Antioxidant and Secondary Metabolite Responses in Wheat under Cereal Leaf Beetle (Oulema melanopus L.) Infestation
Abstract
Wheat is one of the most important cereal crops grown in the Western Himalayas of India but its production is challenged by the insect “cereal leaf beetle (CLB)”. This study explores the impact of domestication and modern crop improvement on wheat's defense mechanisms against the CLB, a global threat to wheat cultivation. Sixteen diverse wheat genotypes having different ploidy levels were investigated, including wild wheat, landraces, mutants, advanced breeding lines, commercial varieties, and a Rye grass genotype. Genotypes with resistance genes, landraces, and wild wheat exhibited the lowest CLB infestation and oxidative damage. These genotypes displayed enhanced antioxidant enzyme activity, such as peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD), and polyphenol oxidase (PPO), reduced reactive oxygen species (ROS) production, and increased plant secondary metabolite (phenol and tannin) production upon CLB infestation. Resistant durum wheat demonstrated moderate responses and exhibited higher levels of flavonoid production. On the other hand, susceptible durum wheat genotypes, commercial variety Shalimar Wheat-02 (SW-2), showed high CLB infestation, ROS production, reduced antioxidant enzyme and secondary metabolite production. This may be because modern varieties like SW-2 were not selected for insect resistance. These findings emphasize the significance of preserving genetic diversity from wild wheat and landraces to bolster wheat breeding programs against pests such as CLB. This study underscores the need to harness ancestral wheat lines to enhance insect pest resistance in modern wheat cultivars.
1 INTRODUCTION
Wheat is one of the most important staple food crops, providing approximately 20% of global food calories and proteins. It is also one of the earliest domesticated crops (Peleg et al., 2011). Various biotic and abiotic stresses challenge wheat production in the world. Among biotic stresses, the Cereal leaf beetle (CLB; Oulema melanopus L.) is a significant pest that threatens global wheat production. The CLB primarily affects cereals such as wheat, oats, barley, and rye. Adult beetles and grubs feed on wheat leaves and scrape the leaf surface, causing reductions in photosynthetic capacity and yield losses of up to 40% (Joukhadar et al., 2013). CLB is considered one of the most devastating pests, causing up to 100% leaf damage to wheat crops in the Kashmir valley of the Western Himalayas, India (Hussain and Ahmad, 2006). The use of chemical insecticides primarily controls the CLB infestation of wheat. However, this approach raises concerns about environmental sustainability and potential risks to human health. Therefore, developing insect-resistant wheat varieties is a sustainable approach to minimizing yield losses and reducing dependence on chemical pesticides.
During evolution, plants developed constitutive and inducible defenses against insect herbivory (Kaplan et al., 2008; War et al., 2012). Constitutive production of plant secondary matabolites (PSMs) serves as the initial line of defense against herbivores. These preexisting chemical compounds deter or harm potential antagonists. Induced production of PSMs, triggered by herbivore attacks or cues, expands the range of interactions between plants and their antagonists (Kessler, 2015; Belete, 2018). A two-step strategy is employed in plants to induce defense mechanisms to counteract various stressors. Initially, plants rely on producing antioxidants as their front line of defense against oxidative stress, and antioxidants function by scavenging and neutralizing ROS, thereby mitigating the potential damage caused by these highly reactive molecules. Subsequently, plants synthesize secondary metabolites, such as phenols, flavonoids and tannins, which serve as additional layers of defense. These secondary metabolites are pivotal in plant protection, acting as deterrents or toxins against herbivores, pathogens, and environmental stressors (Bi and Felton, 1995; Chen, 2008; Kaur et al., 2017; Malik et al., 2023).
Domestication involves the intentional selection and cultivation of wild plants with desirable traits. Over time, this process has favored the development of specific genotypes that exhibit preferred characteristics, such as increased yield, uniformity, and ease of harvest. As a result, the genetic diversity present in wild wheat populations diminished, as only a fraction of the genetic variation present in the wild progenitors was retained in cultivated wheat (Reif et al., 2005; Van de Wouw et al., 2010; Smith et al., 2015; Venske et al., 2019). The reduction in genetic variation in cultivated wheat has several consequences. It diminishes the capacity of wheat plants to respond to changing environmental conditions, including challenges in insect pests and pathogen population pressure (Singh et al., 2013; Shahzad et al., 2021). Modern improved varieties of crops, such as wheat, often exhibit a poor inclusion of insect-resistance genes from their wild relatives (Byerlee, 1996; Keneni, 2012). Incorporating a broader range of resistance genes from diverse genetic sources, including wild relatives, landraces, and unexplored germplasm, can enhance the resilience of modern wheat varieties against evolving insect pests (Tanksley and McCouch, 1997; Chakraborty and Newton, 2011; Lopes et al., 2015).
Given the emergence of CLB as one of the devastating pests for wheat cultivation in the Kashmir valley of the North-western Himalayas, we investigated the potential of antioxidants and PSMs as a source of resistance against CLB using a set of 16 diverse wheat genotypes having different ploidy levels. For instance, diploid A. tauschii, tetraploid durum wheat and hexaploid landraces, mutants, the commercial variety ‘Shalimar Wheat-02’ (SW-2) were used during the present study. In addition, an alternate host of cereal leaf beetle, “Ryegrass”, was also used in this study. We aimed to assess the effectiveness of different wheat genotypes in exhibiting resistance to CLB and to examine the impact of crop improvement on the retention or erosion of insect resistance genes inherited from wild progenitors.
2 MATERIALS AND METHODS
2.1 Plant materials and experiment location
The materials utilized in the present study comprised a diverse set of 16 wheat genotypes having different ploidy levels, including diploid, tetraploid and hexaploid. The 16 genotypes included three accessions of diploid D-genome progenitors of wheat A. tauschii (AT), three tetraploid durum wheat genotypes (DM), three landraces (LR) of hexaploid wheat, one commercially released wheat variety named Shalimar Wheat-02 (SW-2), which exhibited susceptibility to the CLB, three mutants of SW-2, two advanced breeding lines SAB-72 and SAB-74, and a Rye grass genotype (RG-AH) (Table 1). The selection of these genotypes is based on rigorous field screening for CLB resistance from 2021 and 2022. The reaction of these genotypes towards CLB infestation is available (Table 1).
