Photosynthetic and antioxidant responses of Ankistrodesmus flexuosus and Curvastrum pantanale to environmentally relevant copper concentrations
Abstract
Since high concentrations of copper (Cu) are known to negatively affect algae, we investigated the effect of environmentally relevant concentrations of Cu on the metabolism and photosynthesis of microalgae. We investigated the photosynthesis and antioxidant responses of two green microalgae, Ankistrodesmus flexuosus and Curvastrum pantanale, to free Cu (1.7 nM–589.0 nM Cu2+), which included environmentally significant values (1.7 nM–21.4 nM Cu2+). The microalgae had similar growth rates at low Cu2+, but C. pantanale had higher growth rates at 7.4 and 21.4 nM Cu2+, and a higher Chl a/b ratio at 1.7–21.4 nM Cu2+. The Fv/Fo of A. flexuosus was not altered by Cu, whereas its relative maximum electron transport rate (rETRm) began to decrease at 7.4 nM Cu2+. The Fv/Fo and rETRm of C. pantanale increased at 21.4 nM Cu2+. The non-photochemical quenching (NPQ) that was actively dissipated [Y(NPQ)] in A. flexuosus increased with Cu increase, whereas at 589.0 nM Cu2+, the NPQ of C. pantanale decreased and its passive energy dissipation [Y(NO)] increased. C. pantanale had higher photosynthesis at 1.7–21.4 nM Cu2+. Superoxide dismutase (SOD) activity increased in both microalgae at high Cu concentrations, at 21.4 nM Cu2+ for A. flexuosus, and at 589.0 nM Cu2+ for C. pantanale. Thus, environmentally relevant Cu concentrations can favor the survival of tolerant microalgae species, hence shifting the diversity of aquatic biota.
1 INTRODUCTION
Copper (Cu) is an essential micronutrient for microalgae; it is found in electron transport proteins and enzymes involved in photosynthesis (Cid et al. 1995; Peers and Price 2006; Kropat et al. 2015). However, Cu can also be toxic to microalgae when present in high concentrations because it can interfere with various metabolic processes and cause oxidative stress (Hill et al. 1996; Husak 2015; Merchant et al. 2020). The effects of Cu on microalgae depend on several factors, such as species, strain, growth stage, environmental conditions, and exposure duration (Debelius et al. 2009; Andersson et al. 2020).
The effects of Cu on different aspects of microalgal physiology, like growth, biomass, pigments, photosynthesis, and antioxidant enzymes, have been investigated (Sabatini et al. 2009; Guo et al. 2021; Cavalletti et al. 2022). However, most of these studies (Mohanty et al. 1989; Yong et al. 2018) showed that relatively high Cu concentrations (>100 nM), which are unlikely to occur in natural environments, are detrimental to microalgae. Cu often occurs in water bodies at concentrations referred to as environmentally relevant, these are usually non-toxic to organisms, around 10−8–10−7 mol L−1 (US Environmental Protection Agency 2007; Ferreira et al. 2008). Microalgae live in a highly variable environment, ranging from those where Cu levels are at environmentally relevant concentrations to those that receive gradual increases in Cu due to anthropogenic and industrial discharge (Masindi and Muedi 2018; Hubeny et al. 2021). Therefore, microalgae need to acclimate to different conditions in order to grow and multiply.
Although several studies have investigated the effect of Cu on the growth and physiology of microalgae, the relationship between environmentally relevant Cu concentrations, at which microalgae exist in natural waters, and the functionality of their photosystem II (PSII) remains poorly understood. While it is known that high Cu concentrations can cause photoinhibition and decrease PSII efficiency in microalgae (Küpper et al. 2003; Yong et al. 2018; Rocha et al. 2021), the threshold concentration at which these occur and the extent of the effect in different microalgae species/strains are still not well established. In addition, the mechanism and site of action of Cu on photosynthesis are debated.
Cu hinders electron transport (Šeršeň et al. 1997). The PSII's donor side was identified as the site of Cu2+ action by some authors (Kráľova et al. 1994; Arellano et al. 1995; Ouzounidou 1996; Pätsikkä et al. 2001), whereas the acceptor side was indicated by others (Utschig et al. 2001). The PSI has also been suggested as the site of action of Cu2+ (Deng et al. 2014). Additionally, the PSII's D1 protein has been identified as the precise site of Cu2+ action (Vavilin et al. 1995). The site of action and magnitude of Cu on the photosynthesis of microalgae may depend not only on the concentration of copper but also on the species and strains of microalgae.
