Carbon stress causes preferential storage over growth in treeline trees
Abstract
Non-structural carbohydrates (NSCs) play an important functional role in determining plant survival as a backup carbon (C) source, while they also represent a large C sink being traded off against other C sinks when facing stress. However, information regarding how such trade-offs in C allocation under C stress vary among species remains limited. Here, we experimentally induced C stress in juveniles of three tree species by exposing them to in situ artificial shading, and then we quantified the dynamics of whole-tree C allocation patterns between storage and growth. For light-demanding deciduous conifer larch and shade-intolerant deciduous broad-leaved birch, tree growth (represented by relative tree height growth (RHG) and relative basal area increment (RBAI)) was significantly reduced by shading, whereas for shade-tolerant evergreen conifer fir, RHG and RBAI remained constant. Variations of whole-tree NSC reserves were consistent for non-treeline tree species (i.e., birch and fir), showing that shading remarkably reduced NSC storage. In contrast, shading-induced NSC accumulation was found only in treeline tree species (i.e., larch). More importantly, C allocation to NSC storage was prioritized over structural growth only in larch under C stress, suggesting a strong trade-off between survival and growth in treeline trees. Overall, our results discovered different patterns of C allocation in response to C stress across species with different plant functional types, further highlighting the highly conservative survival strategy of treeline tree species. Our study may contribute to an enhanced understanding of how forest biomes mitigate the negative impacts of stressful events in the context of global changes.
1 INTRODUCTION
Ongoing global changes exert pressure across most terrestrial ecosystems, particularly on forest ecosystems that serve efficient carbon (C) sink function (von Arx et al., 2017; Werner et al., 2021). Hence, growing concerns have been raised about how forest biomes react to lessen the survival threats posed by changing environments (Jia et al., 2022; Martinez-Sancho et al., 2022). Trade-offs depend on species-specific strategies for resource allocation, which typically arise when multiple functions of an organism share finite energy simultaneously (Herrera-Ramírez et al., 2020). Trade-offs are especially crucial for long-lived sessile trees, which cannot escape stress through migration and are constrained in active access to resources when facing environmental extremes (Nicotra et al., 2010). It is, therefore, reasonable to assume that the optimal way for trees to persist in a changing environment is through physiological acclimation (Anderegg et al., 2019), which is centred on strategic trade-offs in resource allocation (Huang et al., 2021; Mooney, 1972).
Carbohydrates are assimilated through C source activity (photosynthesis) and are subsequently partitioned to different C sinks (e.g., growth, storage, maintenance of living tissues, biosynthesis of secondary metabolites or rhizodeposition) (Körner, 2006), functioning in a variety of essential physiological processes (Huang et al., 2017). In addition to being partly consumed in cellular respiration or root exudation, photoassimilates are mainly accumulated in plants in the form of structural carbohydrates (including cellulose, hemicellulose, and lignin) (Schädel et al., 2010) and non-structural carbohydrates (NSCs, including soluble sugars and starch) (Martínez-Vilalta et al., 2016). Of the considered two largest C reservoirs in woody plants, structural carbohydrates act as the key components of wood quality (Martínez-Vilalta et al., 2016), while NSCs play an important role in maintaining the routine cellular processes under varying environmental conditions (Hartmann and Trumbore, 2016; Liu et al., 2017). Specifically, stored NSCs can fuel energetically to support vigorous growth during active phenological phases (Montague et al., 2022), safeguard normal survival for plants during fluctuations in C availability (Chapin et al., 1990), and prevent tissue injury or facilitate rapid recovery from damaging events (Long et al., 2021; Sakai et al., 1997). Consequently, revealing the spatio-temporal patterns of NSC variations can provide insights into the C economy of plants and the physiological mechanisms constraining their geographical distributions (Chlumská et al., 2022; Furze et al., 2019).
C-related eco-physiological processes in plants have been extensively studied using NSCs as a reliable indicator over the past decades (Dietze et al., 2014; Handa et al., 2005; Körner, 2003). However, previous studies have mainly focused on organ-specific NSC concentrations to explore the balance of C source-sink relationships (Dawes et al., 2011; Hoch and Körner, 2012; Sveinbjörnsson et al., 2010; Wyka et al., 2016; Zhou et al., 2021), neglecting C allocation at the individual level (e.g., whole-tree level). In fact, measuring NSC concentrations in a single plant tissue alone can hardly provide robust insight into whole-tree C dynamics (Hartmann et al., 2020). NSCs are the dominant currency of C allocation, and upscaling NSCs from organ to individual level can shed new light on revealing C storage in growth processes (Hartmann et al., 2020; Schoonmaker et al., 2021). Moreover, patterns of trade-offs in whole-tree C allocation set the stage for the shifts in survival strategies, with plants either investing more in productivity (represented by growth) or more in longevity (represented by storage) (Wright et al., 2004). Therefore, it is critical to explicitly elucidate how the two main components of a tree's C budget, the C used for structural growth and the C stored in plant tissues as energy substance, are allocated at whole-tree levels to maximize individual fitness under varying environment (Hinman and Fridley, 2018; Long et al., 2021).