| S. No | Ploidy/genome | Accession number | Designation given for present study | Reaction to CLB |
|---|---|---|---|---|
| A. Diploid wheat gene pool | ||||
| 1 | A. tauschii (DD) | TOWWC060 | AT1R | Resistant |
| 2 | A. tauschii (DD) | TOWWC050 | AT2R | Resistant |
| 3 | A. tauschii (DD) | TOWWC069 | AT3S | Susceptible |
| B. Tetraploid wheat gene pool | ||||
| 4 | T. durum (AABB) | NA | DM1R | Resistant |
| 5 | T. durum (AABB) | NA | DM2R | Resistant |
| 6 | T. durum (AABB) | NA | DM3S | Susceptible |
| C. Hexaploid (AABBDD) wheat gene pool | ||||
| C.1 Landraces | ||||
| 7 | Hexaploid (AABBDD) | EC105959 | LR1R | Resistant |
| 8 | Hexaploid (AABBDD) | IC0615576 | LR2R | Resistant |
| 9 | Hexaploid (AABBDD) | IC28544 | LR3S | Susceptible |
| C.2 Advanced breeding line | ||||
| 10 | BC2F4[PBW343-RS111]/3*PBW550)X PBW550 + Yr15 | SAB72 | SAB72 | Resistant |
| 11 | BC2F4[PBW343-RS111]/3*PBW550)X PBW550 + Yr15 | SAB74 | SAB74 | Resistant |
| C.3 Mutant | ||||
| 12 | Shalimar wheat 2 mutant | NA | SW2-M1R | Resistant |
| 13 | Shalimar wheat 2 mutant | NA | SW2-M2R | Resistant |
| 14 | Shalimar wheat 2 mutant | NA | SW2-M3S | Susceptible |
| C.4 Rye grass (RR) | ||||
| 15 | Rye grass | NA | RG-AH | Susceptible |
| C.5 Check | ||||
| 16 | Shalimar wheat 2 | NA | SW2 | Susceptible |
The A. tauschii accessions and two advanced breeding lines were procured from the School of Biotechnology at Punjab Agriculture University (PAU) in Ludhiana, Punjab, India. The advanced breeding lines are known for their combination of high grain weight derived from Rye selection III and the presence of the stripe rust resistance gene Yr15 in the PBW550 background, as described by Kaur et al. (2020). The landraces were acquired from the National Bureau of Plant Genetic Resources (NBPGR), New Delhi, India, SW-2 mutants were developed in the Division of Genetics and Plant Breeding, at Faulty of Agriculture, Sher-e-Kashmir University of Agricultural Sciences and Technology (SKUAST), Wadura campus, Kashmir, India. The material was evaluated in the fields of the Division of Genetics and Plant Breeding at the Faculty of Agriculture, SKUAST-Kashmir, under controlled field conditions. Plots were covered by insect-proof nets to prevent natural infestation and maintain the integrity of the field environment.
2.2 Experimental setup and release of CLB grubs for wheat genotype assessment
All 16 diverse wheat genotypes were evaluated in two lines of 1 meter in length, and replicated three times. A non-infested control was also grown alongside the same genotype infested with the CLB. Stringent measures were taken to prevent natural infestation by covering field plots with insect-proof nets to prevent natural infestation throughout the experiment. At the flag leaf stage, all genotypes were infested with a uniform number of third instar CLB grubs (2 grubs per plant) but a set of plants was left non-infested as control. These grubs were collected from an insect culture maintained on susceptible oat genotype Shalimar fodder oats “SF1 Oats.”. The introduction of the CLB grubs onto the plots was carried out using a delicate camel hairbrush. The grubs were allowed to infest the plants for a designated period of 3 and 5 days after infestation (DAI). The flag leaf samples from control and infested plots were collected for biochemical estimation 3 DAI and the CLB grubs were allowed to continuously infest the plants up to 5 days. Again, the leaf samples from control and infested plots were collected for biochemical estimation 5 DAI. The damaged and normal flag leaf samples soon after detaching from the plants were kept in a portable ice cooler until reaching the laboratory for analysis. Both infested and control plants were closely monitored during the 3- and 5-day intervals for CLB infestation. The severity of CLB infestation was assessed through phenotyping using a standardized scale (1 to 5) provided by Wurschum et al. (2020). In this scale, 1 = no damage; 2 = few lines of feeding damage on the upper leaf side; 3 = several lines of feeding damage; 4 = 50–70% of the flag leaf showing feeding damage; and 5 = more than 70% of the flag leaf showing feeding damage.
2.3 Biochemical assays
At both 3 and 5 days after infestation (DAI), a comprehensive array of biochemical assays was conducted to evaluate the plant's response to CLB infestation. These assays included measurements of oxidative damage markers such as hydrogen peroxide (H2O2) and lipid peroxidation. Furthermore, the activities of crucial antioxidant enzymes, including peroxidase (POD), ascorbate peroxidase (APX), catalase (CAT), superoxide dismutase (SOD), and polyphenol oxidase (PPO), were quantified. Additionally, secondary metabolites such as phenols, flavonoids, and tannins were quantified to determine their induction as a result of CLB infestation. The procedure followed for quantifying various biochemical parameters is available elsewhere (Jan et al., 2023; Shafi et al., 2023) and briefly outlined below:
2.3.1 Quantification of oxidative damages
Quantification of oxidative damage induced by CLB infestation was conducted using two methods. First, the level of H2O2 was determined following the methodology described by Ashraf (2009). Second, the extent of lipid peroxidation due to ROS induced by CLB infestation was assessed by estimating the malondialdehyde (MDA) content following the methodology by Halliwell and Chirico (1993). To accomplish this, the absorbance of each sample was measured at 532 nm and 600 nm using a Spectrophotometer (LABTRONICS LT.29). The nonspecific absorption values at 600 nm were subtracted from the corresponding values at 532 nm. The concentration of MDA was then calculated using the extinction coefficient of 155 mM−1 cm−1 and expressed as micromoles per gram of fresh weight (μmol g−1 FW).
2.3.2 Quantification of antioxidant enzymes
For enzyme extraction, plant tissue (0.2 g) was ground into a fine powder using a chilled mortar and pestle by adding liquid nitrogen. The ground plant tissue was then mixed with extraction buffer (phosphate buffer pH 6.8–7.0 + 3 mM EDTA+5% PVP) to facilitate the release of enzymes. The resulting mixture was typically centrifuged at 22,640 g for 15 min at 4°C to remove cellular debris, and the supernatant containing the enzyme extract was used for subsequent antioxidant enzyme analysis.
Peroxidase (POD) (EC 1.11.1.7) assay: POD activity was quantified based on the change in absorbance over time as described by Castillo et al. (1984). The absorbance of the reaction mixture consisting of 0.1 M sodium phosphate buffer, 12 mM H2O2, 96 mM guaiacol, and 50 μL of enzyme extract was measured at 470 nm using a spectrophotometer at 30-second intervals for a duration of 3 minutes. The extinction coefficient for guaiacol was determined to be 26.6 mM−1 cm−1.
