Bio-positive effects of ionizing radiation on pollen: The role of ROS
Abstract
The concept of ‘hormesis’ is defined as a dose–response relationship whereby low doses of various toxic substances or physical stressors trigger bio-positive effects in diverse biological systems, whereas high doses cause inhibition of cellular performance (e.g. growth, viability). The two-sided phenomenon of specific low-dose stimulation and high-dose inhibition imposed by a ‘hormetic-factor’ has been well documented in toxicology and pharmacology. Multitudinous factors have been identified that correspondingly cause hormetic effects in diverse taxa of animals, fungi, and plants. This study particularly aims to elucidate the molecular basis for stimulatory implications of ionizing radiation (IR) on plant male gametophytes (pollen). Beyond that, this analysis impacts general research on cell growth, plant breeding, radiation protection, and, in a wider sense, medical treatment. For this purpose, IR-related data were surveyed and discussed in connection with the present knowledge about pollen physiology. It is concluded that IR-induced reactive oxygen species (ROS) have a key role here. Moreover, it is hypothesized that IR-exposure shifts the ratio between diverse types of ROS in the cell. The interrelation between ROS, intracellular Ca2+-gradient, NADPH oxidases, ROS-scavengers, actin dynamics, and cell wall properties are most probably involved in IR-hormesis of pollen germination and tube growth. Modulation of gene expression, phytohormone signalling, and cellular antioxidant capacity are also implicated in IR-hormesis.
1 INTRODUCTION
Numerous studies demonstrate bio-positive effects of various toxic substances or physical stressors on diverse biological systems (Calabrese, 2014; Calabrese and Baldwin, 2001; Cedergreen et al., 2006; Luckey, 2006; Macklis and Beresford, 1991). These stimulatory effects are dose-dependently imposed specifically at low doses of the respective noxious factor, until detrimental effects occur when exceeding a specific threshold level (Figure 1A, II). Such fostering influences by low doses are collectively categorized under the general term of ‘hormesis’ (Calabrese, 2014; Luckey, 2006; Macklis and Beresford, 1991). The entire hormesis phenomenon most probably represents a ‘patchwork rug’. Here, diverse chemical and physical stressors (hormetic-factors) influence distinct cellular processes. Yet, considered in their entirety, each one brings about stimulating effects on biological systems in the end. Therefore, it is fascinating to inquire into the molecular fundamentals of individual hormetic-factors and identify the respective biochemical target pathways.
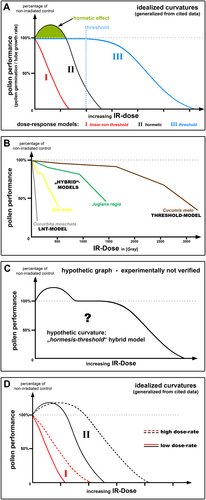
Dose–response models for IR-effects on pollen performance. (A) This diagram depicts idealized model graphs which illustrate the three commonly proposed dose–response models regarding the effects of IR on pollen performance. These generalized curvatures particularly demonstrate a comparison of the approximate proportional relation between the idealized model graphs. Note that IR doses which impose deteriorating effects and the respective lethal dose comparatively increase in the sequence ‘LNT-model’ < ‘hormetic-model’ < ‘threshold-model’. I: “linear no threshold model” (red curve). II: “hormetic model” (black curve). Green marked area shows the bio-positive/stimulatory implications of IR on pollen performance (germination and tube growth). III: “threshold model” (blue curve). Blue dotted line indicates threshold IR dose for occurrence of harmful effects. (B) Data-based graphs representing the widely accepted “LNT-model” and “threshold model”, as well as hypothesized “hybrid-models”. Explanation see text. All graphs were derived and compiled from published data from (Cuny et al., 1993; Kurtar, 2009; Pfahler, 1971; Sadat Hosseini Grouh et al., 2011). (C) This diagram depicts a speculative “hormesis-threshold hybrid graph”, which has not been experimentally verified. (D) This diagram shows four idealized graphs demonstrating the “dose-rate effect” of IR. Developed from data of studies cited in the text. Full lines: low dose rate. Dotted lines: high dose rate. I (red lines): linear non-threshold model. II (black lines): hormetic model.
