Land plant peptide signaling: What we know—and don't know—about its evolution
Abstract
The availability of genome sequences from diverse algal and plant taxa combined with the refinement of comparative genomics tools has begun to reveal how land plant genomes were shaped through duplication, repeat expansion, and gene family gains and losses. Of particular note is a large increase in the complexity and variety of signaling systems in land plants. Among these, signaling through small peptide ligand-receptor interactions has been considered one of the major innovations during land plant evolution. First discovered in angiosperms as mediators of various cell-to-cell communication processes, peptide signaling studies have been expanded to non-angiosperms, including bryophytes. Recent studies point to both common and unique roles for peptide signaling in distantly related species, raising interesting questions about how peptide signaling systems evolved and diversified. While the origin of peptide signaling systems remains elusive, progress in sequencing algal genomes offers clues to understanding the evolution of peptide receptors. This article discusses recent studies of small peptide-mediated signaling systems and highlights current gaps in our knowledge and new avenues for research, which could help us address how peptide signaling systems evolved and contributed to plant terrestrialization.
1 INTRODUCTION
The evolution of land plants permanently transformed ecosystems on Earth. It enabled heterotrophs to expand their niches and flourish on land. It is now largely accepted that extant land plants consist of two monophyletic lineages, namely bryophytes and vascular plants (Figure 1A). In bryophytes, three lineages (hornworts, mosses, and liverworts) diversified within a relatively short time after their common ancestor diverged from ancestral vascular plants (Morris et al., 2018). Among vascular plants, angiosperms diverged more recently and dominate today's land flora (Cheng et al., 2018). Angiosperms include the most intensively studied model plants in the era of biochemistry and molecular genetics. These studies accelerated discoveries of a new class of signaling molecules in plants, endogenous short signaling peptides, and their receptors (Olsson et al., 2019). Small peptide-mediated signaling has been shown to play various roles in morphogenesis, growth regulation, environmental responses, and reproduction. In recent years, comparative genomics and functional characterization have begun to shed light on its wide conservation and roles in non-angiosperm species. Land plants originated from an ancestral lineage of charophytes, a paraphyletic group of freshwater and terrestrial algae (Bowles et al., 2023; Bowman, 2022; Domozych et al., 2016) (Figure 2). Chlorophytes are a sister group to land plants and charophytes (collectively called streptophytes) that are found in marine, freshwater, and terrestrial environments. While multicellularity evolved independently multiple times in green plants, land plants are the only lineage in which complex multicellularity with three-dimensional differentiated tissues evolved (Bowman et al., 2016). The persistence of three-dimensional tissue organization requires coordination of development and physiology among cells that may not be clonally related. This underscores the necessity of intercellular communication during land plant evolution. Indeed, a link between the expansion of signaling components and plant terrestrialization has been suggested (Bowman, 2022; Bowman et al., 2017). Analyses of genome and transcriptome sequences from diverse algal species have also begun to provide insight into how land plant peptide signaling systems originated. This review discusses our growing understanding as well as outstanding questions about peptide signaling systems from a broad molecular evolutionary perspective.
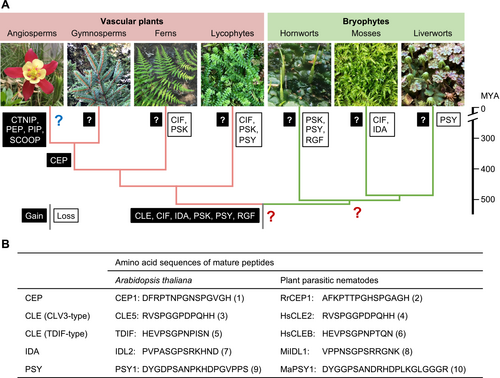
Predicted evolutionary history of land plant signaling peptides and examples of peptide mimics.
(A) Evolutionary gains and losses of signaling peptides were deduced based on the presence or absence of homologs for the Arabidopsis thaliana genes encoding CEP, CIF, CLE, CTNIP/SCREW, IDA, PEP, PIP, PSK, PSY, RGF/GLV/CLEL, and SCOOP peptides (Furumizu and Sawa, 2021a; Furumizu and Aalen, 2023). Question marks in black boxes indicate that it is not known whether unknown peptide signals evolved in non-flowering plants. This raises two more questions (large red question marks). First, it remains unknown whether new peptides evolved in the common ancestor of bryophytes after it diverged from the lineage that led to extant vascular plants. Second, unknown peptide signals may have evolved in the last common ancestor of vascular plants and bryophytes and been subsequently lost in angiosperms (large blue question mark). These lost peptides would have been impossible to find in previous studies focusing on a few angiosperm models. Image credits: hornwort image by Taichi Ikematsu, liverwort image by Hidefumi Shinohara, and other images by Chihiro Furumizu. Divergence dates are based on (Hu et al., 2023). MYA, million years ago.
