Integrated transcriptome and metabolome analysis reveals the podophyllotoxins accumulation and formation mechanisms in Juniperus sabina L. leaves
Abstract
Podophyllotoxin is an important secondary metabolite because of its remarkable anticancer and anti-Condyloma acuminatum activities. Juniperus sabina L. (J. sabina) has been proven as a new plant source of podophyllotoxins. However, the mechanism of podophyllotoxins accumulation and synthesis in J. sabina is unclear, which greatly limits their applications. Based on this, transcriptomic and metabolomic analyses were performed on different developmental stages of J. sabina leaves to determine the molecular mechanism of the biosynthesis of podophyllotoxins in this study. Firstly, 8 podophyllotoxins and 54 differentially accumulated compounds were identified from metabolomic analysis of J. sabina leaves at different developmental stages. The total lignans and podophyllotoxin contents were determined by HPLC, clarifying that May to September was the critical period for the accumulation of podophyllotoxins in J. sabina leaves. Transcriptome and metabolic analysis showed that 47 DEGs encoded 16 key enzymes involved in podophyllotoxin biosynthesis, among which 8 key enzyme genes (PAL, HCT, CCR, C4H, DIR, PLR, OMT and CYP82) were identified. Furthermore, JsPLR was proven to be a key rate-limiting enzyme cytoplasmic-localized. JsPLR overexpression significantly increased matairesinol and podophyllotoxin content.. The findings of qRT-PCR study indicated that the differential expression of JsPLR gene might be a key factor leading to the differential accumulation of podophyllotoxin. Overall, the study brings new insight into the accumulation and formation mechanisms of podophyllotoxins and the comprehensive development and utilization of J. sabina resources.
1 INTRODUCTION
Podophyllotoxin, a natural product, is an aryl tetrahydronaphthalene lignan derived from Berberidaceae plants and has been proven to be an effective anticancer drug (Guerram et al., 2012; Zi et al., 2019). In international research since the 1980s, the efficacy of etoposide produced from podophyllotoxin as a precursor chemical for the therapy of ovarian cancer, breast cancer, and small cell lung cancer has been proven (Wang et al., 2010; Qiu et al., 2019). With the increasing incidence of cancer, the market demand for etoposide will continue to increase (Canel et al., 2000). The huge commercial value of podophyllotoxin leads to large-scale illegal collection, which puts some Sinopodophyllum species on the verge of extinction (Nadeem et al., 2000; Kumari et al., 2017). As a classic example of creating novel medications from natural ingredients, podophyllotoxin now relies on natural extraction, and the supply–demand gap is growing.
More and more studies on podophyllotoxin metabolic pathway have been completed, thus providing a rough understanding of podophyllotoxin biosynthesis (Lau & Sattely, 2015). The biosynthesis of podophyllotoxins includes three stages. (1) First, coniferyl alcohol, the precursor of lignans, is synthesized from phenylpropane, and then (2) podophyllotoxins with diverse structures are synthesized from coniferyl alcohol. (3) Finally, podophyllotoxins are glycosylated and modified to form podophyllotoxin glycosides (Li et al., 2018).
In detail, phenylalanine is first converted into coniferyl alcohol in several steps, which is a synthetic precursor compound for podophyllotoxin (Tsuyama et al., 2013). Direct protein oxidase (DIR) catalyzes the dimerization of two coniferyl alcohols to form (+)-pinoresinol (Seneviratne et al., 2015). The enzyme pinoresinol-lariciresinol reductase (PLR) may catalyze the conversion of (+)-pinoresinol to (+)-lariciresinol and then to (−)-secoisolariciresinol, which is the major rate-limiting enzyme in the manufacture of podophyllotoxins (Dinkova-Kostova et al., 1996). (−)-Secoisolariciresinol is catalyzed specifically by secoisolariciresinol dehydrogenase (SDH) to produce (−)-matairesinol (Xia et al., 2001). Marques et al. (2013) identified two Cytochrome P450s (CYPs), CYP719A23 and CYP719A24, that can catalyze (−)-matairesinol to (−)-pluviatolide. Finally, 2-oxoglutarate/Fe (II)-Dependent dioxygenase (2-ODD) catalyzes the closed-loop formation of (−)-deoxypodophyllotoxin from (−)-yatein C-1 and C-9 (Lau & Sattely, 2015). Among them, the enzyme that catalyzes the transformation of deoxypodophyllotoxin into podophyllotoxin has yet to be identified, and this step has become one of the bottlenecks in the study of podophyllotoxin biosynthesis pathway. Podophyllotoxins exist mainly in glycosylated form in plants due to the activity of glycosyltransferase (UGT) (Zhao et al., 2001).