The present study analyses hormetic influences, particularly of the environmental stress factor ionizing radiation (IR), which have been correspondingly reported regarding various cell types of fungi (Jakobsen et al., 2021), plants (Miller and Miller, 1987; Stephan, 2021), insects (Vimal et al., 2022), and mammals (Luckey, 2006). Varying levels of different ionizing radiations, such as X-rays, γ-rays, α-particles or β-particles, normally originate from natural sources (geological activities and cosmic radiation), as well as artificially from human activities (e.g. radioactive waste, nuclear accidents, and nuclear weapons). While high total absorbed doses of IR consistently impose detrimental effects on biological systems, low doses, on the other hand, have been reported to induce either harmful, stimulatory or no effects (Stephan, 2021; Tang et al., 2017). These observations resulted in three IR dose–response models, which are still debated in the radiobiological community today (Doss, 2018): 1) the ‘linear no threshold model’ (Figure 1A, I); 2) the ‘hormetic model’ (Figure 1A, II); and 3) the ‘threshold model’ (Figure 1A, III). Here, it should be mentioned that especially ‘radiation hormesis’, in contrast to the widely accepted ‘chemical hormesis’, has been a matter of critical discussion for decades (Calabrese and Baldwin, 2000; Luckey, 2006).
IR-induced hormesis has been observed after exposure to low doses of irradiation throughout all three eukaryotic kingdoms as enhancement of cellular survivability, as well as increase of growth and accelerated development (Lehrer and Rosenzweig, 2015; Tugay et al., 2006). However, the present study particularly aims to elucidate IR-related hormesis in plants. It has been variously reported that diverse low doses of IR impose bio-positive effects on numerous aspects of plant performance, for example, stimulation of pollen and seed germination as well as the growth of pollen tubes, seedlings, roots and shoots (Figure 1A, II; Figure 2) (Maity et al., 2005; Marcu et al., 2013; Miller and Miller, 1987; Nayak et al., 2015; Qi et al., 2015; Stephan, 2021; Thapa, 2004; Zanzibar and Sudrajat, 2016). To date, still too little is known about the molecular responses to abiotic stress in plants. Therefore, the present theoretical work seeks to pave the way for further analyses of molecular fundamentals concerning stimulatory implications of the stressor IR, particularly on plant male gametophytes (pollen) (Figure 1A, II). To this end, the effects specifically of ‘low doses’ from total absorbed ‘acute high-dose IR’ were surveyed. Nevertheless, hormesis by ‘low doses’ has generally been reported for ‘chronic low-dose IR’ as well. Hence, in the present work, a survey of currently available data was conducted to carve out the most plausible hypothesis concerning the molecular basis of IR-hormesis in plants, specifically in pollen. In this connection, reactive oxygen species (ROS) are hypothesized to play a key role.
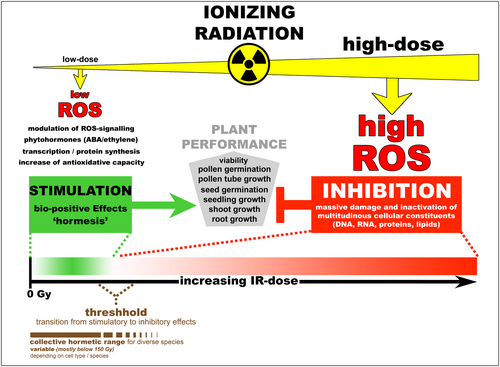
Schematic overview showing the connection between IR dose, ROS-level, and the occurrence of stimulatory or inhibitory effects on biological systems. This graphical abstraction summarizes the relation between low/high IR doses and the amount of IR-induced ROS. Moreover, the effects of different IR/ROS doses on plant performance are indicated. Green box and arrow indicate bio-positive effects (hormesis), whereas the red box and T-shaped indication represent inhibitory effects. The dose–response relationship for the “hormetic model” is depicted in a “coloured gradient bar graph”. Green: stimulation. White: no implications. Red: inhibition.
For a comprehensive review of the negative as well as positive effects of IR on plants in general, see (Caplin and Willey, 2018), and especially on pollen germination and tube growth, see (Stephan, 2021).
