Hormone and transcriptomic analysis revealed that ABA and BR are key factors in the formation of inter-subgeneric hybridization barrier in water lily
Abstract
Understanding the process of signal communication between pollen and stigma is of significant importance for plant sexual reproduction. In the case of inter-subgeneric hybridization in water lily, a pre-fertilization hybridization barrier exists, the regulatory mechanism of which remains unclear. In this study, we conducted hormone and transcriptome analyses of unpollinated stigmas (Mock), self-pollinated stigmas (SP), cross-pollinated stigmas within the same subgenus (CP), and inter-subgenus cross-pollination stigmas (ISCP) in water lily to elucidate the formation mechanism of the inter-subgeneric hybridization barrier. Our results indicated that the lack of abscisic acid (ABA) and brassinolide (BR) in ISCP stigmas are key factors contributing to the formation of the inter-subgeneric hybridization barrier in water lily. Exogenous application of ABA and BR can help overcome the barrier between inter-subgeneric water lily crosses. Through transcriptome analysis, we identified nine candidate genes involved in regulating the inter-subgeneric hybridization barrier in water lily. In addition, we further demonstrated the importance of the NCED2-mediated ABA synthesis pathway in the pollination process through AS-ODN technology. Our study confirms that ABA and BR are critical for breaking the inter-subgeneric hybridization barrier. The identification of the nine candidate genes provides important clues for further research on the hybridization recognition mechanism in water lily.
1 INTRODUCTION
Water lily is a collective name for plants of the Nymphaea, which are widely used in landscaping, medicine and water restoration (Yu et al. 2018, Parveen et al. 2022, Xiong et al. 2023). To date, more than 50 species of water lilies have been discovered, which can be categorized into tropical and hardy water lily according to their geographical distribution (Perry and Robinson 1997, Sun et al. 2021). Tropical water lily is favored for their diverse flower colors, including red, blue, purple, white, yellow and other complex hues (Yu et al. 2018). However, tropical water lily is intolerant to low temperatures and struggles to survive harsh winters in higher latitudes (Hao et al. 2022). On the other hand, hardy water lily, found in temperate regions, possess unique cold tolerance (Sun et al. 2021, Sun et al. 2023). Nevertheless, the flower colors of hardy water lily are limited to red, white and yellow, and their flower stalks do not emerge above the water surface, reducing their ornamental value (Sun et al. 2021, Sun et al. 2023). Therefore, hybrid breeding between tropical and hardy water lily holds great significance to obtain cultivars that are both cold-tolerant and possess diverse flower colors. However, the hybridization between tropical and hardy water lily has proven to be challenging, with low seed set and inefficient pollination (Sun et al. 2018, Sun et al. 2019, Hao et al. 2022). Studies have shown that pre-fertilization hybridization barriers during the cross between tropical and hardy water lily are the main hurdles in achieving successful seed production (Sun et al. 2018, Sun et al. 2019, Hao et al. 2022), with low pollen germination rate being a commonly observed phenomenon. Additionally, abnormalities in pollen tube growth, such as bending or swelling at the tip and failure to penetrate the ovule, have been observed (Hao et al. 2022).
Studies have shown that indoleacetic acid (IAA), zeatin riboside (ZR) and abscisic acid (ABA) are the key hormones involved in plant fertilization (Mesejo et al. 2013, Breygina et al. 2023). In pollen germination experiments, IAA and gibberellic acid (GA) stimulate pollen tube growth, while ABA inhibits pollen tube growth, and ZR has no significant effect on this process (Breygina et al. 2023). Low levels of IAA can lead to abnormalities in pollen tube growth (Ma et al. 2013). IAA levels positively correlated with the speed of fertilization, ABA promoted fertilization and ZR was mainly related to sperm-egg fusion and seed development (Yang et al. 2022). In tobacco, IAA has been reported to participate in the interaction between pollen and stigma (Chen and Zhao 2008). Jasmonic acid (JA) and salicylic acid (SA), closely related to biotic stress, have also been reported to participate in regulating plant pollination and fertilization processes (Shi et al. 2017, Schubert et al. 2019, Lu et al. 2021, Wang et al. 2021a). In pear, it has been reported that pollination can affect the synthesis of JA and ABA in the pistil. Pollination combinations with hybridization barriers activate the expression of genes related to phytohormone signaling pathways and plant-pathogen interaction pathways (Shi et al. 2017). It is believed that the JA-mediated defense pathway may be an important pathway leading to self-incompatibility in pears (Shi et al. 2017).
In this study, we examined changes in endogenous hormone contents in water lily stigmas after unpollinated (Mock), self-pollinated (SP), cross-pollinated in the same subgenus (CP) and inter-subgenus cross-pollination (ISCP) treatments. Changes in the contents of ABA, GA3, brassinolide (BR), MeJA and SAG were considered to be the main factors contributing to the formation of cross-subgenus hybridization barriers in water lily. Potential genes regulating the formation of cross-subgenus hybridization disorder in water lily were excavated by RNA-seq analysis. The role of NCED2 in regulating the cross-subgenus hybridization disorder was further verified. The mining of this information provides a theoretical basis for understanding the mechanism of the formation of inter-subgenus hybridization barriers and improving the efficiency of inter-subgenus hybridization in water lily.