The microalgae species used in this study belong to the Chlorophyta and the family Selenastraceae (Mori et al. 2018). Members of this family are common in lakes, wetlands, reservoirs, and rivers (Krienitz et al. 2001; Yee 2016), and they exhibit high morphological diversity (Krienitz and Bock 2012). They are important indicator species for ecosystem health and function (Da Silva et al. 2017). Their diversity and ability to survive in most freshwater environments make them valuable for studying changing environmental factors, such as Cu concentrations. Ankistrodesmus spp. have been used as model species in many physiological studies; these species have been shown to be more susceptible to Cu in terms of growth than other green algae, such as Scenedesmus sp. (Swartzman et al. 1990; Magdaleno et al. 2014), Chlorella sp. and Monoraphidium sp. (Magdaleno et al. 2014). The effects of Cu on the photosynthesis of Ankistrodesmus spp. have also been studied (Shioi et al. 1978; Whitelam and Codd 1984; Rocha and Espíndola 2021). However, most studies have not considered environmentally relevant concentrations of Cu, and their effects on the photosynthesis of Ankistrodesmus flexuosus and Curvastrum pantanale, a recently described species (Da Silva et al. 2017), have not been studied. In this study, we provide important information on the photosynthesis and antioxidant responses of these species to environmentally relevant Cu. Even though both species belong to the same family, they may have different tolerances to Cu in terms of their growth, biomass, and photosynthetic responses. Studying the differences in photosynthetic activity between algal species could facilitate the understanding of how different algal species can contribute to primary productivity in aquatic ecosystems (Juneau and Harrison 2005). Having such information will help in ecological management, understanding biodiversity and could also provide background information for the choice of microalgae species for biotechnological applications.
The study of the dynamic effects of environmentally relevant Cu on the photosynthesis of microalgae species is crucial for several reasons. First and foremost, microalgae play a vital role in the Earth's ecosystems by contributing to almost 50% of the planet's photosynthesis (Shen and Yin 2022). Thus, understanding the impact of Cu on microalgae photosynthesis is important because it can directly affect the overall productivity as well as the global climate. It is therefore essential to investigate why certain species exhibit greater sensitivity to Cu than others. Questions such as: is there a threshold concentration of Cu that, when exceeded, leads to a significant decline in microalgae photosynthesis? Do environmentally relevant concentrations of Cu cause some microalgae species to use light more efficiently? In microalgae, what part of their PSII is affected by Cu and how does this impact their overall photosynthetic capacity? Answering these questions can contribute to the understanding of the dynamics and resilience of aquatic ecosystems.
2 MATERIALS AND METHODS
2.1 Microalgae strains used for this study
The green microalgae used, Ankistrodesmus flexuosus (CCMA-UFSCar 083) and Curvastrum pantanale (CCMA-UFSCar 350), were kindly donated by Prof. Inessa L. Bagatini, from the Department of Botany, Federal University of São Carlos, Brazil (SISGEN A5EF193). Both microalgae were cultured in modified BG-11 media (Rippka et al. 1979) with a pH of 7.0. The BG-11 media were sterilized by autoclaving (121°C, 15 psi, 20 min) before microalgae inoculation.
2.2 Treatments of culture media with Cu
For the experimental cultures, BG-11 media were prepared without any copper added. Copper (as Cu standard for atomic absorption spectroscopy; #38996, Sigma-Aldrich) was added to the BG-11 media after the media had been autoclaved and allowed to cool. Seven different stock concentrations of Cu made from a Cu standard (1000 ppm) were added to the autoclaved media to achieve the nominal concentrations as presented in Table 1 [Nominal (Total added) copper]. Cu treatments were made in triplicate, and the Cu-treated media were allowed to equilibrate for 24 h before the microalgae were inoculated.
| Nominal (Total added) copper (nM Cu) | Free copper ions (nM Cu2+) |
|---|---|
| 80 | 1.7 |
| 120 | 2.5 |
| 160 | 3.4 |
| 180 | 3.8 |
| 350 | 7.4 |
| 1000 | 21.4 |
| 19600 | 589.0 |
The concentration of free copper ions (Cu2+) (Table 1; Free copper ions) that correspond to each nominal Cu in the media at the beginning of the experiments, were calculated using the Visual MINTEQ 3.1 (Gustafsson 2013) free chemical equilibrium calculation software. All results are presented as free Cu2+ concentrations.
2.3 Microalgae culture conditions
The cultures were grown in 1 L polystyrene tissue culture flasks (4.5 cm optical path). Cultures were started with an initial cell density of 104 cells/mL and a volume of 550 mL by inoculating exponentially growing cells. The cultures were continuously bubbled with filtered air (using 0.22 μm syringe filters) to ensure good mixing of CO2 conditions for the cells, and were kept at a temperature of 23 ± 2°C, for an experimental time of 96 h. Cultures were illuminated in a 12:12 h light/dark cycle with a light intensity of 230 μmol photons m−2 s−1 for A. flexuosus, and 250 μmol photons m−2 s−1 for C. pantanale. The respective light intensities represent the saturation irradiances of these species that were obtained from preliminary studies through rapid light curves.
2.4 Experimental data collection
2.4.1 Biomass and growth measurements
Cell counts of A. flexuosus and C. pantanale were made using a Fuchs Rosenthal chamber. We plotted a growth curve with the natural logarithms of the cell counts against experimental time (days), and the linear part of this curve represents the exponential growth phase of the microalgae. A straight line (0–48 h) representing the exponential growth phase was fitted using linear regression using Microsoft Excel 2013 for Windows. We used the slope of this fitted line as the specific growth rates.
2.4.2 Photosynthesis parameters
Chl a fluorescence measurements serve as sensitive and prompt indicators of metal toxicity, and have been used to monitor the onset, development, extent and even recovery from metal toxicity. A pulse amplitude modulated fluorometer (Phyto-PAM-II compact, Heinz Walz GmbH), which measures Chl a fluorescence, controlled by the PamWin V3.12w software (Heinz Walz GmbH) was used to measure the photosynthesis parameters.