2 IR-BASED HORMESIS ON POLLEN PERFORMANCE
It is important to note that a specific hormetic-factor, such as low-dose IR, can have various implications on diverse cells. The same factor can stimulate one cell type or species, whereas it solely impairs the performance of another. This has been reported repeatedly, for example, regarding the differential implications of IR on the performance of plant male gametophytes (Stephan, 2021). Altogether, the surveyed studies about pollen of diverse taxa reveal manifold IR-dose response curvatures, which represent the three ‘standard models of dose-response’. A generalized comparison of the three idealized curvatures is given in Figure 1A. In summary, it can be concluded that IR-doses which impose deteriorating effects on pollen performance increase in the sequence ‘LNT-model’ < ‘hormetic-model’ < ‘threshold-model’ (Figure 1A). Lethal doses increase similarly (Figure 1A).
However, looking at data of individual species, some of these stand out because they demonstrate highly individual curve characteristics, which can be interpreted as ‘hybrid-type models’, specifically representing ‘step by step threshold curvatures’ (Figure 1B) (Pfahler, 1971; Sadat Hosseini Grouh et al., 2011). Such negative IR-effects on pollen performance in multiple stages can be explained by the distinct gradual inactivation of diverse cellular processes. This might either be caused by direct physical IR effects on bio-molecules or, as this study especially suggests in the following, through IR-generated ROS, which are produced by radiolysis of water. To pre-empt the next section (see section 3), elements of individual biochemical pathways are differentially susceptible to the quantity and composition of the cellular ROS-collective, which is changed in relation to the received radiation dose. Hormesis might be based on ‘multiple-step actions’, which means that low IR doses produce specific ROS levels that either have no effects or positively trigger signalling effectors, whereas increasing IR/ROS levels impair enzymatic activity (see section 3).
Most interestingly, among all surveyed data, a presumed ‘hormesis-threshold hybrid graph’ (Figure 1C) could not be identified in particular (Stephan, 2021). Why can such a hypothetical graph not be validated experimentally? This might indicate that ‘homeostasis of cellular performance’ (threshold model) as well as ‘stimulation of cellular performance’ (hormetic model), which both occur up to a certain threshold IR level, are based on the same molecular pathway system. Both graph types (Figure 1A, II and Figure 1A, III) might represent special variants of the same molecular network: Here, potentially cell-specific conditions of the individual ROS-homeostasis system.
Altogether, it can be stated that plant response to IR, in general, dramatically varies. Thus, from the entirety of all observations, the conclusion must be drawn that the action of IR on biological systems very likely depends on the distinct properties of species, individuals, tissues, cell types and their respective physiological conditions. This corresponds to a scenario which depicts IR effects as differential concerning the inherent molecular characteristics of diverse cells (Reisz et al., 2014; Stephan, 2023). In short, biochemical diversity and individual physiological status most probably represent the cause for the unequal impact of IR on various cell types.
Stimulatory effects on pollen germination and tube growth by low-dose IR have been documented for diverse gymnosperm and angiosperm species (Chauhan and Katiyar, 1998; Chauhan and Katiyar, 1990; Lecuyer et al., 1991; Livingston and Stettler, 1973; Møller and Mousseau, 2017; Pandey and Kumar, 2013; Seibold et al., 1979; Yigit et al., 2009; Zelles and Seibold, 1976), reviewed in (Stephan, 2021) (Figure 1A). Corresponding to pollen, radiation hormesis has also been reported regarding performance of sporophytic plant tissues, in particular seed germination, seedling growth, root growth, flag leaf area, photosynthetic rate, stomatal conductance, and plant nutrition (Abdel-Hady et al., 2008; Kim et al., 2005; Maity et al., 2005; Marcu et al., 2013; Nayak et al., 2015; Qi et al., 2015; Singh and Datta, 2010b; Singh and Datta, 2010a; Thapa, 2004; Zanzibar and Sudrajat, 2016; Fornalski et al., 2012).
By comparison, bio-positive effects on plant gametophyte and sporophyte primarily occur in a dose range below ~150 Gy (for acute doses), however, those various cellular systems do not exhibit a specific, consistent dose for maximal stimulation (Figure 2). IR doses exceeding that range are mostly harmful, yet, some cases of IR-hormesis at considerably higher IR-levels have also been documented for pollen (Chauhan and Katiyar, 1990; Falque et al., 1992; Livingston and Stettler, 1973; Pandey and Kumar, 2013) and the sporophyte (Sidrak and Suess, 1974; Singh, 1974).