Juniperus sabina is a plant rich in podophyllotoxins. At present, there are 22 podophyllotoxins identified in the leaves of J. sabina (Li et al., 2006). In previous research from our group, it was found that the highest podophyllotoxin content in J. sabina leaves was 9.268 mg g−1 DW (weight of dry sample), which is the highest podophyllotoxin content in Juniperus plants reported so far (Xu et al., 2022). J. sabina is abundant and its leaves are a potential plant source of podophyllotoxins. However, the accumulation and synthesis characteristics of podophyllotoxins in J. sabina leaves are still unclear, which restricts the understanding of the molecular mechanism of podophyllotoxins biosynthesis and hinders the application of J. sabina resources.
On that basis, this study intends to conduct a multi-omics joint analysis on podophyllotoxins of J. sabina at different developmental stages to clarify the content and accumulation characteristics of podophyllotoxins in J. sabina. Finally, the key genes of podophyllotoxins biosynthesis were excavated by multi-omics analysis. This study can provide a basis for the efficient utilization of J. sabina resources, the directional cultivation of J. sabina with high podophyllotoxin content and the creation of new germplasm, and can also provide a reference for the biosynthesis and regulation of podophyllotoxins in other Juniperus plants.
2 MATERIALS AND METHODS
2.1 Plant materials
Juniperus sabina leaves were collected from the Northwest Agriculture and Forestry University Expo Park (Yangling, Shaanxi, China, 34°18′3″E,108°2′56″N). In mid-March 2021, the newly grown leaves began to be sampled until February of the following year, and samples were collected in the middle of each month. At the same time, the new leaves of different branches of the same plant are marked to facilitate the collection of subsequent samples. In addition, three periods (May, July, September) with significant differences in podophyllotoxin accumulation during the growth period of J. sabina (leaf age of GA: 60 days, GB: 120 days, GC: 180 days) were selected (Figure S1), collected in centrifuge tubes, rapidly frozen with liquid nitrogen, and stored in an ultra-low-temperature refrigerator at −80°C. Three J. sabina plants were selected as biological replicates to collect leaves in each period (May, July, September).
2.2 Untargeted metabolomic profiling
Leaves of J sabina from different growth periods (GA: 60D, GB: 120D, GC: 180D) were used for metabolomic analysis (Figure S2). The UHPLC separation was carried out using an EXIONLC System (SCIEX), and the column was a Waters Acquity UPLC HSS T3 (2.1 mm x 100 mm, 1.8 μm, Waters, USA). The mobile phases were 0.1% formic acid-water solution (A) and acetonitrile (B) with the following gradients: 0–0.5 min, 2% B; 0.5–10 min, 2%–50% B; 10–11 min, 50%–95% B; 11–13 min, 95% B; 13–13.1 min, 95%–2% B; 13.1–15 min, 2% B. The column temperature was set at 40°C and the flow rate was 0.4 mLL min−1. The auto-sampler temperature was set at 4°C and the injection volume was 2 μL.
A SCIEX 6500 QTRAP+ triple quadrupole mass spectrometer equipped with IonDrive Turbo V ESI ion source was used for the mass spectrometry analysis in multiple reaction monitoring (MRM) modes. The ion source parameters were as follows: IonSpray Voltage: +5500/−4500 V, Curtain Gas: 35 psi, Temperature: 400°C, Ion Source Gas 1:60 psi, Ion Source Gas 2: 60 psi, DP: ± 100 V.
Analyst Work Station Software (Version 1.6.3, SCIEX) was used for all mass spectrometry data acquisition and quantitative analysis of target compounds. The MS conventer was used to convert the raw mass spectra into TXT format. Then the R program package was used for peak extraction and annotation in combination with a self-created database.
2.3 Quantification of podophyllotoxin
The extraction (technological conditions: RLM 1:40, 90% methanol, ultrasonic time 7 minutes, extraction time 1.5 h) and HPLC measurement of podophyllotoxin were performed according to the method reported by Xu et al. (2022). High-performance liquid chromatography (HPLC, Agilent 1260, Agilent Technologies Inc.) was used to make the determination using a C18 (250 mm × 4.6 mm, 5 m; Agilent Technologies Inc.) column. The mobile phases were methanol and a 2% formic acid-water solution with the following intervals: 10%–30% methanol (0–10 min), 30%–55% methanol (10–20 min), 55%–80% methanol (20–35 min), 80%–84% methanol (35–40 min), 84%–100% methanol (40–45 min). The HPLC parameters are as follows: the column temperature 35°C, flow rate 0.8 mL min−1, injection volume 20 μL, and the UV detection wavelength 290 nm.
