated LUC activity, and notably, the impact of SlPHL1 on LUC expression, driven by proSlPAP7(I), proSlPAP10b(I), proSlPAP12(I), proSlPAP15(I), and proSlPAP17b(I), was considerably greater than that of EV2 (Figure 4B, C). Altogether, these findings indicate that SlPHL1 can directly stimulate the expression of SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b by binding to their promoters.
3.3 The P1BS motifs located on the promoters of SlPAP10b and SlPAP15 play important roles for activation of the transcription by SlPHL1
To investigate the potential importance of P1BS motifs in regulating the transcription of SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b, we mutated all P1BS motifs (5’-GNATATNC-3′ to 5’-GNggggNC-3′) in proSlPAP7(I)::LacZ, proSlPAP10b(I)::LacZ, proSlPAP12(I)::LacZ, proSlPAP15(I)::LacZ, and proSlPAP17b(I)::LacZ (Figure S3), followed by Y1H analysis. Consistent with the above results, the yeast cells co-transformed by each wild I-fragment with GAD-cSlPHL1 but not GAD displayed a blue color in the presence of X-gal (Figure 5). However, the blue color of the yeast cells decreased substantially when the P1BS motifs in proSlPAP7(I), proSlPAP12(I) and proSlPAP17b(I) were mutated (Figure 5). Furthermore, upon mutating the P1BS motifs in proSlPAP10b(I) and proSlPAP15(I), the yeast cells displayed no trace of blue color (Figure 5). These findings suggest that the P1BS motifs have limited functionality in the interaction of SlPHL1 with proSlPAP7(I), proSlPAP12(I) and proSlPAP17b(I), whereas they are critical for the binding of SlPHL1 with proSlPAP10b(I) and proSlPAP15(I).

To ascertain the precise binding sites of SlPHL1 on proSlPAP10b(I) and proSlPAP15(I), as well as the indispensability of P1BS motifs in this interaction, we conducted a comprehensive EMSA test. Figure 6A shows the strong interaction between cSlPHL1 and probes P2, P3, P5, and P6, consistent with the experimental observations in Figure 3E. Nevertheless, it should be noted that the signal of the retained bands was diminished when the amount of MBP-cSlPHL1 was reduced to one-tenth or when a non-biotin-labeled probe (cold probe) was applied at 100- and 500-fold concentrations (Figure 6A). Importantly, MBP-cSlPHL1 showed little binding affinity towards P2, P3, P5, and P6 containing mutant P1BS motifs (5’-GNggggNC-3′) (Figure 6A). We set out to learn if the P1BS motifs are also required for SlPHL1 transcriptional activation of SlPAP10b and SlPAP15. The TTEA results indicate that the co-transformation of SlPHL1 and a wild promoter fragment (either proSlPAP10b(I) or proSlPAP15(I)) into tobacco leaves produced strong LUC fluorescence signals (Figure 6B). However, mutation of the P1BS motifs from 5’-GNATATNC-3′ to 5’-GNggggNC-3′ obviously attenuated the fluorescence signals (Figure 6B), and the fluorescence signal was observed to decrease by 77.4 and 70.0%, respectively, upon mutation of the P1BS motifs in proSlPAP10b(I) and proSlPAP15(I) (Figure 6C). Overall, these data demonstrate that the P1BS motifs located in the promoters of SlPAP10b and SlPAP15 are necessary to activate their transcription by SlPHL1 directly.