(B) Examples of peptide mimics found in plant parasitic nematodes. RrCEP1 is from Rotylenchulus reniformis, HsCLE2 and HsCLEB are from Heterodera schachtii, MiIDL1 is from Meloidogyne incognita, and MaPSY1 is from Meloidogyne arenaria. Post-translational modifications are not indicated. Numbers in parentheses indicate the following references: 1, (Ohyama et al., 2008); 2, (Eves-van den Akker et al., 2016); 3, (Cock and McCormick, 2001); 4, (Wang et al., 2011); 5, (Ito et al., 2006); 6, (Guo et al., 2017); 7, (Vie et al., 2015); 8, (Kim et al., 2018); 9, (Amano et al., 2007); and 10, (Yimer et al., 2023).
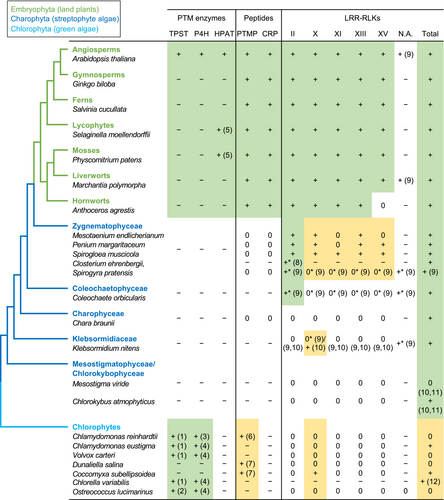
Conservation of peptide signaling-related genes in green plants.
The phylogenetic relationships are based on Jiao et al. (2020), Li et al. (2020), and Wang et al. (2020b). N.A. refers to LRR-RLKs that are not assigned to a specific subfamily. The gene conservation status is indicated as follows: +, presence of homologs; 0, absence of homologs; −, data not available; *, data based on transcripts. Numbers in parentheses indicate the following references: 1, EC:2.8.2.20 entries in UniProt (https://www.uniprot.org); 2, (Zhou et al., 2010); 3, (Velasquez et al., 2012); 4, EC:1.14.11.2 entries in UniProt; 5, (MacAlister et al., 2016); 6, (Oelkers et al., 2008); 7, (Zhang et al., 2020b); 8, (Sasaki et al., 2007); 9, (Bowman et al., 2017); 10, (Ngou et al., 2022); 11, (Gong and Han, 2021); and 12, (Dievart et al., 2011). The LRR-RLK data are based on reference 10 (Ngou et al., 2022) unless otherwise noted. Green boxes indicate that homologs of the given family are likely conserved. Yellow boxes indicate that the presence of a homolog is ambiguous. Note that LRR-RLKs of other subfamilies have also been reported to engage in peptide signaling.
2 SMALL SIGNALING PEPTIDES IN LAND PLANTS
Signaling peptides are generated from prepropeptides through multiple maturation steps and classified into two categories: post-translationally modified peptides (PTMPs) and cysteine-rich peptides (CRPs) (Figure 3A) (Olsson et al., 2019; Tavormina et al., 2015). Mature PTMPs are 5–30 amino acids long and characterized by enzymatic post-translational modifications, such as proline hydroxylation and glycosylation and tyrosine sulfation (Stührwohldt and Schaller, 2019). These modifications may affect PTMP activities via increased binding or stability. It is noteworthy that the mature forms have been determined only for a handful of peptides isolated from angiosperms; it is possible that other modifications remain to be discovered. CRPs are longer and characterized by an even number of at least four cysteine residues that form disulfide bridges necessary for proper secondary structures and activities. CRPs have diverse primary sequences and functions. Only a subset of CRPs, ranging from 40–160 amino acids, act as signaling molecules (Olsson et al., 2019; Tavormina et al., 2015). Hereafter, we focus mainly on PTMPs to discuss the evolutionary histories of signaling peptides.