2.4 Determination of total lignans
The total lignan content (TLC) of the extracts was calculated by UV spectrophotometry (Burcu et al., 2014). Briefly, serial dilutions of podophyllotoxin (PPT) were used to prepare standard calibration curves. The absorbance of extracts (1.25 mg mL−1) and podophyllotoxin dilutions was measured with a UV spectrophotometer (UV mini-1240, Shimadzu) at 290 nm. Analysis was performed by colorimetric assay (regression equation Y = 0.0037 X + 0.101; R2 = 0.9992; Y, absorbance at 290 nm; X, the content of total lignans, mg PPT mL−1 DW).
2.5 Transcriptome sequencing analysis
2.5.1 Preparation of cDNA libraries and sequencing by Illumina
Total RNA was extracted from J. sabina samples using an Omega RNA extraction kit (OMEGA R6827, Omega Biotek). The specific requirements for total RNA samples are as follows: OD 1.7 ≤ OD260/280 ≤ 2.1, RNA concentration > 400 ng μL−1, RIN (RNA integrity number) value ≥6.5, 28S/18S ≥ 1.0, to ensure that the quality of RNA samples meets RNA-Seq requirements. Following that, an aliquot (3 μg) of each was utilized to prepare cDNA. The sequencing library was created using the NEB Next UltraRNA Library PrepKit kit following the manufacturer's instructions (New England BioLabs, Ltd.). The library concentration was determined using the Step One Plus real-time PCR technique (Applied Biosystems Inc.). The construction and sequencing of the cDNA library was entrusted to Beijing Baimark Biotechnology Co. The cDNA library was submitted to 2 × 150 bp double-ended sequencing using the Illumina Hiseq high-throughput sequencing platform after passing the quality inspection, and three copies of each sample were selected for biological repetition.
2.5.2 Sequence assembly and functional notes
To produce high-quality sequences, the raw sequences were filtered to eliminate splice-containing reads and low-quality reads (clean reads). Due to the absence of a reference genome for J. sabina, the clean reads were merged to get the transcript sequences using Trinity v2.0.6, and the non-redundant sequences (unigene) were screened for further study.
For similarity comparison, the sequences of all assembled unigenes were examined using BLAST (E-value ≤10−5) with NR, KOG, COG, eggNOG 4.5, KEGG, and Swiss-Prot databases. KOBAS 2.0 and InterProScan were used to assess KEGG Orthology (KO) and Gene Ontology (GO) data for functional genes. Lastly, using HMMER (E-value ≤10−10), the predicted unigenes were matched to the Pfam database to acquire annotated details for each unigene.
2.5.3 Differential expression genes (DEGs) analysis
Each transcript's expression levels were estimated using a previously described scheme (Sun et al., 2019). For each sample, the levels of gene expression were assessed with RSEM (version 1.2.31). The mapped reads of individual transcripts were standardized to reads per kilobase (RPKM) values to calculate the differential expression level. DEGs were identified using the |log2 (fold changes)| ≥ 1 and p-value ≤0.05 criteria.
2.6 qRT-PCR analysis verification
Total RNA of J. sabina samples was extracted by an Omega RNA extraction kit (OMEGA R6827, Omega Biotek). Its concentration and purity were detected by Nanodrop 1000 (Nanodrop Technologies). According to the instructions of the FastKing RT Kit (with gDNase and KR116, Suzhou Yuheng Biotechnology Co., Ltd.), a reverse-transcription reaction was carried out to synthesize cDNA. The relative expression of genes was calculated using UBIQUITIN C (UBC) as an internal standard (Willems et al., 2008), and the primers were designed by Premier 5.0 (Table S1). Oligo 6.0 was used to verify the designed primers. The samples used in qRT-PCR analysis are the same as the sequencing samples, and the reaction system and degree are carried out following the instructions of the Biomarker 2 × SYBR Green Fast qPCR Mix fluorescence quantitative kit. The relative expression of each unigene was assessed using the 2 −ΔΔCt method (Lu et al., 2023).
2.7 Construction of JsPLR vector
The CDS sequence of J. sabina PLR (JsPLR), gene involved in podophyllotoxin synthesis, was obtained based on the transcriptome sequencing outcomes. JsPLR nucleic acid sequence was analyzed by TB Tools V1.098726 (a toolbox for biologists), and the complete amino acid coding region (ORF sequence) was selected. Using the target gene as a template, the overexpression (Cambia 2300-PLR; 35S: PLR) and subcellular localization (Cambia 2300-GFP-PLR; 35S: GFP-PLR) vectors was constructed by double enzyme digestion, and the constructed vector was transferred to Agrobacterium tumefaciens (GV3101, Table S2 lists the primers used).