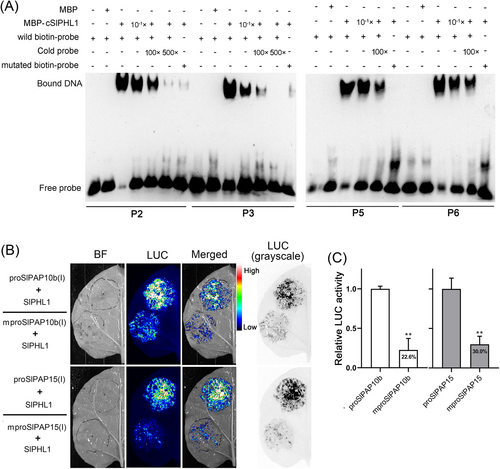
3.4 Overexpression of SlPAP15 could increase APase activity and stimulate plant growth
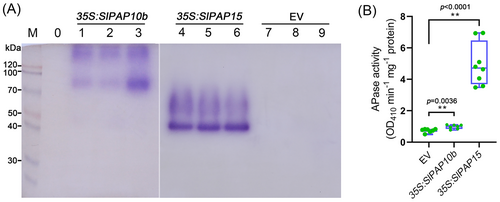
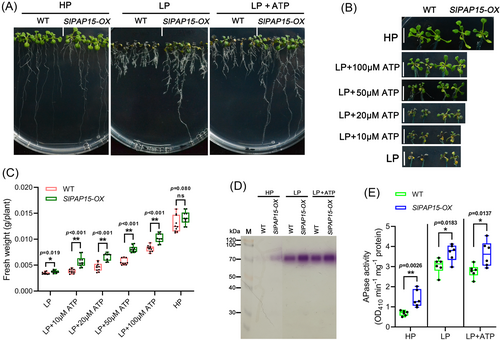
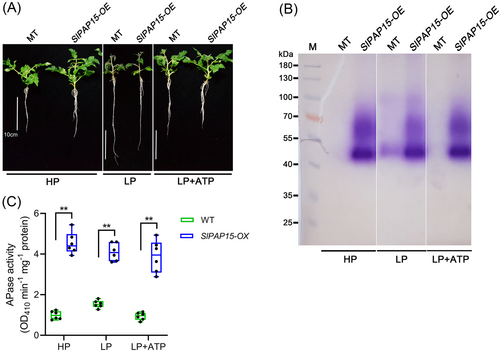
SlPAP10b and SlPAP15 encode two PAPs whose transcription is induced by Pi deficiency, but these data were mainly derived from amino acid sequence alignment and RT-qPCR analysis (Suen et al., 2015; Srivastava et al., 2020; Zhang et al., 2021). Whether they have APase functions remains to be elucidated. We used the following three approaches to investigate the potential APase activities of SlPAP10b and SlPAP15 and their roles in regulating plant PSR. (1) The first technique was to transform the overexpression vectors of SlPAP10b and SlPAP15 into N. benthamiana leaves by Agrobacterium, transiently express them, extract the proteins, and analyze the APase activity. As depicted in Figure 7A and B, Agrobacterium infiltration with the construct overexpressing either SlPAP10b or SlPAP15 substantially enhanced APase activities compared to Agrobacterium infiltration with the empty vector, regardless of β-NAP or pNPP as substrate. Specifically, it appeared that overexpression of SlPAP15 produced a stronger APase activity than overexpression of SlPAP10b (Figure 7). (2) Transgenic Arabidopsis overexpressing SlPAP15 was obtained by Agrobacterium-mediated transformation of Col-0, named SlPAP15-OX, which was utilized for LP stress experiments after confirmation by genotyping PCR and reverse transcription PCR (Figure S4). Five-day-old seedlings of Col-0 and SlPAP15-OX were transferred to media of HP, LP, and LP plus different concentrations of ATP for another 7 days of growth, there was no significant difference between WT and SlPAP15-OX under HP conditions, however, SlPAP15-OX grew better than WT under LP and LP applied ATP conditions, characterized by longer primary roots and higher fresh weight (Figure 8A-C). Furthermore, the APase activities of SlPAP15-OX shoots were higher than those of Col-0 under HP, LP, and LP applied 50 μM ATP (Figure 8D and E). Also, root-associated APase activity appears greater in SlPAP15-OX compared to WT (Figure S5). (3) The third technique is to construct and acquire SlPAP15-OE, a stable overexpressing plant of SlPAP15 in the background of MT (Figure S4). After two weeks of growth under HP, LP, and LP supplemented with 50 μM ATP conditions, SlPAP15-OE seedlings appeared to grow better than MT seedlings under HP and LP + 50 μM ATP (Figure 9A). After quantification of fresh weight, it was found that SlPAP15-OE values were slightly higher than MT under HP and LP conditions, while SlPAP15-OE value was significantly higher than MT under LP + 50 μM ATP conditions (Figure S6). As expected, the APase activities of SlPAP15-OE leaves were dramatically higher than those of MT (Figure 9B, C). Similarly, the root APase activity in SlPAP15-OE was also clearly greater than in MT (Figure S7). Taken together, these data indicate that SlPAP10b and SlPAP15 both encode APases and that overexpression of SlPAP15 could promote plant growth.