Additionally, there is no consensus on how NSC accumulation in plant tissues is achieved, and two conflicting hypotheses regarding passive and active C accumulation have emerged (Chapin et al., 1990). According to the hypothesis of passive C accumulation, NSC storage is a plastic and lowest-priority C sink, which only increases if other C sink demands are satiated (Sala et al., 2012). For instance, several studies have confirmed that low temperatures typically restrict growth to a greater extent than photosynthesis, potentially leading to residual C (i.e., the C in excess of growth demand) (Hoch and Körner, 2009; Körner, 2015). Alternatively, the active C accumulation hypothesis posits that NSC storage is a heritable and highly competitive C sink activity, which could be achieved by up-regulating its own or down-regulating structural growth (Blumstein et al., 2022; Reich, 2014). Therefore, the growth process of a tree over its long life history is closely associated with its NSC reserves (Landhäusser et al., 2012). Indeed, a growing number of empirical research has demonstrated that the responses of long-lived trees to a multitude of stress are mirrored by actively maintaining or even enhancing the C investments into NSC stores at the expense of structural growth (Adams et al., 2017; Anderegg et al., 2012; Hudgeons et al., 2007; O'Brien et al., 2017; Piper et al., 2015). Nonetheless, a major concern is that environmental constraints in several experimental manipulations (e.g., water limitation or foliage removal) could directly incur both reduced rates of photosynthesis and biosynthesis (Hartmann et al., 2018; Weber et al., 2019; Wiley et al., 2017a), leading to the question that whether tree growth is source or sink limited (Fatichi et al., 2014; Fatichi et al., 2019).
Considering that sufficient light is necessary for NSC production (Kozlowski, 1992), subjecting trees to shade conditions would separate C source- and sink-related constraints and induce a negative C balance (Weber et al., 2019; Wiley et al., 2017b). As suggested by Agrawal (2020), understanding allocation trade-offs across species is a core biological problem, bearing on the evolution of ecological strategies as well as forecasting various responses to environmental changes. Herein, we set up an in-situ shading experiment to evaluate whether trade-offs in whole-tree C allocation between NSC storage and structural growth were detectable under C stress in the Qinling Mountains of north-central China. Juveniles from one treeline tree species (Larix chinensis) and two non-treeline tree species (Betula albo-sinensis and Abies fargesii) were exposed to shade, and NSC pools at both organ and whole-tree levels were tracked, and tree growth was monitored during the shading experiment. We hypothesized that treeline tree species survived with a highly conservative C allocation strategy relative to non-treeline tree species if a preferential C allocation to NSC storage rather than structural growth under C stress was detected only in the treeline tree species larch.
2 MATERIALS AND METHODS
2.1 Study area and species
This study was conducted in the Taibaishan National Forest Park (33°57′-34°09'N, 107°42′-107°58′E) and the Taibaishan National Nature Reserve (33°49′-34°05'N, 107°22′-107°51′E) on the northern slope of the Qinling Mountains, north-central China. The Qinling Mountains are an obvious geographical demarcation line between northern and southern China and the most critical boundary for vegetation distribution between the warm-temperate and subtropical zones in eastern mainland China (Liu et al., 2009). The northern slope of the Qinling Mountains is characterized by a warm-temperate climate with relatively dry summers and cold winters (Liu et al., 2021). Mean annual temperatures in this region range from 1 to 6°C above 2000 m and from 6 to 11°C below 2000 m a.s.l, with a lapse rate of 0.50 ± 0.02°C/100 m (Tang and Fang, 2006). Annual precipitation averages 980 mm, mostly in summer (June–September). Snow cover generally lasts for more than five months.
Five distinct natural vegetation zones distribute along the elevational gradient on the northern slope of the Qinling Mountains, including Quercus forest (below 2200 m a.s.l.), Betula forest (2250–2850 m a.s.l.), Abies forest (2850–3150 m a.s.l.), Larix forest (3150–3400 m a.s.l.), and subalpine meadow (above 3400 m a.s.l.) (Dang et al., 2015). Three tree species with different plant functional types (i.e., shade-intolerant deciduous broad-leaved birch (Betula albo-sinensis), shade-tolerant evergreen conifer fir (Abies fargesii), and light-demanding deciduous conifer larch (Larix chinensis)) form their evident upper distribution range limits along elevation on the northern slope of the Qinling Mountains. Birch reaches its upper range limit at the elevation of 2850 m by forming an ecotone with subalpine fir, while fir reaches its upper range limit at 3150 m by forming an ecotone with subalpine larch. Larch, which shows good adaptation to extreme cold habitats, forms an altitudinal treeline at the elevation of 3400 m. It is generally accepted that the growing seasons for all the three tree species begin in early June and extend to late September when the first severe frost occurs.
2.2 Experimental design and sampling
In-situ shading manipulation experiments were conducted throughout the growing season of 2021 at the lower distribution elevation, middle distribution elevation, and upper range limit for each of the three studied tree species, i.e., 2370 m for B. albo-sinensis, 2860 m for A. fargesii, and 3160 m for L. chinensis at their lower distribution elevations; 2610 m for B. albo-sinensis, 3010 m for A. fargesii, and 3280 m for L. chinensis at their middle distribution elevations; and 2840 m for B. albo-sinensis, 3150 m for A. fargesii, and 3410 m for L. chinensis at their upper range limits (Table 1). For each tree species at each distribution elevation, fourteen healthy individuals (juvenile trees <1.3 m in height) with similar size were selected, eight shaded and six treated as controls. In total, 126 individuals were selected in the shading experiment. Juvenile individuals of the three tree species were selected based on the following criteria: (1) vigorous appearance, (2) no distinct signs of herbivore damage, and (3) no close neighbours (Piper et al., 2014). Artificial shading was achieved by covering foliage with black nylon shading nets. To examine the effect of shading nets on light availability, a quantum photo-synthetically active radiation (PAR) meter (AZ-8584) was used to detect the photosynthetic photon flux density (PPFD) for shaded and non-shaded conditions.