2.8 Transient over-expression and subcellular localization
The constructed overexpression vector Cambia 2300-PLR was transformed into J. sabina leaves by injection. The control group was injected with a heavy suspension of Agrobacterium tumefaciens GV3101 transformed with the empty vector Cambia 2300. J. sabina leaves were collected at 0, 2, 4 and 6 days after transformation, and stored in the refrigerator at −80 °C for later use. After that, 50 mg of preserved leaves were taken, frozen and fully ground in a mortar with liquid nitrogen, RNA was extracted and the expression of genes related to podophyllotoxin synthesis was detected by qRT-PCR in the transgenic materials. At the same time, 1 g of freeze-dried powder of the above experimental materials leaves was extracted with methanol, and the volume was adjusted to 20 mg mL−1, and the content of podophyllotoxins was evaluated by HPLC. Three J. sabina plants of uniform growth were selected as replicates for each treatment group.
The vector Cambia 2300-GFP-PLR was transiently expressed in 45-day-old tobacco plants using a 1 mL syringe without needle removed. After 48 h, the GFP fluorescent signals were captured by a confocal laser scanning microscope (LSM510, Karl Zeiss) using an excitation light of 488 nm and an emission light of 500 to 530 nm. The GFP empty vector was used as a control.
2.9 Statistics and sequence analysis
The pearson correlation coefficient (r) was calculated using SPSS 16.0. Phylogenetic analysis was carried out using the bootstrap adjacency phylogenetic tree with 1000 bootstrap repetitions by MEGA 7.0. DNAMAN was used to align amino acid sequences.
3 RESULTS
3.1 Dynamic accumulation patterns of lignans and podophyllotoxins
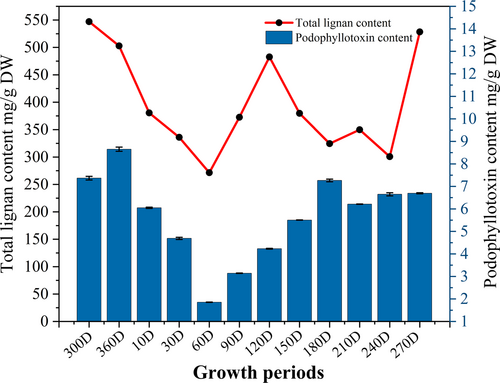
To clarify the annual accumulation law of podophyllotoxins in J. sabina, the contents of total lignans (TLC) and podophyllotoxin in different growth stages of J. sabina were dynamically detected. As shown in Figure 1, the contents of TLC and PPT in the leaves of J. sabina in different months were between 271.42 ~ 528.36 mg g−1 DW and 1.85 ~ 8.65 mg g−1 DW, respectively, and their contents changed in a “W” shape with the seasonal changes. From February to May, the accumulation of PPT decreased linearly, then increased linearly from May to September. From September to January, the accumulation of PPT in the leaves of J. sabina showed a steady trend and fluctuated slightly. Based on the above research, the critical period for the transformation and accumulation of PPT in J. sabina leaves is from May to September.
3.2 Metabonomics analysis of J. sabina leaves
The PLS-DA model scoring chart demonstrates that the three groups of samples (GA, GB, and GC) are highly separated (Figure. 2A). A comprehensive targeted metabolomics method was used to assess the dynamic distribution of cellular metabolites during lignans conversion in J. sabina leaves in order to better understand the changes in cellular metabolism. In total, 1022 cellular metabolites were discovered and divided into 20 different groups (Figure 2B, Table S1). 60% of these are phenols (17%), alkaloids (15%), terpenoids (14%), flavonoids (8%), and lignans and coumarins (6%). Fifty-four metabolites showed significant changes over the three periods and belonged to five classes: fifteen phenols and flavonoids, eight alkaloids, eight amino acids, five terpenoids, and two lignans and coumarins (Table S2). The accumulation of metabolites in the leaves of twenty species of J. sabina at different growth stages was assessed using stacked plots, with the area of the stacks reflecting the proportion of each compound in the total compounds (Figure 2C). The accumulation of flavonoids reduced at different stages of J. sabina growth, whereas the accumulation of phenols and lignans belonging to the phenylpropane metabolic pathway increased.