2 MATERIALS AND METHODS
2.1 Plant materials
This study used hardy water lily (Nymphaea. ‘Peter Slocum’) and tropical water lily (Nymphaea. ‘NangKwaug Fah’) as experimental materials. The water lily plants were preserved in the Bai Ma Education Demonstration Base of Nanjing Agricultural University, China. There are two main reasons for choosing these two cultivars for this study. Firstly, both cultivars have high fruit set rates and good pollen vitality, eliminating the influence of fruit set rates and pollen vitality in the experiment. Secondly, both cultivars exhibit obvious pre-fertilization hybridization barriers. N. ‘Peter Slocum’ and N. ‘NangKwaug Fah’ were used as the female and male parents, respectively. The hybridization was conducted following the methods described by Sun et al. (2018, 2019).
2.2 Phytohormone detection by LC–MS/MS
The stigma of water lily was collected 6 h after pollination, including self-pollination (SP), cross-pollinated in the same subgenus (CP), and inter-subgenus cross-pollination (ISCP) treatments. An unpollinated sample (Mock) was used as control. Each sample had 4 biological replicates. After being flash-frozen in liquid nitrogen, the samples were stored at −80°C. The levels of ABA, IAA, GA, JA, SA, and BR, which belong to the six major classes of hormones, were detected in the stigmas of water lily.
The stigmas were frozen in liquid nitrogen, ground into fine powder, and 50 mg of powder was taken for extraction in a mixture of methanol:water:formic acid (15:4:1, v/v/v). The extraction solution was filtered through a 0.22 μm PTFE membrane (Anpel) and then analyzed using LC-ESI-MS/MS (Hui et al. 2018). The obtained mass spectrometry data were qualitatively analyzed by establishing the MWDB plant hormone database based on relevant standards (Šimura et al. 2018). The hormone content detection experiment was conducted at Suzhou Panomic Bio-medical Technology Co., Ltd..
2.3 Hormone treatments and pollen tube visualization
The stamens of the first day blooming waterlily flower were removed, and then 30 μM ABA, 0.3 mM GA or 0.5 μM BR were added into the pistil fluid of the waterlily flower. The hormone concentrations used in the experiment were modified by reference to the hormone treatment concentrations in previous articles (Kovaleva et al. 2005, Kovaleva et al. 2017, Breygina et al. 2023, Wang et al. 2023). After about 10 minutes of exogenous hormone application, pollination was performed.
Six hours after pollination, the stigmas were fixed in FAA fixative for 6 hours, then soften overnight in 1 mM NaOH. The softened stigmas were stained with aniline blue using a previously detailed experimental protocol (Duan et al. 2014, Su et al. 2020, Zhang et al. 2021) and observed under a fluorescence microscope at a wavelength of 405 nm. Images were captured using a DS-Ri2 digital camera.
For the calculation of fruiting percentage, we did 3 independent experiments, each consisting of 6 independent pollination events. The ratio of the number of water lily fruits obtained in each experiment to the total number of flowers in the treatment was calculated as the fruition rate. For the calculation of the number of seeds, we had six replications for each treatment and went through three experiments. The average number of mature seeds harvested from each water lily fruit in each trial was calculated.
2.4 Antioxidant enzyme activity assay
For the determination of antioxidant enzyme activity, 1 mL of ice-cold 25 mM HEPES buffer (pH = 7.8) containing 0.2 mM EDTA, 2 mM ascorbic acid and 2% polyvinylpyrrolidone was used to extract total protein from 0.1 g of waterlily stigmas. The homogenate was centrifuged at 12,000 g for 20 min at 4°C, and then the supernatant was carefully collected for enzyme activity assays. The enzyme activities of superoxide dismutase (SOD), peroxidase (POD) and catalase (CAT) were measured. The measurement of enzyme activity follows the method described by Wang et al. (2011).
2.5 RNA extraction and sequencing
The samples used in this experiment were the same as the samples described in the hormone detection. The FastPure Universal Plant Total RNA Isolation Kit (Novazyme Biotechnology) was used to extract total RNA from plants, following the instructions of the kit. The integrity of the total RNA was determined by 1% agarose gel electrophoresis. The integrity and concentration of the RNA were accurately measured using the Agilent 2100 RNA Nano 6000 Assay Kit (Agilent Technologies). The detection indicators included RIN value, 28S/18S ratio, presence of baseline uplift in the profile and 5S peak. RNA-seq was performed by Suzhou Panomic Bio-medical Technology Co., Ltd (Suzhou, China) on the Illumina Novaseq6000 platform.
2.6 RNA sequencing data processing
After filtering adapter sequences and low-quality regions, the clean reads were aligned to the N. colorata genome using HISAT2 (Kim et al. 2015, Pertea et al. 2016). The gene expression levels for each sample were calculated using featureCounts (Liao et al. 2014) as FPKM (fragments per kilobase of exon per million mapped fragments). An expression matrix was generated using Trinity to assess differential gene expression among different samples (Grabherr et al. 2011). To identify enriched differentially expressed genes (DEGs) in terms of GO terms and metabolic pathways, functional enrichment analyses, including GO and KEGG, were performed relative to the whole transcriptome background using a bonferroni-corrected P-value threshold of <0.05. For GO functional enrichment and KEGG pathway analysis, the R package ClusterProfiler was utilized (Wu et al. 2021).
2.7 Co-expression network analysis
We performed co-expression network analysis of hormones and gene expression using the weighted gene co-expression network analysis (WGCNA) R package (Langfelder and Horvath 2008). To determine the relationship between gene expression and hormone levels, we analyzed the normalized gene expression data along with the levels of ABA, GA3, Me-JA, SAG, and BR. We visualized module correlations and co-expression networks using the LinkET R package and Cytoscape software (v3.9.1) (Smoot et al. 2011, Huang 2021). The obtained genes were further analyzed for correlation with genes involved in hormone synthesis, and annotation of the final genes was performed using hmmscan. Based on the results of qRT-PCR, we selected the most feasible genes for follow experiments (Johnson et al. 2010).