| Species | Group | Control | Shaded | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Date | Jun | Sep | Jun | Sep | Jun | Sep | Jun | Sep | Jun | Sep | Jun | Sep | |
| Sampling Site | Upper | Middle | Lower | Upper | Middle | Lower | |||||||
| L. chinensis | Elevation (m) | 3410 | 3280 | 3160 | 3410 | 3280 | 3160 | ||||||
| DB (mm) | 25.3 (8.2) | 27.9 (8.3) | 28.1 (7.5) | 30.7 (7.2) | 20.4 (4.4) | 22.6 (4.4) | 20.2 (4.1) | 21.7 (4.3) | 34.1 (9.6) | 35.9 (9.3) | 19.3 (6.9) | 21.2 (6.7) | |
| H (cm) | 108.6 (29.8) | 113.9 (32) | 123.1 (12.4) | 132.9 (15.6) | 171.8 (50.5) | 181.7 (48.7) | 104.1 (24.8) | 106 (25.1) | 147.6 (31.2) | 150.9 (31.4) | 158.4 (64.4) | 161.7 (65.5) | |
| A. fargesii | Elevation (m) | 3150 | 3010 | 2860 | 3150 | 3010 | 2860 | ||||||
| DB (mm) | 31.5 (10.6) | 33.8 (10.5) | 41 (7.9) | 43 (7.9) | 21.3 (5.8) | 24.6 (5.7) | 32.2 (5.8) | 34 (5.5) | 33.2 (7.9) | 34.5 (7.8) | 22.1 (7.5) | 24.2 (7.4) | |
| H (cm) | 157.7 (38.1) | 165.7 (37.4) | 185.9 (21.9) | 196.7 (21.4) | 88.4 (15.4) | 100.3 (15.2) | 153 (21.2) | 158.5 (20.7) | 143.4 (33) | 148.8 (32.6) | 94.1 (19.3) | 101.1 (19.1) | |
| B. albo-sinensis | Elevation (m) | 2840 | 2610 | 2370 | 2840 | 2610 | 2370 | ||||||
| DB (mm) | 12.8 (2.5) | 14.9 (2.5) | 16.4 (4.4) | 18.9 (4.6) | 19.3 (6.3) | 24.9 (9.6) | 11.8 (3.2) | 13.6 (3.2) | 14.5 (3.6) | 16.1 (4) | 11.7 (3.1) | 13.5 (3.5) | |
| H (cm) | 130.8 (34.6) | 135.6 (35.6) | 162.1 (36.2) | 169.8 (37.6) | 166.7 (40.2) | 179.6 (43.9) | 135.4 (31.1) | 138.1 (30.7) | 132.8 (33.1) | 136.6 (33.8) | 114.8 (26.5) | 117.7 (26.6) | |
- Data are mean values and standard deviations (in parentheses); DB, diameter at the stem base (approximately 5 cm above the root crown); H, tree height.
The diameter at the stem base (DB, approximately 5 cm above the root crown) and tree height (H) of all selected individuals were measured in the early (June) and late (September) growing season in four directions, 90° apart from each other. Tissues (including foliage, twig, root and stem) of all selected individuals were also sampled in June and September, respectively. To minimize the effects of diurnal fluctuations in foliage NSCs due to the differences in photosynthetic activity, tissue sampling was carried out around noon. Foliage and twigs were collected from the leading twigs on the upslope canopy side using a pole cutter (Li et al., 2001). Stem samples were collected by mixing the outermost 1 cm xylem tissue of two increment cores extracted from opposite directions using an increment borer. Roots with a diameter between 0.5 and 2 cm were collected. The phloem and bark were removed from the twig, stem and root samples in the field with a knife (Chantuma et al., 2009; Hartmann and Trumbore, 2016). All samples were immediately microwaved (three 20-s cycles at 600 W) within 6 h after sampling to denature oxidizing enzymes and were then put into an ice cooler to reduce tissue respiration for transportation. In the laboratory, they were oven-dried at 80°C to a constant weight, ground into fine powder, and stored in well-sealed plastic vials for later chemical analysis.
2.3 Measurements of leaf photosynthetic capacity and analyses of NSC
Photosynthetic gas exchange was measured on detached twigs from all sampled juveniles in September 2021, with a highly portable ambient photosynthesis system (LCi-T, ADC Bioscientific Ltd.). The measurements were made on net photosynthetic rate (A) from 10:00 to 11:30 a.m. on clear days under a saturation light intensity of 1600 μmol m−2 s −1 and a CO2 concentration of 400 ppm, with air temperature inside the cuvette set for 25°C.
Non-structural carbohydrates (NSCs) were defined as the sum of total soluble sugars (including glucose, sucrose and fructose) plus starch. NSC concentrations of each organ (foliage, twig, root and stem) of all the sampled individuals were analyzed using the anthrone method (Li et al., 2008; Yemm and Willis, 1954). Total soluble sugar concentrations were calculated from the standard regression equation based on glucose standard solutions, and starch concentrations were calculated by multiplying the glucose concentration by the conversion factor of 0.9 (Osaki et al., 1991). The concentrations of NSC, total soluble sugar and starch were expressed per unit of dry matter weight (mg g−1).