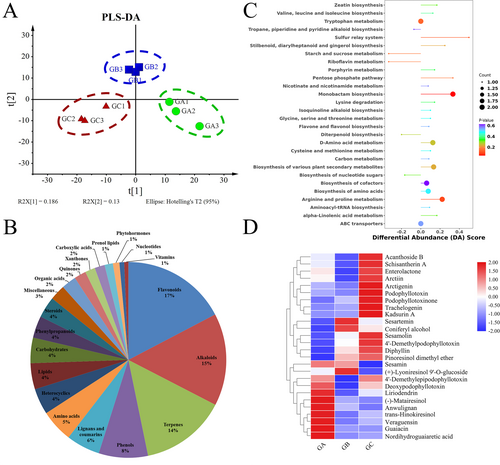
The samples in the GC group are clustered together, unlike the samples in the GA and GB groups (Figure S2). These findings suggested that the months of July to September may be critical for the accumulation and transformation of intracellular metabolites in J. sabina leaves. The results of Differential Abundance Score (DA Score) between GB group and GC group showed that ‘Arginine and proline metabolismproline’ and ‘Biosynthesis of various plant secondary metabolites’ are up-regulated, while primary metabolisms such as ‘Starch and sucrose metabolism’ and ‘Riboflavin metabolism’ are down-regulated (Figure 2D).
3.3 Analysis of related components of podophyllotoxin
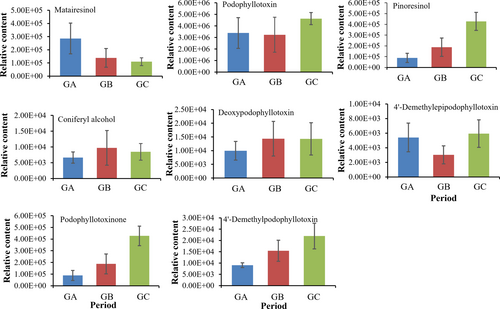
The linked components of podophyllotoxins were then investigated based on the metabolites acquired by metabolomics, and eight podophyllotoxins and its precursor compounds were found (Figure 3). The results of metabolic spectrum analysis showed that, compared with GA and GB groups, the contents of five podophyllotoxins (deoxypodophyllotoxin, podophyllotoxin, podophyllotoxin, epipodophyllotoxin and dimethyl-epipodophyllotoxin), and the precursor piniresinol were significantly increased in GC group. In addition, the precursors of podophyllotoxin synthesis, piniresinol and matairesinol, showed opposite accumulation characteristics, i.e., increased levels in the GA and GB groups and significantly decreased levels in the GC group. This may explain the high consumption of the precursor compounds as substrates during the accumulation of podophyllotoxins and, therefore, the decrease in content of both. In summary, May to September is the key period for the accumulation and transformation of secondary metabolites in J. sabina, which is consistent with our previous speculation.
3.4 Transcriptome profiles of J. sabina leaves
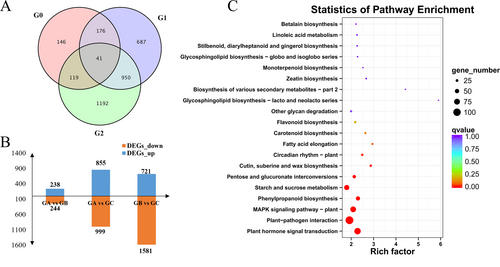
Three biological repeats of each sample were sequenced and nine cDNA libraries were constructed to better understand the gene expression profile of J. sabina at three different growth stages. This study collected 93.60 GB of clean data (Q30 > 93.42%), and the average GC content of all libraries ranged between 44.17% and 44.49%. These clean data were assembled into 100,642 unigenes, which corresponded to 172,545 distinct assembled transcripts. The average unigene length is 903.02 bp, and the N50 is 1,748 bp, indicating that the assembly integrity is high (Table S3). As a result, the analysis of DEGs is trustworthy. DESeq2 analysis was used to further investigate the potential genes involved in podophyllotoxin biosynthesis in J. sabina. There were 3311 DEGs found in G0 (GA vs GB), G1 (GA vs GC), and G2 (GB vs GC). The DEG statistics revealed the greatest differences in gene expression between the GB and GC, including 2302 DEGs (Figure 4A), of which 721 genes were up-regulated and 1581 genes were down-regulated (Figure 4B). The four pathways with the most DEGs in the combination of GA vs GB vs GC, according to KEGG enrichment analysis, were plant hormone signal transduction, plant-pathogen interaction, MAPK signal pathway-plant, and phenylpropane biosynthesis (Figure 4C).