2.8 Quantitative real-time PCR
HiScript III RT SuperMix for qPCR (gDNA wiper) kit (Novazyme Biotechnology) was used to synthesize the first strand of cDNA. 1 μg total RNA was used in each 20-μL reaction. The cDNA products were diluted 10 times with deionized water before use. qRT-PCR experiments were conducted on a CFX96 Touch™ Real-Time PCR detection system (Bio-Rad) using the ChamQ SYBR qPCR Master Mix kit (Novazyme Biotechnology). Each reaction used 2 μL of diluted cDNA, and other reaction components and conditions were carried out according to the manufacturer's instructions. Specific primers were designed for qRT-PCR (Table S4). The ACTIN11 gene of waterlily was used as the internal reference gene (Luo et al. 2010), and three biological and three technical replicates were performed for each treatment. The relative expression levels were calculated using the 2−ΔΔCt method (Livak and Schmittgen 2001, Xu et al. 2012).
2.9 Oligonucleotide design and treatment
Design positive (sense phosphorothioate oligodeoxynucleotides, S-ODN) and antisense (antisense phosphorothioate oligodeoxynucleotides, AS-ODN) oligonucleotides based on the cDNA sequence of NCED2 using the Sfold database (http://sfold.wadsworth.org/cgi-bin/soligo.pl) (Su et al. 2020, Zhao et al. 2020, Zhang et al. 2021, Li et al. 2022)(Table S5). Assess potential off-target effects using the BLAST program (https://blast.ncbi.nlm.nih.gov/Blast.cgi). The 5′ and 3′ ends of S-ODN and AS-ODN were modified with thiol groups to maintain stability. S-ODN and AS-ODN were synthesized by Beijing Tsingke Biotech Co., Ltd.
Before ODN treatment, the stamens were removed from selected water lily flowers first. Then, the designed ODN (the secretions from water lily stigmas were diluted to a concentration of 20 μM) was added to the stigmas of water lily flowers. After 1 h of ODN treatment, the pollination procedure was performed.
2.10 Statistics
For the analysis of intergroup significant differences, a one-way analysis of variance (ANOVA) was used. When P < 0.05, Tukey's test was employed for post-hoc comparisons. Two-group comparisons were conducted using a two-tailed Student's t-test. All analyses were performed using GraphPad Prism 8 software.
3 RESULTS
3.1 Hormones play an important role in pollen and stigma recognition in water lily
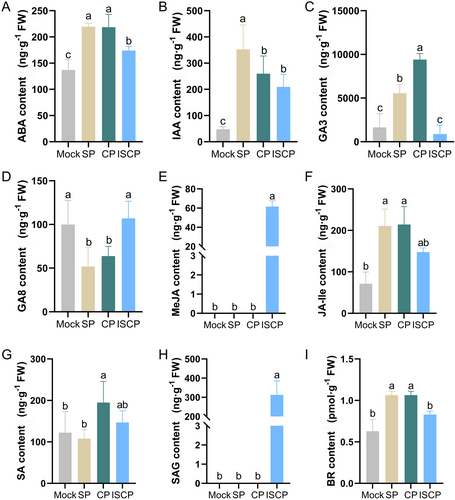
To understand the regulation of plant hormones on pollination in water lily, we analyzed the hormone levels in the stigma (Table S1). The results revealed a significant accumulation of ABA, GA3, jasmonic acid-isoleucine (JA-lle) and BR in stigmas from self-pollination (SP) and cross-pollination with the same subgenus (CP). In contrast, the levels of these four hormones were lower in the unpollinated (Mock) stigma and stigma from inter-subgenus cross-pollination (ISCP) (Figure 1). Interestingly, the content of GA8 in the stigma exhibited an opposing trend to that of GA3. This could be attributed to the conversion of active gibberellins to inactive gibberellins in the stigma after Mock and ISCP pollination. Methyl jasmonate (MeJA) and glycosylated salicylic acid (SAG), which are closely associated with biotic stress, were only detected in ISCP-pollinated stigmas. This suggests that certain regulatory networks associated with disease resistance in water lily stigmas were activated after ISCP treatment (Figure 1). In addition, other hormones such as IAA, SA and JA appeared to have no direct correlation with the different pollination systems (Figures 1 and S1; Table S1).
3.2 ABA and BR treatments increase fruit set after hybridization between water lily subgenera
To further investigate the role of hormones in the pollination hybridization process of water lily, we applied ABA, GA and BR onto the ISCP-pollinated stigmas. Six hours after pollination, we collected the stigma and observed the pollen tubes' germination. The results showed a significant germination of pollen tubes on stigmas treated with SP and CP, while no pollen tube germination was observed on stigmas treated with ISCP (Figure 2A,B). When exogenous ABA and BR were applied, we observed that they promoted the germination of pollen tubes on ISCP-pollinated stigmas, while GA treatment did not (Figure 2A,B).