2.4 Calculations of tree growth, NSC pool and structural biomass
Biomass of each organ (i.e., foliage, twig, root and stem) for each sampled juvenile was calculated using the allometric growth equations of L. chinensis (Fu, 1994), A. fargesii (Tang and Xu, 1993), and B. albo-sinensis (Chen and Peng, 1996b), respectively. The NSC pool for each organ was calculated by multiplying organ NSC concentration by organ biomass. The whole-tree biomass was then obtained by summing each organ biomass and the whole-tree NSC pool was achieved by summing each organ NSC pool (Piper, 2015). The whole-tree structural biomass was determined by subtracting the whole-tree NSC pool from the whole-tree biomass. Then, the ratio of the NSC pool to structural biomass at the whole-tree level was calculated to evaluate the C allocation between storage and growth (see Supplemental Table S1).
2.5 Statistical analyses
One-way analysis of variance (ANOVA) was first used to compare PPFD, A, RHG and RBAI between control and shaded groups in each tree species at each elevation to determine the effects of shading on light availability, leaf photosynthesis and tree growth. The Kruskal-Wallis test was applied if the data were not normally distributed.
To further explore the effects of shading on C allocation (including NSC, sugar, starch pool, sugar: starch ratio at both organ and whole-tree level and NSC pool: structural biomass ratio at whole-tree level) for each tree species at each elevation, one-way analysis of covariance (ANCOVA) was repeatedly conducted. Organ and whole-tree biomass were included as covariates in ANCOVA due to the positive correlations of biomass with NSC pool and structural biomass.
In addition, three-way ANOVA was repeatedly performed to analyze the total effects of shading, tree species, elevation, and their interactions on (1) PPFD, A, RHG and RBAI; (2) NSC, sugar, starch pool, and sugar: starch ratio at both organ and whole-tree level; and (3) NSC pool: structural biomass ratio at whole-tree level. Then, two-way ANOVA was repeatedly employed to detect further the effects of shading, elevation, and their interaction on these responsive variables for single species.
All the data were tested for normality using the Shapiro–Wilk test and for homogeneity of variances using the Bartlett test. Data were log or square-root transformed to fulfil these two assumptions when applying ANOVA. Significant differences (P < 0.05) in dependent variables were detected using the Tukey–Kramer Honest Significance Difference (HSD) test. All the statistical tests were performed with the software R (R Core Team, 2021; www.R-project.org; version 4.1.2) and its base packages.
3 RESULTS
3.1 Effects of shading on light availability, leaf photosynthesis and tree structural growth
Shade treatment had significantly negative effects on light availability and leaf photosynthesis in each tree species at each elevation (Tables 2 and 3; Figure 1; Supplemental Table S3). Specifically, the photosynthetic photon flux density (PPFD) for shaded conditions reached only 19.54, 15.38, and 15.98% of the full light at the lower, middle, and upper elevations of larch individuals (P < 0.001; Figure 1a); only 20.72, 17.80, and 17.30% of the full light at the lower, middle, and upper elevations of fir individuals (P < 0.001; Figure 1b); and only 15.11, 15.01, and 15.28% of the full light at the lower, middle, and upper elevations of birch individuals (P < 0.001; Figure 1c), respectively. Additionally, the net photosynthetic rate (A) decreased by 61% at the lower elevation (P < 0.01), 70% at the middle elevation (P < 0.01), and 69% at the upper elevation (P < 0.001) in the shaded larch individuals (Figure 1d); by 74% at the lower elevation (P < 0.001), 55% at the middle elevation (P < 0.001), and 45% at the upper elevation (P < 0.001) in the shaded fir individuals (Figure 1e); and by 92% at the lower elevation (P < 0.001), 64% at the middle elevation (P < 0.001), and 91% at the upper elevation (P < 0.01) in the shaded birch individuals (Figure 1f), respectively.
| Source of variation | Species | Elevation | Treatment | Species×Elevation | Species×Treatment | Elevation×Treatment | Species×Elevation×Treatment | |
|---|---|---|---|---|---|---|---|---|
| PPFD | F | 770.842 | 393.616 | 44436.779 | 140.301 | 696.506 | 324.598 | 99.805 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| A | F | 27.941 | 1.721 | 487.492 | 12.587 | 11.863 | 3.4063 | 14.904 |
| p | 0.000 | 0.184 | 0.000 | 0.000 | 0.000 | 0.037 | 0.000 | |
| RHG | F | 18.855 | 17.180 | 67.193 | 7.412 | 0.964 | 4.575 | 1.298 |
| p | 0.000 | 0.000 | 0.000 | 0.000 | 0.385 | 0.012 | 0.276 | |
| RBAI | F | 23.717 | 12.484 | 14.777 | 0.587 | 1.388 | 1.358 | 1.706 |
| p | 0.000 | 0.000 | 0.000 | 0.673 | 0.254 | 0.262 | 0.154 | |
| NSC | F | 89.137 | 13.611 | 3.233 | 7.289 | 7.178 | 0.086 | 3.806 |
| p | 0.000 | 0.000 | 0.075 | 0.000 | 0.001 | 0.917 | 0.006 | |
| Soluble sugar | F | 61.339 | 11.078 | 6.033 | 10.410 | 6.558 | 0.267 | 4.149 |
| p | 0.000 | 0.000 | 0.016 | 0.000 | 0.002 | 0.766 | 0.004 | |
| Starch | F | 112.271 | 13.594 | 0.925 | 5.778 | 6.376 | 0.912 | 3.177 |
| p | 0.000 | 0.000 | 0.338 | 0.000 | 0.002 | 0.405 | 0.016 | |
| Sugar: starch | F | 80.322 | 2.323 | 0.060 | 7.081 | 0.149 | 1.130 | 2.048 |
| p | 0.000 | 0.103 | 0.806 | 0.000 | 0.862 | 0.327 | 0.093 | |
| NSC pool: structural biomass | F | 60.402 | 17.805 | 8.256 | 0.997 | 27.812 | 7.606 | 5.139 |
| p | 0.000 | 0.000 | 0.005 | 0.413 | 0.000 | 0.000 | 0.000 | |
| df | 2 | 2 | 1 | 4 | 2 | 2 | 4 |
- Note: PPFD, photosynthetic photon flux density; A, net photosynthetic rate; RHG, relative tree height growth; RBAI, relative basal area increment; NSC, non-structural carbohydrate pool at whole-tree level.