By and large, IR doses which induce hormesis relate to the respective lethal dose (LD) of individual taxa. LD widely vary, particularly between cell types of diverse kingdoms. This means concerning animals that hormesis occurs in a very low dose range (<1 Gy) because animal physiology is highly IR-sensitive (LD ~ 10 Gy). Whereas pollen represents extremely IR-tolerant systems (on average above 1000 Gy), correspondingly, their hormetic dose range is significantly higher (Stephan, 2021).
In this context, it should be highlighted, particularly concerning IR effects in general, that the exposure dose rates (Gy/h) are important. An equal total IR dose which is received at chronic low dose-rates (LDR) imposes stronger inhibitory effects on pollen performance than when received at acute high dose-rates (HDR) (Kovalchuk et al., 2007; Møller and Mousseau, 2017; Møller et al., 2016; Zelles and Seibold, 1976), for pollen reviewed by (Stephan, 2021) (Figure 1D). Likewise, bio-positive IR-effects demonstrate a similar dependence on the applied dose-rate (Stephan, 2021; Zelles and Seibold, 1976) (Figure 1D). Both dose–response curve shapes (LNT and hormetic model) are affected by changes of dose-rate (Figure 1D). In summary, it can be concluded that increase of IR exposure-rates particularly shifts harmful effects to higher IR-doses, hence leading to higher LD (Figure 1D). Interestingly, the IR-dose range for hormesis is likewise shifted to higher doses (Figure 1D). It should be highlighted that, to date, still too little is known about the influence of dose-rate concerning IR-exposure of biological systems (Lowe et al., 2022).
Yet, how can this shift to higher doses be explained? Several studies demonstrate IR-induced changes in transcription profiles in diverse plant cells (reviewed in (Stephan, 2023)), yet studies about pollen are still missing. The available data demonstrate that a substantial number of genes that are related to oxidative stress and the enzymatic antioxidant system of plant cells were particularly deregulated by IR, as well as heat shock proteins and chaperones. The performed microarray experiments on irradiated plants show that the number and mRNA levels of transcriptionally deregulated genes greatly vary according to irradiation conditions: acute or chronic and high or low IR doses (Gicquel et al., 2012; Kovalchuk et al., 2007; Stephan, 2023). This complicates experimentation, just as different developmental conditions or cell cycle status in plant tissues during IR exposure. All these obstructions are most probably the cause for variation between manifold reports about IR-hormesis.
All things considered, specifically regarding acute high dose rates, cells are forced to give a more rapid and powerful response than chronic low dose rates in comparison. Therefore, a greater burst of antioxidants and heat shock proteins is required, which might be responsible for the extension of hormetic effects and LD to higher doses (Figure 1D).
Moreover, different ROS-scavengers are specifically produced corresponding to IR dose (low: antioxidant compounds/metabolites; high: antioxidant enzymes) (Gicquel et al., 2012). Hence, depending on IR exposure variations, the composition of the total cellular ROS-collective varies.
3 THE ROLE OF REACTIVE OXYGEN SPECIES IN IR-HORMESIS
Regarding biological systems, the detrimental consequences of exposure to IR are many-faceted. Their molecular constituents are directly damaged by radiation through energy transfer. In addition, IR induces the generation of a variety of harmful molecules, such as free radicals, which affect physiological processes as an indirect consequence of irradiation. Particularly, one class is of utmost importance due to their ubiquity. ROS are produced by radiolysis of intra- and extracellular water and represent a diverse group of highly reactive chemicals (hydroxyl radicals, hydrogen peroxide, superoxide radicals, singlet oxygen) (Figure 3) (Desouky et al., 2015; Hall and Giaccia, 2006; Hutchinson, 1966; Reisz et al., 2014; Riley, 1994; Saha, 2012; Singh and Singh, 1982; Wardmann, 2009).