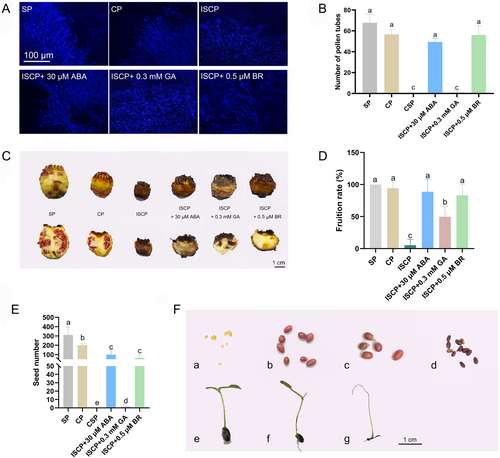
To further understand the relationship between ABA, BR, GA and the fruit set rate in water lily hybridization, we collected the hybrid fruits of water lily 25 days after pollination. The results revealed that water lily treated with SP and CP successfully produced fruits with a high fruit set rate, and the seeds developed normally. However, water lily treated with ISCP did not show fruit enlargement and experienced rotting. Interestingly, when ABA and BR were applied to water lily's ISCP-pollinated stigmas, it partially prevented the rotting of the ovary, significantly improving the fruit set rate and yielding a certain number of hybrid seeds. On the other hand, GA treatment, despite not promoting pollen tube germination on ISCP-pollinated stigmas, stimulated the enlargement of water lily ovaries, resulting in an increased fruit set rate. However, the majority of seeds in the fruits showed abortion (Figure 2C-E).
The germination experiment was conducted on the obtained seeds, it was found that the SP- and CP-derived seeds exhibited a higher germination rate. Additionally, the hybrid seeds obtained through ISCP with exogenously applied ABA and BR were also able to germinate successfully (Figure 2F). However, compared to the seeds obtained through SP and CP, the hybrid seeds obtained through ISCP required a longer germination time and exhibited a significantly slower plant growth rate (Figure 2F).
3.3 ABA and BR synergistically promote antioxidant enzyme activities in water lily stigmas
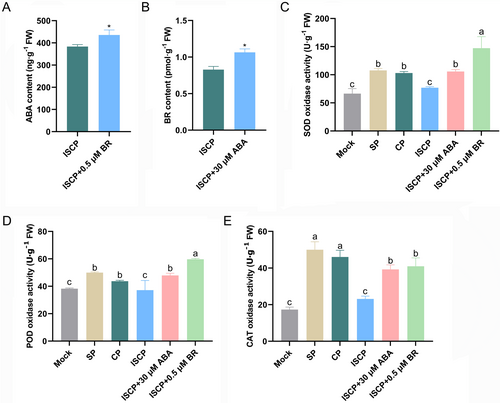
Reactive oxygen species (ROS) play a significant role in regulating the interaction between water lily pollen and the stigma as also previously demonstrated (Sun et al. 2019, Sun et al. 2023). SOD, POD and CAT are crucial antioxidant enzymes that directly regulate ROS accumulation (Kao et al. 2018). To gain further insights into how ABA and BR promote pollen germination on ISCP water lily, we assessed the activity of antioxidant enzymes in the stigma following pollination. Our results showed that higher SOD, POD and CAT activity were observed in the SP and CP stigmas than in the in the Mock and ISCP stigmas. Furthermore, exogenous application of ABA and BR on the ISCP stigma promoted the enzyme activity of SOD, POD and CAT (Figure 3C-E). As studies have shown that there is a synergistic regulation between ABA and BR (Li et al. 2021, An et al. 2023), we further measured their levels in the stigma. The results revealed that BR application on the ISCP stigma promoted the accumulation of endogenous ABA content, while ABA application on the ISCP stigma increased the endogenous BR content (Figure 3A,B). This suggests a potential synergistic regulation of ABA and BR in the process of hybridization in water lily.
3.4 Transcription profiles of water lily stigmas from different pollination combinations
In order to gain a deeper understanding of the specific mechanisms underlying the inter-subgeneric hybridization barriers in water lily, we utilized RNA-seq technology to investigate the gene transcription levels in Mock, SP, CP and ISCP stigmas. The experimental design consisted of four sample groups, with four independent biological replicates for each sample, resulting in a total of 16 RNA-seq libraries. On average, each library generated 45.81 million single-end reads, ranging from 41.76 million to 50.44 million. The average number of clean reads was 43.92 million, ranging from 40.06 million to 49.56 million. Among these reads, 97.81% had a base quality score of Q20, and 93.65% had a base quality score of Q30 (Table S2). Principal component analysis (PCA) based on FPKM values revealed that the first principal component (PC1) explained 40.8% of the variance in the dataset, while the second principal component (PC2) explained 25.3% of the variance. The Mock, SP, CP, and ISCP treatments formed distinct clusters, with the SP and CP groups exhibiting relatively closer clustering, and the clustering of all biological replicates was well-differentiated (Figure S2). These data indicate the reproducibility and reliability of the RNA-seq data.