| Source of variation | Larix chinensis | Abies fargesii | Betula albo-sinensis | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Elevation | Treatment | Elevation×Treatment | Elevation | Treatment | Elevation×Treatment | Elevation | Treatment | Elevation×Treatment | ||
| PPFD | F | 539.21 | 14266.81 | 423.69 | 5.460 | 10629.050 | 12.793 | 70.911 | 19888.624 | 43.993 |
| p | 0.000 | 0.000 | 0.000 | 0.008 | 0.000 | 0.000 | 0.000 | 0.000 | 0.000 | |
| A | F | 7.473 | 49.652 | 3.385 | 5.823 | 207.215 | 4.823 | 22.001 | 707.021 | 60.534 |
| p | 0.002 | 0.000 | 0.045 | 0.006 | 0.000 | 0.014 | 0.000 | 0.000 | 0.000 | |
| RHG | F | 1.640 | 35.591 | 1.093 | 18.975 | 13.851 | 2.660 | 3.981 | 26.189 | 3.836 |
| p | 0.208 | 0.000 | 0.346 | 0.000 | 0.000 | 0.084 | 0.027 | 0.000 | 0.031 | |
| RBAI | F | 2.517 | 2.872 | 0.715 | 12.986 | 5.370 | 1.601 | 2.569 | 7.046 | 1.892 |
| p | 0.095 | 0.099 | 0.496 | 0.000 | 0.026 | 0.216 | 0.091 | 0.012 | 0.165 | |
| NSC | F | 4.504 | 3.411 | 3.130 | 12.591 | 6.515 | 1.950 | 2.253 | 13.211 | 6.249 |
| p | 0.018 | 0.073 | 0.056 | 0.000 | 0.015 | 0.157 | 0.120 | 0.000 | 0.005 | |
| Soluble sugar | F | 0.928 | 2.378 | 1.519 | 15.493 | 6.974 | 2.960 | 1.634 | 13.499 | 5.940 |
| p | 0.405 | 0.132 | 0.233 | 0.000 | 0.012 | 0.065 | 0.209 | 0.000 | 0.006 | |
| Starch | F | 8.149 | 3.308 | 3.759 | 8.809 | 5.340 | 1.074 | 3.618 | 11.367 | 6.282 |
| p | 0.001 | 0.077 | 0.033 | 0.000 | 0.027 | 0.352 | 0.037 | 0.002 | 0.005 | |
| Sugar: starch | F | 6.798 | 0.093 | 0.723 | 0.513 | 0.226 | 1.221 | 9.352 | 0.037 | 2.284 |
| p | 0.003 | 0.762 | 0.492 | 0.603 | 0.637 | 0.307 | 0.000 | 0.849 | 0.116 | |
| NSC pool: structural biomass | F | 4.451 | 23.753 | 7.315 | 9.328 | 22.712 | 6.776 | 6.214 | 18.903 | 4.624 |
| p | 0.019 | 0.000 | 0.002 | 0.000 | 0.000 | 0.003 | 0.005 | 0.000 | 0.016 | |
| df | 2 | 1 | 2 | 2 | 1 | 2 | 2 | 1 | 2 |
- Note: PPFD, photosynthetic photon flux density; A, net photosynthetic rate; RHG, relative tree height growth; RBAI, relative basal area increment; NSC, non-structural carbohydrate pool at whole-tree level.
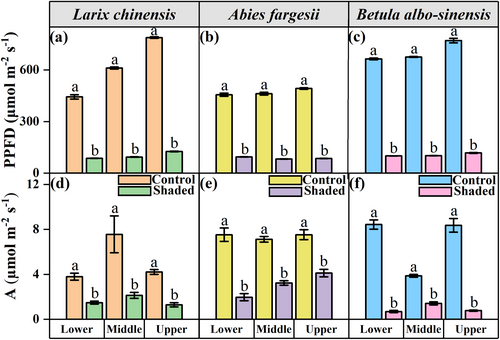
Larch and birch individuals grew significantly more slowly during shading, as was evidenced by their decreased RHG and RBAI (Table 3; Figure 2). Significant reductions in RHG were observed at each elevation in the two deciduous tree species (P < 0.01; Figure 2), with more pronounced declines in the treeline larch individuals than in the birch individuals (70 vs 40% at the middle elevation and 61 vs 41% at the upper elevation; Figure 2 a, c). Similarly, the RBAI of birch was also significantly reduced by shading (P < 0.05 at the lower elevation; Figure 2f).