3.5 Genes related to podophyllotoxin biosynthesis
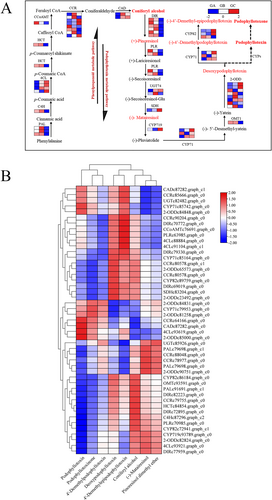
The current study identified differences in podophyllotoxin synthesis pathway in different developmental stages of J. sabina leaves by combining metabonomics and transcriptomics analysis, as reported by Lau and Sattely (2015). Transcriptomic analysis revealed 47 differential genes encoding 16 putative podophyllotoxin biosynthetic enzymes, the majority of which (PAL, C4H, HCT, CAD, DIR, PLR, and OMT1) showed up-regulated (GA and GB stages) and then down-regulated (GC stage) in J. sabina leaves. The high expression of these genes in GA and GB provided sufficient substrates for podophyllotoxins biosynthesis in J. sabina.
For further determining the key genes involved in podophyllotoxin biosynthesis, the Pearson correlation coefficient between the expression level of these genes and the relative level of podophyllotoxin metabolites was calculated (Figure 5B). The relative expression levels of PAL, HCT, CCR, C4H, DIR, PLR, OMT and CYP82 were correlated with the relative levels of most of the podophyllotoxin metabolites and their precursors in J. sabina leaves. These results indicated that these 8 structural genes may play a key role in the accumulation of podophyllotoxin metabolites in the leaves of J. sabina. Among them, PLR encodes the enzyme that catalyzes the two-step reduction reaction in the process of podophyllotoxin synthesis, and is the key rate-limiting enzyme in podophyllotoxin synthesis (Corbin et al., 2017).
3.6 qRT-PCR verification of differential expression
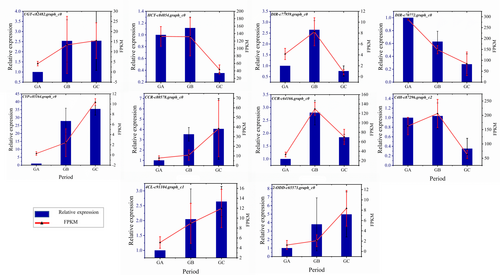
To validate the accuracy of transcriptome data, ten DEGs associated with podophyllotoxin synthesis were selected with UBC as internal reference gene for qRT-PCR verification. The validation results indicated that a high degree of concordance and association was demonstrated for the selected genes between qRT-PCR and transcriptomic datasets (Figure 6).
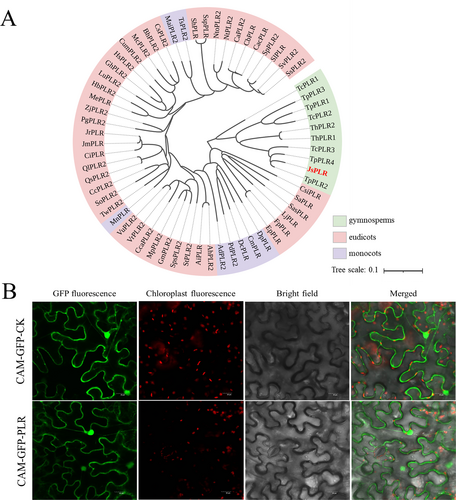
3.7 Phylogenetic tree construction and subcellular localization of JsPLR gene
The PLR transcripts from transcriptome sequencing results were combined with BLAST comparisons using the NCBI database in this study, and the top 62 similar sequences were chosen for phylogenetic tree construction (Figure 7A; sequence information referenced in Table S3). A phylogenetic tree indicated that the protein sequence of J. sabina PLR (JsPLR) was highly similar to its Cupressaceae homologue (TpPLR2), with a Per. Ident value of 94.23%. To investigate the subcellular localization of PLR, a the JsPLR-GFP fusion construct driven by the 35S promoter was transiently expressed in tobacco leaves. A strong GFP signal was localized to both the cytoplasm and the nucleus (Figure 7B), which, in combination with previously reported results, suggests that JsPLR is preferentially located in the cytoplasm (Chou and Shen, 2010).
3.8 Gene expression amounts and podophyllotoxins contents in transgenic J. sabina
JsPLR was transiently overexpressed into J. sabina (Figure 8A). After two, four and six days of expression, JsPLR expression level was measured by qRT-PCR, and confirmed that PLR expression level was higher in J. sabina leaves overexpressing JsPLR than in the control (8 times higher after 2 days; Figure 8B). A decline in expression occurred over time, with a significant decrease on day four, followed by a plateau. In addition, the content of podophyllotoxin and its related precursors in transgenic J. sabina leaves was determined by HPLC.