3.5 Both SlSPX1 and SlSPX4 physically interact with SlPHL1
SPXs have been shown to interact with PHRs and inhibit their transcriptional activities to downstream PSI genes in Arabidopsis and rice (Lv et al., 2014; Puga et al., 2014; Wang et al., 2014b; Zhong et al., 2018; Wang et al., 2023), but it is unclear whether the SlSPX proteins identified by Liao et al. (2022) interact with SlPHL1. To that objective, a Y2H assay was initially carried out. Because the N-terminus of SlPHL1 demonstrates autoactivation in yeast, and cSlPHL1 has the MYB-CC domain and is sufficient to bind the P1BS motif (Zhang et al., 2021; Wu et al., 2023), cSlPHL1 was employed in the Y2H assay. Yeast cells co-transformed by BD-cSlPHL1/AD-SlSPX1 grew well on both SD/−T/−L/−H and SD/−T/−L/−H/−A media, as did yeast cells co-transformed by BD-cSlPHL1/AD-SlSPX4 on SD/−T/−L/−H media (Figure 10A). In contrast, yeast cells transformed by other combinations did not exhibit growth on the selective media (Figure 10A). Additionally, the coexpression of either BD-cSlPHL1/AD-nSlSPX1 or BD-cSlPHL1/AD-nSlSPX4 resulted in growth on the selective media SD/−T/−L/−H/−A. However, the coexpression of BD-cSlPHL1/AD, BD-cSlPHL1/AD-nSlSPX2, BD-cSlPHL1/AD-nSlSPX3, or BD-cSlPHL1/AD-nSlSPX5 did not exhibit any growth on the same media (Figure 10B). Furthermore, the coexpression of BD-cSlPHL1 with any AD-fused C-terminal SlSPX did not cause yeast growth on the selective media (Figure 10B). According to these findings, both nSlSPX1 and nSlSPX4 interact with cSlPHL1.
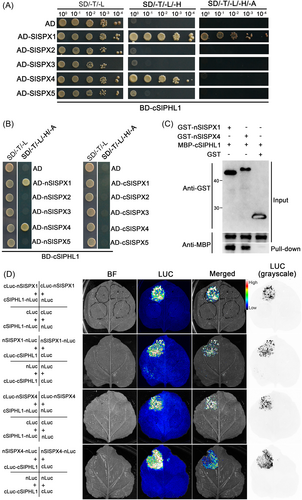
To further verify the results, in addition to MBP-cSlPHL1, we purified recombinant GST-nSlSPX1 and GST-nSlSPX4 (Figure S8) and used them together in a pull-down assay. As expected, MBP-cSlPHL1 was pulled down by GST-nSlSPX1 and GST-nSlSPX4 but not by GST (Figure 10C), and MBP alone was neither pulled down by GST-nSlSPX1 nor GST-nSlSPX4 (data not shown), suggesting that both nSlSPX1 and nSlSPX4 interact with cSlPHL1 in vitro. To assess the interactions in vivo, N. benthamiana leaves were subjected to an LCI assay (Figure S9). As demonstrated in Figure 10D, coexpression of cLuc-SlSPX1 with cSlPHL1-nLuc resulted in substantial LUC complementation, as did coexpression of nSlSPX1-nLuc with cLuc-cSlPHL1, cLuc-SlSPX4 with cSlPHL1-nLuc, and nSlSPX4-nLuc with cLuc-cSlPHL1. Conversely, the coexpression of other combinations failed to produce the same level of LUC complementation (Figure 10D). Overall, these results indicate a specific interaction between SlSPX1/4 and SlPHL1.