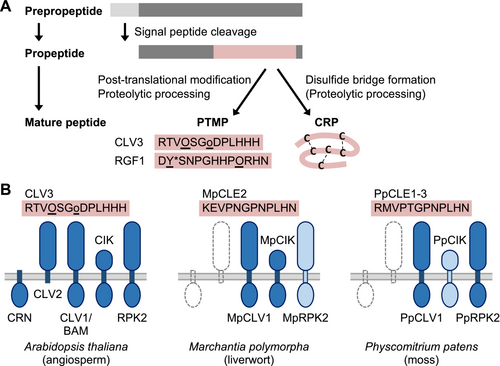
Biogenesis of signaling peptides and proteins involved in signal perception.
(A) Biogenesis of post-translationally modified peptides (PTMPs) and cysteine-rich peptides (CRPs) from prepropeptides. After the cleavage of signal peptides, PTMP propeptides undergo proteolytic processing and enzymatic modifications. Mature peptide sequences of Arabidopsis thaliana CLV3 and RGF1 are shown with known modified residues underlined. O, hydroxyproline; o, triarabinosylated hydroxyproline; and Y*, sulfated tyrosine. CRPs are defined by intramolecular disulfide bridge formation and may undergo proteolytic processing.
(B) PTMPs signal through specific LRR-RLKs and LRR-RLPs at the plasma membrane (light gray lines). (Left) Receptor components are summarized for A. thaliana CLV3 (Hirakawa, 2021). CLV1/BAM (LRR-RLKs XI) interacts with a co-recptor, CIK (LRR-RLK II). RPK2 (LRR-RLK XV) likely acts independently. CLV2, an LRR-RLP lacking a cytoplasmic domain, and CRN, a membrane-associated protein kinase, act together to transmit the CLV3 signal. In Marchantia polymorpha (middle) and Physcomitrium patens (right), homologous LRR-RLKs (dark blue) are known to mediate MpCLE2 and PpCLE signaling, respectively (Takahashi et al., 2021; Whitewoods et al., 2018). Functional characterization of MpRPK2 or PpCIK (pale blue) has not been reported. Dotted lines indicate that CLV2 and CRN homologs are absent in M. polymorpha and P. patens.
2.1 Evolution of small signaling peptides
All PTMPs with known functions were originally discovered in angiosperms through purification of secretory bioactive compounds, characterization of causative genes in developmental mutants, or in silico prediction. Later, they were shown to be conserved across species, to varying degrees [for some examples of pioneering studies, refer to (Cock and McCormick, 2001; Lorbiecke and Sauter, 2002)]. The phylogenetic distribution of prepropeptide-encoding genes indicates that six peptide families, namely CLE [CLAVATA3/EMBRYO SURROUNDING REGION-RELATED; (Cock and McCormick, 2001)], CIF [CASPARIAN STRIP INTEGRITY FACTOR (Okuda et al., 2020)], IDA [INFLORESCENCE DEFICIENT IN ABSCISSION; (Vie et al., 2015)], PSK [PHYTOSULFOKINE; (Yang et al., 2001)], PSY [PLANT PEPTIDE CONTAINING SULFATED TYROSINE; (Amano et al., 2007; Ogawa-Ohnishi et al., 2022)], and RGF/GLV/CLEL [ROOT MERISTEM GROWTH FACTOR/GOLVEN/CLE-LIKE; (Matsuzaki et al., 2010; Meng et al., 2012; Whitford et al., 2012)], were present in the most recent common ancestor of vascular plants and bryophytes (Figure 1). These peptide families have followed contrasting evolutionary trajectories between vascular plants and bryophytes (Table 1). Overall, peptide gene families have expanded substantially in vascular plants (e.g., Olsson et al., 2019; Whitewoods et al., 2018; Zhang et al., 2020b). Moreover, the variety of signaling peptides has increased in vascular plants; at least five peptide families evolved within seed plants (angiosperms and gymnosperms) (Figure 1A). In contrast, bryophytes have small peptide families, consistent with the low genetic redundancy in these plants. For example, two groups of CLE peptides are each represented by a single gene in the hornwort Anthoceros agrestis and the liverwort Marchantia polymorpha. Uniquely, the TDIF (tracheary element differentiation inhibitory factor)-type CLE genes are not found in mosses, while the number of CLAVATA3 (CLV3)-type CLE genes greatly increased in Physcomitrium patens, at least partly due to whole genome duplications in the moss lineage (Whitewoods et al., 2018; Zhang et al., 2020b). Reduced-expression analyses of seven CLE genes in P. patens suggested that these genes have redundant roles in the development of gametophores (multicellular haploid bodies formed during vegetative growth) (Whitewoods et al., 2018). It remains unknown whether the moss CLE genes underwent functional diversification/specification. Molecular genetics studies revealed that CLV3-type and TDIF-type CLE signaling are required for proper growth and development of the haploid body of M. polymorpha (Hirakawa et al., 2020; Hirakawa et al., 2019). These and other studies uncovered the functional analogy in CLE signaling between the diploid sporophytic meristems in angiosperms and the haploid gametophytic meristems in bryophytes (Whitewoods, 2021). Studies in non-angiosperms have also suggested neofunctionalization in CLE signaling. Namely, vascular patterning roles for TDIF are not conserved in lycophytes and likely evolved in euphyllophytes (ferns and seed plants) (Hirakawa and Bowman, 2015). The stem cell restricting activities of the angiosperm CLE genes could be derived characters rather than ancestral functions (Hirakawa, 2022). A better understanding of the functional and mechanistic diversity of CLE signaling may provide insight into the evolution of new cells, tissues, and organs.