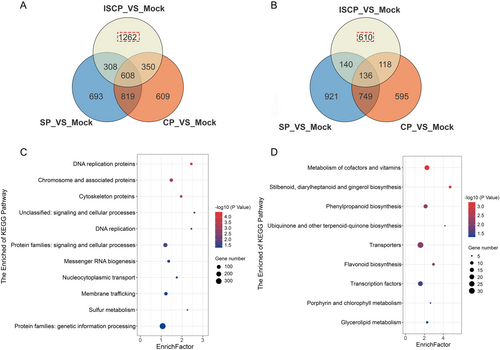
Venn plot analysis revealed that in the ISCP_vs_Mock comparison, there were 1262 upregulated differentially expressed genes (DEGs) and 610 downregulated DEGs that were unique to this group (Figure 4A,B). These DEGs may play an important role in the inter-subgeneric hybridization barriers in water lily. To gain further insights into the functions of these 1872 DEGs, we conducted GO and KEGG analyses. The KEGG results showed that the 1262 upregulated genes were mainly involved in signal transduction, membrane transport, and cellular cytoskeleton (Figure 4C). Additionally, the GO analysis indicated that the upregulated genes were mainly enriched in kinase activity and signal transduction, with Ca2+ transport and Ca2+ signaling exhibiting significant enrichment (Figure S3A). On the other hand, the 610 downregulated genes were primarily enriched in secondary metabolites, such as phenylpropanoid biosynthesis and flavonoid biosynthesis (Figure 4D). The GO analysis revealed that the downregulated genes were mainly enriched in processes such as abscisic acid metabolism, carotenoid metabolism, and protein phosphorylation (Figure S3B). DEGs in SP_vs_Mock and CP_vs_Mock cross groups are considered to be keys in promoting pollen germination on the water lily's stigma. Through GO and KEGG enrichment analysis, we found that the 819 DEGs upregulated were mainly related to carbohydrate synthesis and metabolic process, hormone synthesis and the synthesis of some secondary metabolites related to antioxidants (e.g., flavonoids, carotenoids, etc.) (Figure S4A,B). The 749 down-regulated DEGs are mainly associated with signaling, cell wall formation, and biological stress (Figure S4C,D).
3.5 Co-expression network analysis and key gene identification
The above results indicated that the changes in hormone levels play a significant role in regulating the formation of inter-subgeneric hybridization barriers in water lily. To understand the regulatory mechanisms behind the variations in hormone levels, we performed weighted gene co-expression network analysis (WGCNA) using the changes in ABA, GA3, MeJA, SAG and BR contents as phenotype data. The genes were divided into 26 co-expression modules using the WGCNA package (Figure S5 A,B,C). Genes within the same module exhibited highly similar expression patterns and were considered to be tightly co-regulated. The correlation between the phenotype data and the modules was visualized using the LinkET R package (Figure 5A). The results showed that the magenta, turquoise, blue and yellow modules were positively correlated with MeJA and SAG contents, while the lightcyan module was negatively correlated with MeJA and SAG contents. Modules associated with ABA, GA3 and BR contents exhibited high similarity, such as the yellow, tan, black, brown and darkturquoise modules, which were positively correlated with these three hormones. On the other hand, the green, turquoise, blue and orange modules showed negative correlations with ABA and BR contents (Figures 5A and S5D).
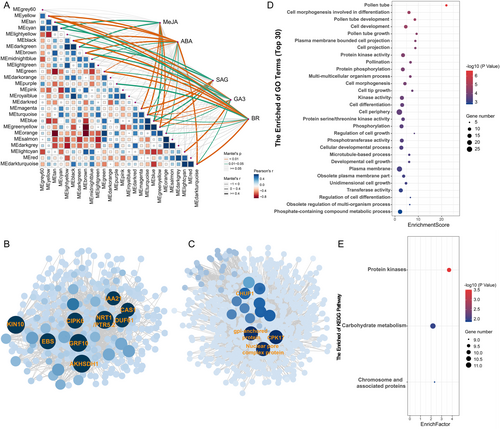
It is interesting that two modules, blue and turquoise, showed correlations with all five hormones (Figure 5A). Therefore, we selected the blue and turquoise modules for further analysis. GO and KEGG enrichment analysis revealed that genes in the blue module were mainly enriched in cellular cytoskeleton movement, signal transduction, kinase activity, and phosphorylation (Figure S6A,C). On the other hand, genes in the turquoise module were primarily enriched in regulating pollen tube growth, organ development, cell growth and kinase activity (Figure S6B,D). We constructed a gene regulatory network using WGCNA and visualized the network using Cytoscape software (Figure 5B,C). We extracted the genes with the most connections, referred to as hub genes, and performed GO and KEGG analysis on them. The GO enrichment analysis showed that these hub genes were primarily enriched in regulating pollen tube growth, cell development, kinase activity, and phosphorylation (Figure 5D). KEGG enrichment analysis revealed enrichment in protein kinases, carbohydrate metabolism, and chromatin-related proteins (Figure 5E). In addition, we performed GO and KEGG enrichment analysis for DEGs in the module with significant positive correlation between MeJA and SAG content. The results showed that these DEGs were mainly related to flavonoid synthesis, pathogen defense and hormone signaling (Figure S7A,B). These clues suggested that when stigmata is exposed to incompatible pollen, stigmata misinterprets the pollen as a foreign invading microorganism, and then activates the expression of genes related to biological stress response, and rejects the incompatible pollen.
3.6 Identification of genes involved in hormone biosynthesis
To further understand the relationship between the obtained hub genes and the levels of these five hormones (ABA, GA3, BR, MeJA, and SAG), we analysed the expression of structural genes involved in their biosynthesis (Figure 6A) and performed a correlation analysis with the hub genes. The results revealed that key genes involved in ABA biosynthesis, named ZEP, NCEDs, ABA2.1, and AAO3, exhibited high expression levels in the stigmas of SP and CP, which was consistent with the observed changes in ABA content. KAO, GA3ox and GA20X1 were identified as potential important factors contributing to the accumulation of GA3 in the stigmas of SP and CP. Within the BR synthesis pathway, most structural genes, such as CYP90A1 and CYP85A1/2, were directly associated with the accumulation of BR in the stigmas of SP and CP. The specific expression of LOX and OPCL in ISCP stigma is the main reason for the specific presence of MeJA in ISCP-pollinated stigmas. In the SA synthesis pathway, both the branched-chain pathway and the phenylalanine pathway appeared to participate in regulating the synthesis of SAG in ISCP stigmas. Genes such as ICS in the branched-chain pathway and PAL1.4 in the phenylalanine pathway were identified as potentially important factors leading to the specific accumulation of SAG in the transmitting tract of the pistil.