Ascorbate peroxidase (APX) (EC 1.11. 1.11): The assay for APX, based on the methodology established by Nakano and Asada (1981), involved a 3 mL reaction mixture consisting of 0.1 M sodium phosphate buffer, 3 mM ascorbic acid, 3 mM ethylene diamine tetra acetic acid (EDTA), 100 μL of enzyme extract and 3 mM H2O2 added at the end to initiate the reaction. The absorbance of the reaction mixture was measured at 290 nm using a spectrophotometer at 30-second intervals over a period of 3 minutes. The recorded data demonstrated a decrease in absorbance over time, indicating the enzymatic activity of ascorbate peroxidase.
Catalase (CAT) (EC 1.11. 1.6): The CAT assay was performed by following the methodology described by Teranishi et al. (1974). The reaction was initiated by adding 75 mM H2O2 to 0.1 M sodium phosphate buffer and 50 μL of enzyme extract. The absorbance of the reaction mixture was measured at 240 nm using a spectrophotometer at 30-second intervals for 3 minutes. The decrease in absorbance over time reflects the enzymatic activity of catalase.
Superoxide dismutase (SOD) (EC 1.15.1.1): The SOD assay was performed based on the methodology described by Dhindsa et al. (1981). A 3 mL reaction mixture comprising 0.1 M sodium phosphate buffer, 0.2 M methionine, 2.25 mM nitroblue tetrazolium (NBT), 3 mM ethylene diamine tetra acetic acid (EDTA), 1.5 M sodium carbonate, 2 mM riboflavin, and 100 μL of enzyme extract was incubated in light for 20 minutes, followed by cessation of the reaction by keeping it in the dark. The absorbance of the reaction mixture was then measured at 560 nm using a spectrophotometer.
Polyphenol oxidase (PPO) (EC 1.10.3.1): PPO activity was assessed using the method outlined by Archana et al. (2011). The reaction mixture consisted of 0.1 M sodium phosphate buffer, 50 μL enzyme extract, and 50 mM catechol. The enzymatic activity was quantified by measuring the change in absorbance at 495 nm per minute.
2.3.3 Quantification of secondary metabolites
One gram of crushed leaf sample was mixed with 2 mL of 100% ice-cold methanol. The mixture was then incubated in darkness for 48 hours at room temperature and then centrifuged at 906 g for 10 minutes. The resulting supernatant was collected and used for further assays of phenols, flavonoids, and tannins.
Phenol: The phenolic assay was based on the methodology described by Van der Rest (2006). The phenol assay involved mixing 2% sodium carbonate and 0.1 mL of ethanol extract. After 5 minutes, the addition of FC reagent caused a color change to dark green, indicating the phenol concentration. Absorbance at 760 nm was measured and compared to a gallic acid standard, expressed as mg g−1 FW of leaf.
Flavonoid: Flavonoid assay was based on the methodology described by Hapsari et al. (2022). The flavonoid assay involved combining 10% aluminum chloride, 1 M sodium carbonate, 5% sodium nitrite, and 0.2 mL of ethanol extract. The yellow-colored mixture was analyzed for flavonoid content by measuring absorbance at 510 nm. Absorbance was compared to a rutin standard and expressed as mg g−1 FW of leaf.
Tannin: The tannin assay was based on the methodology described by Sadasivam and Manickam (1992). The tannin assay involved combining 8% HCl, 4% vannalin and 0.5 mL of ethanol extract. After a 20-minute incubation, absorbance at 500 nm was measured and compared to a tannic acid standard to quantify tannin concentration and expressed as mg g−1 FW of leaf.
2.3.4 Quantification of plant photosynthetic pigments
Where ‘A' is absorbance (nm), ‘V' is the volume of the sample (ml) and ‘W' is the fresh weight of the sample (g).
2.4 Statistical analysis
Following the collection of experimental data, a three-way repeated-measures ANOVA was employed to discern statistically significant differences (p < 0.05) among genotypes, groups, and time intervals. To evaluate the impact of CLB infestation on oxidative damage (H2O2 and lipid peroxidation), antioxidant enzyme activities, secondary metabolites, and plant photosynthetic pigments among the 16 diverse wheat genotypes, multiple pairwise comparisons were conducted using the Student–Newman–Keuls (SNK) test.
The analysis was executed utilizing R software version 4.3.1. Additionally, principal component analysis (PCA) was performed to examine the levels of both constitutive and induced biochemical resistance across the diverse genotypes. This comprehensive statistical approach facilitated the determination of noteworthy effects stemming from CLB infestation. It also enabled the identification of patterns within the biochemical responses and resistance mechanisms exhibited by the varied wheat genotypes within the diversity panel.
3 RESULTS
3.1 Cereal leaf beetle infestation, ROS production and oxidative damage
The three-way repeated ANOVA on CLB infestation, H2O2 production, and lipid peroxidation in a set of 16 diverse wheat genotypes having different ploidy levels revealed statistically significant differences (p < 0.001) among infested or control groups (group), time after infestation, i.e., 3 DAI and 5 DAI (time), and among different wheat genotypes (genotypes) (Table 2). Significant interactions were also observed between “group and genotypes,” “time and genotypes,” and “group and time” in the infestation and oxidative damage analyses. However, no significant interactions were found between “time and genotypes" or among “group, time, and genotypes" while analyzing the data of H2O2 and lipid peroxidation assays.
| Source of Variation | df | MDA | H2O2 | CLB Infestation Score | POD | APX | CAT | SOD | PPO | Total chlorophyll | Phenol | Tannin | Flavonoids | Carotenoids |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Group | 1 | 15.35*** | 98.94*** | 34.1*** | 1.31** | 0.10*** | 11.94*** | 0.186*** | 0.69*** | 48.46*** | 21368*** | 0.01*** | 41758*** | 2.48*** |
| Time | 1 | 96.29*** | 232.75*** | 0.7*** | 1.06** | 0.02*** | 1.187*** | 0.235*** | 0.91*** | 9.74*** | 49*** | 0.007*** | 95*** | 0.43*** |
| Genotypes | 15 | 3.08*** | 9.58*** | 0.32*** | 0.35** | 0.11*** | 0.198*** | 0.393*** | 0.12** | 40.36*** | 2500*** | 0.0001 | 5635*** | 0.86*** |
| Group: Time | 1 | 0.007*** | 4.94*** | 1.29*** | 0.13 | 0.005*** | 0.17*** | 0.032*** | 0.06 | 12.84*** | 14*** | 0.001*** | 45*** | 0.14*** |
| Group: Genotypes | 15 | 0.81*** | 1.81*** | 0.53*** | 0.10 | 0.006*** | 0.038*** | 0.142*** | 0.02 | 3.66*** | 594*** | 0.00 | 1585*** | 0.12*** |
| Time: Genotypes | 15 | 0.0004 | 0.0005 | 0.08*** | 0.04 | 0.0007*** | 0.0003 | 0.005*** | 0.004 | 0.03*** | 0.005 | 0.00001 | 7*** | 0.03*** |
| Group: Time:Genotypes | 15 | 0.00005 | 0.00003 | 0.13*** | 0.01 | 0.0001*** | 0.005 | 0.009*** | 0.001 | 0.05*** | 0.0007 | 0.000.6 | 11*** | 0.05*** |
| Residuals | 64 | 0.0001 | 0.04 | 0.0002 | 0.11 | 0.0001 | 0.008 | 0.0003 | 0.049 | 0.000.7 | 0.0006 | 0.009 | 0.0008 | 0.0003 |
- Significance levels:
- * (p < 0.05),
- ** (p < 0.01), and.