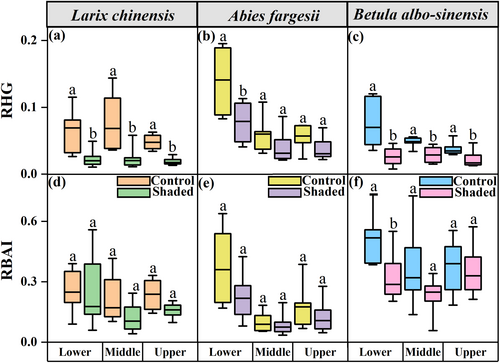
In contrast to the two deciduous tree species (i.e., larch and birch), shading did not induce significant reduction in RBAI or RHG of the evergreen fir trees, with the only exception that fir's RHG decreased significantly at the lower elevation (P < 0.05; Figure 2b).
3.2 Effects of shading on NSC pool
NSC pools were significantly affected by the shading treatment, species type, elevation, and their interactions at both organ and whole-tree levels (Table 2; Supplemental Tables S2 and S3). For organ-level NSC reserves in the treeline tree species larch, shading led to remarkable increases in late-season NSC pool in foliage at the lower elevation (P < 0.05; Figure 3a), in twig at the middle (P < 0.01; Figure 3b) and upper elevations (P < 0.05; Figure 3b), and in stem at the middle elevation (P < 0.05; Figure 3d); while it significantly decreased NSC pool in root at the lower (P < 0.01; Figure 3c) and upper elevations (P < 0.05; Figure 3c), and in foliage (P < 0.05; Figure 3a) and stem (P < 0.01; Figure 3d) at the upper elevation. Nonetheless, shading exhibited significantly negative effects on organ-level NSC pools in the non-treeline tree species fir and birch. Specifically, NSC pool in the organs of twig and stem of fir individuals experienced remarkable decreases during shading (P < 0.05 at the lower elevation and P < 0.001 at the upper elevation; Figure 3 f, h), and NSC pool in all organs of birch individuals showed significant reductions (P < 0.001 for foliage at the lower and middle elevations and for stem at the lower elevation, P < 0.01 for twig at the lower elevation, and P < 0.05 for root at the upper elevation; Figure 3 i, j, k, l).
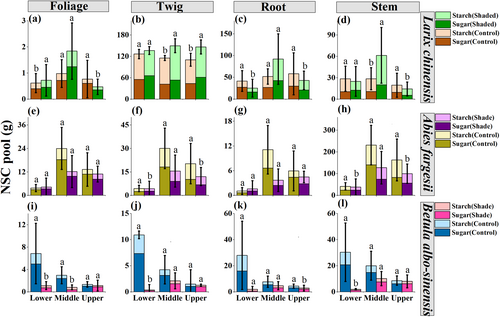
For the whole-tree NSC pool, shading significantly increased NSC reserves in the treeline tree species larch (Figure 4). Specifically, NSC accumulation in larch was mirrored by the significant increases in starch pool at the middle elevation (P < 0.01; Figure 4c), in sugar pool at the upper elevation (P < 0.001; Figure 4b), and in NSC pool at both the middle (P < 0.01) and upper elevations (P < 0.05; Figure 4a). For the non-treeline tree species fir and birch, however, shading led to the significant reductions in NSC reserves (Figure 4). The decline in NSC pool of fir trees was mainly attributable to the decreases in starch pool at the lower elevation (P < 0.05 for NSC and starch) and in sugar and starch pool at the upper elevation (P < 0.001 for NSC, P < 0.01 for sugar, and P < 0.05 for starch; Figure 4 e, f, g), and the decrease in NSC pool of birch trees was primarily attributed to the reduced sugar pool at the lower elevation (P < 0.01 for NSC and P < 0.001 for sugar; Figure 4 i, j). In addition, the significantly decreased ratio of sugar pool to starch pool was only found in the shaded evergreen fir at the middle elevation (P < 0.05; Figure 4h).

3.3 Effects of shading on carbon allocation between storage and growth
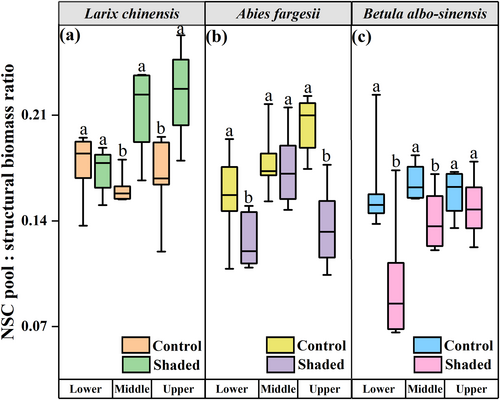
Significant interaction of shading and elevation was observed for whole-tree C allocation among all the three tree species (Tables 2 and 3; Supplemental Table S3). For treeline tree species larch, the ratio of NSC pool to structural biomass increased remarkably during shading (Table 3; F = 23.753, P < 0.001). Though the ratio was not significantly different between shaded individuals and the controls for the lower-elevation larch individuals, it increased remarkably at both the middle and upper elevations (P < 0.01; Figure 5a). Shading decreased C allocation to storage for the non-treeline tree species, showing that NSC pool: structural biomass ratios were significantly lower in the fir trees at the lower (P < 0.05) and upper elevations (P < 0.001; Figure 5b), and in the birch trees at the lower (P < 0.05) and middle elevations (P < 0.05; Figure 5c).