Before measuring the minimum fluorescence (Fo), maximum fluorescence (Fm), and rapid light curves (RLC), the microalgae samples were dark-acclimated for 20 min to ensure open PSII centers and relaxed NPQ. We measured the Fo with a weak measuring beam (1 μmol photons m−2 s−1) and the Fm with a high-intensity saturation pulse (5000 μmol photons m−2 s−1). The difference between Fm and Fo was used as the variable fluorescence (Fv).
The rapid light curves (RLCs) were made by illuminating the microalgae samples with 21 steps (20 s each) of increasing PAR (0–1120 μmol photons m−2 s−1). Each PAR was multiplied by its corresponding Fv’/Fm′ to get the relative electron transport rate (rETR) at each illumination step. The RLCs were plotted as rETR vs PAR and fitted using the equation of Platt et al. (1980). Alpha (α), the initial slope of the RLC, which indicates how effectively light energy is absorbed and used for photochemistry (Henley 1993), and rETRm (maximum relative electron transport rate) were obtained from the RLC. The saturation irradiance (Ek) was calculated as rETRm/α.
The samples were acclimated to fixed light intensities (230 μmol photons m−2 s−1 for A. flexuosus and 250 μmol photons m−2 s−1 for C. pantanale), which were applied for a duration of 0.2 s after every 20 s for a total of 6 min. Afterward, the Fs (steady-state fluorescence) and Fm′ (maximum fluorescence at light-adapted state) were measured, and from these, the Fq'/Fm′ (PSII operating efficiency, also known as ) (Genty et al. 1989; Baker 2008) was calculated.
2.4.3 Quantification of Superoxide dismutase (SOD) activity
A 10 mL culture aliquot from each Cu treatment was centrifuged (at 2597 × g for 15 min), and the resulting algal pellets were extracted. The extraction was done in 3 mL cold phosphate buffer (0.05 M, pH 7.8), using glass beads (3 mm in diameter) and vortexing for 3 min. After vortexing, the extracts were centrifuged (at 2597 × g for 15 min at 4°C), and the resulting supernatant was used to quantify SOD activity. Superoxide dismutase (SOD) activity was assayed according to the method of Beauchamp and Fridovich (1971), with modifications. The reaction mixture consisted of 0.1 mL of algal enzyme extract, 2.35 mL phosphate buffer (pH 7.8; 50 mM), 0.2 mL Na2EDTA (10 mM), 0.1 mL nitroblue tetrazolium (NBT; 1 mM), 0.3 mL methionine (100 mM), and 0.05 mL riboflavin (0.2 mM) in a test tube. The test tubes were incubated in light (~ 688 μmol photons m−2 s−1) in an aluminum foil-lined box for 10 min. Then, the absorbance was read at 560 nm in a microplate reader (Epoch, Bio Tek Instruments, Inc.). One unit of SOD (U) activity was defined as the amount of enzyme required to cause 50% inhibition of NBT reduction, monitored at 560 nm (Yilancioglu et al. 2014).
2.5 Data analyses
Two-way analysis of variance (ANOVA) was used to determine differences in the measured parameters, with Cu concentration and microalgae as factors. Tukey's HSD test was used for multiple comparisons of the means. The assumptions of normality and homoscedasticity were confirmed using Shapiro–Wilk test and Levene's test, respectively, before doing the ANOVA. Principal component analysis (PCA) using a correlation matrix was used to analyze the relationship between the measured parameters and the microalgae species. Statistical analyses were done using R for Windows (R Development Core Team 2020).
3 RESULTS
3.1 Growth and biomass responses of A. flexuosus and C. pantanale to Cu treatments
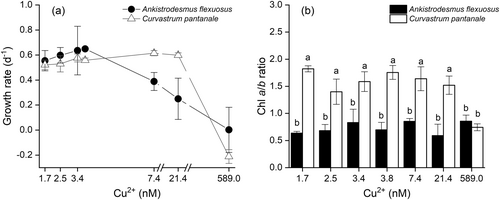
A. flexuosus and C. pantanale responded differently to Cu treatments in terms of their growth (interaction effect: F6,28 = 6.63, P = 0.0002, Figure 1a), and Chl a/b ratio (interaction effect: F6,28 = 11.00, P < 0.0001, Figure 1b). The growth of A. flexuosus started to decrease at 7.4 nM Cu2+, whereas that of C. pantanale only decreased at 589.0 nM Cu2+ (the highest Cu concentration). The Chl a/b ratio of C. pantanale was significantly (P < 0.0001) higher than that of A. flexuosus at the 1.7–21.4 nM Cu2+ range. At 589.0 nM Cu2+, the Chl a/b of the two species did not differ significantly (P = 0.3784).
3.2 Individual photosynthesis responses of A. flexuosus and C. pantanale to Cu concentrations
The Fv/Fo of A. flexuosus was not significantly (F6,28 = 2.76, P = 0.03, Figure 2a) affected by Cu treatment, but Cu higher than 7.4 nM Cu2+ decreased its Ek (Figure 2b) and its rETRm (Figure 2c). Its alpha (Figure 2d), Fv/Fm (Figure 2e), Fq'/Fm′ (Figure 2f), and qP (Figure 3a) were all significantly lower at 589.0 nM Cu2+.