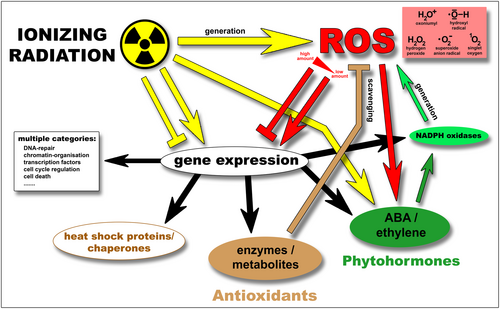
Diagram indicating the effects of IR and associated ROS on various cellular constituents. This schematic overview outlines implications of IR, thereby showing mutual effects and interrelations of IR-induced ROS on gene expression, biosynthesis, and functionality of antioxidant enzymes, antioxidants, HSPs/chaperones, and NOX. For detailed explanation, see text chapter 3. Arrows indicate positive effects (e.g., activation, induction, stabilization), whereas the T-shaped indications represent inhibitory effects (repression, damage). Colours correspond to the respective source of effect. Yellow: Ionizing radiation (IR). Red: Reactive oxygen species (ROS). Brown: Antioxidant enzymes, antioxidative compounds/metabolites. Light green: NADPH oxidases (NOX). Dark green: Phytohormones (abscisic acid, ethylene).
ROS can be toxic to cells, however, they also play regulatory roles in numerous physiological processes, for example, in cell growth, cell division, differentiation and development, as well as responses to biotic and abiotic stimuli (Czarnocka and Karpinski, 2018; Foyer and Noctor, 2016). It has been demonstrated that ROS act as signalling molecules in the regulation of processes like pollen germination and tip growth of pollen tubes (Liu et al., 2009; Maksimov et al., 2018; Pasqualini et al., 2015; Speranza et al., 2012) as well as root hairs (Carol and Dolan, 2006; Carol et al., 2005; Foreman et al., 2003; Zhang et al., 2022). In this connection, ROS are endogenously generated by cellular NADPH oxidases (NOX) (Figure 3) (Cardenas et al., 2006; Foreman et al., 2003; Kaya et al., 2015; Kaya et al., 2014; Kaya et al., 2019; Lassig et al., 2014; Potocky et al., 2007). Whereas ROS-scavenging enzymes (superoxide dismutase, catalase) antagonize the NOX-generated ROS (Figure 3). Consequently, a ROS-homeostasis system maintains the intracellular and apoplastic steady-state of endogenous ROS-production and ROS-scavenging, thereby generating a tip-directed ROS-gradient that is required for orderly elongation of pollen tubes (Duan et al., 2014; Maksimov et al., 2018). ROS influence the tip growth of pollen and root hairs by promoting rigidification of the cell wall and activation of Ca2+-channels (Foreman et al., 2003; Maksimov et al., 2018; Monshausen et al., 2007). Hereby, ROS affect the tip-directed gradients of Ca2+ and pH, which are connected to actin dynamics (Carol and Dolan, 2006; Mori and Schroeder, 2004; Pasqualini et al., 2015). As a consequence of high ROS levels or elevated Ca2+ concentration, the actin architecture is massively reorganized, thereby correspondingly forming “actin punctate foci” in pollen tubes (Snowman et al., 2002; Wilkins et al., 2011).
Biogenic synthesis of ROS in pollen, particularly hydrogen peroxide (H2O2), has been associated with early stages of germination, and the same applies to exogenous ROS, which are produced by the female stigma (Breygina and Klimenko, 2020). ROS play a role in intercellular communication between male and female gametophytes during tube growth in the pistil (Zhang et al., 2020). Hence, pollen is susceptible to internal and external ROS cues, which both influence its performance in plant sexual reproduction.
The physiological ROS levels, which play a role in plant signalling, are tightly controlled and localized at specific subcellular areas (Duan et al., 2014; Kaya et al., 2015; Kaya et al., 2014; Kaya et al., 2019; Lassig et al., 2014; Potocky et al., 2007; Speranza et al., 2012; Wudick and Feijo, 2014). However, in contrast, the excessive large amounts of ROS that are ubiquitously induced by acute high-dose IR impose devastating effects globally on a wide variety of cellular constituents, such as DNA, RNA, proteins, and lipids, thereby dramatically impairing cellular viability (Stephan, 2021).
By this study, it is hypothesized that, on the other hand, specifically, the ‘low to moderate amounts’ of ROS, which are exogenously induced by low-dose IR, are the major cause of stimulatory IR effects on plant growth and development (IR-hormesis). Most likely by deregulation of ROS-related cell-signalling (Figure 2, Figure 3). Alteration of actin dynamics through ROS might play a role here. In addition, also modulation of cellular antioxidant capacity, such as increased activities of SOD, APX, and ascorbate (Kim et al., 2005), which leads to increased stress resistance, most probably improves cellular performance (Figure 3).