- *** (p < 0.001).
The extent of CLB infestation across the diverse genotypes at different time intervals is illustrated in Figure 1a. No significant difference was found among the genotypes in the control groups since no insect was released on control genotypes. However, significant differences were noticed among the genotypes after the infestation with CLB at 3DAI and 5DAI. For instance, at 3 DAI, the highest level of infestation was on the susceptible genotypes SW-2-S, RG-AH, SW2 M3-S, and DM3S. Conversely, the least infestation was observed on the resistant genotypes SAB-72, SAB-74, LR-1R, and AT-1R. This trend was similarly evident at 5 DAI, where the level of CLB infestation was persistently higher in susceptible genotypes compared to resistant genotypes (Figure 1a).
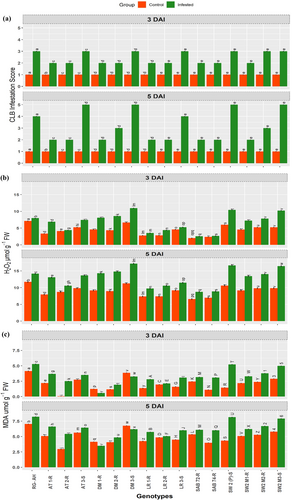
The escalation of ROS, specifically H2O2, triggered by CLB infestation across 16 genotypes at distinct time points is depicted in Figure 1b). Notably, at both 3 DAI and 5 DAI, H2O2 exhibited a statistically significant surge in all the infested versus the corresponding controls (Figure 1b). At 5DAI, among the genotypes, the highest levels of H2O2 were measured in DM-3S (17.17 μg g−1 FW), closely followed by SW-2 (16.65 μg g−1 FW), SW2 M3S (16.44 μg g−1 FW), DM-1R (14.81 μg g−1 FW) and DM-2R (14.31 μg g−1 FW) and then with RG-AH (14.19 μg g−1 FW) and AT3S (13.65 μg g−1 FW) (Figure 1b). In contrast, the lowest levels of H2O2 were recorded in the resistant genotypes SAB 74-R (8.88 μg g−1 FW), SAB 72-R (8.75 μg g−1 FW), LR-1R (9.74 μg g−1 FW), and AT-2R (10.03 μg g−1 FW).
When checking the impact of CLB-induced oxidative damage, we used MDA as a proxy to assess lipid peroxidation across the diverse wheat genotypes (Figure 1c). At both 3DAI and 5DAI, elevated levels of lipid peroxidation were evident in CLB-infested genotypes compared to their respective controls. Interestingly, in the susceptible genotype “DM-3S", the level of MDA was observed more in control genotypes than in the infested genotypes due to CLB infestation and it is maybe genotype-specific (Figure 1c). At 3DAI, enhanced MDA was observed only in the susceptible genotypes. Notably, SW-2, SW2 M3-S, RG-AG, AT 1R, and AT 3S exhibited MDA values ranging from 5.208 to 3.687 μmol g−1 FW, distinguishing them from other infested samples. In contrast, the lowest lipid peroxidation level was recorded in the genotypes DM-1R, DM-2R followed by LR-2R and AT-2R, LR-1R. On the other hand, moderate levels of lipid peroxidation were observed in genotypes SAB-72 and SAB-74. Similar trend of MDA activity was observed at 5 DAI but with higher MDA level than at 3 DAI (Figure 1c; Table S1-S3).
3.2 Quantification of antioxidants induced due to cereal leaf beetle infestation
The three-way repeated-measures ANOVAs analyses on CLB-induced antioxidative enzymes, including POD, CAT, APX, PPO and SOD activities across diverse wheat genotypes, revealed significant effects for group (infested or control), time (time after infestation), and genotypes (different wheat genotypes) (p < 0.001) (Table 2). Notably, significant interactions were observed between group and time, group and genotypes, and infestation and genotype in the case of APX, CAT, and SOD antioxidant enzyme activity. However, no statistically significant interactions were found for POD and PPO responses to CLB infestation. Additionally, non-significant interactions were observed between group, time, and genotypes for all antioxidant enzymes, except for SOD and APX.
While analyzing the data for peroxidase enzyme activity in two groups of genotypes (infested vs control), we noticed that POD activity shows a more substantial increase under CLB infestation than the control (Figure 2a). However, the opposite was true for genotypes “SW2 M2-R, SW2 M3-S (3DAI) and genotype “SW2 M3-S" (5DAI).
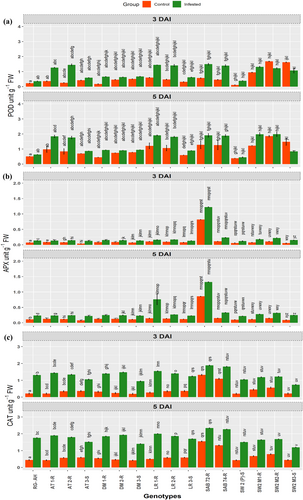
At 3 DAI, the most significant increase in POD activity was observed in infested genotype SAB 72-R (1.496 unit g−1 FW) followed by infested genotypes AT-2R (1.443 unit g−1 FW), LR-1R (1.443 unit g−1 FW) and LR-2R (1.406 unit g−1 FW). Conversely, lower POD activity was recorded in other susceptible genotypes (Figure 2a). Similarly, at 5 DAI, the infested group demonstrated the highest POD content compared to 3 DAI time point. The highest POD activity was observed for genotypes SW-2 M2-R (1.99 unit g−1 FW) and SW-2 M1-R (1.98 unit g−1 FW), followed by genotypes LR-1R (1.91 unit g−1 FW), SAB 72-R (1.91 unit g−1 FW), and SAB 74-R (1.88 unit g−1 FW). The mutant genotypes “SW2-M2R” at 3 DAI and “SW2-M3S, at both 3 DAI and 5 DAI, showed higher POD activity under controlled conditions than under CLB-infested conditions. This intriguing variation could be attributed to the downregulation of defense responses in these mutants in response to CLB infestation.