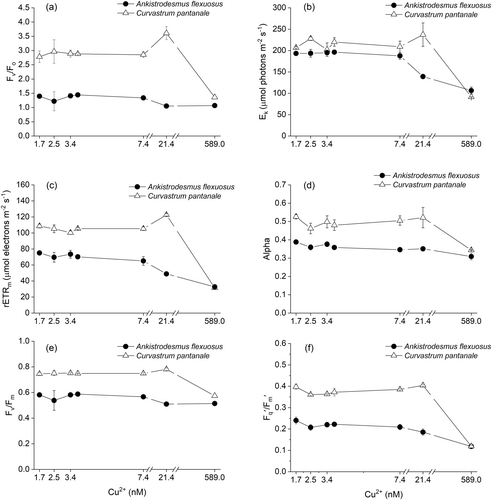
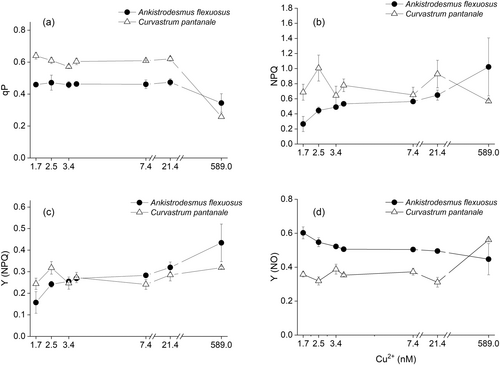
For C. pantanale, Fv/Fo (P < 0.001, Figure 2a) and rETRm (P < 0.001, Figure 2c) increased significantly at 21.4 nM Cu2+ and decreased at 589.0 nM Cu2+. Its Ek (Figure 2b), alpha (Figure 2d), Fv/Fm (Figure 2e), Fq'/Fm′ (Figure 2f), and qP (Figure 3a) were mostly uniform between 1.7–21.4 nM Cu2+, however, all these parameters significantly (P < 0.001) decreased at 589.0 nM Cu2+.
The NPQ of A. flexuosus significantly (F6,28 = 8.44, P < 0.0001) increased from 1.7 to 589.0 nM Cu2+ in a progressive manner (Figure 3b). On the other hand, the NPQ of C. pantanale was similar across Cu treatments; it was significantly (P < 0.05) reduced only at 589.0 nM Cu2+ in comparison to 2.5 nM Cu2+ (Figure 3b).
The Y (NPQ) of A. flexuosus (Figure 3c) significantly increased in response to increasing Cu concentration, while the Y(NPQ) of C. pantanale (Figure 3c) was not affected by any Cu concentration. In A. flexuosus, Y (NO) decreased significantly (P = 0.0001, Figure 3d) with an increase in Cu concentration, being lowest at 589.0 nM Cu2+ (Figure 3d). Y (NO) was significantly (P < 0.0001) higher in C. pantanale at 589.0 nM Cu2+ (Figure 3d).
3.3 Comparison between the photosynthesis responses of A. flexuosus and C. pantanale to Cu concentrations
The Fv/Fo (interaction effect: F6,28 = 22.90, P < 0.0001, Figure 2a), Ek (interaction effect: F6,28 = 16.35, P < 0.0001, Figure 2b), rETRm (interaction effect: F6,28 = 70.52, P < 0.0001, Figure 2c), and alpha (coefficient of light usage; interaction effect: F6,28 = 5.41, P = 0.0008, Figure 2d) of A. flexuosus and C. pantanale were affected by Cu differently. Curvastrum pantanale had the highest (P < 0.05) Fv/Fo at all the Cu concentrations tested. It also had significantly higher (P < 0.0001) rETRm and alpha than A. flexuosus at 1.7–21.4 nM Cu2+. For Ek, C. pantanale had significantly (P < 0.05) higher values than A. flexuosus at 2.5 and 3.8–21.4 nM Cu2+.
The Fv/Fm (interaction effect: F6,28 = 12.82, P < 0.0001, Figure 2e) and Fq'/Fm′ (interaction effect: F6,28 = 61.19, P < 0.0001, Figure 2f) of A. flexuosus and C. pantanale also responded differently to Cu treatments. C. pantanale had significantly (P < 0.0001) higher values of both Fv/Fm and Fq'/Fm′ at all Cu concentrations, except at 589.0 nM Cu2+ where their values decreased and were similar in C. pantanale and A. flexuosus.
The qP (photochemical quenching) of A. flexuosus and C. pantanale were significantly (interaction effect: F6,28 = 18.73, P < 0.0001, Figure 3a) different at all Cu concentrations. C. pantanale had significantly (P < 0.0001) higher qP than A. flexuosus at 1.7–21.4 nM Cu2+. However, the qP of C. pantanale decreased at 589.0 nM Cu2+, with A. flexuosus having a significantly (P = 0.003) higher qP at this Cu concentration.