Regarding the role of ROS in radiation-hormesis of pollen, differences between angiosperms and gymnosperms are of significance. ROS levels that stimulate the performance of gymnosperm pollen are higher than for angiosperm pollen in comparison (Maksimov et al., 2018; Smirnova et al., 2014). This particularly corresponds to the comparably higher radiation tolerance of gymnosperm gametophytes (Stephan, 2021).
It should be highlighted that the general term ROS represents a multiplicity of highly reactive molecules (Figure 3). Therefore, not only the total amount but the ratio between individual components of this multifaceted molecular collective is of significance. It should be considered that the proportional composition of total cellular ROS is crucial for pollen germination and tube growth. For example, hydroxyl radicals (.OH) loosen the coherence of cell wall polysaccharides, whereas H2O2 molecules increase cell wall integrity (Breygina and Klimenko, 2020; Smirnova et al., 2014).
IR generates a specifically composed group of non-biogenic extra- and intracellular ROS, which affect the cell's redox state and disturb the natural ROS-collective. Under aqueous conditions, the major class of ROS generated by IR are hydroxyl radicals (.OH) and ionized water (H2O+). Whereas H2O2, superoxide anion radicals (.O2−), and diverse organic radicals are secondary products of IR-induced ROS, whose intracellular stoichiometry is influenced by chemical reactions of molecular constituents in the cell, e.g. metal ions (Reisz et al., 2014; Riley, 1994). Accordingly, it can be deduced that IR not only increases the total cellular ROS quantity but IR shifts the molecular composition of the intrinsic ROS-collective. As a consequence, various essential processes during pollen germination and growth are potentially affected, for example, the regulation of mechanical properties of the cell wall by .OH and H2O2.
Furthermore, the IR-induced alteration of ROS quantity and ratio has the potential to deregulate gene transcription (Figure 3). Gadjev et al. show that specific transcription factors are deregulated by different types of ROS (hydrogen peroxide, superoxide, and singlet oxygen) (Gadjev et al., 2006) (Figure 3).
Moreover, Nagata et al. relate the stimulation of root hair elongation through IR (γ-rays) to the induced ROS, which activate biosynthesis of the phytohormone ethylene (Nagata et al., 2004). Moreover, they suggest that gamma-irradiation induces trichome formation via its normal developmental pathway (Nagata et al., 1999). Here, an IR-responsive interrelation between ROS, the TTG1 (WD40) protein, and the antioxidants ascorbic acid and anthocyanin might play a role (Nagata et al., 1999; Nagata et al., 2003) (Figure 3).
Regarding phytohormones, Qi et al. demonstrate that Arabidopsis seed germination, seedling and root growth were stimulated by low-dose γ-irradiation, with maximal bio-positive effects at 50 Gy (Qi et al., 2015). Under these IR conditions cellular H2O2 levels were increased, and the phytohormone abscisic acid (ABA) was induced (Figure 3). Elevated H2O2 levels or bio-positive effects were not observed in ABA-deficient aba2-1 mutants (Qi et al., 2015). Transcript levels of NADPH oxidases NOX were increased ~30% at 50 Gy (Qi et al., 2015), which has been described similarly by additional studies (Cho et al., 2000; Kim et al., 2007; Kim et al., 2021) (Figure 3). Moreover, this IR-range induced transcriptional upregulation of various ABA-biosynthesis genes, ABA-transporters, and ABA-regulators (Kim et al., 2014; Kim et al., 2007; Qi et al., 2015; Ya et al., 2012) (Figure 3).
In concordance with known plant responses to other stressors, this suggests that the signalling molecules ABA, NOX, and H2O2 are very likely to be involved in IR response and, potentially, also in triggering of bio-positive IR-effects (Figure 3). However, regarding this scenario, the important question remains how IR might be sensed by the ABA stress pathway. Why is expression of ABA-related genes upregulated by IR?
Unfortunately, Qi et al. solely examined H2O2 and disregarded the multiplicity of different ROS which are produced by IR. They entirely allocate the elevated cellular ROS to endogenous production by NOX. However, it is very unlikely that 50 Gy γ-irradiation did not generate different IR-related ROS. It should be emphasized that ABA has been associated with oxidative stress in plants, thereby inducing the signal molecule H2O2 and the expression of antioxidant genes as well (Gietler et al., 2020). In recognition of this fact, it is far more likely that primarily IR induces specific types of ROS, which subsequently elicit the “ABA stress-pathway”.