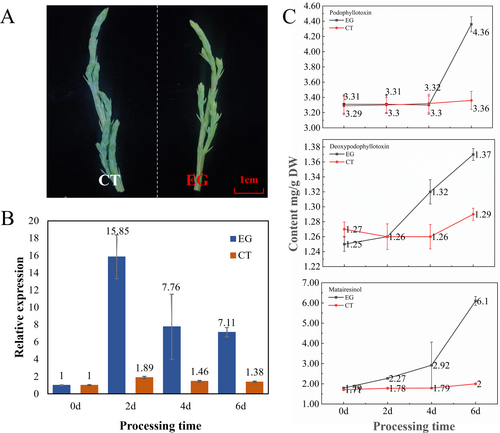
The findings showed that the content of podophyllotoxin, deoxypodophyllotoxin, and (−)-matairesinol increased significantly in JsPLR-overexpressing J. sabina leaves. The content of (−)-matairesinol showed a progressive increase on day two of the transgenesis, and the content increased by three times that of the control group on day six (Figure 8C). The levels of deoxypodophyllotoxin and podophyllotoxin (Figure S3) on day six were 1.06 and 1.30 times higher than those of the control, respectively. So, it is speculated that PLR gene plays a key role in the synthesis of podophyllotoxin from J. sabina.
3.9 Analysis of the expression pattern of the JsPLR gene
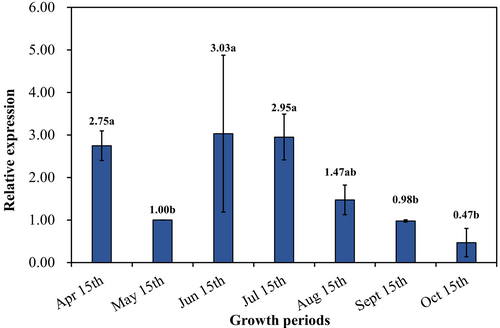
The expression of JsPLR gene in J. sabina leaves at different growth stages was quantified by qRT-PCR, and it showed a “V” pattern in accordance with the accumulation pattern of podophyllotoxin (Figure 9). The expression of JsPLR gene was higher in June, July and August than in May, indicating that the high expression of the gene during this period promoted the accumulation of the synthesis of the podophyllotoxins, while the expression of the gene decreased significantly in September and October, when the J. sabina entered the late growth period, and the accumulation of podophyllotoxin during this period showed a stable trend. The above results show that the expression of JsPLR gene was positively correlated with the accumulation of podophyllotoxins in J. sabina leaves.
4 DISCUSSION
J. sabina is an evergreen plant, and its perennial characteristics make its metabolite variation complicated (Bielecka et al., 2019). Podophyllotoxins are the main anticancer medicinal components in J. sabina leaves (Zheljazkov et al., 2021). This study analyzed the dynamic changes of metabolites in different growth stages of J. sabina leaves, demonstrated the accumulation and formation mechanisms of podophyllotoxins, and laid a theoretical foundation for the comprehensive development and utilization of J. sabina resources.
The accumulation of secondary metabolites in plants is influenced by external environments (Lee et al., 2021). In fact, podophyllotoxin's seasonal accumulation has been found in Sinopodophylum hexandrum (Li et al., 2015). This study reports that the accumulation of podophyllotoxin and total lignans in J. sabina leaves showed a “W” type change (Figure 1). J. sabina leaf buds begin to sprout in March, and the leaves continue to elongate from April to September. Consequently, during the growth phase, primary metabolic activities gradually intensify to produce large amounts of organic matters, resulting in less substrate for secondary metabolic production, and leading to a decrease in podophyllotoxin levels between March and May. Thereafter, primary metabolic activity in the early growth stages provided sufficient substrate for secondary metabolism, which may also account for the increase in podophyllotoxin content from May to September (Figure 1). During dormancy, primary and secondary metabolic activities diminish and the podophyllotoxin content reaches a steady state. Therefore, May to September is the key period for podophyllotoxin accumulation in J. sabina leaves.
In this study, the differential metabolites of J. sabina leaves at different growth stages were evaluated by metabonomics (Figure 2). The results showed that the contents of phenols, alkaloids, lignans, and coumarins increased during the growth and development of J. sabina leaves, while the proportion of flavonoids and their derivatives, amino acid derivatives, and fatty acid derivatives decreased. These results showed that the accumulation of flavonoids in J. sabina leaves decreased greatly in the late growth stage, which may be due to the synthesis of lignans (Lau & Sattely, 2015) by various enzymes in the phenylpropane metabolism pathway, thus increasing the accumulation of lignans.