| Representative mature peptide sequence* | Number of PTMP-encoding genes† | ||||
|---|---|---|---|---|---|
| Angiosperm | Hornwort | Moss | Liverwort | ||
| CEP | DFRPTNPGNSPGVGH | 15 (1, 2, 3) | 0 | 0 | 0 |
| CIF | DYGNNSPSPRLERPPFKLIPN | 4 (4) | 2‡ (22) | 0 | 1‡ (22) |
CLE (CLV3-type) |
RLVPSGPNPLHN | 29 (5, 6) | 1 (22, 23, 24) | 7 (25) | 1 (26) |
CLE (TDIF-type) |
HEVPSGPNPISN | 4 (7) | 1 (23) | 0 | 1 (26) |
| CTNIP/SCREW | GPVSGSGPNGCTNIPRGTPRCHG | 5 (8, 9) | 0 | 0 | 0 |
| IDA + IDL | PIPPSAPSKRHN | 9 (10) | 1 (22) | 0 | 2 (22) |
| PEP | ATKVKAKQRGKEKVSSGRPGQHN | 8 (11, 12) | 0 | 0 | 0 |
| PIP + PIPL | ASGPSRRGAGH | 11 (10, 13) | 0 | 0 | 0 |
| PSK | DYIYT | 7 (14, 15) | 0 | 1‡ (22) | 1‡ (22) |
| PSY | DYGDPSANPKHDPGVPPS | 9 (16) | 0 | 1 (22) | 0 |
| RGF/GLV/CLEL | DYSNPGHHPPRHN | 11 (17, 18, 19) | 0 | 0 | 1 (22) |
| SCOOP | AVETPPSRSRRGGGG | 28 (20, 21) | 0 | 0 | 0 |
- Numbers in parentheses indicate the following references: 1, (Ohyama et al., 2008); 2, (Delay et al., 2013); 3, (Roberts et al., 2013); 4, (Okuda et al., 2020); 5, (Cock and McCormick, 2001); 6, (Carbonnel et al., 2023); 7, (Ito et al., 2006); 8, (Rhodes et al., 2022); 9, {Liu, 2022 #5389}; 10, (Vie et al., 2015); 11, (Huffaker et al., 2006); 12, (Bartels et al., 2013); 13, (Toyokura et al., 2019); 14, (Yang et al., 2001); 15, (Kaufmann and Sauter, 2019); 16, (Ogawa-Ohnishi et al., 2022); 17, (Matsuzaki et al., 2010); 18, (Meng et al., 2012); 19, (Whitford et al., 2012); 20, (Gully et al., 2019); 21, (Hou et al., 2021); 22, (Furumizu et al., 2021); 23, (Li et al., 2020); 24, (Zhang et al., 2020a); 25, (Whitewoods et al., 2018); and 26, (Bowman et al., 2017).
- * The following Arabidopsis thaliana sequences are shown: CEP1 (At1g47485), CIF1 (At2g16385), CLE9 (At1g26600), CLE41 (At3g24770), CTNIP1 (At1g06135), IDA (At1g68765), PEP1 (At5g64900), PIPL3/TOLS2 (At4g37295), PSK1 (At1g13590), PSY1 (At5g58650), RGF1 (At5g60810), SCOOP1 (At5g44565). Note that post-translational modifications are not indicated.
- † The number of genes encoding PTMPs are shown for the angiosperm Arabidopsis thaliana, the hornwort Anthoceros agrestis, the moss Physcomitrium patens, and the liverwort Marchantia polymorpha.