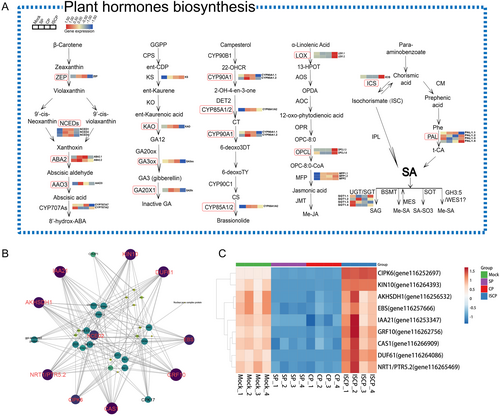
The top 13 genes with the highest connectivity in the blue and turquoise modules were subjected to correlation analysis with the structural genes involved in the synthesis of the five hormones (Figure 6B). The results revealed that the expression of nine genes was highly correlated with the expression of the structural genes involved in the synthesis of the five hormones. Additionally, we observed that NCED2 had the highest number of connections among all the structural genes, suggesting that NCED2 may be a key node gene for the regulation of these nine candidate genes. Visualization of the expression of these nine genes showed that their expression was low in the stigmas of SP and CP, but high in the stigmas of Mock and ISCP (Figure 6C).
To further validate the response of candidate genes to different pollination in water lily, qRT-PCR was used to confirm the expression of the candidate genes. The results showed that the expression of most genes was consistent with the transcriptome results (Figure 7). However, there were still some differences between the expression levels of some genes and the transcriptome data, such as NCED1, CIPK6, KIN10, and GRF10. Interestingly, CIPK6 and KIN10 are specifically expressed in the stigma of ISCP, which seems to suggest that these two genes play an important role in promoting the formation of intergeneric hybridization disorder of waterlily.

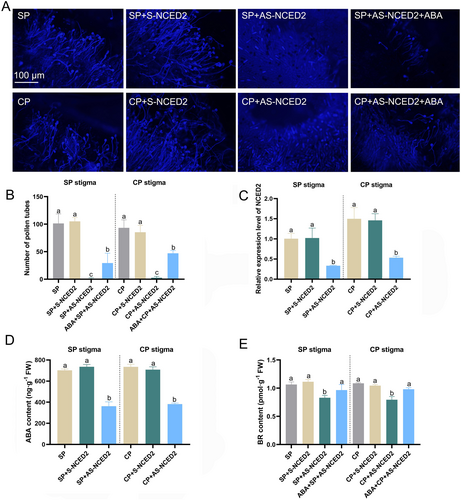
3.7 Silencing of NCED2 on SP and CP stigmas inhibits pollen tube germination
The high connectivity of NCED2 among all the structural genes in the network caught our interest. We further investigated the expression of NCED2 in the stigmas of SP and CP. The antisense oligonucleotide (ASO)-mediated was used to transiently silence the expression of NCED2 (Figure 8C). When NCED2 was silenced, the abundant pollen tube germination on the stigmas of SP and CP disappeared (Figure 8A,B), accompanied by a decrease in ABA content (Figure 8D). However, when exogenous ABA was applied, pollen tube germination resumed on the stigmas (Figure 8A,B). These results indicated that NCED2 is an important rate-limiting enzyme in ABA synthesis in the water lily pistil and that NCED2 promotes pollen germination by increasing ABA accumulation on the stigmas. In addition, the BR content on the stigma of SP and CP water lily was also downregulated after NCED2 silencing. Interestingly, the BR content could be restored by exogenous ABA treatment (Figure 8E).
4 DISCUSSION
Plant hormones play important regulatory roles in the pollination and fertilization processes of plants, and research in this area has received widespread attention (Yang et al. 2022). Previous studies have shown that when the stigma receives compatible pollen, hormones related to growth and development significantly increase, such as auxin, gibberellins (GA), and brassinosteroids (BR) (Wu et al. 2008, Ye et al. 2010, Shi et al. 2017, Qian et al. 2021, Wang et al. 2023). On the other hand, when the stigma receives incompatible pollen, hormones associated with plant stress tolerance significantly increase, such as ethylene, jasmonic acid (JA) and salicylic acid (SA) (Shi et al. 2017, Jegadeesan et al. 2018, Liu et al. 2022). In order to reveal the relationship between inter-subgeneric hybridization barriers and hormones in water lily, comprehensive transcriptomic and plant hormone analyses were conducted on the stigmas pollinated with Mock, SP, CP or ISCP. Additionally, we investigated the role of hormones in regulating inter-subgeneric hybridization barriers in water lily, with particular emphasis on the importance of ABA in overcoming these barriers (Figures 2 and 8).