In addition, the interrelation between actin and ROS (Stephan, 2023) might play a role concerning the observed bio-positive effects of low IR doses on pollen tube growth. The positive feedback loop between NOX(RBOH)-produced ROS, ROS-dependent Ca2+-channel activation, and Ca2+-dependent NOX(RBOH) activation altogether have to be fine-tuned for orderly polarized growth (Kaya et al., 2014; Wudick and Feijo, 2014). Here, the apical-directed Ca2+-gradient influences actin cytoskeleton dynamics through the regulation of ABPs and protein kinases (CDPKs) (Allwood et al., 2001; Holdaway-Clarke and Hepler, 2003; Yang et al., 2021). In turn, the actin cytoskeleton correspondingly affects cellular Ca2+-homeostasis (Cardenas et al., 2008; Qian and Xiang, 2019; Scholz et al., 2020; Wang et al., 2004). As a consequence, specifically, those ROS which are induced by low-dose IR potentially deregulate this tightly controlled signalling system and thus hormetically promote growth, whereas high IR doses and their associated ROS primarily impose detrimental effects.
In this connection, it is interesting that Morré suggests NOX as a molecular target for chemical hormesis of cell growth (Morre, 2000).
In summary, low IR doses induce supplementing ROS, which support the cellular ROS-homeostasis system and enhance ROS-signalling. On the other hand, high IR levels generate excessive ROS which massively disturb the cell's fine-tuned ROS entity and, above all, comprehensively damage numerous cellular constituents.
4 CONCLUSION AND PERSPECTIVES
All in all, this study hypothesizes molecular fundamentals for the radiation-hormesis phenomenon, particularly regarding IR (X- and γ-rays, α-particles). One should bear in mind that due to protecting environmental conditions, such as the earth's shielding atmosphere, no biological system evolved a direct molecular detection system for these ionizing radiations. So, how can cells detect radiation? How are transcription of specific genes or other cellular processes deregulated by IR? Which are the molecular targets and mediators of IR? In this connection, IR-induced ROS are major stressors in IR-exposed cells and hence might represent one possibility for organisms to measure IR levels indirectly. Above all, particularly low amounts of specific ROS represent the most plausible facilitators for IR-hormesis in plant gametophytes, sporophytes, and, very likely, in most other cellular systems. This article draws attention to the various types of ROS which are induced by IR. Their composition and mutual ratio most probably influence cellular response to IR. Yet, the different qualities and quantities of diverse ionizing radiations and their associated ROS types should be more closely observed to exactly determine the basis of IR effects, for example, IR-hormesis. It is concluded that deregulation of the cellular ROS-homeostasis system through those ROS, which are specifically generated by low-dose IR, represents a key element. Especially cellular processes facilitating pollen germination and polarized tube growth are affected. In this context, the interrelation between ROS and the actin cytoskeleton is of potential significance. The particular ROS, which are induced by low IR doses, influence Ca2+-levels. These changes in Ca2+- and ROS-signalling thereby trigger the reorganization of actin architecture and influence tip-directed membrane transport. Moreover, exogenous ROS modify the cell wall properties that are essential for turgor-driven pollen tube elongation. Unfortunately, to this day, there are still numerous open questions concerning the role of ROS in actin remodelling of plants. Specifically, the effects of IR-associated ROS have been insufficiently investigated by only a few studies reviewed by (Stephan, 2023; Stephan, 2021). The interplay of eclectic network components which take part in the regulation of pollen tube germination and growth demonstrates itself as complex, and despite numerous studies on this subject, our picture is still fragmental. Specifically regarding IR-hormesis, numerous open questions remain, which should be answered because this intriguing phenomenon holds the potential to contribute to many beneficial areas for human society. For example, the improvement of agriculture and radiation protection but also, in a wider view, the advancement of human medicine.
ACKNOWLEDGEMENTS
The author thanks Hildegard Stephan for helpful comments.
CONFLICT OF INTEREST STATEMENT
The author declares that there is no conflict of interest concerning the research performed in this study.
Open Research
DATA AVAILABILITY STATEMENT
Data sharing is not applicable to this article as no new data were created or analyzed in this study.