- ‡ These sequences are atypical and lack some of the highly conserved residues. Further verification is needed to confirm their authenticity.
Gene losses also contributed to shaping lineage-specific catalogs of signaling peptides (Figure 1A). RGF/GLV/CLEL (hereafter RGF for brevity) peptides play diverse roles in angiosperms, such as maintaining root apical meristem activities, lateral root development, root gravitropism, and immunity (Kaufmann and Sauter, 2019; Rzemieniewski and Stegmann, 2022). In silico searches for RGF-like sequences identified potentially homologous peptides from both vascular plants and bryophytes (Furumizu and Sawa, 2021b). In bryophytes, while the RGF peptide family is highly conserved in liverworts, RGF-like sequences are found only in early-diverging lineages in mosses and are absent in P. patens and the hornwort species that have been examined. Consistently, RGF receptor homologs are present in M. polymorpha but absent in P. patens and A. agrestis, supporting the hypothesis that bryophyte RGF peptides signal through homologs of known RGF receptors (Furumizu et al., 2021). The effect of heterologous expression of M. polymorpha RGF in A. thaliana suggested that signaling activities are conserved in the liverwort RGF (Furumizu and Sawa, 2021b). Functional characterization of this M. polymorpha RGF could be instrumental in inferring ancestral functions of RGF signaling.
2.2 Dark matter—missing peptides to be uncovered?
Superficially, it appears that new peptide signals evolved only in three phases: in the common ancestor of vascular plants and bryophytes, in the common ancestor of seed plants, and during the evolution of angiosperms (refer to section 3.2 for further discussion). This may be a biased view, arising from the fact that all known signaling peptides were discovered in a few angiosperm model plants, preventing us from appreciating the full range of peptide signals; the possibility that novel peptide signals evolved within and outside of angiosperms has not yet been fully explored. Interestingly, sequences similar to plant peptide signals of both the CRP and PTMP classes are also found in organisms that interact with plants, including symbiotic fungi as well as bacterial, fungal, and nematode pathogens (Wu et al., 2021; Le Marquer et al., 2019). For example, small peptides that resemble CEP [C-TERMINALLY ENCODED PEPTIDE; (Ohyama et al., 2008)], CLE, IDA, or PSY were found in molecules secreted by plant parasitic nematodes (Gheysen and Mitchum, 2019; Yimer et al., 2023) (Figure 1B). These nematode peptides can act as plant peptide mimics and modulate plant development or immunity. A PSY-like peptide, RaxX, is produced by the pathogenic bacterium Xanthomonas oryzae (Pruitt et al., 2015). The origins and modes of action of genes encoding plant peptide mimics are not well understood. Importantly though, considering that mature signaling peptides are short, it is possible that mimetic peptide-encoding genes evolved de novo. It is similarly feasible that new signaling peptide-encoding genes evolved independently in each land plant lineage (Figure 1A). Congruently, discoveries of small secreted peptides with unknown receptors have been reported in angiosperm models and recently in the moss P. patens (e.g., Chen et al., 2014; Lyapina et al., 2021; Palit et al., 2023; Wang et al., 2020a). Elucidating their modes of action could provide evolutionary insight into the diversity of peptide-mediated intercellular communication. While our understanding of peptide signaling in angiosperms has advanced considerably, much less is known about peptide signaling systems in non-model plants and in non-angiosperms. The deep evolutionary nodes of divergence of the three bryophyte lineages and non-seed vascular plants warrant further biochemical and functional investigations in these groups.