Catalase enzyme activity increased significantly in all diverse genotypes upon CLB infestation compared to the non-infested controls (Figure 2c). The increase in catalase activity was more pronounced in infested genotypes at 5 DAI than in infested genotypes at 3 DAI. While comparing the catalase levels between different genotypes, markedly elevated catalase content was noticed in advanced breeding lines SAB 72-R and SAB 74-R, followed by the landraces LR-1R, wild wheat AT-1R, and durum wheat DM-1R and DM-2R. Resistant mutants, such as SW2 M1-R, SW2 M2-R, and RG-AH, exhibited a moderate level of catalase activity in response to CLB infestation relative to other genotypes (Figure 2c). Conversely, SW-2, DM-3S, SW2 M3-S, and AT-3S displayed lower catalase activity levels in response to CLB infestation (Figure 2c).
APX activity responded prominently to CLB infestation across diverse genotypes at 3 DAI and 5 DAI compared to the non-infested controls (Figure 2b). At 3DAI, APX slightly increased in all the genotypes with respect to control, except in DM-1R, SAB 74-R, which had non-significant APX activity. Furthermore, the highest APX activity was observed in SAB 72-R (1.22 unit g−1 FW), followed by LR-1R (0.757 unit g−1 FW). In addition, SAB 74-R, SW2 M2-R, and SW-2 M1-R displayed moderate APX activity levels, i.e., 0.232, 0.221, and 0.180 unit g−1 FW, respectively. The remaining genotypes showed a minimal response in APX activity upon CLB infestation (Figure 2b). Conversely, at 5 DAI, the susceptible genotypes of wild wheat AT-3S and durum wheat DM-3S as well as resistant genotypes including AR-2R, DM-1R exhibited non-significant APX activity compared to their controls. Furthermore, comparable trends in APX activity were observed among the other genotypes. The highest activity levels were recorded in SAB 72-R (1.324 unit g−1 FW), followed by SAB 74-R, SW2 M2-R, and SW2 M1-R (0.331, 0.320, and 0.279 unit g−1 FW, respectively). LR-1R and AT-2R displayed moderate APX activity levels, i.e., 0.757 and 0.242 unit g−1 FW, respectively. Conversely, other susceptible genotypes, including RG-AH, exhibited relatively lower ascorbate peroxidase activity. Moreover, it is interesting to highlight that SAB-72 exhibited the highest APX activity among the genotypes, which could potentially be attributed to its genetic background (Figure 2b).
The impact of CLB infestation on PPO activity exhibited statistically significant differences across all genotypes over control at both 3 DAI and 5 DAI (Figure 3a). At 3 DAI, among the genotypes, the highest PPO activity was observed in SAB-72, SAB-74 followed by LR -1R, DM-2R and AT-1R (Figure 3a). In contrast, the lowest PPO activity was recorded in susceptible genotypes, including SW2 M3-S, SW-2, AT-3S and DM-3S. A similar trend of PPO activity was observed at 5 DAI in response to CLB infestation.

Significant variation in SOD activity in response to CLB infestation was observed among all the 16 genotypes. At 3 DAI, the highest SOD activity was observed in genotypes including AT-2R, SAB-72, AT-1R, LR-2R, DM-1R, followed by a moderate level of SOD activity observed in genotypes DM-2R, and LR-1R. Less significant SOD activity was observed in susceptible genotypes, including AT-3S, DM-3S and RG-AG (Figure 3b). Other genotypes exhibited non-significant changes in SOD activity compared to their non-infested controls (Figure 3b). Similar trend of SOD activity was recorded at 5 DAI, it is pertinent to mention that the SOD activity increased with increased infestation time from 3 DAI to 5 DAI (Figure 3b; Tables S1-S3).
3.3 Photosynthetic pigments and secondary metabolites in wheat genotypes infested with cereal leaf beetle
The three-way repeated measure ANOVAs on total chlorophyll content, carotenoid levels, phenolic response, tannin content, and flavonoid response in 16 diverse contrasting wheat genotypes subjected to CLB infestation revealed significant effects for Group, Time, and Genotypes (p < 2e-16; Table 2) in all analyses. Interactions between the factors were also significant, indicating the combined influence of infestation, time, and genotype on these biochemical responses. Furthermore, in tannin and phenol, the three-way interaction was statistically insignificant (Table 2).
Total chlorophyll and carotenoids: A significant reduction in total chlorophyll levels across all genotypes was observed upon CLB infestation compared to the non-infested controls (Figure 4a). At 3 DAI, the reduction in total chlorophyll content due to CLB infestation was more pronounced in susceptible genotypes, including LR-3S, DM-3S, AT-3S, SW-2 and their susceptible mutant SW2 M3-S, than in the resistant genotypes. However, it is interesting to note that in the case of durum wheat genotypes DM-2R and DM-3S, which includes a susceptible one, total chlorophyll content increased after CLB infestation at 3 DAI. Moreover, as infestation progressed to 5 DAI, total chlorophyll declined in the susceptible DM-3S, while the resistant DM-2R maintained a consistent level of chlorophyll (Figure 4a). Furthermore, by 5 DAI, a greater extent of chlorophyll damage was observed, where the susceptible genotypes continued to exhibit significant reductions in chlorophyll content, while the resistant genotypes displayed comparatively less damage in response to CLB infestation.
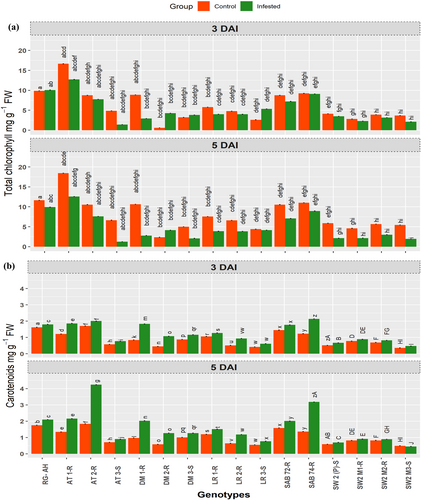
The response of diverse genotypes to CLB infestation revealed an increase in carotenoid content compared to their respective control genotypes, corresponding to the degree of infestation (Figure 4b). The genotypes exhibiting the highest levels of carotenoids were among the resistant genotypes, including SAB-74 and AT-1R. Moderate carotenoid levels were observed in genotypes such as DM-1R, LR-1R, and RG-AH. Conversely, the lowest levels of carotenoids were recorded in LR-3S, SW-2, and the susceptible mutant SW2 M3-S. A similar trend was observed at 5 DAI, with carotenoid concentrations progressively increasing with CLB infestation (Figure 4b).
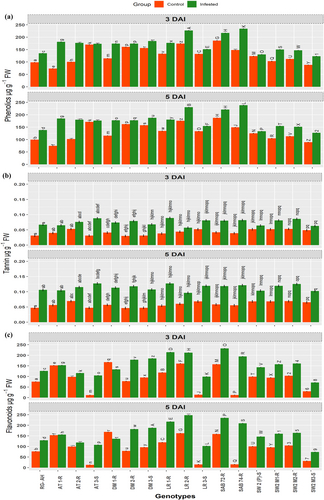
Secondary metabolites: The levels of secondary metabolites, such as phenols, tannins, and flavonoids increased across all genotypes in response to the degree of CLB infestation compared to their non-infested controls. Significant increases in these secondary metabolites were observed in SAB-72, SAB-74, LR-1R, LR-2R, AT-1R, and AT-2R, followed by SW2-M1-R and SW2-M2-R upon CLB infestation (Figure 5 ac). Conversely, a less significant increase was noted in RG-AH, SW-2, and SW2 M3-S (Figure 5a-c; Tables S1-S3).