4 DISCUSSION
4.1 Shading-induced source limitation occurred in non-treeline tree species
Our results indicated that shading had significant negative effects on tree growth in winter-deciduous trees (larch and birch) (Table 3; Figure 2), which were in line with previous studies reporting that shading induced substantial reductions in tree growth (Myers and Kitajima, 2007; Portsmuth and Niinemets, 2007; Yang et al., 2021). For example, a long-term deep-shade experiment showed that reduced light strongly decreased the growth of above-ground organs in a range of temperate tree species (Weber et al., 2019). The same results were also found in a short-term progressive (from moderate to heavy) shading in deciduous tree species (Wang and Wang, 2023). It is widely accepted that light is a key factor driving tree growth and that declines in tree height and radial increments under light deprivation (Table 3; Figure 2) are the consequences of reduced C availability (Figure 1 d, f) and constrained meristem activity (Sevillano et al., 2016; Wyckoff and Clark, 2005; Yang et al., 2021). Surprisingly, in this study, evergreen fir trees did not experience a significant growth reduction during shading (Figure 2 b, e). The distinct growth responses to shading found in our study might be attributable to the contrasting tolerance to shade of the studied tree species (Myers and Kitajima, 2007; Pagès et al., 2003; Portsmuth and Niinemets, 2007; Walters et al., 2014; Zhou et al., 2021).
The preponderance of evidence has demonstrated that woody plants exhibited significant reductions in growth under C stress, which were often accompanied by substantial decreases in NSC contents (Maguire and Kobe, 2015; Piper et al., 2009; Sapes et al., 2021; Veneklaas and den Ouden, 2005; Yang et al., 2021). Our findings of the non-treeline birch trees were consistent with these studies, showing that birch distributed at the lower, middle and upper elevations all experienced significant reductions in the NSC pool at both organ and individual levels (Figures 3 and 4). The consistency of declines in photosynthetic capacity, NSC levels and tree growth (Figures 1, 2, 3 and 4) is indicative of source limitation (Weber et al., 2019), suggesting that shading caused C demand of birch overweighing the supply of newly assimilated C (El Omari, 2022; Wang and Wang, 2023; Weber et al., 2018). In contrast to the evergreen fir trees which remained substantial NSC stores in root and foliage under C stress, however, the winter-deciduous birch trees experienced significant NSC reductions in these two tissues (Figure 3). It is generally accepted that deciduous tree species are more resilient to environmental stress than evergreens owing to their better performance in utilizing resources (Grelet et al., 2001; Piper and Fajardo, 2016; Piper et al., 2014). Therefore, decreases in foliage and root NSC reserves might be attributed to the ability of deciduous birch to transport NSCs from foliage and mobilize NSCs from roots to support normal physiological processes under C stress (Martínez-Vilalta et al., 2016).
Interestingly, our results showed that the decreased tree growth was accompanied by an increased whole-tree NSC pool in the treeline tree species larch under C stress (Figures 2 and 4). Specifically, shading resulted in a preferential allocation of NSCs to twigs rather than to below-ground organs (Figure 3). The positive effect of shading on NSC accumulation in twigs could be interpreted for the following reasons. Firstly, foliage augmented the NSC translocation to twigs to ensure the subsequent twig elongation, thereby facilitating the maximum space occupation and light-harvesting for light-demanding larch under C stress (Li et al., 2018b; Zheng et al., 2023). Secondly, twigs can act as a “bridge” (Hoch et al., 2002), performing a buffer function between organs assimilating C (foliage) and consuming C (stems and roots) to reduce the costs of NSC transportation under C stress (Dietze et al., 2014; He et al., 2022). In addition, soluble sugars in the larch trees at the treeline elevation increased following shading (Figure 4), implying that soluble sugars, rather than starch, were mainly responsible for the enhanced NSC accumulation in the treeline larch trees under C stress. This finding may be attributed to the specific functions of starch and soluble sugars, with the former representing a recalcitrant C reserve and the latter acting as an immediate energy source (Du et al., 2020; Hartmann and Trumbore, 2016). Moreover, it has been proposed that trees growing at altitudinal treeline ecotone have to endure extreme harsh winters and severe late-spring freeze events, especially under C stress, and rely on sugar pool to improve their resistance and resilience to abiotic stressors for successful survival (Li et al., 2018a; Zhu et al., 2012).
4.2 Treeline tree species exhibited strategic trade-offs between survival and growth
Unlike the non-treeline tree species (i.e., birch and fir) (Figure 5 b, c), an increased ratio of NSC pool to structural biomass was observed in the treeline tree species larch under C stress (Figure 5a). It has been suggested that the relationship between variations in storage and growth under C stress is not relevant to species-specific shade tolerance, indicating more complex mechanisms beyond shade tolerance (Weber et al., 2019). There is a potential tendency for woody plants to trade off among the ecologically important traits under stress (Agrawal, 2020). Therefore, the existence of a strategic trade-off might hold the answer to the increasing C storage accompanied by decreasing C assimilation capacity and growth in the treeline larch trees under C stress (Herrera-Ramírez et al., 2021).