The germination and development of plant pollen are closely regulated by hormones (Calabrese and Agathokleous 2021). IAA and ZR play a favorable role in the affinity between pollen and pistil (Yang et al. 2009). IAA plays an important role in promoting pollen tube growth (Wu et al. 2008). ZR, as the main natural active ingredient of cytokinins, is closely related to plant fertilization (Yang et al. 2022). Higher IAA, ZR levels and (IAA + ZR) / ABA levels favored pollination, while lower ABA levels were detrimental during pollen-stigma recognition, adhesion and germination (Yang et al. 2022). In the process of hybrid breeding in water lily, the difficulty of pollen germination on the stigma is the main factor leading to inter-subgeneric hybrid barriers (Sun et al. 2018, Sun et al. 2019, Hao et al. 2022, Sun et al. 2023). Spraying exogenous hormones can be used to promote and increase the success rate of pollination, ensuring the progress of hybrid seed production (Daza et al. 2021).
Our results seemed to be consistent with the findings described above. Hormones associated with growth and development mainly accumulate in SP and CP stigmas, such as GA3 and BR. However, hormones accumulated in ISCP stigma are mainly related to biotic stress, such as MeJA and SAG (Figure 1). Interestingly, in our results, ABA appears to positively regulate the affinity of pollen and stigma in waterlily, mainly accumulating in SP and CP stigmas (Figure 1). Studies have shown that maintaining a steady-state level of ABA is important for pollen germination and promoting pollen growth on the stigma (Lu et al. 2023). Higher levels of ABA hinder the normal development of the anther, thus reducing male fertility (Oliver et al. 2005, Oshino et al. 2007, Sharma and Nayyar 2014). Increased levels of ABA in anthers lead to excessive accumulation of ROS, causing oxidative damage to anther tissues and disrupting the normal process of programmed cell death (PCD) in the chorioallantoic layer, which in turn affects the fertility of males (Sinha et al. 2021), while lower levels of ABA resulted in abnormal pollen development, thus affecting fertility (Kim et al. 2020, Lu et al. 2023). Endogenous ABA negatively affects pollen germination (Kovaleva et al. 2005), but exogenous ABA promotes pollen germination and pollen tube elongation in P. hybrida L self-compatible clones (Kovaleva et al. 2017). By exogenously applying ABA, GA and BR to ISCP stigmas, we found that both ABA and BR treatments can promote pollen germination on ISCP stigmas and facilitate the production of seeds through inter-subgeneric hybridization in water lily (Figure 2). Previous studies have shown that low concentrations of ABA can promote BR accumulation. In addition, BZR1, a key transcription factor for the corresponding BR signaling, has also been reported to promote ABA accumulation (Li et al. 2021, An et al. 2023). Our results showed that exogenous application of ABA could promote the accumulation of BR in the stigma of water lily and, vice versa, exogenous application of BR could also promote the accumulation of ABA in the stigma of water lily (Figure 3A,B). Therefore, we believed that ABA and BR could synergically promote the activity of antioxidant enzymes. They helped to alleviate ROS accumulation on ISCP stigmas by promoting the activities of antioxidant enzymes such as SOD, POD and CAT, thus breaking the inter-subgeneric hybridization barriers in water lily (Figure 3).
Pollen-pistil recognition involves complex signal communication, which is the first step for pollen germination on the stigma, and ultimately leads to successful plant hybridization (Chapman and Goring 2010, Jany et al. 2019, Johnson et al. 2019). To further understand the recognition mechanism between pollen and stigma during water lily hybridization and uncover the regulatory mechanisms involved in inter-subgeneric hybridization barriers in water lily, we conducted RNA-seq analysis of water lily stigmas unpollinated or pollinated with SP, CP or ISCP treatments. A total of 1262 genes specifically upregulated in the stigma of ISCP treatment and 610 genes specifically downregulated in the stigma of ISCP treatment were identified (Figure 4A,B).
Among the 1262 upregulated genes, their functions are mainly enriched in signal transduction pathways, such as Ca2+ transport, Ca2+ signal transduction and kinase activity. Conversely, the downregulated genes are primarily enriched in the synthesis of certain metabolites, including flavonoid synthesis, carotenoids and ABA synthesis (Figures 4C,D and S3). Calcium (Ca2+) serves as a crucial regulator in mediating multiple signal transduction pathways involved in pollen germination and pollen tube growth (Nie et al. 2023). In pear hybridization, disruption of cytoplasmic Ca2+ oscillations in incompatible pollen and disturbance of the focused Ca2+ gradient at the tip of developing pollen tubes severely impair its polar growth (Jiang et al. 2014, Qu et al. 2016). High levels of Ca2+ can induce programmed cell death events in incompatible pollen on the stigma of Papaveraceae (Wilkins et al. 2014). In Brassicaceae, the self-incompatibility response triggered an immediate increase in Ca2+ concentration within the stigma, promoting extensive callose deposition, thereby suppressing the germination and pollen tube elongation of incompatible pollen (Iwano et al. 2015). These findings suggested a negative regulatory role for Ca2+ in the communication process between pollen and the stigma. Interestingly, in our findings, genes associated with flavonoid synthesis, carotenoid synthesis, ABA synthesis and other related pathways showed a downregulation, and these metabolites are involved in the clearance of ROS.