3.6 Negative regulation of SlPAP10b and SlPAP15 expression and APase activity by SlSPX1
To ascertain the potential functionality of SlSPX1 via this interaction, a mY1H assay was performed. As shown in Figure 11A, yeast cells transformed with GAD-cSlPHL1 and AD, rather than GAD and AD, exhibited a distinct blue color on X-gal-containing media when either proSlPAP10b(I)::LacZ or proSlPAP15(I)::LacZ was present. It is worth mentioning that the blue hue considerably reduced when AD was substituted with AD-nSlSPX1 (Figure 11A). These findings might suggest that SlSPX1 exerts an inhibitory effect on SlPHL1 binding to both proSlPAP10b(I) and proSlPAP15(I). A TTEA experiment was conducted in N. benthamiana leaves to validate such an effect further. The results showed that infiltration of Agrobacteria expressing SlPHL1 and the empty vector EV2 remarkably triggered LUC fluorescence signals in the presence of either proSlPAP10b(I) or proSlPAP15(I); however, the imaging signals were decreased when EV2 was replaced by SlSPX1 (Figure 11B).
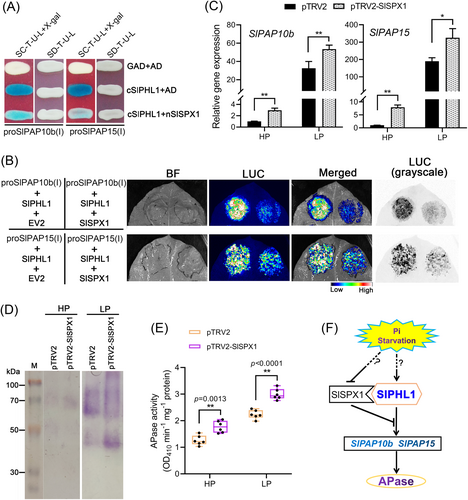
In this case, it has been postulated that SlSPX1 functions as a suppressor of the expression of SlPAP10b and SlPAP15, as well as of the APase activity. To this end, the VIGS experiment targeting SlSPX1 was conducted on MT seedlings. The results from the RT-qPCR analysis revealed a significant decrease in the transcript abundance of SlSPX1 in pTRV2-SlSPX1 compared to pTRV2 under both HP and LP conditions (Figure S10), suggesting that the chosen DNA fragment successfully inhibited the expression of SlSPX1 in tomato leaves. It is important to note that the expression levels of SlPAP10b and SlPAP15 were substantially higher in pTRV2-SlSPX1 than in pTRV2 (Figure 11C). Additionally, the protein was extracted from the leaves, and APase activities were evaluated there as well. After SlSPX1 was silenced, the APase activities were uniformly enhanced in both HP and LP, regardless of whether the substrate was β-NAP or pNPP (Figure 11D, E). Finally, SlSPX1 suppresses APase activity by reducing SlPHL1 transcriptional activation to SlPAP10b and SlPAP15 (Figure 11F).
4 DISCUSSION
One of the defining characteristics of PSR is elevated PAP activity (Bhadouria and Giri, 2022). Numerous studies have identified multiple PAPs that are primarily associated with P remobilization within the plant and P acquisition from the soil by hydrolyzing organic P compounds, but only a few have addressed their regulatory mechanisms (Tian and Liao, 2015; Wang and Liu, 2018; Bhadouria and Giri, 2022). Elucidating the regulatory mechanism of PAP not only aids in understanding the molecular mechanism of plant response to LP stress but also facilitates the improvement of crop P acquisition and utilization efficiency via biotechnology tools, thus reducing the overuse of nonrenewable P-containing fertilizers (Tian and Liao, 2015). The present study demonstrated that SlPHL1 stimulates the expression of two tomato genes, SlPAP10b and SlPAP15, by directly binding to the P1BS elements on their respective promoters (Figures 1-6). SlPAP10b and SlPAP15, which are sequence-analyzed as PAP, also perform the physiological function of APase (Figures 7-9). These findings established a molecular pathway underlying SlPHL1's response to LP stress by directly modulating the transcription of SlPAP10b and SlPAP15 to induce APase activity in tomato.