Eight podophyllotoxins and their synthetic precursors in J. sabina leaves were identified by metabonomics. The accumulation of precursors and intermediates involved in the synthesis of podophyllotoxin and its derivatives tends to decrease to varying degrees as they accumulate in J. sabina leaves during plant growth and development (Figure 3). This indicates that during the growth and development of J. sabina leaves, the precursors and intermediates related to podophyllotoxin synthesis, such as coniferyl alcohol, terpineol and arhats, may synthesize podophyllotoxin and its derivatives downstream under the enzymatic action.
At present, the biosynthetic pathway of podophyllotoxin has been preliminarily revealed, which mainly includes three stages: phenylpropanoid synthesis, lignans synthesis and podophyllotoxin synthesis (Li et al., 2018). In this study, eight key enzyme genes (PAL, HCT, CCR, C4H, DIR, PLR, OMT and CYP82) were screened by combining the data of the transcriptome and metabolome (Figure 5B). Among them, PLR encodes an enzyme catalyzing the two-step reduction reaction during podophyllotoxin synthesis, and is the key rate-limiting enzyme gene for podophyllotoxin synthesis (Corbin et al., 2017). In this study, the fluorescent signal of GFP-tagged JsPLR was found in the nucleus in addition to the cytoplasm (Figure 7B). Previous studies have also shown that PLR enzyme may play a role in cytoplasm (Markulin et al., 2019).
The expression of PLR has been shown to be instrumental in the synthesis of podophyllotoxin, and PLR has been identified in many plants (Markulin et al., 2019). At present, the PLR coding sequence has been isolated from different plant genus, such as Forsythia, Pinus and Linum, and its expression and function have been studied (Hwang et al., 2020; Kumari et al., 2022; Bayindir et al., 2020). PLR enzyme can catalyze the reduction of (+)-pinoresinol to (+)-lariciresinol, and then further reduce it to (−)-secoisolariciresinol, showing strong enantioselectivity (Wankhede et al., 2013; Markulin et al., 2019; Hemmati et al., 2007). It is worth noting that human intestinal bacteria can convert (+)-pinoresinol to (+)-lariciresinol, suggesting that PLRs-like enzymes also exist in bacteria (Clavel et al., 2006; Xie et al., 2003).
Although the PLR coding gene from (+)-pinoresinol to (−)-matairesinol has been cloned and expressed in vitro, there is no report about its gene expression and link with podophyllotoxin yield (Suzuki and Umezawa, 2007). Here, we show a clear link between PLR expression and the yield of podophyllotoxin (Figure 8). However, the knowledge base about PLR should be expended through biotechnology (Malik et al., 2014) or enzyme-assisted chemistry (Ricklefs et al., 2016) to generate higher production of podophyllotoxins for cancer treatment.
5 CONCLUSIONS
In this study, the leaves of J. sabina were used as experimental materials, and the accumulation of podophyllotoxins in J. sabina at different developmental stages was studied for the first time by transcriptomics and metabonomics. Metabonomics analysis identified 8 podophyllotoxins and 54 differential compounds. The accumulation of total lignans and podophyllotoxin in J. sabina leaves at different growth stages showed that May–September was the rapid accumulation period of podophyllotoxin in J. sabina leaves. Eight key enzyme genes of podophyllotoxin biosynthesis in J. sabina were screened by combined analysis of transcriptome and metabolism. By cloning and functional verification of the key regulatory gene JsPLR, the relationship between JsPLR gene and podophyllotoxin and its precursors (deoxypodophyllotoxin and (−)-matairesinol) was explained for the first time. The results of qRT-PCR showed the positive regulation of JsPLR gene in JsPLR leaves at different growth stages. This study brings insights into the accumulation mechanism of podophyllotoxins and promotes the comprehensive utilization of J. sabina.
AUTHOR CONTRIBUTIONS
Shengnan Xu: Methodology, Software, Formal analysis, Writing - Original Draft, Huizhong Hu: Software, Formal analysis, Resources, Writing - Review & Editing, Ziyi Wang: Resources, Data Curation, Zhifang Yang: Formal analysis, Investigation, Supervision, Xiaocha Wei: Resources, Data Curation. Dengwu Li: Conceptualization, Methodology, Supervision.
ACKNOWLEDGMENTS
This work was supported by the special fund for the Key Research and Development Program of Shaanxi Province (2023-YBNY-001) and the program from the National Natural Science Foundation of China (No. 31570655).
Open Research
DATA AVAILABILITY STATEMENT
The datasets generated for this study can be found in the NCBI (BioProject ID PRJNA961969).