3.4 Comparative analysis of biochemical defense mechanisms in diverse wheat genotypes against CLB infestation
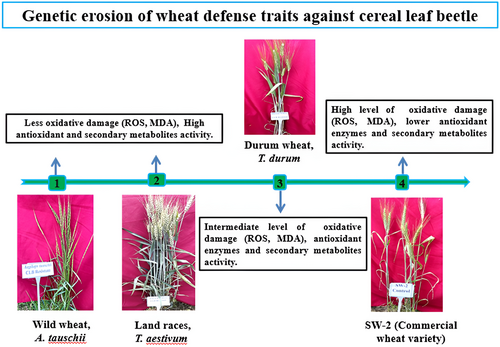
The comprehensive evaluation of oxidative damage, antioxidant enzyme activity, and PSMs across different ploidy levels of wheat, including diploid wild wheat (A. tauschii), tetraploid durum wheat, hexaploid landraces, mutants, alternate host ryegrass, and the commercial variety Shalimar Wheat-02 (SW-2), highlighted significant variations in their response to CLB infestation.
Among the different genotypes, diploid A. tauschii and hexaploid wheat landraces exhibited the least susceptibility to oxidative damage caused by CLB infestation, along with the highest levels of antioxidant enzyme activity and PSM production. Notably, SOD activity was markedly higher in diploid wild wheat than in other genotypes. On the other hand, tetraploid durum wheat displayed a moderate level of resistance to oxidative damage, antioxidant enzyme activity and PSM production. Interestingly, the mutant SW-2 displayed moderate resistance to oxidative damage and elevated peroxidase activity, comparable to the land races. However, the commercial wheat variety Shalimar Wheat-02 (SW-2) and ryegrass genotypes “RG-AH” showed heightened susceptibility to oxidative damage caused by CLB infestation, with lower levels of antioxidant enzyme activity and PSM production compared to other genotypes. Remarkably, PPO levels were higher in ryegrass, followed by landraces and A. tauschii.
When comparing resistant and susceptible genotypes within the diverse wheat genotypes, the resistant genotypes, such as diploid A. tauschii, hexaploid wheat landraces, and SW-2 mutants, demonstrated the least susceptibility to oxidative damage caused by CLB infestation. This resistance was found to be on par with that of the SAB-74 advanced breeding line containing various resistance genes. Conversely, the susceptible genotypes exhibited greater oxidative damage due to CLB infestation, including susceptible genotypes of diploid A. tauschii, tetraploid durum wheat, landraces, and mutants. Notably, the SW-2 commercial variety exhibited a particularly high susceptibility to oxidative damage caused by CLB infestation.
Furthermore, the resistant genotypes, including diploid A. tauschii, hexaploid wheat landraces, and SW-2 mutants, displayed elevated levels of antioxidant enzyme activity and PSM production. Durum genotypes exhibited intermediate levels of these responses, while the commercial variety “SW-2” exhibited the lowest levels (Figure 6; Tables S1-S3).
3.5 Principal component analysis
Principal component analysis (PCA) was conducted to explore the relationship between ROS, antioxidant enzymes, photosynthetic pigments, and PSM in wheat genotypes with different ploidy levels under both constitutive and cereal leaf beetle-infested conditions. Out of the 15 eigenvalues representing principal components (PCs), the first four PCs with eigenvalues greater than 1 were selected under CLB infestation conditions. These four PCs accounted for 79.45% of the total variance. PC1 mainly explained the variance in CAT, H2O2, and CLB infestation scale, while PC2 was associated with phenolics, POD, PPO, flavonoids, and carotenoids. Similarly, in the control condition, the first five PCs with eigenvalues >1 explained 83.28% of the cumulative variance. PC1 was linked to photosynthetic pigments, while PC2 was related to phenolics, CAT, APX, SOD, and H2O2 (Tables S4 and S5).
The PCA biplot (Figure 7a) demonstrated varying responses to CLB infestation in different genotypes. Susceptible genotypes formed one cluster with higher CLB infestation, H2O2, and MDA levels. In contrast, resistant genotypes such as SAB-72, SAB-74, and wild wheat displayed elevated antioxidant enzyme activity and secondary metabolites formed another separate cluster. Additionally, the PCA biplot (Figure 7b) for the control condition showed lower oxidative damage, higher antioxidant enzyme activity, and varied photosynthetic pigments and secondary metabolites. Notably, the proportion of these components was significantly lower in the wheat diversity panel compared to when induced by cereal leaf beetle infestation.
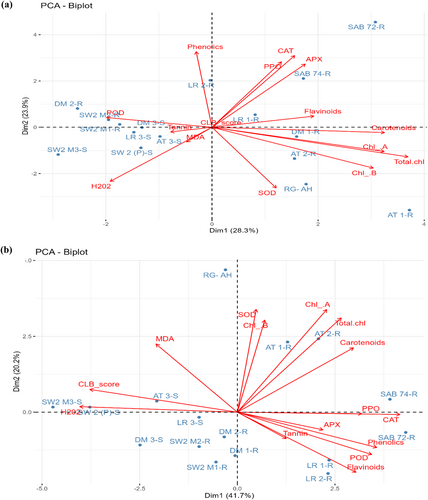
From the PCA results, potential CLB-tolerant lines were identified based on criteria such as low CLB infestation scores, decreased MDA values, lower H2O2 content and higher activities of essential antioxidant enzymes. Furthermore, a preference was given to genotypes with higher concentrations of secondary metabolites, including phenolics, flavonoids, and tannins.
4 DISCUSSION
4.1 Defensive traits against cereal leaf beetle infestation in wheat
Wheat is a cornerstone of global food security. It experiences an array of biotic and abiotic stresses during its cultivation. Particularly in the Western Himalayas of India, the menace of CLB infestation looms large. However, a troubling trend of genetic erosion in improved wheat varieties threatens their natural defenses. This is particularly notable in modern cultivars that have undergone rigorous breeding for yield and agronomic traits, often at the expense of essential defense mechanisms. As a consequence, these crops become vulnerable to a spectrum of challenges, including pests, diseases, and environmental fluctuations (Rosenthal and Dirzo 1997).