Twenty-five SlPAP genes are present in tomato (Srivastava et al., 2020), and our investigation revealed that the application of LP treatment remarkably increased the transcriptional expression of sixteen PAP genes (Figure 1). Notably, five specific PAP genes, namely SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b, were found to be regulated by SlPHL1 (Figures 1 and 2). Intriguingly, there is at least one P1BS motif in each promoter close to the translation start site ‘ATG’ (Figure 3B), and SlPHL1 can bind to these P1BS elements (Figure 3E). These results suggest that these five genes are targets of SlPHL1. Overexpression of SlPHL1 increased SlPAP9a's expression (Figure 1), and its promoter sequence contains four P1BS motifs within 2 kb upstream of the ‘ATG’ (data not shown), but silencing of SlPHL1 had no effect on SlPAP9a's expression (Figure 2). Due to our lack of understanding of this discrepancy and our failure to focus on this gene in further study, we cannot say for certain whether or not it is a direct target of SlPHL1. In addition, there are additional PSI PAP genes, such as SlPAP26a, SlPAP26b, and SlPAP27c, whose promoter regions include P1BS motifs; however, SlPHL1 does not appear to be responsible for regulating the expression of these genes (Figure 1). This may imply that other SlPHR proteins, perhaps SlPHR1 and SlPHL2 as previously described (Zhang et al., 2021), are also involved in the regulation of tomato APase activity, with SlPAP26a, SlPAP26b, and SlPAP27c as targets. Both AtPHR1 and AtPHL2 can directly regulate the transcriptional expression of AtPAP10 to enhance APase activity in response to LP stress (Sun et al., 2016). Also, AtPHR1 was thought to be a key regulator involved in the regulation of LP-induced anthocyanin synthesis (Bustos et al., 2010; He et al., 2021; Liu et al., 2022), but it was recently discovered to be involved in the process of jasmonate-induced anthocyanin synthesis along with its homologs AtPHL1, AtPHL2, and AtPHL3 (He et al., 2023). Similarly, Liao et al. (2022) recently demonstrated that multiple SlPHR proteins play a role in the suppression of AM symbiosis by LP stress in tomato. It is, therefore, highly likely that SlPHL1 and other SlPHRs work together to regulate APase activity in a functionally redundant manner.
Despite the mutations in the P1BS motifs of proSlPAP7(I), proSlPAP12(I), and proSlPAP17b(I), SlPHL1 was still able to interact with these fragments, albeit to a lesser extent (Figure 5). This observation aligns with our prior finding that SlPHL1 binds to the SlPT7 promoter region, but the P1BS motif does not appear to be required (Zhang et al., 2021), perhaps suggesting that SlPHL1 interacts with the promoters of its target genes not only through P1BS motifs but also through other elements such as P1BS-like motifs. According to Sun et al. (2016), two P1BS-like elements adjacent to each other and located near ‘ATG’ in the promoter of AtPAP10 were shown to be more relevant for LP-induced expression than the neighboring P1BS motif. In addition, data from EMSA indicate that AtPHL2 is capable of binding to P1BS-like motifs, such as 5’-AAATATCC-3′ and 5’-ACATATTC-3′ (Sun et al., 2016). Moreover, RLI1, a putative MYB transcription factor exhibiting resemblance to PHRs, demonstrates affinity not just for the P1BS motif but also for the P1BS-like elements present on the promoters of its designated target genes (Ruan et al., 2018). The P1BS-like elements are also present at the promoters of the genes regulated by SlPHL1, including SlPAP7, SlPAP10b, SlPAP12, SlPAP15, and SlPAP17b (data not shown). As a result, we posit that SlPHL1 modulates PSI genes by binding additional elements besides the P1BS motifs; this will be a principal focus of our ongoing investigation.