A significant (interaction effect: F6,28 = 8.10, P < 0.0001, Figure 3b) difference in NPQ in response to Cu treatment was recorded in A. flexuosus and C. pantanale. C. pantanale had higher NPQ at most of the Cu concentrations tested; however, at 589.0 nM Cu2+, A. flexuosus had higher NPQ (P = 0.0004).
C. pantanale had significantly higher Y(NPQ) than A. flexuosus at 1.7–2.5 nM Cu2+ (P < 0.05, Figure 3c), but at 589.0 nM Cu2+, A. flexuosus had a significantly (P = 0.002) higher Y(NPQ). For the Y(NO), A. flexuosus had significantly higher (P < 0.0001, Figure 3d) values at 1.7–21.4 nM Cu2+, but this trend was reversed at 589.0 nM Cu2+ where C. pantanale had a significantly (P = 0.0001) higher Y(NO).
3.4 Superoxide dismustase activities of A. flexuosus and C. pantanale in response to Cu concentrations
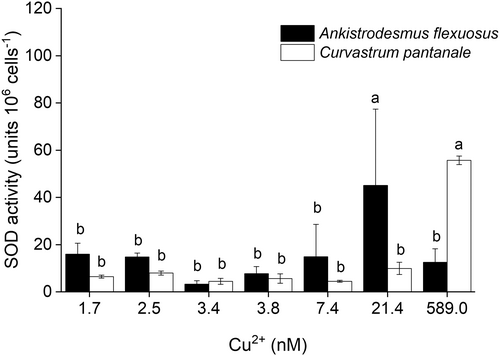
The SOD activities of A. flexuosus and C. pantanale in response to Cu treatment were significantly different (interaction effect: F6,28 = 8.85, P < 0.0001, Figure 4). The SOD activity of A. flexuosus significantly (F1,28 = 19.91, P = 0.001) increased at 21.4 nM Cu2+, while that of C. pantanale significantly (F1,28 = 30.00, P < 0.001) increased just at 589.0 nM Cu2+.
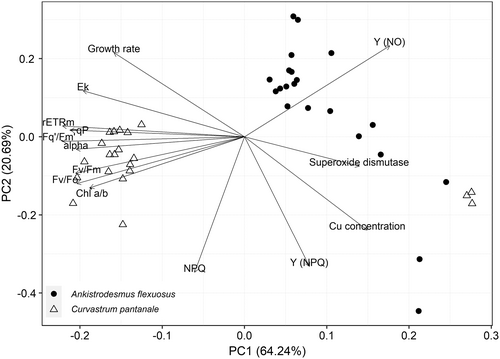
The relationships of the two microalgal species to the measured parameters using principal components analysis (PCA) biplot are presented in Figure 5. The first and second principal components (PCs) explained 84.93% of the total variation in the data. C. pantanale had higher Chl a/b, rETRm, qP, Fq'/Fm′, alpha, Fv/Fm, Fv/Fo, and Ek than A. flexuosus, whereas A. flexuosus had a higher Y (NO). Y (NO) is negatively correlated with Chl a/b. An increase in Y (NPQ) was strongly correlated with an increase in Cu concentration in A. flexuosus. The PCA analysis confirmed that an increase in SOD activity was strongly correlated with an increase in Cu concentration in both A. flexuosus and C. pantanale.
4 DISCUSSION
Copper in high concentrations is generally detrimental to the growth, biomass, and overall metabolism of microalgae, despite being an essential nutrient (Hill et al. 1996; Husak 2015; Merchant et al. 2020). The growth and biomass of different species and strains of microalgae have different tolerance to Cu levels (Debelius et al. 2009; Andersson et al. 2020). Similarly, the two species studied here also showed different tolerance to Cu levels with regard to their growth rate (Figure 1a). The population of C. pantanale withstood higher Cu concentrations than A. flexuosus and, at the same time, C. pantanale had approximately 3 times higher Chl a/b ratio (Figure 1b). The cells of A. flexuosus had a low Chl a/b ratio. Higher Chl b than Chl a is common among picophytoplankton species, who have a Chl a/b ratio in the range of 0.6 to 0.8, which is usually lower than those of the light-harvesting complex II (LHC II) of most Chlorophyte microalgae (Ohki and Honjho 1997). Although the background Chl a/b ratio of C. pantanale was higher than that of A. flexuosus, C. pantanale was susceptible to high Cu since, at 589.0 nM Cu2+, its Chl a/b ratio decreased from its background levels. On the other hand, A. flexuosus was more resistant to high Cu, as its Chl a/b ratio was not altered at high Cu.
The higher sensitivity of A. flexuosus to Cu agrees with the results of Magdaleno et al. (2014), who exposed four green microalgae (Ankistrodesmus fusiformis, Chlorella ellipsoidea, Monoraphidium contortum, and Scenedesmus acuminatus) to Cu, and showed that Ankistrodesmus fusiformis was the most sensitive species. The decrease in the Chl a/b ratio of C. pantanale could have been due to the drastic decrease in its Chl a in comparison to its Chl b. This may have resulted from the faster degradation of the Chl a and the conversion of some of it to Chl b by high Cu. Under oxidative stress, Chl a is converted to Chl b by the oxidation of the methyl group on the ring II (Chettri et al. 1998). This was corroborated by the findings of Prasad et al. (2001), who recorded a slower degradation of Chl b due to Cu-induced photooxidative damage. Additionally, a higher degradation of PSI in comparison to PSII has been reported to cause a decrease in Chl a/b ratio because PSI has a higher Chl a/b ratio than PSII (Dinç et al. 2012).