In response to these concerns, the present study comprehensively analyzed induced or upregulated biochemical resistance to CLB infestation across different wheat genotypes with different ploidy levels. The aim is to identify resources and mechanisms that confer insect resistance, a critical target of wheat improvement programs. The diverse set of wheat genotypes used in this study includes wheat D-sub-genome progenitor A. tauschii genotypes, AT 1R, AT 2R, and AT 3S, tetraploid durum wheat genotypes, DM 1R, DM 2R, and DM 3S, and hexaploid wheat landraces LR 1R, LR 2R, and LR 3S. Within each group, there were both resistant and susceptible genotypes. Notably, specific genotypes exhibited considerably lower levels of CLB infestation. Among them are hexaploid advanced breeding lines SAB-72 and SAB-74, landraces LR-R and LR-2R, and diploid wheat progenitors AT-1R and AT-2R.
These resistant genotypes also manifest reduced oxidative damage, as evident from lower H2O2 production and lipid peroxidation, compared to local wheat varieties such as SW2 and its derived mutantsSW2-M3-S. In contrast, resistant mutants in the SW2-M1-R and SW2-M2-R background display an intermediate response to CLB infestation. This divergence in susceptibility suggests that resistance in landraces and wild wheat is rooted in their distinctive genetic backgrounds. The unusual behavior/trends observed in lipid peroxidation in some durum wheat genotypes may be due to different genetic backgrounds and, hence, different responses toward insect infestation. This study corroborates previous research by Lukasik and Golawska (2019), which showcased the utility of H2O2 and MDA as biochemical markers for assessing oxidative stress in triticale seedlings challenged with cereal aphids.
Similarly, Subramanian et al. (2019) highlighted utilizing multiple molecular defense strategies, including the generation of ROS and H2O2 in susceptible Brachypodium distachyon genotypes to Hessian fly larvae infestation. These insights provide valuable tools for understanding the physiological responses of susceptible wheat varieties to pest attacks. In conclusion, this study underscores the imperative of retaining and harnessing defensive traits from wild genotypes and landraces to develop modern wheat varieties against the growing threats posed by insect pests and environmental uncertainties.
4.2 Unveiling antioxidant enzyme responses to cereal leaf beetle infestation in diverse wheat genotypes
It is well documented that a cohort of antioxidant enzymes, including SOD, CAT, POD, and APX, collaboratively combat and neutralize ROS generated during insect feeding (Caverzan et al., 2016). Specifically, SOD transforms superoxide radicals into H2O2, which CAT and APX subsequently disassemble into water and oxygen. Meanwhile, POD emerges as a guardian enzyme against oxidative stress, neutralizing H2O2 and other peroxides. The enhanced activity of these enzymes in resistant wheat genotypes seems pivotal in curbing oxidative damage triggered by cereal leaf beetle infestation. By efficiently scavenging ROS, these enzymes maintain cellular redox equilibrium and minimize harm.
In the present study, we delved into the differential response of antioxidant enzymes, SOD, APX, CAT, and PPO across a panel of 16 diverse wheat genotypes upon CLB infestation. Notably, genotypes bearing resistance genes, such as SAB-72 and SAB-74, landraces (LR-1R and LR-2R), and wild wheat (AT-1R and AT-2R), exhibited enhanced antioxidant activity. Conversely, the modern wheat variety SW-2 and derived susceptible mutants displayed lower antioxidant activity. It is worth mentioning that durum wheat, resulting from the hybridization of two wild wheat progenitors, exhibited intermediate levels of antioxidative enzymes. It is pertinent to mention that in susceptible mutant SW2-3 M-S, the CLB infestation leads to lower POD activity compared to control. This is attributed to the downregulation of defense responses in this mutant during CLB infestation. In particular, the resistant mutant displayed elevated PPO activity, suggesting that resistance to CLB infestation could be linked to specific genes in landraces and wild wheat genotypes.
This underlines a potential loss of insect antioxidant-responsive genes from wild wheat and landraces in improved wheat varieties such as SW-2, which was selected as an early maturing wheat genotype for Western Himalayan region of Kashmir valley. Our findings align with previous research, as several studies have demonstrated differential expression and activity of antioxidant enzymes as defense mechanisms against herbivorous insects in various plant species, including wheat (Taggar et al., 2012; War et al., 2013; Kaur et al., 2017; Pant and Huang, 2021; Malik et al., 2023). Incorporating genetic resources from wild relatives, such as Aegilops ssp., into wheat breeding programs could bolster resistance against economically significant insect pests (Migui and Lamb, 2003; Smith et al., 2004).
Furthermore, Pour-Aboughadareh et al. (2020) observed enhanced activities of antioxidant enzymes in wild wheat relatives exposed to water deficit conditions, indicating their potential role in stress resilience. This study also unveiled altered expression levels of key antioxidant enzyme-encoding genes under water deficit stress. Such insights underline the importance of exploring diverse wheat genotypes and their innate defense mechanisms to ensure sustainable crop health in the face of biotic stresses.
4.3 Variation in secondary metabolite and photosynthetic pigment responses to cereal leaf beetle infestation in diverse wheat genotypes
Our analysis of secondary metabolites and photosynthetic pigments across 16 diverse wheat genotypes in response to CLB infestation uncovered substantial variation. Secondary metabolites, including phenols, tannins, and flavonoids, which are crucial for plant defense against pests, exhibited diverse levels among the genotypes. Phenols reached their peak in SAB-72 and resistant landraces, indicating their potential role in conferring resistance against CLB. Tannins, abundant in landraces and wild wheat genotypes, have antifeedant properties, enhancing resistance. Meanwhile, flavonoids, known for antioxidant and antimicrobial properties, predominated in resistant genotypes, particularly SAB-72 and resistant durum wheat. Conversely, susceptible genotypes showed diminished secondary metabolite levels, suggesting vulnerability to CLB due to compromised defensive compound production.
Furthermore, we quantified photosynthetic pigments and carotenoids across the genotypes in response to CLB infestation. As CLB infestation escalated, susceptible genotypes exhibited reduced photosynthetic pigments, including total chlorophyll content. This decline could be attributed to CLB-induced damage impairing photosynthesis and chlorophyll degradation. However, the reverse trend in two durum wheat genotypes was unusual and can be attributed to localized darkening at the infestation site. The same has been also observed in an earlier study involving the infestation of wheat crop with Hessian fly, which results in dark green coloration and, hence, increased chlorophyll content in susceptible wheat genotypes (Cartwright and LaHue, 1944). In contrast, carotenoids, which are vital for photoprotection and antioxidant defense, surged in wild wheat A. tauschii (AT 1–30 and AT 2–31), SAB-74, resistant landraces (LR-1R and LR 2R), durum wheat, and ryegrass compared to susceptible mutants and improved varieties such as SW2. This suggests that these genotypes possess different mechanisms/genes/QTLs to enhance carotenoid production in response to CLB infestation.