A substantial increase in APase activity was detected in tobacco leaves by transient overexpression of either SlPAP10b or SlPAP15 and in Arabidopsis and tomato by stable overexpression of SlPAP15 (Figures 7-9). These experimental results at least suggest that SlPAP10b and SlPAP15 function as APases and that pNPP and β-NAP are substrates for their action. Furthermore, Arabidopsis and tomato plants overexpressing SlPAP15 grew better when ATP was applied at low Pi levels (Figures 8A and 9A). In addition, overexpression of SlPAP15 in Arabidopsis elevated the activity of root-associated APase observed in the presence of BCIP (Figure S5). Based on these results, it appears that ATP and BCIP could also serve as SlPAP15 substrates. Amino acid sequence analysis by Srivastava et al. (2020) indicates that both ADP and ATP are potential substrates for SlPAP15. Thus, SlPAP10b and particularly SlPAP15 ought to be substrate-non-specific APase enzymes. Similarly, overexpression of either OsPAP10c or OsPAP21b resulted in increased APase activity towards pNPP, β-NAP, and BCIP, as well as facilitated ATP degradation and plant growth, as evidenced by higher shoot weight and biomass in comparison to WT (Lu et al., 2016; Mehra et al., 2017; Deng et al., 2020).
Both SlPAP10b and SlPAP15 are members of the I group of PAPs that are around 55 kDa in size and include 470 and 555 amino acids, respectively (Srivastava et al., 2020). However, in-gel profiling from our research revealed that overexpression of either SlPAP10b or SlPAP15 generates several isozyme bands, often greater than 55 kDa (Figure 7). One possible explanation is that more disulfide bridges are formed between PAP subunits, leading to the emergence of homo- or heterodimers, as described previously (Bhadouria and Giri, 2022). It is noteworthy that the APase isozyme bands generated by the transient expression of SlPAP15 in tobacco leaves are similar to those observed in tomato overexpressing SlPAP15, and these bands manifest predominantly at around 50 and 70 kDa (Figures 7A and 9B). Nonetheless, overexpression of SlPAP15 in Arabidopsis resulted in the development of a single dominant isozyme band with an approximate molecular weight of 70 kDa (Figure 8D). Thus, it is conceivable that SlPAP15 possesses a diverse range of biochemical profiles. Additionally, in contrast to the earlier discovery that SlPAP15 is predominantly expressed in roots (Srivastava et al., 2020), we found that LP also has a strong inductive effect on SlPAP15 transcription in leaves (Figure 1). The most likely explanation for the divergence is the use of different experimental materials: they utilized PR, while we used MT. Several experimental designs, such as the Pi concentration for the LP treatment and the sample time, could also be the cause. Overexpression of SlPAP15 enhanced both intracellular and root-associated APase activity (Figures 7-9 and S7), and SlPAP15 was proposed to have the secretory property (Srivastava et al., 2020), thus further suggesting that SlPAP15 is an APase with multiple biochemical functions. In conclusion, despite the biochemical mechanism of action and subcellular localization of SlPAP15 have not yet been clarified, it is evident from the available data that SlPAP15 is an important APase located downstream of SlPHL1 and involved in the regulation of the PSR, with the potential to be a useful candidate for improving plant PUE.