3 EVOLUTION OF PEPTIDE RECEPTORS
3.1 Receptors for signaling peptides
Signaling peptides are perceived by specific receptors; plasma membrane-localized receptor-like kinases (RLKs) with a variable number of leucine-rich repeats (LRRs) in the extracellular domain have been identified as major receptor components. LRR-RLKs are assigned to distinct subfamilies according to the number and organization of LRRs (Shiu and Bleecker, 2001). LRR-RLKs of the subfamilies X, XI, XIII, and XV have been identified as the main receptors. They bind signaling peptides in their large ectodomains. LRR-RLKs of subfamily II have small ectodomains and act as co-receptors. For example, in Arabidopsis thaliana CLV3 signaling, LRR-RLKs XI [CLV1 and BARELY ANY MERISTEM (BAM)], LRR-RLKs II [CLV3 INSENSITIVE RECEPTOR KINASE (CIK)/CLE-RESISTANT RECEPTOR KINASE (CLERK)/NSP-INTERACTING KINASE (NIK)], and an LRR-RLK XV [RECEPTOR LIKE PROTEIN KINASE 2 (RPK2)/TOADSTOOL2 (TOAD2)] have been identified as receptor complex components (Hirakawa, 2021) (Figure 3B, left). These proteins are conserved in the liverwort M. polymorpha and the moss P. patens, and some of them have been shown to act as receptors of the homologous CLE peptides (Takahashi et al., 2021; Whitewoods et al., 2018) (Figure 3B, middle and right). Additionally, an LRR-receptor-like protein (LRR-RLP) that lacks a cytoplasmic domain (CLV2) and a membrane-associated protein kinase (CORYNE, CRN) are involved in CLV3 signal transduction in A. thaliana (Hirakawa, 2021). CLV2 and CRN are not conserved in bryophytes and likely evolved in the vascular plant lineage (Whitewoods et al., 2018). They may have enabled more precise control of CLV3 signaling or altered its function, as we discussed in section 2.1.
LRR-RLK subfamilies with large ectodomains have different ligand preferences. In A. thaliana, LRR-RLKs XI and XV act as PTMP receptors, while LRR-RLKs XIII act as CRP receptors (Olsson et al., 2019). Intriguingly, LRR-RLKs X have been shown to have more diverse signaling roles and include receptors for both PTMPs (PSK receptor, PSKR) and CRPs [TAPETUM DETERMINANT1 (TPD1) receptor, EXCESS MICROSPOROCYTES1 (EMS1)/EXTRA SPOROGENOUS CELLS (EXS)] as well as brassinosteroids (Furumizu and Sawa, 2021a; Olsson et al., 2019). Phylogenetic analyses have shown that major receptor groups within LRR-RLKs X or XI were already established in the common ancestor of vascular plants and bryophytes (Furumizu et al., 2021; Furumizu and Sawa, 2021a). It remains an open question how the LRR-containing ectodomain evolved and diversified in each LRR-RLK subfamily. Because of this diversity of the ectodomain sequences, inter-subfamily phylogenetic analyses rely on the short conserved cytoplasmic kinase domains, where the number of informative characters is limited. This led to little to no statistical support for deep nodes that represent phylogenetic relationships between different subfamilies (e.g., Liu et al., 2017a). Consequently, early evolutionary histories of peptide receptors remain elusive and present a major and exciting challenge.
3.2 A peptide-and-receptor riddle: Which came first?
As we discussed above, several signaling peptide families evolved within seed plants (Figure 1A). Among these new peptides, CEP, CTNIP/SCREW (SMALL PHYTOCYTOKINES REGULATING DEFENSE AND WATER LOSS), and PAMP-INDUCED SECRETED PEPTIDE (PIP) are similar to IDA in that the sequences are rich in proline, serine, and glycine residues (Table 1). These peptides are recognized by homologous LRR-RLKs XI represented by CEP RECEPTOR (CEPR), HAESA-LIKE 3 (HSL3), RECEPTOR-LIKE KINASE 7 (RLK7), and HAESA (HAE), respectively, in A. thaliana [reviewed in (Furumizu and Aalen, 2023)]. These receptors evolved and diversified through gene duplications in seed plants after their divergence from ferns (Furumizu and Aalen, 2023). That is, the evolution of new signaling peptides largely coincides with the diversification of pre-existing receptors. Receptor diversification via gene duplication may underlie subfunctionalization and neofunctionalization. Because ligands and receptors have co-evolved through physical interactions, both evolution of new signaling peptides and evolution of receptors can contribute to the elaboration of peptide-receptor pairs. Pertinent to this, two questions arise. First, are genes encoding similar signaling peptides homologous? Second, what are the ligands for homologous receptors in non-seed plants? It is challenging to address the first question because the sequence conservation is mostly limited to the short regions corresponding to mature peptides, making it hard to test their homology. Consideration of residues required for prepropeptide processing and/or refinement of analytic techniques using machine learning may improve our understanding (Srikant et al., 2023; Zhang et al., 2020b). In answering the second question, the IDA family peptides are widely conserved beyond seed plants and thus are strong candidates for the ligands. Notably, in the moss P. patens, IDA-like peptides were not found, but LRR-RLKs homologous to the HAE clade receptors are present (Figure 1A) (Bowman et al., 2017; Furumizu et al., 2021). It is both important and interesting to characterize the moss HAE-like LRR-RLKs and identify their ligands. In the next section, we take a step deeper and explore the origin of the peptide-receptor signaling system.