As proposed by Agrawal (2020) and Blumstein et al. (2022), strategic trade-off in interrelated functions is a subset of habitat-specific adaptive specialization, which arises as a heritable trait evolving under natural selection. Therefore, our findings of the diverse responses in allocation patterns to C stress (Figure 5) might be attributed to the relative stress tolerances of the studied tree species formed during their long-term ecological evolution (Hartmann et al., 2020; Maguire and Kobe, 2015; Schoonmaker et al., 2021). Trade-offs in C allocation could mediate plant growth, with only conservative species growing slower and accumulating more resource reserves to deal with stressful conditions (Meira-Neto et al., 2019; Muller-Landau, 2010). Therefore, it is reasonable to assume that, compared with the non-treeline trees (i.e., birch and fir) inhabiting the relatively warm environments at the lower elevations, treeline larch trees, which are exposed to frequent and unpredictable low-temperature extremes could exhibit higher stress-tolerance by employing a more conservative C allocation strategy (Chen et al., 2021; He et al., 2022). This idea is also supported to a certain extent by the distinct effect of the interaction of elevation and shading on the ratio of NSC pool to structural biomass (Tables 2 and 3; Supplemental Table S3), indicating that the species-specific differences in changes in the assignment of C between storage and growth might be primarily driven by elevation gradients. Additionally, C allocation is functional in physiologically determining survival and predicting the success of woody plants in ecological communities under unfavourable habitats (Dietze et al., 2014; Wiley et al., 2017a). As reported previously, treeline trees respond by storing C upon exposure to freezing temperatures (Wiley, 2020) and the trade-off strategy is likely an important mechanism contributing to the enduring success of settlement at the treeline elevation (Chen et al., 2021). Moreover, by allocating more C to storage, the treeline tree, larch, could reduce the risk of C depletion during long-term stress episodes and allow for a more rapid recovery from damaging events (e.g., extreme low temperature and light deprivation) (Piper, 2011; Wiley, 2020; Yang et al., 2015).
It is worth noting that a small increase in the investment of C storage can come at a large cost to competitive C sink in growth over a tree's lifetime (Blumstein et al., 2022; Chen et al., 2021; Piper et al., 2009; Wiley and Helliker, 2012). Our results showed that the treeline larch trees larch were also likely mitigating C stress effects by saving C without sustaining growth (Figures 2, 4 and 5), indicating their survival with a relatively more conservative and safer strategy. Although higher C reserves might mitigate the impairments of normal plant physiological functions under stress, the concomitant reduction in growth can also compromise the competitive ability of trees (Tilman, 2004). Consequently, active NSC storage at the expense of growth constitutes a “bet hedging” strategy (Richardson et al., 2013; Yang et al., 2015). In this study, treeline tree species larch allocated proportionally less C to structural growth, which might decrease its short-term competitive ability; while the build-up of a larger NSC pool was likely to increase its long-term safety, thereby fostering future tree performance (Figures 2, 4 and 5) (von Arx et al., 2017; Blumstein et al., 2022; Sala et al., 2012; Wiley and Helliker, 2012).
In fact, C allocation to ecological traits of structural growth and NSC storage is determined by a trade-off between growth and survival, which mirrors a principal axis of variations in survival strategies among species (Atkinson et al., 2014). C allocation to NSC storage in the treeline tree species under C stress contributes to the growth-survival trade-off through its positive effect on survival, which might also reflect a shift from a productive to a conservative strategy. Investing less in productivity (represented by structural biomass) and more in longevity (represented by NSC reserves) facilitates treeline trees to cope with unpredictable adverse factors encountered in their long-life history (Hartmann et al., 2020; Sala et al., 2012; Wiley and Helliker, 2012). In addition, trade-offs between survival and growth have been reported for several tree species, where species allocating more resources to high survival traits (such as NSC reserves) tend to grow slower in the face of environmental stress (Adler et al., 2014; Philipson et al., 2014). For example, experimental studies on both temperate and tropical tree species have suggested an essential role of NSCs in the physiological mechanism of mortality, as plants with larger NSC reserves have higher survivorship (Adams et al., 2013; O'Brien et al., 2014; Signori-Müller et al., 2021). Our results of C allocation patterns in the treeline tree species larch also agree with the previous findings, indicating that NSC levels are associated positively with survival rates and negatively with growth rates (Gora and Esquivel-Muelbert, 2021; O'Brien et al., 2015; Poorter and Kitajima, 2007).
A better understanding of C allocation patterns within trees holds importance at the scale of species, communities, and forest ecosystems more broadly (Wiley et al., 2017a). Collectively, our results point towards a more conservative (storage-first) survival strategy employed by the treeline tree species larch, which has to survive in extreme cold conditions compared to the non-treeline tree species birch and fir, which inhabit relatively warm environments. Our research shows new evidence of environmentally-driven C allocation and is of great implication for further understanding the complex interactions between environmental factors and C allocation in perennial woody plants.
AUTHOR CONTRIBUTIONS
RH and HD conceived this study. RH and HS performed the investigation and statistical analyses. RH wrote the original draft. All authors contributed to manuscript editing and approved the final manuscript.
ACKNOWLEDGEMENTS
We would like to thank Ms. Wenting Zhu, Mr. Dongyue Yu and Mr. Haikun Liu for their help in the field work. We also thank Mr. Linhong Yu, Ms. Ying Ai, Ms. Yuzhen Han and Ms. Nana Wang for their help in the sample analysis. We thank the two anonymous reviewers for their helpful comments on an early version of this manuscript. This work was supported by the National Natural Science Foundation of China (31971491, 32201371).
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