There exist five different SPX proteins in tomato, of which only SlSPX1 and SlSPX4 interact with SlPHL1 (Figure 10). Furthermore, the N-terminus of SPX1/4 and the C-terminus of SlPHL1 serve as the specific regions responsible for the binding (Figure 10). The interaction between SlPHL1 and SlSPX2 was recently demonstrated, but no interaction between SlPHL1 and SlSPX1 was observed (Singh et al., 2023). Following a comprehensive examination, it was found that they opted to clone the full-length CDS of SlPHL1 instead of its C-terminus as bait and employed the yeast strain PJ469A instead of AH109. Our previous study has shown that full-length SlPHL1 exhibits self-activation (Zhang et al., 2021), making it inappropriate for direct cloning into pGBKT7 for Y2H. The pull-down analyses and LCI tests confirmed that nSlSPX1 and nSlSPX4 interact with cSlPHL1 (Figure 10). Thus, our differing results from Singh et al. (2023) are most likely due to distinct experimental designs. Likewise, it is the C-terminus of OsPHR2 that is capable of binding to OsSPX1, OsSPX2, SPX4, and SPX6 (Lv et al., 2014; Wang et al., 2014b; Zhong et al., 2018). The presence of SlSPX1 shows a negative effect for SlPHL1 transcriptionally activating the transcription of SlPAP10b and SlPAP15 (Figure 11A, 11B). Furthermore, silencing of SlSPX1 enhanced the transcript levels of SlPAP10b and SlPAP15 as well as the APase activity (Figure 11). Also, silencing of SlSPX1 was found to promote plant growth and root mycorrhization in tomato (Singh et al., 2023). Similarly, OsSPX1, OsSPX2, OsSPX4, and OsSPX6 inhibit OsPHR2 transcription of PSI genes (Lv et al., 2014; Wang et al., 2014b; Zhong et al., 2018). Therefore, SlSPX1 is most likely an important inhibitory factor in the regulation of tomato PSR by SlPHL1. OsSPX4 inhibits the binding of OsPHR2 to its cis-element and reduces the targeting of OsPHR2 to the nucleus (Lv et al., 2014), and OsSPX6 hinders the translocation of OsPHR2 to the nucleus and inhibits the binding of OsPHR2 to the P1BS element (Zhong et al., 2018). In this way, the mechanism of SlSPX1 inhibiting the transcriptional activity of SlPHL1 on SlPAP10b and SlPAP15 deserves further study. Additionally, it should be noted that Pi itself does not bind to the SPXs (Wild et al., 2016; Dong et al., 2019). Instead, InsP8 is the bona fide signaling molecule that promotes the association between SPXs and PHRs (Ried et al., 2021). So, whether InsP8 is the true mediator between SlSPX1 and SlPHL1 is another question that needs to be focused on.
In summary, we have established a molecular mechanism of SlPHL1-dependent LP enhancement of APase activity in tomato, as schematically illustrated in Figure 11F: Under Pi-deficient conditions, SlPHL1 binds directly to the promoters of SlPAP10b and SlPAP15 through the P1BS motif to activate their transcriptional expression, thereby enhancing the corresponding PAP activity; whereas under Pi-sufficient conditions, SlSPX1 (and possibly SlSPX4) binds to SlPHL1, resulting in decreased transcriptional activity of SlPHL1 on SlPAP10b and SlPAP15, thereby reducing APase activity.
AUTHOR CONTRIBUTIONS
The experiments were conceived and designed by Y. Zhang, Z. Liu, and Y. Liu. The experiments were carried out by Y. Liu, C. Li, D. Zhang, S. Huang, E. Wang, and Y. Wang. The data were analyzed by Y. Liu, C. Li, Z. Liu, and Y. Zhang. Y. Zhang and Z. Liu drafted the manuscript. All authors discussed the results and approved the final version of the manuscript.
ACKNOWLEDGEMENTS
This work was supported by the National Natural Science Foundation of China (31701985 and 31700256), Natural Science Foundation of Fujian Province, China (2021 J01092), National College Students Innovation and Entrepreneurship Training Program (S202310389015 and X202310389311).
CONFLICT OF INTEREST STATEMENT
The authors declare that they have no conflict of interest.
Open Research
DATA AVAILABILITY STATEMENT
All data generated or analyzed during this study are included in this article or the supplementary material. The materials generated during the current study are available from the corresponding author on reasonable request.