Chl a/b ratio is related to the light absorption efficiency of photosynthesis or the PSII antenna size (Kume et al. 2018) of microalgae; the lower the ratio, the larger the antenna size (Kirst et al. 2012; Perrine et al. 2012). Although low Chl a/b ratio is linked to large antenna size, it does not lead to higher photosynthesis (Negi et al. 2020; Dauda and Lombardi 2023), as recorded in this study (Figure 1b). A similar decrease in Chl a/b, as observed in C. pantanale here, was also recorded in Monoraphidium sp. treated with the same Cu concentration (Dauda and Lombardi 2023). Although much more resistant to Cu than the species studied here, the Chl a/b ratio of Scenedesmus vacuolatus also decreased at 210 μM Cu2+ (Sabatini et al. 2009), 420 times higher Cu concentration than in our study. Nevertheless, it is accepted that the sensitivity of microalgae to Cu ions is species-specific and related to the previous history of the cells (Lopez et al. 2019; Andersson et al. 2020).
The unaltered Fv/Fo by Cu treatment in A. flexuosus suggests that the efficiency of electron donation to QA by the donor side of the PSII (Babani and Lichtenthaler 1996; Lichtenthaler et al. 2005) was not affected by the Cu concentrations used in this study (Figure 2a). Cu started to affect the PSII of A. flexuosus at 7.4 nM Cu2+ because it was at this concentration that the rate of electron transport (rETRm) through the electron transport chain (ETC) started to reduce in this microalga (Figure 2c). The reduction in electron transport also coincided with the reduction in the intensity of irradiance (Ek) needed to saturate the photosynthetic rate (Figure 2b,c). The reduction in the rETRm in A. flexuosus at 7.4 nM Cu2+ could have been due to the increased protection provided by the non-photochemical quenching (NPQ), which is characterized by energy dissipation primarily as heat (Maxwell and Johnson 2000; Govindjee 2002; Baker 2008; Ruban 2016). This is because the activity of the NPQ and the yield of the regulated non-photochemical quenching [Y(NPQ)] (Klughammer and Schreiber 2008) in A. flexuosus started to peak at 7.4 nM Cu2+ (Figure 3b,c), which suggests that the decreases in rETRm and Ek were due to the energy dissipation through the NPQ (Figures 2b,c and 3b). Although Cu concentration at 7.4 nM Cu2+ and above triggered the NPQ mechanism and reduced electron transport through the ETC, the yields of the PSII (Fv/Fm and Fq'/Fm′) and the oxidation state of QA (qP) were not affected by Cu until 589.0 nM Cu2+ (Figures 2e,f and 3a). These results show that the different photosynthetic processes of the PSII of A. flexuosus had different sensitivities to Cu concentration. The rETRm of A. flexuosus in this study decreased at lower Cu concentrations in comparison to Monoraphidium sp. (Dauda and Lombardi 2023), which decreased at 589 nM Cu2+, and Chlorolobion braunii (Baracho et al. 2019), which decreased at 5000 nM Cu2+. In contrast to our results, the Ek of Scenedesmus quadricauda showed very high resilience to Cu ions (Yong et al. 2018), which may be related to the genus.
In C. pantanale, the increase in Fv/Fo at 21.4 nM Cu2+ suggests a higher efficiency of electron donation from the donor side (water-splitting complex) of the PSII to QA (Figure 2a). We recorded an increase in rETRm at this Cu concentration as well, which suggests that more electrons passed through the ETC of C. pantanale at 21.4 nM Cu2+ due to the increased Fv/Fo at this Cu concentration (Figure 2a,c). It could also be that in addition to the PSII, other components downstream of the PSII may have acclimated to this Cu level, hence the increase in rETRm. A similar increase in rETRm at 7.4 nM Cu2+ was reported in Monoraphidium sp. (Dauda and Lombardi 2023). The Fv/Fo is a good indicator of stress condition because it immediately shows high amplitude changes even when small changes occur in Fv and Fo (Lichtenthaler et al. 2005). Therefore, our recorded decrease in Fv/Fo at 589.0 nM Cu2+ suggests an impairment in the ability of the donor side of the PSII to donate electrons, which is an indication of structural alterations in the PSII (Joshi and Mohanty 2004). This is also related to the decreased amount of Chl a, as shown by the low Chl a/b ratio at this Cu level (Figure 1b).