4 TRACING THE ALGAL ORIGIN OF PEPTIDE SIGNALING COMPONENTS
4.1 Inferring the algal ancestry of the signaling peptide biogenesis machinery
As discussed above, the ancestry of some signaling peptide families dates back to before the divergence between vascular plants and bryophytes. These ancient origins hint that signaling peptides may have been already present in an ancestral algal lineage within which land plants evolved. Thus far, no conclusive evidence has been reported for land plant peptide homologs in algae. Bioinformatic searches found CLE-like sequences in the unicellular chlorophyte algae, Chlamydomonas reinhardtii, Coccomyxa subellipsoidea, and Dunaliella salina (Oelkers et al., 2008; Zhang et al., 2020b) (Figure 3). However, their presumptive CLE motif amino acid sequences (e.g., RGLPAGETPEHH and RVVIIGQDPYHN from C. subellipsoidea and LDVPQGPNPLER from D. salina) are divergent from authentic mature CLE peptides (Figures 1B and 3) and, unlike land plant CLEs, are not located near the carboxy termini of the predicted prepropeptides (Zhang et al., 2020b). Whether they function as secreted signaling molecules remains unknown. Similarly, while one study reported the RALF-family of CRPs in a chlorophyte alga, Volvox carteri, a more focused search for RALF-like sequences found none in the chlorophytes analyzed (Campbell and Turner, 2017; Liu et al., 2017b). Given the widespread presence of CRPs with diverse sequences and functions in eukaryotes, exploring the diversity and possible signaling functions of CRPs in charophytes could provide insights into how peptide-mediated signaling systems evolved.
The chemical and functional diversity of signaling peptides can be increased by post-translational modifications (Figure 2A). Notably, certain modification enzyme homologs are present in algae. For instance, the tyrosylprotein sulfotransferase (TPST) originally identified in A. thaliana is responsible for tyrosine sulfation of signaling peptides (Komori et al., 2009). Subsequent bioinformatic searches revealed that TPST is conserved from angiosperms to the chlorophyte alga Ostreococcus lucimarinus (Zhou et al., 2010) (Figure 2). To date, tyrosine sulfation in land plants has been described only in signaling peptides. This implies that every plant species has the potential to produce sulfated peptides. Another prominent modification in signaling peptides is proline hydroxylation. While the enzymes responsible for this modification of signaling peptide precursors have not been identified, prolyl 4-hydroxylases (P4Hs) are regarded as candidates. P4H catalyzes the conversion of proline to hydroxyproline and has been characterized in various plants, ranging from angiosperms to the chlorophyte alga Chlamydomonas reinhardtii (Velasquez et al., 2012) (Figure 2). Hydroxyproline residues in signaling peptides can be further arabinosylated, as found in some CLV3/CLE peptides (Figure 3A) (Ohyama et al., 2009). This modification is initiated by hydroxyproline O-arabinosyltransferase (HPAT), which was also identified in A. thaliana (Ogawa-Ohnishi et al., 2013). Unlike TPST and P4H, HPAT orthologs are found exclusively in land plants (MacAlister et al., 2016) (Figure 2). In addition to post-translational modification enzymes, several proteases that mediate proteolytic processing of precursor peptides have been identified. Subtilases (SBTs) have been implicated as major proteases in plant signaling peptide biogenesis (Stührwohldt and Schaller, 2019, Olsson et al., 2019). Genes encoding SBTs form a large gene family, and only a few SBTs have been reported to engage in the processing of signaling peptides. Some of the signaling peptide processing SBTs belong to subfamilies that have significantly expanded by whole genome duplications or gene duplications in specific species/lineages, which could have contributed to differential regulation or biogenesis of signaling peptides (e.g., Taylor and Qiu, 2017; Xu et al., 2019). Phylogenetic analyses revealed that SBTs involved in signaling peptide biogenesis are conserved in charophytes and chlorophytes (Taylor and Qiu, 2017; Xu et al., 2019). Considering the phylogenetic distribution of all these processing enzymes, the biogenesis machinery of land plant signaling peptides appears to be an assemblage of components of algal origin and those specific to land plants.