Further exploration of the pathways governing the production and regulation of these compounds could yield insights for breeding programs aiming to improve CLB resistance in wheat. Our findings align with Rao et al. (2017), emphasizing the pivotal role of plant secondary metabolites in defense mechanisms against herbivores. Wheat, a critical global cereal crop, has evolved various chemical compounds, such as phenols, flavonoids, and tannins, to deter herbivory and safeguard against potential harm (Bennett and Wallsgrove, 1994). Phenols, with antioxidant properties, are synthesized in response to herbivore attacks, reinforcing cell walls and deterring feeding. Flavonoids, which are diverse in function, disrupt herbivore digestion, interfere with hormones, and act as antifeedants. Tannins, polyphenolic compounds, inhibit protein digestion, influencing herbivore nutrient acquisition (Alamgir and Alamgir, 2018). The dynamic interplay of these compounds underscores wheat's sophisticated defense mechanisms against CLB and underscores the potential for genetic enhancement.
4.4 Implications of domestication and crop improvement on defense response against cereal leaf beetle in wheat
The study of genetic mechanisms that govern resistance to CLB infestation is a critical endeavor with far-reaching implications for sustainable agriculture. As proposed by Rosenthal and Dirzo (1997), changes in plant life history, domestication, and agronomic selection can impact plant defenses against insect pests. Their research on maize and its wild relatives demonstrated that the transition from perennial to annual life history, domestication, and agronomic selection caused plant defenses to diminish due to trade-offs with increased crop yield.
In line with this model, our study has brought to light a significant observation: a decline in insect herbivore resistance from wild relatives to cultivars. Our results coincided with the outcomes of the comprehensive meta-analysis performed by Fernandez et al. (2021), who investigated the intricate relationship between defense traits, nutritional composition, and herbivore susceptibility in fruit and seed crops. Their analysis revealed that cultivated crops are more susceptible to herbivory than their wild counterparts. Additionally, cultivated crops exhibited lower nutritional quality, which may contribute to increased herbivore consumption and subsequent biomass loss. These findings underscore the crucial need for a balanced approach in crop breeding that considers yield enhancement and insect pest resistance.
The value of landraces and wild varieties in breeding programs cannot be overstated. These varieties possess a unique advantage due to their long coexistence with pests in their native habitats. Over generations, these plant populations have undergone natural selection, developing defense mechanisms to withstand insect attacks. These mechanisms include physical barriers, secondary metabolite production, and the induction of defense-related genes (Tadesse et al., 2022; Yumurtaci, 2015; Bouhssini et al., 2009; Varella et al., 2017). Modern cultivars can be fortified against insect pests by harnessing these traits through breeding. A prime advantage lies in the genetic diversity exhibited by landraces and wild genotypes, unlike modern cultivars, which often have limited genetic variability due to extensive selection for specific traits (Friebe et al., 1996; Warburton et al., 2006). Breeding programs incorporating landraces and wild genotypes can tap into this reservoir of genetic diversity to develop new cultivars with improved insect resistance (Mondal et al., 2016).
Furthermore, landraces and wild varieties are well adapted to local environmental conditions, including insect pest pressures. Incorporating these adaptive traits into modern cultivars can improve overall fitness and resilience in crops within specific agroecosystems. This strategy can reduce dependence on chemical insecticides, promoting sustainable and environmentally friendly pest management practices. The importance of prioritizing landraces and wild genotypes in breeding programs cannot be overlooked. It promises to create resilient and sustainable crop systems that can withstand insect pressures, thus ensuring long-term food security. The findings of this study resonate with Rao et al. (2017), who highlighted the crucial role of plant secondary metabolites in defense mechanisms against herbivores. Wheat, a pivotal cereal crop globally, has evolved an array of chemical compounds, including phenols, flavonoids, and tannins, to deter herbivory and safeguard itself from potential damage (Bennett and Wallsgrove, 1994).
Phenols, characterized by their antioxidant properties, are synthesized in response to herbivore attacks and reinforce cell walls, rendering them more resistant to mechanical damage caused by feeding (Kumari et al., 2021; Kumar et al., 2020; Lattanzio et al., 2006). Flavonoids, with their diverse functions, including UV protection and pigmentation, can make plant tissues unpalatable or toxic to herbivores, thus acting as feeding deterrents (Jan et al., 2021). Tannins, found in many plant species, including wheat, inhibit protein digestion in herbivores by forming complexes with proteins in their gut, thereby impeding nutrient acquisition (Alamgir and Alamgir, 2018).
In summary, investigating the genetic mechanisms underlying resistance to CLB infestation opens new avenues for sustainable crop protection. This underscores the importance of preserving resistance genes while enhancing yield and quality in crop breeding. The integration of landraces and wild varieties, rich in natural defenses, holds promise for developing robust cultivars that can thrive in the face of evolving pest pressures, thus ensuring global food security.
5 CONCLUSIONS AND PERSPECTIVE
A set of 16 diverse wheat genotypes having different ploidy levels exhibited varying levels of oxidative damage and antioxidant enzyme and secondary metabolite activity in response to CLB infestation. The genotypes with higher antioxidant activity, particularly landraces and wild wheat genotypes, demonstrated resistance against CLB infestation. Among the diverse wheat genotypes, which include landraces, wild relatives, and commercial varieties, it was observed that improved varieties often display poor retention of insect pest resistance genes and, hence, less resistance towards insect pests. This phenomenon has significant implications for the long-term sustainability and resilience of wheat crops. Overall, the study underscores the importance of preserving and utilizing genetic resources from landraces and wild wheat genotypes to enhance resistance against CLB. Future studies should focus on unraveling the genetic aspects of resistance to further enhance our understanding of CLB resistance in wheat. The genotypes identified and the mechanism discovered for their resistance against CLB could prove useful in their use as genetic resources for wheat breeding programs aimed at enhancing CLB resistance in wheat.
AUTHOR CONTRIBUTIONS
Manikannan Parthiban: Conducted experiments, wrote manuscript. Farkhandah Jan: Conducted experiments. Mohammad Ayuob Mantoo: Conceived idea, wrote manuscript.Satinder Kaur: Wrote manuscript, corrected manuscript. Sachin Rustgi:Wrote manuscript, corrected manuscript. Fehim Jeelani Wani: Conducted statistical analysis. Rajeev Kumar Varshney: Wrote manuscript, corrected manuscript. Reyazul Rouf Mir: Conceived idea, mentored work, guidance, wrote manuscript, and corrected manuscript.
ACKNOWLEDGMENTS
Dr. R.R. Mir is highly thankful to the Department of Biotechnology, Govt. of India for providing grants (BT/Ag/Network/Wheat/2019-20), and Sachin Rustgi is thankful to the USDA, National Institute of Food and Agriculture (GRANT13715753) for the grants.
Open Research
DATA AVAILABILITY STATEMENT
The data that support the findings of this study are available from the corresponding author upon reasonable request.