Previous studies have suggested that the high accumulation of ROS in the stigma of water lily is an important factor contributing to reproductive barriers in inter-subgeneric hybrids (Sun et al. 2019, Sun et al. 2023). Furthermore, elevated ROS levels have also been shown to promote self-incompatibility and cross-incompatibility in other species. For example, in Brassicaceae, the levels of ROS in the stigma play a role in regulating both self-incompatibility and interspecies hybrid barriers (Zhang et al. 2021, Huang et al. 2023). In Arabidopsis, variations in ROS levels in the stigma directly impact pollen hydration and germination on the transmitting tract cells (Liu et al. 2021). Therefore, we propose that the accumulation of ROS mediated by Ca2+ signaling in the stigma of water lily may be one of the key factors contributing to the formation of reproductive barriers in inter-subgeneric hybrids. Further, WGCNA analysis was performed and two modules, blue and turquoise, were screened from 26 expression modules (Figure S5). These two modules showed a significant correlation with the content of MeJA, SAG, ABA, GA3 and BR (Figure 5A). Nine candidate genes (CIPK6, KIN10, IAA21, AKHSDH1, EBS, DUF61, NRT1/PTR5.2 and CAS1) were finally obtained by correlation analysis. Among these genes, Ca2+ signaling (CIPK6), AUX response (IAA21), and hormone transporter association (NRT1/PTR5.2) may play a more important role. Furthermore, these results provide further evidence for the significance of Ca2+ and hormone signaling in the formation of hybridization barriers in waterlily. In previous studies, CIPK6 and KIN10 have been reported to be involved in regulating ABA synthesis and ABA signaling processes. For example, in Arabidopsis and Brassica napus, CBL-interacting protein kinase (CIPK6) can participate in the regulation of plant responses to salt stress due to drought by participating in ABA signaling (Chen et al. 2012, Chen et al. 2013). In the study of KIN10, it was found that overexpression of α subunit (KIN10) of SNF1-related protein kinase (SnRK1) in potatoes can promote the growth of buds on potato tubers by hindering ABA accumulation (Song et al. 2021, Wang et al. 2020). KIN10 can also participate in ABA signaling through interaction with SnRK2s, a key gene in ABA signaling (Belda-Palazon et al. 2020, Belda-Palazón et al. 2022). In addition, the CBL-interacting protein kinase family was also reported to interact with SnRK1 to participate in the response to ABA (Yan et al. 2014, Zhang et al. 2008). These clues suggest that CIPK6 and KIN10 may play an important role in ABA accumulation and ABA signaling in water lily stigmas, which provides a research direction for understanding the regulation of ABA on the formation of inter-generic hybridization disorder of waterlily.
NCED is a key rate-limiting enzyme in ABA synthesis that catalyzes the oxidative cleavage of 9-cis-violaxanthin or 9-cis-neoxanthin, producing xanthoxin in the plastids (Gupta et al. 2022). RNAi-mediated silencing of the NCED gene reduces ABA levels and inhibits pollen development in transgenic tomatoes (Dai et al. 2018). Interestingly, overexpression of NCED also suppresses pollen development, despite the increase in ABA levels, indicating that pollen development is sensitive to ABA homeostasis (Dai et al. 2018, Wang et al. 2021b). In our results, the reduction in ABA levels in the stigma of water lily (ISCP stigma) is an important factor contributing to reproductive barriers in inter-subgeneric hybrids. Additionally, NCED2 plays a crucial role in ABA synthesis in the stigma of water lily (Figures 6A,B and 7A). To investigate the regulatory role of NCED2 in reproductive barriers in water lily, we treated the SP and CP stigmas with antisense oligodeoxynucleotides (AS-ODNs), significantly reducing the expression of NCED2 (Figure 8D,E). Moreover, silencing of NCED2 hindered pollen germination on the stigma, while ABA treatment restored pollen germination (Figure 8A).
5 CONCLUSION
In this study, hormonal and transcriptomic analyses were performed on unpollinated stigmas, self-pollinated stigmas, cross-pollinated stigmas of the same subgenus and inter-subgenus cross-pollinated stigmas of water lily. Our results suggest that changes in hormone levels in water lily stigmas are key factors in the formation of inter-subgenus hybridization barriers in water lily, with changes in ABA and BR levels being the key breakthrough point in breaking the hybridization barrier between water lily subgenera. Nine potential regulatory genes were identified by WGCNA analysis, which are associated with Ca2+, hormone and other signaling. In addition, we verified that the low expression of NCED2 in ISCP stigmas is a key factor in the reduction of ABA in ISCP stigmas, as well as a key gene contributing to the formation of inter-subgenus hybridization barriers of water lily. This work provides a theoretical basis for understanding the mechanism of formation of inter-subgenus hybridization barriers in water lily. It also provides a direction to break the hybridization barrier among water lily subgenera.
AUTHOR CONTRIBUTIONS
Ping Zhou and Yingchun Xu conceived the original idea and designed the experiments; Ping Zhou, Jingwen Li, Huiyan Jiang, Zhijuan Yang and Hongyan Wang performed the experiments; Yanjie Wang provided the experimental materials; Ping Zhou analyzed the data and wrote the manuscript; Chunqing Sun, Qun Su and Qijiang Jin revised the manuscript. All authors have read and approved the manuscript.
ACKNOWLEDGMENTS
This work was supported by the National Natural Science Foundation of China (Grant No. U1803104 and U2003113), Hainan Natural Science Foundation (No. 2021JJLH0031, 322MS158 and 321RC670) and Guangxi Natural Science Foundation (No. 2022GXNSFBA035635). We would like to thank Suzhou Panomics Biomedical Technology Co., Ltd (Suzhou, China) for providing hormone and transcriptome testing services.
Open Research
DATA AVAILABILITY STATEMENT
The RNA-seq data can be obtained from the BIG Data Center (http://bigd.big.ac.cn) with the BioProject accession PRJCA018998.