4.2 LRR-RLK evolution in algae
It has been suggested that the structural combination of the extracellular LRR domain and the cytoplasmic kinase domain evolved independently in multiple eukaryotic lineages (Dievart et al., 2011). In the green plant lineage, LRR-RLKs are uniformly conserved in land plants and present in most charophyte species examined (Figure 2). In chlorophytes, sporadic occurrence of LRR-RLK genes has been reported (Dievart et al., 2011; Dievart et al., 2020; Liu et al., 2017a; Ngou et al., 2022). These chlorophyte LRR-RLKs were suggested to have evolved independently from those in streptophytes (Dievart et al., 2011; Dievart et al., 2020). Increased sequence sampling from diverse species facilitates more accurate comparisons and helps explain the sporadic distribution pattern in chlorophytes through independent gains or losses of LRR-RLK genes.
Intriguingly, it was recently suggested that some of the charophyte LRR-RLKs belong to the subfamilies that include land plant peptide receptors (Ngou et al., 2022) (Figure 2). This finding opens an exciting possibility that the birth of peptide receptors may have preceded the evolution of land plants. However, these data contradict another study, which did not assign algal LRR-RLKs with large ectodomains to a specific subfamily X, XI, XIII, or XV (Bowman et al., 2017). As we discussed above, phylogenetic relationships between different subfamilies have not been clearly resolved (Liu et al., 2017a; Man et al., 2020). Therefore, manual curation of candidate algal sequences and further phylogenetic analyses are required to elucidate the evolutionary relationships between land plant peptide receptors and similar LRR-RLKs found in charophytes.
On the other hand, LRR-RLKs II unambiguously evolved in charophytes (Figure 2). Angiosperm LRR-RLKs II function not only in endogenous peptide signaling as co-receptors but also in other signaling pathways, such as brassinosteroid signaling and detecting bacterial pathogen-associated molecular patterns (PAMPs) (Fontes, 2023; Liu et al., 2020). For multicellular eukaryotes to survive and flourish in any ecological niches, those with efficient innate immunity systems that control microbial colonization must have been naturally selected. Therefore, an interesting hypothesis is that one of the earliest functions of streptophyte LRR-RLKs II descended from pre-existing systems used to recognize pathogens or associated damage-associated molecular patterns (DAMPs). As far as we are aware, no functional or expression data have been reported for algal LRR-RLKs II. Elucidating their biological roles and modes of action could provide key insights into the evolution of land plant peptide signaling systems, which may have been assembled stepwise, similar to other signaling pathways such as auxin and ethylene (Bowman et al., 2019).
5 PERSPECTIVES
Chlorophytes and charophytes have independently adapted to terrestrial environments. It is therefore unlikely that peptide signaling, as we know it in land plants, was a prerequisite for terrestrialization. It is still possible that peptide signaling systems evolved in response to the development of complex multicellularity in land plants. Besides, peptides can act more efficiently as intercellular signaling molecules in terrestrial environments, leading to the expansion of peptide signaling systems in land plants. An open question is whether this expansion was preceded by the evolution of homologous peptide signaling systems in algae. While peptide receptor-like LRR-RLKs are present in extant charophytes, known signaling peptide families are likely specific to land plants (Figure 2). We think that this warrants further investigation given that unidentified signaling peptides may remain in less characterized lineages (Figure 1A). Indeed, a holistic approach would be needed to trace the land plant peptide signaling system to its origin. That is, we need to learn more about green algae and to further expand investigations into peptide signaling systems to include underexplored land plant lineages, combining various approaches from molecular genetics and biochemistry to genomics and informatics. In addition, peptide mimics that evolved possibly independently in microbes could provide insight into how genes encoding signaling peptides arise. In turn, molecular communication mediated by small signaling peptides could illustrate how species interact and evolve to shape complex ecosystems.
AUTHOR CONTRIBUTIONS
CF conceived the manuscript through discussions with HS. CF drafted the manuscript with input from HS. CF and HS finalized the manuscript.
ACKNOWLEDGMENTS
The authors apologize to those whose studies were relevant for this manuscript but not included due to space limitation. CF thanks Reidunn Birgitta Aalen for inspiring discussions over recent years. We thank John L. Bowman for valuable feedback on the manuscript. Investigations by CF were supported by grants from Sumitomo Foundation, LOTTE Foundation, Foundation of Kinoshita Memorial Enterprise, and the Graduate School of Integrated Sciences for Life at Hiroshima University. Investigations by HS were supported by grants from Grants-in-Aid for Scientific Research on Innovative Areas (20H05410, 22H04730), Kato Memorial Bioscience Foundation, Toyota Physical and Chemical Research Institute, Takeda Science Foundation, The Naito Grant for the advancement of natural science, and Sumitomo Foundation.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