Even though we recorded an increase in Fv/Fo and rETRm at 21.4 nM Cu2+ in C. pantanale, its Ek, Fv/Fm, Fq'/Fm′, and qP were largely unaltered between 1.7–21.4 nM Cu2+, which show that the oxidation state of QA and the photosynthesis yield of this microalga were unaffected by Cu within this Cu range (Figures 2a-c, e-f and 3a). Photosynthesis was reduced in C. pantanale at 589.0 nM Cu2+, as shown by the decrease in most of the photosynthesis parameters measured at this Cu level, as well as a decrease of almost 50% in Chl a/b (Figures 1b, 2, and 3a). Energy dissipation as heat and fluorescence through passive means [Y(NO)] (Klughammer and Schreiber 2008; Masojídek et al. 2013) increased at 589.0 nM Cu2+ in C. pantanale, when its Chl a/b decreased (Figures 3d and 1b). Therefore, we hypothesize that an increase in Y(NO) may be linked with an increase in Chl b in the photosynthetic apparatus of this microalga. The increase in Y(NO) was responsible for protecting the photosynthetic apparatus of A. flexuosus at low (1.7–3.4 nM Cu2+) Cu concentrations.
Most of the light absorbed by A. flexuosus at 1.7 nM Cu2+ was used for photosynthesis, this is shown by the lowest NPQ and Y(NPQ) (Figure 3b,c). At the start of illumination, when a full trans-thylakoid ΔpH is not yet established, the Y(NO) is mostly responsible for protection, and it is responsible for reduced Fq'/Fm′ (also referred to as Y(II)). However, when the ΔpH becomes established, the Y(NPQ) takes over protection (Townsend et al. 2018).
Comparing the photosynthesis of A. flexuosus and C. pantanale in response to Cu, C. pantanale had higher values of Fv/Fo, Ek, rETRm, alpha, Fv/Fm, Fq'/Fm′, and qP (Figures 2, 3, and 5). These indicate that the donor side of the PSII of C. pantanale had a higher efficiency of electron donation to QA, and more of its QA was oxidized (i.e., “open”), which led to a higher electron transport rate through its ETC. All these ultimately led to C. pantanale having higher PSII yields in comparison to A. flexuosus. The differences in photosynthesis responses of these two microalgae demonstrate that they respond differently to Cu and suggest that they have different photosynthesis capacities. Even though the photosynthesis of C. pantanale was higher between 1.7–21.4 nM Cu2+, A. flexuosus was more resilient. This is because the steepest decrease in photosynthesis was observed in C. pantanale at 589.0 nM Cu2+, where the photosynthesis of both microalgae was significantly reduced. Our results also demonstrate the use of different protective mechanisms by A. flexuosus and C. pantanale in response to high Cu stress. As A. flexuosus used the regulated NPQ (Y NPQ) for protection against light effect at high Cu, C. pantanale rather used the unregulated or passive mechanism of energy dissipation [Y (NO)] (Figure 3c,d). Although the same light intensity was used for each microalga throughout this study, the light intensity could have been high to induce stress in the microalgae cells at high Cu treatment (589.0 nM Cu2+). This is because, at this high Cu concentration, the microalgae had reduced growth, and they also exhibited oxidative stress (Figures 1a and 4).
The formation of a proton gradient across the thylakoid membrane (ΔpH) is responsible for activating the NPQ (Krause and Jahns 2004; Pfündel et al. 2008; Murchie and Ruban 2020). Therefore, the low Y(NPQ) at 1.7 nM Cu2+ in A. flexuosus suggests that Cu did not increase the ΔpH, hence our recording of low NPQ and Y(NPQ) (Figure 3b,c). Rather, the Y(NO) was responsible for dissipating the light energy at this low Cu (Figure 3d). However, an increase in Cu concentration up to 589.0 nM Cu2+ in A. flexuosus, raised the ΔpH, thereby increasing the Y(NPQ), which substituted the Y(NO) in dissipating energy. This means that, at high Cu concentration, the photosynthesis apparatus of A. flexuosus was actively dissipating absorbed light energy to protect its cells. The high ΔpH at 589.0 nM Cu2+ could have resulted from processes downstream of the PSII, such as the Cyt b6f complex. On the other hand, the unaltered Y(NPQ) of C. pantanale at all Cu concentrations, and its increased Y(NO) at 589.0 nM Cu2+, suggests that even at 589.0 nM Cu2+, C. pantanale did not use the regulated or active dissipation mechanism [Y(NPQ)]; instead, the cells used the unregulated energy dissipation mechanism [Y(NO)]. Y(NO) consists of the NPQ due to the photo-inactivation of PSII and constitutive thermal dissipation that is resistant to environmental stresses (Busch et al. 2009; Huang et al. 2012). However, high Y(NO) reflects the inability of cells to protect themselves against damage to excess light (Huang et al. 2012), a situation which we suggest happened with C. pantanale in this condition. High energy dissipations, Y(NPQ) in A. flexuosus and Y(NO) in C. pantanale, caused by high Cu concentration (589.0 nM Cu2+) could have led to the reductions in Fq'/Fm′ in these microalgae.
The photosynthetic outcomes elucidated in this study carry important ecological and environmental implications, particularly concerning the response of microalgae to Cu concentrations. Cu concentrations around 21.4 nM Cu2+ could favor photosynthesis in some species of microalgae, as seen in C. pantanale in this study, whereas Cu up to 589.0 nM Cu2+ inhibits photosynthesis in microalgae (Figures 2 and 5). Furthermore, the utilization of distinct protective mechanisms in response to Cu stress highlights the adaptability of these microalgae, shedding light on their potential ecological roles in natural environments. The protective mechanisms used by these microalgae, such as non-photochemical quenching (NPQ), provide insights into their resilience strategies in the face of Cu stress, contributing to our broader understanding of the ecological consequences of Cu contamination in aquatic ecosystems. Our findings elucidate the dynamic nature of these microalgae photosynthetic processes in the presence of varying Cu concentrations; different microalgae species could have different photosynthetic responses to Cu levels.
Superoxide dismutase (SOD) is an antioxidant enzyme that scavenges superoxide anion, a type of reactive oxygen species (ROS) in aerobic cells, and converts it to peroxide (Kumar et al. 2014; Khorobrykh et al. 2020). Copper enhances the production of superoxide anions in microalgae cells by binding to thiols and disrupting the cell's redox status (Dietz et al. 1999; Smith et al. 2014). This, in turn, makes them susceptible to Cu stress. An increase in SOD indicates an increase in the synthesis of superoxide free radicals (McCord 2008; Nowicka 2022). The increase in SOD activities in the cells of A. flexuosus at 21.4 nM Cu2+, and C. pantanale at 589.0 nM Cu2+, shows that with an increase in Cu, A. flexuosus was more susceptible to higher superoxide anion synthesis than C. pantanale (Figure 4). This is because the cells of A. flexuosus upregulated their SOD activity at a lower Cu concentration than in C. pantanale. However, the cells of A. flexuosus could not sustain an increase in SOD activity at 589.0 nM Cu2+, which suggests that this protective mechanism was lost in this microalga at high Cu concentrations. Such an effect could have been due to the inhibition of the SOD enzyme in A. flexuosus by high production of ROS caused by high Cu toxicity (Anu et al. 2016).
In comparison with our results, SOD activities in Scenedesmus vacuolatus and Chlorella kessleri increased at 46.7 μM Cu2+ (Sabatini et al. 2009), a much higher (~93x higher) Cu concentration than the ones we used in this study. SOD activities were also increased at high Cu in Chorella ehrenbergii (Wang et al. 2018) and Chlorococcum sp. (Qiu et al. 2022), similar to our recorded increase in SOD activity at high Cu in C. pantanale. These suggest that different microalgae species or strains have different antioxidant sensitivities to Cu. Species that are more tolerant to Cu, like C. pantanale could serve as bioindicators of high Cu.
The finding that certain environmental concentrations of Cu can boost the photosynthesis of some species of microalgae (Dauda and Lombardi 2023) suggests that the microalgal biota in aquatic environments may change according to slight variations the Cu levels. The two microalgae studied here showed some distinct differences in response to environmentally relevant Cu levels. These differences demonstrate that in the aquatic habitat, where such levels of Cu exist, microalgae will potentially react to their presence by adjusting their photosynthesis and oxidative responses. Microalgae tolerant to these levels of Cu, as seen here in C. pantanale, could dominate the phytoplankton biomass due to their higher growth, photosynthesis, and antioxidant protection, in contrast to species that are more sensitive, such as A. flexuosus. Such differential responses of microalgae could alter the abundance of aquatic primary producers, an effect that can potentially affect the stability of the aquatic ecosystem.
5 CONCLUSION
Ankistrodesmus flexuosus and Curvastrum pantanale showed different tolerance to Cu concentrations, as observed from their growth rates, chlorophyll a/b ratios, and photosynthesis responses. C. pantanale was, in general, more resilient to Cu than A. flexuosus. This was confirmed by the different photosynthetic behavior of the two green algae when exposed to Cu. The NPQ mechanism protected the cells of A. flexuosus from damage by light, even at high Cu concentrations. While A. flexuosus used the active/regulated energy dissipation mechanism [Y(NPQ)] for photo-protection, C. pantanale used the passive dissipation of energy [Y(NO)] at high Cu. Superoxide dismutase (SOD) activity increased at 21.4 nM Cu2+ for A. flexuosus, and at 589.0 nM Cu2+ for C. pantanale.
Assessments of how microalgae species are affected by not just toxic Cu levels, but also by the nuances in environmental Cu levels will provide better understanding and environmental predictions under present and future climate scenarios. This information becomes increasingly important if environmental Cu loading continues to rise due to anthropogenic activities. A situation that can favor the dominance of only better-adapted species, leading to reduced species diversity and primary productivity.
AUTHOR CONTRIBUTIONS
Suleiman Dauda: Conceptualization, Methodology, Experiment, Data collection, curation, and analyses, Validation, Visualization, Writing – original draft, review & editing.
Ana Teresa Lombardi: Conceptualization, Funding acquisition, Methodology, Resources, Supervision, Validation, Writing – review & editing.
All authors read and approved the final manuscript.
ACKNOWLEDGEMENTS
SD is grateful to The World Academy of Sciences & Conselho Nacional de Desenvolvimento Científico e Tecnológico (TWAS-CNPq; grant number 121853/2017-9) for the doctoral fellowship, and ABU Zaria for research funding. ATL is grateful to Fundação de Amparo à Pesquisa do Estado de São Paulo (FAPESP 2018/07988-5) and Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq 304280/2019-4) for research grants. The Ankistrodesmus flexuosus and Curvastrum pantanale strains were a gift from Prof. Inessa L. Bagatini, of the Department of Botany, Federal University of São Carlos, Brazil.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




